Stress Corrosion Cracking Probability of Selective Laser Melted 316L Austenitic Stainless Steel under the Effect of Grinding Induced Residual Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Residual Stress Measurement
2.3. Corrosion and SCC Susceptibility Tests
2.4. Microstructural and Chemical Characterization
3. Results and Discussion
3.1. SLM Specimen Characterization
3.2. Residual Stress Analysis
3.3. Corrosion Behavior through Potentiodynamic Polarization Tests
3.4. Galvanostatic Behavior
3.5. Microstructural Analysis
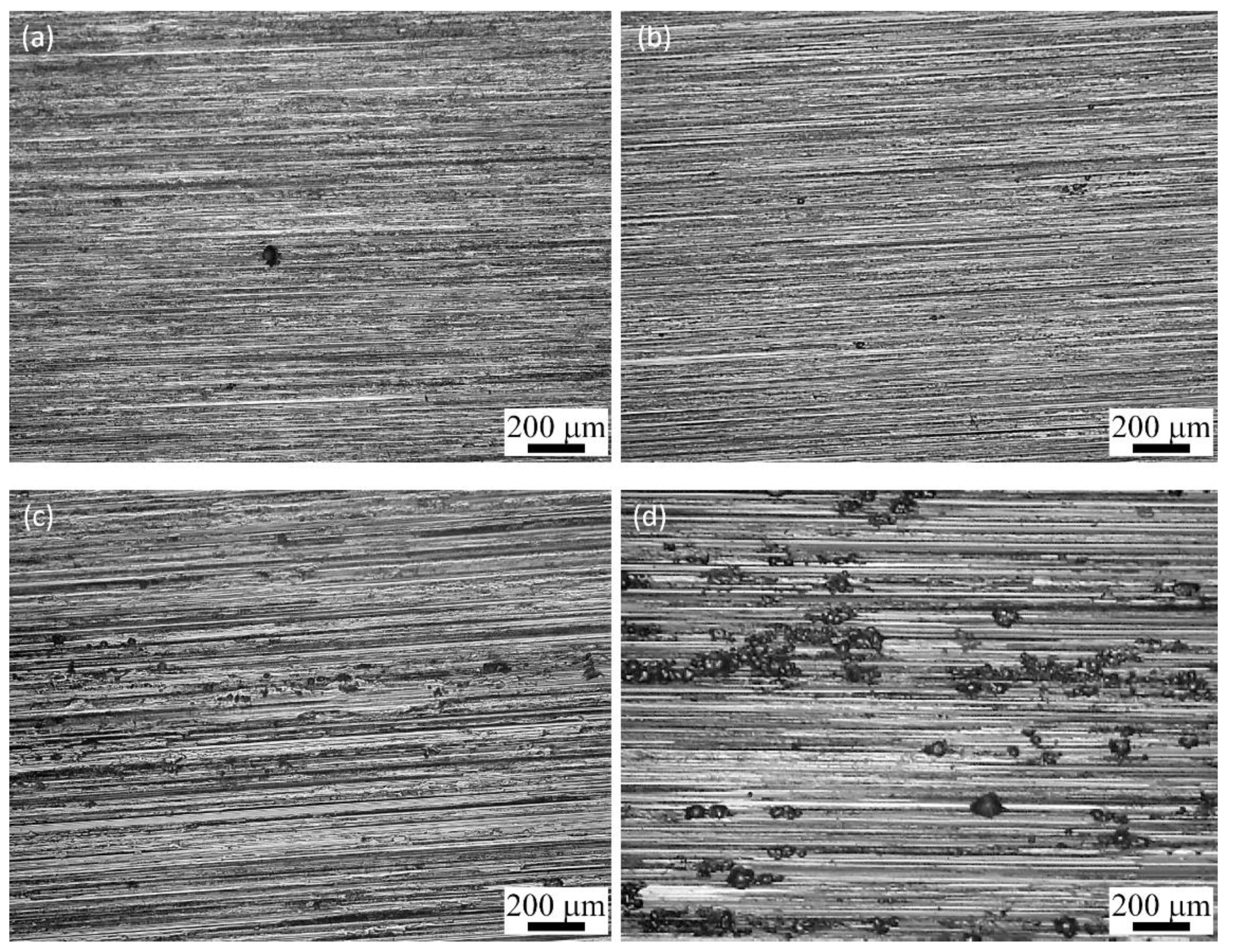
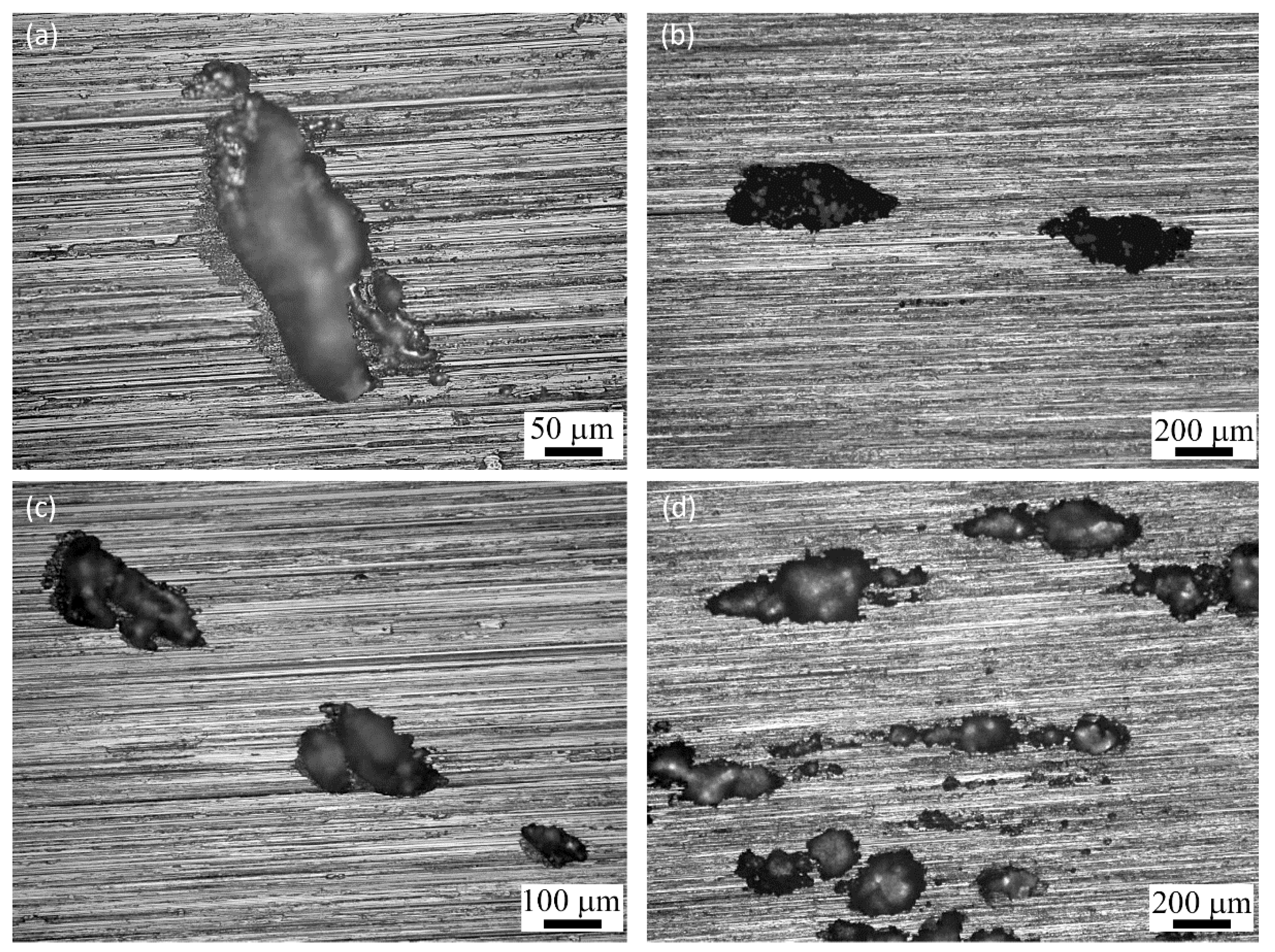
3.5.1. Machining Surface Analysis after Galvanostatic Tests
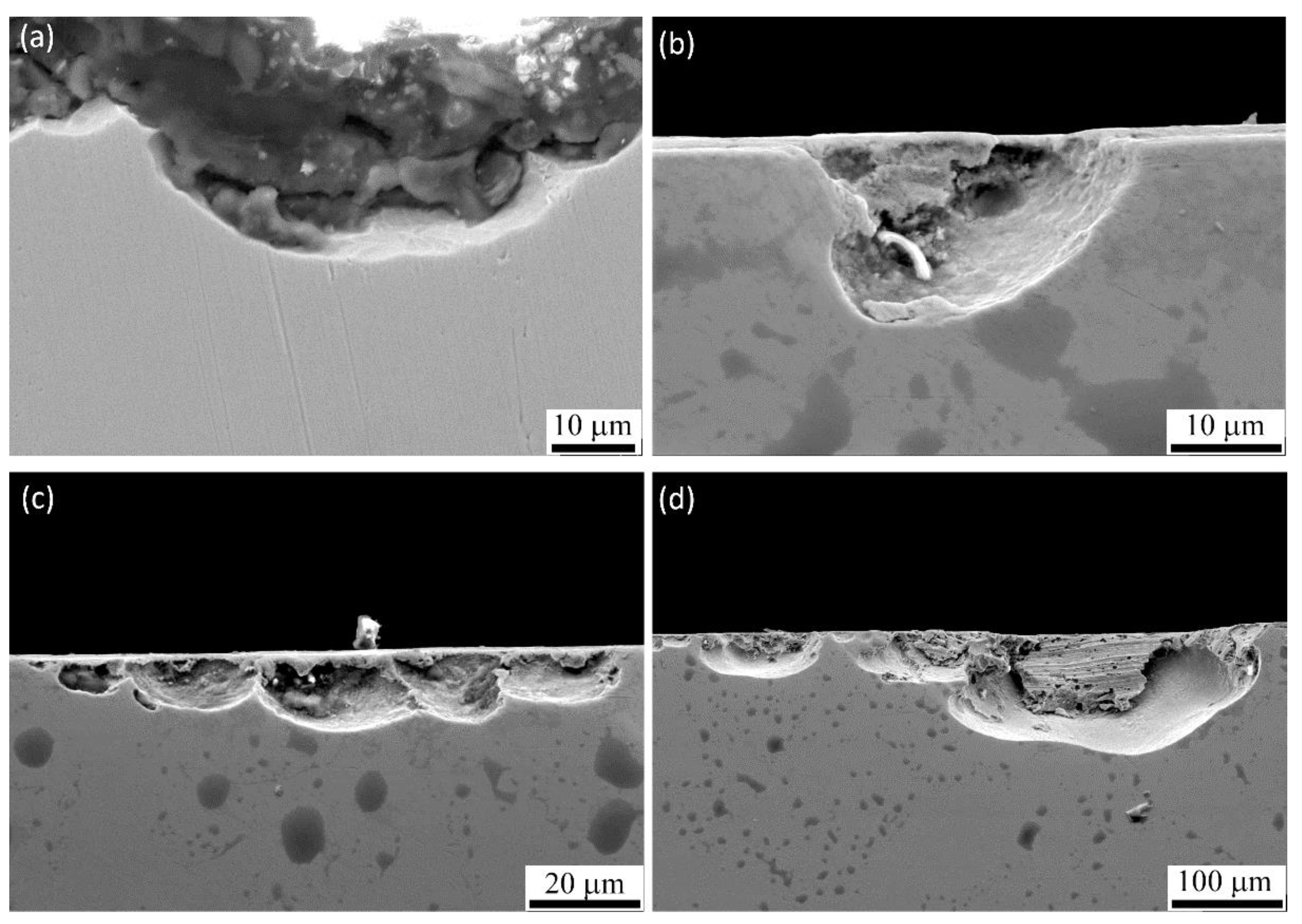
3.5.2. SCC Initiation
3.5.3. SCC Propagation
4. Conclusions
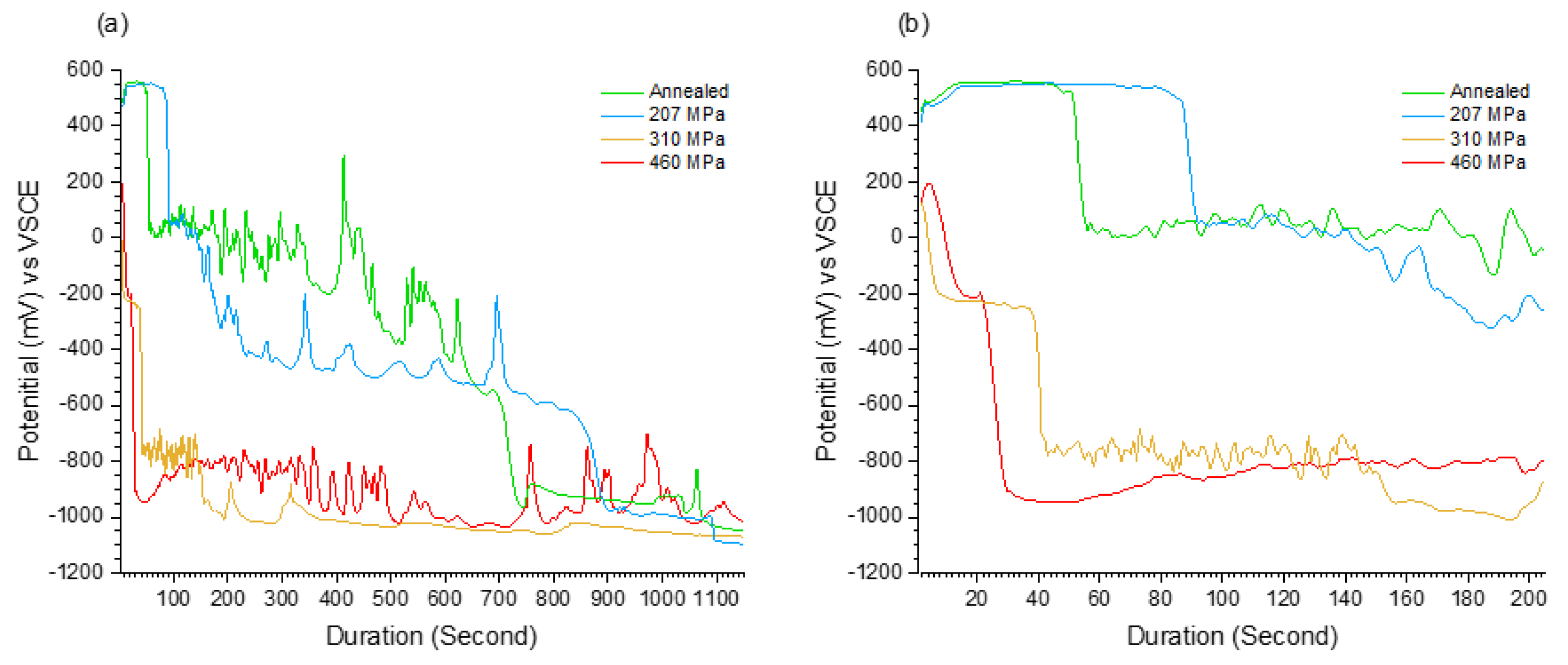
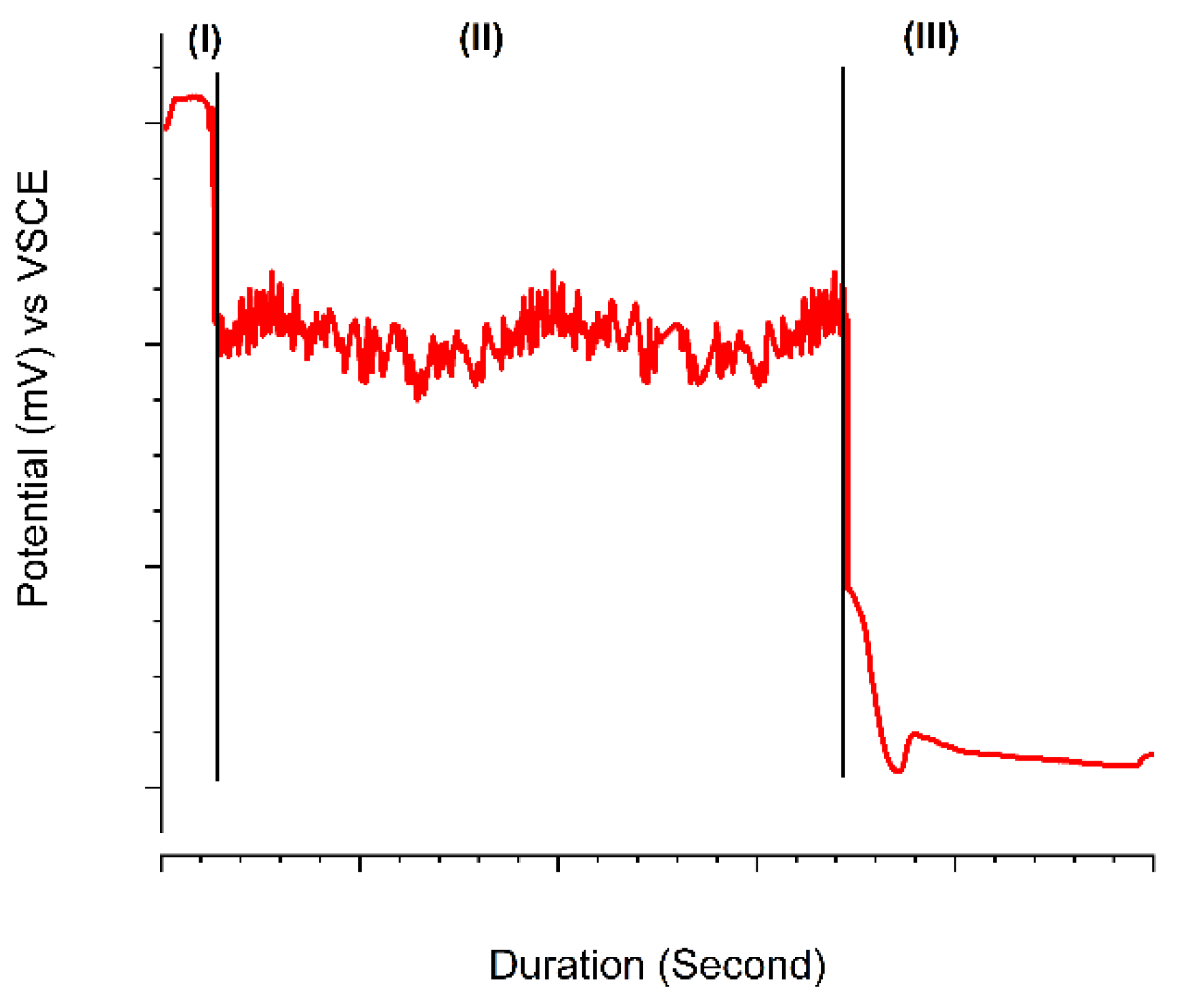
- Galvanostatic measurements showed three distinctive regions, namely incubation, metastable, and stable regions, followed by a sudden potential drop on each stage.
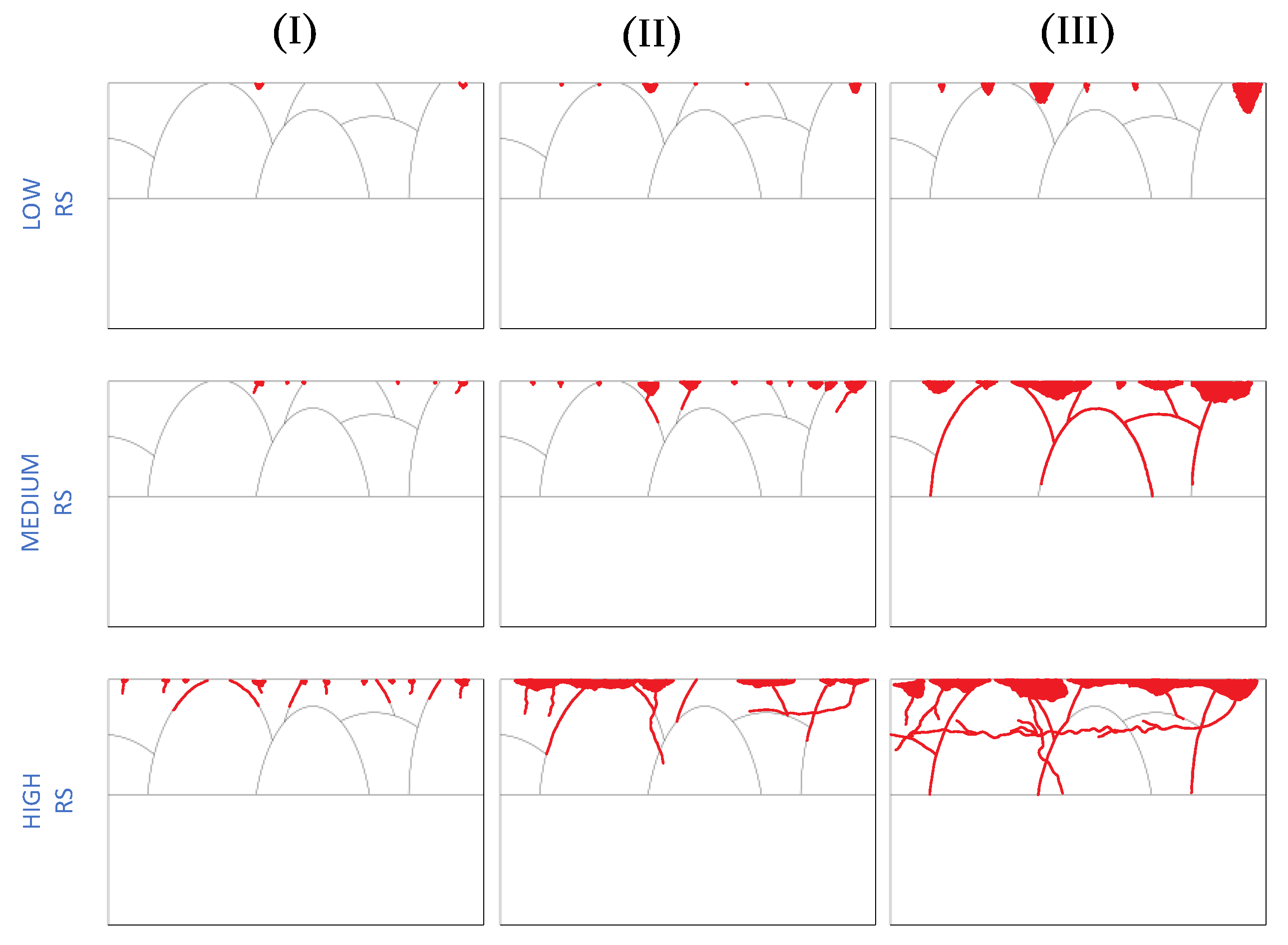
- There was a significant correlation between the measured RS magnitude and the time and potential of incubation and metastable regions. For higher RS magnitudes the potential drops occurred dramatically in a shorter period.
- For annealed specimens and specimens with a low magnitude of RS, the dominant corrosion defect observed was pitting without any sign of SCC.
- For specimens with higher RS magnitudes, cracks were initiated from porosities, machining marks, melt pool boundaries, and transgranular form within grains. The most remarkable correlation was the priority of initiation sites with the magnitude of tensile RS.
- Cracks were initiated from surface pores for medium magnitudes of RS and melt pool boundaries and machining marks for specimens with the highest measured RS magnitude.
- SCC propagation was along the melt pool boundaries that showed high susceptibility of melt pool boundaries to SCC propagation followed by transverse propagation in a longitudinal direction near to the surface and transgranular propagation.
- Transgranular propagation in build direction was observed for specimens with high RS magnitude that was mostly in the columnar microstructure.
- High solidification rate and inverse segregation of Molybdenum along the melt pool boundaries combined with the machining induced tensile RS lead to the high susceptibility of SLM microstructure and especially melt pool boundaries to SCC initiation and propagation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourell, D.L. Perspectives on Additive Manufacturing. Annu. Rev. Mater. Res. 2016, 46, 1–18. [Google Scholar] [CrossRef]
- Rosen, D.W. A review of synthesis methods for additive manufacturing. Virtual Phys. Prototyp. 2016, 11, 305–317. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Roberts, I.A.; Wang, C.J.; Esterlein, R.; Stanford, M.; Mynors, D.J. A three-dimensional finite element analysis of the temperature field during laser melting of metal powders in additive layer manufacturing. Int. J. Mach. Tools Manuf. 2009, 49, 916–923. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Parametric analysis of thermal behavior during selective laser melting additive manufacturing of aluminum alloy powder. Mater. Design 2014, 63, 856–867. [Google Scholar] [CrossRef]
- Sames, W.J.; Unocic, K.A.; Dehoff, R.R.; Lolla, T.; Babu, S.S. Thermal effects on microstructural heterogeneity of Inconel 718 materials fabricated by electron beam melting. J. Mater. Res. 2014, 29, 1920–1930. [Google Scholar] [CrossRef]
- Shen, N.; Chou, K. Numerical thermal analysis in electron beam additive manufacturing with preheating effects. In Proceedings of the 23rd Solid Freeform Fabrication Symposium, Austin, TX, USA, 6–8 August 2012; pp. 774–784. [Google Scholar]
- Jia, Q.; Gu, D. Selective laser melting additive manufacturing of Inconel 718 superalloy parts: Densification, microstructure and properties. J. Alloys Compd. 2014, 585, 713–721. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Yao, J.; Cheng, X.; Li, X. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, Y.; Wang, L.; Jiang, W. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int. J. Adv. Manuf. Technol. 2012, 59, 1025–1035. [Google Scholar] [CrossRef]
- Sanviemvongsak, T.; Monceau, D.; Macquaire, B. High temperature oxidation of IN 718 manufactured by laser beam melting and electron beam melting: Effect of surface topography. Corros. Sci. 2018, 141, 127–145. [Google Scholar] [CrossRef]
- Sieradzki, K.; Newman, R. Stress-corrosion cracking. J. Phys. Chem. Solids 1987, 48, 1101–1113. [Google Scholar] [CrossRef]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.; Singer, L.; Thomas, S.; Kelly, R.; Scully, J.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A Review. Corrozsion 2018, 74, 1318–1350. [Google Scholar] [CrossRef]
- Örnek, C. Additive manufacturing—A general corrosion perspective. Corros. Eng. Sci. Technol. 2018, 53, 531–535. [Google Scholar] [CrossRef]
- Turnbull, A. Corrosion pitting and environmentally assisted small crack growth. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014, 470, 20140254. [Google Scholar] [CrossRef]
- Dietzel, W.; Turnbull, A. Stress Corrosion Cracking; GKSS-Forschungszentrum Geesthacht GmbH: Geesthacht, Germany, 2007. [Google Scholar]
- Tian, Y.; Tomus, D.; Rometsch, P.; Wu, X. Influences of processing parameters on surface roughness of Hastelloy X produced by selective laser melting. Addit. Manuf. 2017, 13, 103–112. [Google Scholar] [CrossRef]
- Strano, G.; Hao, L.; Everson, R.M.; Evans, K.E. Surface roughness analysis, modelling and prediction in selective laser melting. J. Mater. Process. Technol. 2013, 213, 589–597. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Goodridge, R.D.; Hague, R.J.; Christie, S.D.; Edmondson, S. The effect of laser remelting on the surface chemistry of Ti6al4V components fabricated by selective laser melting. J. Mater. Process. Technol. 2016, 232, 1–8. [Google Scholar] [CrossRef]
- Damon, J.; Dietrich, S.; Vollert, F.; Gibmeier, J.; Schulze, V. Process dependent porosity and the influence of shot peening on porosity morphology regarding selective laser melted AlSi10Mg parts. Addit. Manuf. 2018, 20, 77–89. [Google Scholar] [CrossRef]
- Haušild, P.; Davydov, V.; Drahokoupil, J.; Landa, M.; Pilvin, P. Characterization of strain-induced martensitic transformation in a metastable austenitic stainless steel. Mater. Des. 2010, 31, 1821–1827. [Google Scholar] [CrossRef]
- Kruszyński, B.W.; Wójcik, R. Residual stress in grinding. J. Mater. Process. Technol. 2001, 109, 254–257. [Google Scholar] [CrossRef]
- Balart, M.; Bouzina, A.; Edwards, L.; Fitzpatrick, M. The onset of tensile residual stresses in grinding of hardened steels. Mater. Sci. Eng. A 2004, 367, 132–142. [Google Scholar] [CrossRef]
- Hauk, V.; Oudelhoven, R.; Vaessen, G. The state of residual stress in the near surface region of homogeneous and heterogeneous materials after grinding. Met. Mater. Trans. A 1982, 13, 1239–1244. [Google Scholar] [CrossRef]
- Withers, P. Residual stress and its role in failure. Rep. Prog. Phys. 2007, 70, 2211. [Google Scholar] [CrossRef]
- Bruschi, S.; Pezzato, L.; Ghiotti, A.; Dabalà, M.; Bertolini, R. Effectiveness of using low-temperature coolants in machining to enhance durability of AISI 316L stainless steel for reusable biomedical devices. J. Manuf. Process. 2019, 39, 295–304. [Google Scholar] [CrossRef]
- Acharyya, S.; Khandelwal, A.; Kain, V.; Kumar, A.; Samajdar, I. Surface working of 304L stainless steel: Impact on microstructure, electrochemical behavior and SCC resistance. Mater. Charact. 2012, 72, 68–76. [Google Scholar] [CrossRef]
- Zhou, S.; Turnbull, A. Effect of stress transients on the crack propagation rate in steam turbine disc steel. Corrosion 2006, 62, 508–513. [Google Scholar] [CrossRef]
- Bland, L.G.; Locke, J.S.W. Chemical and electrochemical conditions within stress corrosion and corrosion fatigue cracks. npj Mater. Degrad. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, K.; Hu, Y.; Wang, S.; Wang, X. Effect of machining-induced surface residual stress on initiation of stress corrosion cracking in 316 austenitic stainless steel. Corros. Sci. 2016, 108, 173–184. [Google Scholar] [CrossRef]
- Turnbull, A.; Mingard, K.; Lord, J.; Roebuck, B.; Tice, D.; Mottershead, K.; Fairweather, N.; Bradbury, A. Sensitivity of stress corrosion cracking of stainless steel to surface machining and grinding procedure. Corros. Sci. 2011, 53, 3398–3415. [Google Scholar] [CrossRef]
- Volpe, L.; Burke, M.G.; Scenini, F. Understanding the role of Diffusion Induced Grain Boundary Migration on the preferential intergranular oxidation behaviour of Alloy 600 via advanced microstructural characterization. Acta Mater. 2019, 175, 238–249. [Google Scholar] [CrossRef]
- Chang, L.; Volpe, L.; Wang, Y.L.; Burke, M.G.; Maurotto, A.; Tice, D.; Lozano-Perez, S.; Scenini, F. Effect of machining on stress corrosion crack initiation in warm-forged type 304L stainless steel in high temperature water. Acta Mater. 2019, 165, 203–214. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Biglari, F.R.; Arezoodar, A.F.; Dabalà, M. Role of grinding induced surface residual stress on probability of stress corrosion cracks initiation in 316L austenitic stainless steel in 3.5% sodium chloride aqueous solution. Corros. Eng. Sci. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- ASTM. F138-08 Standard Specification for Wrought 18Chromium-14Nickel-2.5 Molybdenum Stainless Steel Bar and Wire for Surgical Implants (UNS S31673). ASTM F138 (Medical Device Standards and Implant Standards). 2003. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/F138-08.htm (accessed on 20 January 2021).
- Fitzpatric, M.; Fry, A. Measurement Good Practice Guide No. 52 Determination of Residual Stresses by X-ray Diffraction-Issue 2; NPL-National Physics Laboratory: London, UK, 2013. [Google Scholar]
- ASTM. G 59-97 (2014)—Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Gunenthiram, V.; Peyre, P.; Schneider, M.; Dal, M.; Coste, F.; Fabbro, R. Analysis of laser–melt pool–powder bed interaction during the selective laser melting of a stainless steel. J. Laser Appl. 2017, 29, 022303. [Google Scholar] [CrossRef]
- Qiu, C.; Kindi, M.A.; Aladawi, A.S.; Hatmi, I.A. A comprehensive study on microstructure and tensile behaviour of a selectively laser melted stainless steel. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kain, V. Effect of surface machining and cold working on the ambient temperature chloride stress corrosion cracking susceptibility of AISI 304L stainless steel. Mater. Sci. Eng. A 2010, 527, 679–683. [Google Scholar] [CrossRef]
- Hong, T.; Nagumo, M. Effect of surface roughness on early stages of pitting corrosion of type 301 stainless steel. Corros. Sci. 1997, 39, 1665–1672. [Google Scholar] [CrossRef]
- Lou, X.; Song, M.; Emigh, P.W.; Othon, M.A.; Andresen, P.L. On the stress corrosion crack growth behaviour in high temperature water of 316L stainless steel made by laser powder bed fusion additive manufacturing. Corros. Sci. 2017, 128, 140–153. [Google Scholar] [CrossRef]
- Lou, X.; Andresen, P.L.; Rebak, R.B. Oxide inclusions in laser additive manufactured stainless steel and their effects on impact toughness and stress corrosion cracking behavior. J. Nucl. Mater. 2018, 499, 182–190. [Google Scholar] [CrossRef]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C. On the corrosion and metastable pitting characteristics of 316L stainless steel produced by selective laser melting. J. Electrochem. Soc. 2017, 164, C250–C257. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Li, X. Corrosion of metallic materials fabricated by selective laser melting. npj Mater. Degrad. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Nguyen, Q.B.; Ng, F.L.; An, X.H.; Liao, X.Z.; Liaw, P.K.; Nai, S.M.L.; Wei, J. Hierarchical microstructure and strengthening mechanisms of a CoCrFeNiMn high entropy alloy additively manufactured by selective laser melting. Scr. Mater. 2018, 154, 20–24. [Google Scholar] [CrossRef]
- Lin, L.; Chao, C.; Macdonald, D. A point defect model for anodic passive films II. Chemical breakdown and pit initiation. J. Electrochem. Soc. 1981, 128, 1194–1198. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Soltani, F.; Shirsalimi, F.; Ezadi, B.; Attarzadeh, N. The semiconducting properties of passive films formed on AISI 316 L and AISI 321 stainless steels: A test of the point defect model (PDM). Corros. Sci. 2011, 53, 3186–3192. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Peyre, P.; Carboni, C.; Forget, P.; Beranger, G.; Lemaitre, C.; Stuart, D. Influence of thermal and mechanical surface modifications induced by laser shock processing on the initiation of corrosion pits in 316L stainless steel. J. Mater. Sci. 2007, 42, 6866–6877. [Google Scholar] [CrossRef]
- Liu, X.; Frankel, G. Effects of compressive stress on localized corrosion in AA2024-T3. Corros. Sci. 2006, 48, 3309–3329. [Google Scholar] [CrossRef]
- Van Boven, G.; Chen, W.; Rogge, R.; Sutherby, R. The Effect of Residual Stress on Pitting and Stress Corrosion Cracking of High Pressure Natural Gas Pipelines. Acta Mater. 2007, 55, 29–43. [Google Scholar] [CrossRef]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Hodgson, P.D.; Fabijanic, D. On the enhanced corrosion resistance of a selective laser melted austenitic stainless steel. Scr. Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Zehnder, A.T. Fracture Mechanics; Lecture Notes in Applied and Computational Mechanics; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-007-2594-2. [Google Scholar]
- Anderson, T.L. Fracture Mechanics: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Antony, K.; Arivazhagan, N. Studies on energy penetration and marangoni effect during laser melting process. J. Eng. Sci. Technol. 2015, 10, 509–525. [Google Scholar]
- Rombouts, M.; Kruth, J.P.; Froyen, L.; Mercelis, P. Fundamentals of Selective Laser Melting of alloyed steel powders. CIRP Ann. 2006, 55, 187–192. [Google Scholar] [CrossRef]
- Man, C.; Duan, Z.; Cui, Z.; Dong, C.; Kong, D.; Liu, T.; Chen, S.; Wang, X. The effect of sub-grain structure on intergranular corrosion of 316L stainless steel fabricated via selective laser melting. Mater. Lett. 2019, 243, 157–160. [Google Scholar] [CrossRef]
- Martin, M.; Weber, S.; Izawa, C.; Wagner, S.; Pundt, A.; Theisen, W. Influence of machining-induced martensite on hydrogen-assisted fracture of AISI type 304 austenitic stainless steel. Int. J. Hydrogen Energy 2011, 36, 11195–11206. [Google Scholar] [CrossRef]
- Wang, X.; Carter, L.N.; Pang, B.; Attallah, M.M.; Loretto, M.H. Microstructure and yield strength of SLM-fabricated CM247LC Ni-Superalloy. Acta Mater. 2017, 128, 87–95. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Marcus, P. Corrosion Mechanisms in Theory and Practice; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Spencer, D.T.; Edwards, M.R.; Wenman, M.R.; Tsitsios, C.; Scatigno, G.G.; Chard-Tuckey, P.R. The initiation and propagation of chloride-induced transgranular stress-corrosion cracking (TGSCC) of 304L austenitic stainless steel under atmospheric conditions. Corros. Sci. 2014, 88, 76–88. [Google Scholar] [CrossRef]



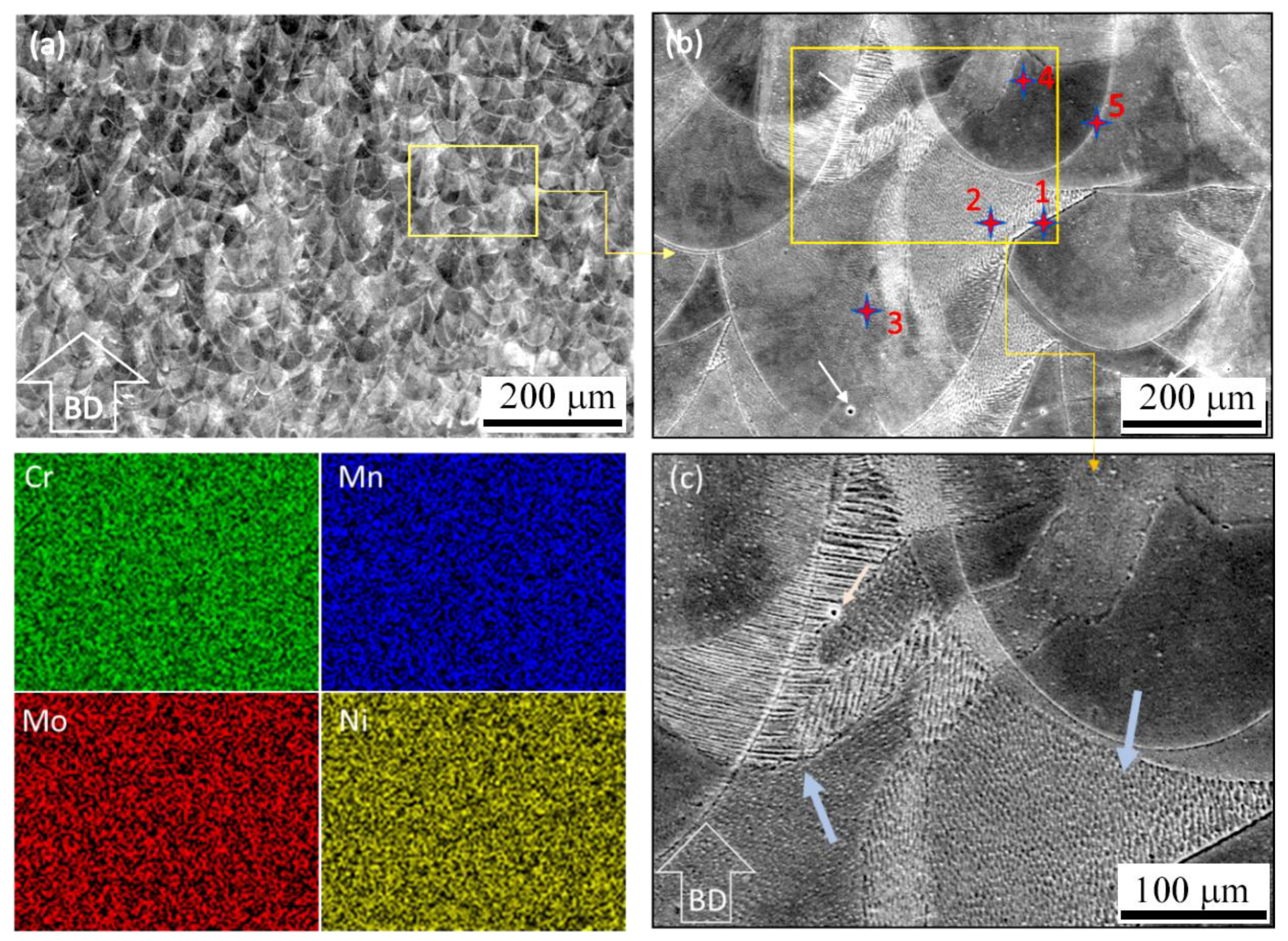




| Element | Region 1 (%) | Region 2 (%) | Region 3 (%) | Region 4 (%) | Region 5 (%) |
|---|---|---|---|---|---|
| Mo | 2.2 | 2.4 | 3.3 | 3.4 | 2.1 |
| Cr | 18.3 | 18.5 | 18.7 | 18.2 | 18.5 |
| Mn | 1.6 | 1.9 | 1.8 | 1.9 | 1.7 |
| Fe | bal. | bal. | bal. | bal. | bal. |
| Ni | 13.90 | 13.54 | 13.70 | 14.14 | 13.89 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RS (MPa) | 247 ± 4 | 207 ± 5 | 203 ± 6 | 306 ± 5 | 310 ± 3 | 328 ± 5 | 411 ± 6 | 463 ± 9 | 465 ± 3 | −130 ± 9 | −65 ± 12 |
| Depth of cut (µm) | 30 | 30 | 30 | 60 | 60 | 60 | 90 | 90 | 90 | As built | Annealed |
| Residual Stress (MPa) | Stage 1 | Stage 2 | Stage 3 | |||
|---|---|---|---|---|---|---|
| Average Potential (mV) | Duration (s) | Average Potential (mV) | Duration (s) | Average Potential (mV) | Duration (s) | |
| Annealed | 559 ± 8 | 51 | −165 ± 5 | 639 | −951 ± 10 | 510 |
| 207 | 553 ± 9 | 78 | −461 ± 10 | 749 | −988 ± 12 | 373 |
| 310 | 141 ± 6 | 5 | −227 ± 8 | 34 | −876 ± 9 | 1161 |
| 460 | 195 ± 4 | 3 | −211 ± 7 | 18 | −913 ± 11 | 1179 |
| Element | Near Melt Pool Boundary (%) | Center of Melt Pool (%) |
|---|---|---|
| Mo | 1.6 | 3.8 |
| Cr | 19.9 | 18.5 |
| Mn | 2.3 | 1.9 |
| Fe | 63.6 | 60.3 |
| Ni | 12.4 | 14.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdanpanah, A.; Lago, M.; Gennari, C.; Dabalà, M. Stress Corrosion Cracking Probability of Selective Laser Melted 316L Austenitic Stainless Steel under the Effect of Grinding Induced Residual Stresses. Metals 2021, 11, 327. https://doi.org/10.3390/met11020327
Yazdanpanah A, Lago M, Gennari C, Dabalà M. Stress Corrosion Cracking Probability of Selective Laser Melted 316L Austenitic Stainless Steel under the Effect of Grinding Induced Residual Stresses. Metals. 2021; 11(2):327. https://doi.org/10.3390/met11020327
Chicago/Turabian StyleYazdanpanah, Arshad, Mattia Lago, Claudio Gennari, and Manuele Dabalà. 2021. "Stress Corrosion Cracking Probability of Selective Laser Melted 316L Austenitic Stainless Steel under the Effect of Grinding Induced Residual Stresses" Metals 11, no. 2: 327. https://doi.org/10.3390/met11020327
APA StyleYazdanpanah, A., Lago, M., Gennari, C., & Dabalà, M. (2021). Stress Corrosion Cracking Probability of Selective Laser Melted 316L Austenitic Stainless Steel under the Effect of Grinding Induced Residual Stresses. Metals, 11(2), 327. https://doi.org/10.3390/met11020327








