Solvent Extraction of Iron(III) from Al Chloride Solution of Bauxite HCl Leaching by Mixture of Aliphatic Alcohol and Ketone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Analysis
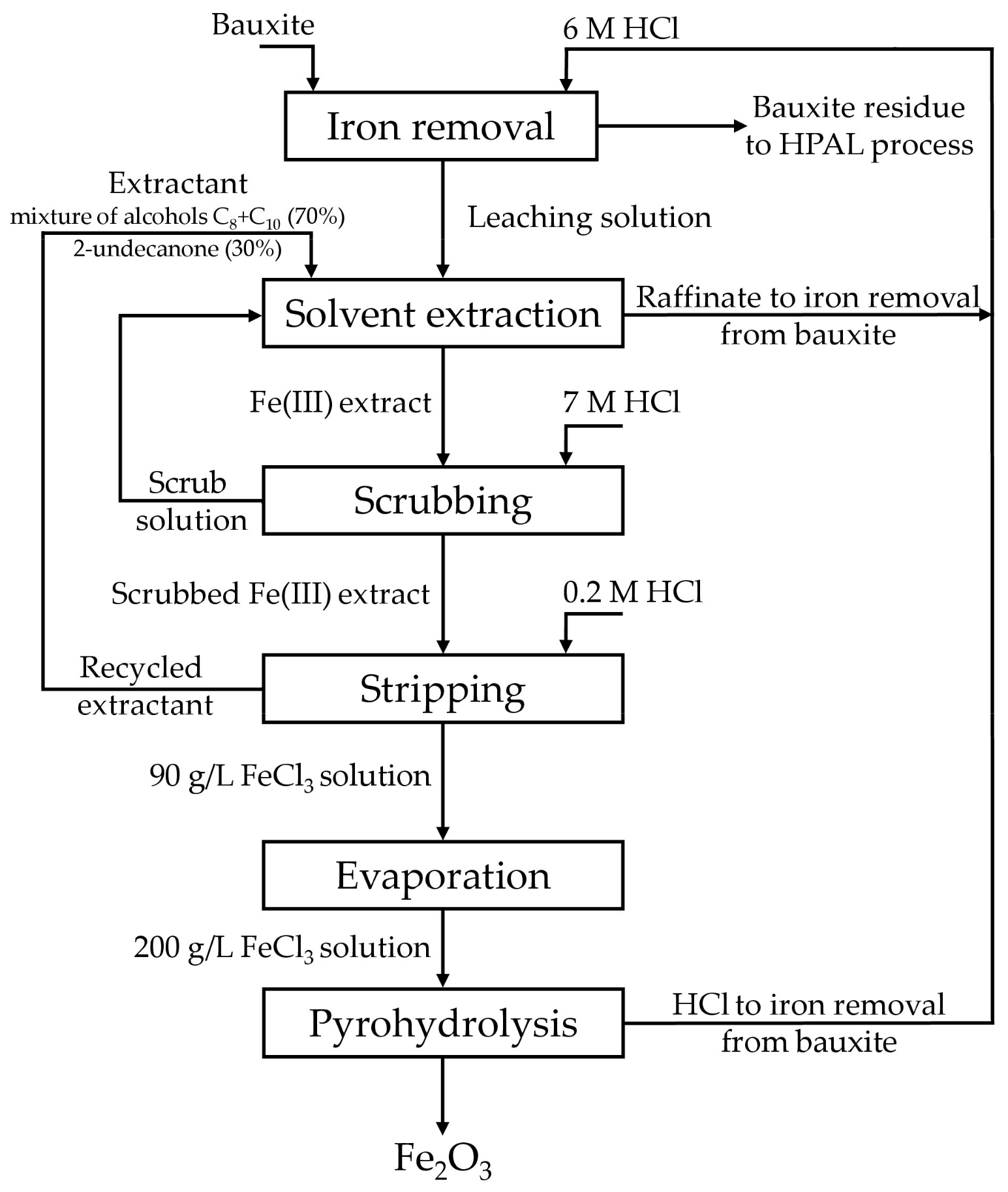
2.3. Experiments
3. Results
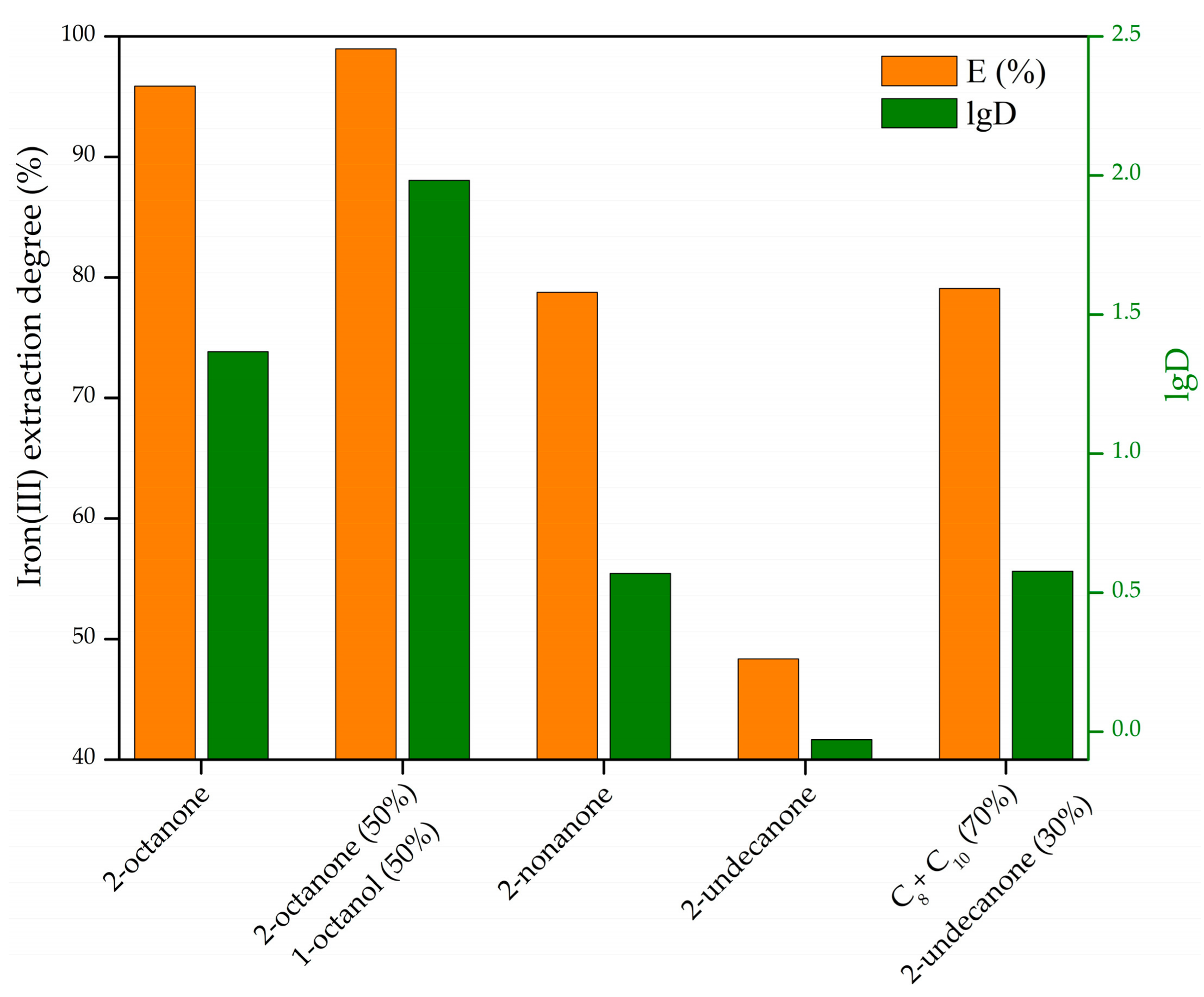
3.1. Iron(III) Extraction by Neutral Oxygen-Containing Extractants from HCl Solution
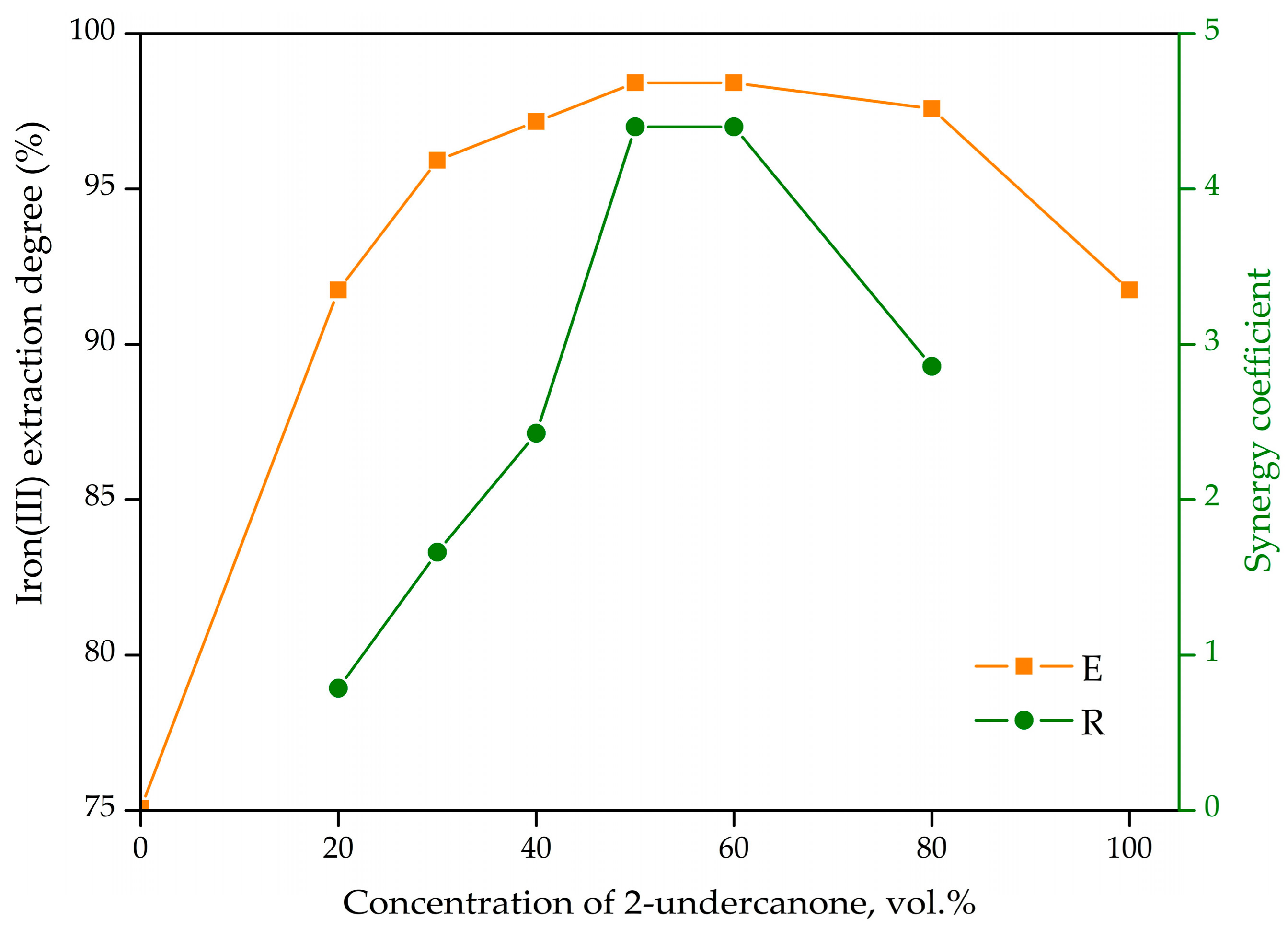
3.1.1. Iron(III) Extraction by Mixtures of 2-Undecanone and C8 + C10 Alcohols
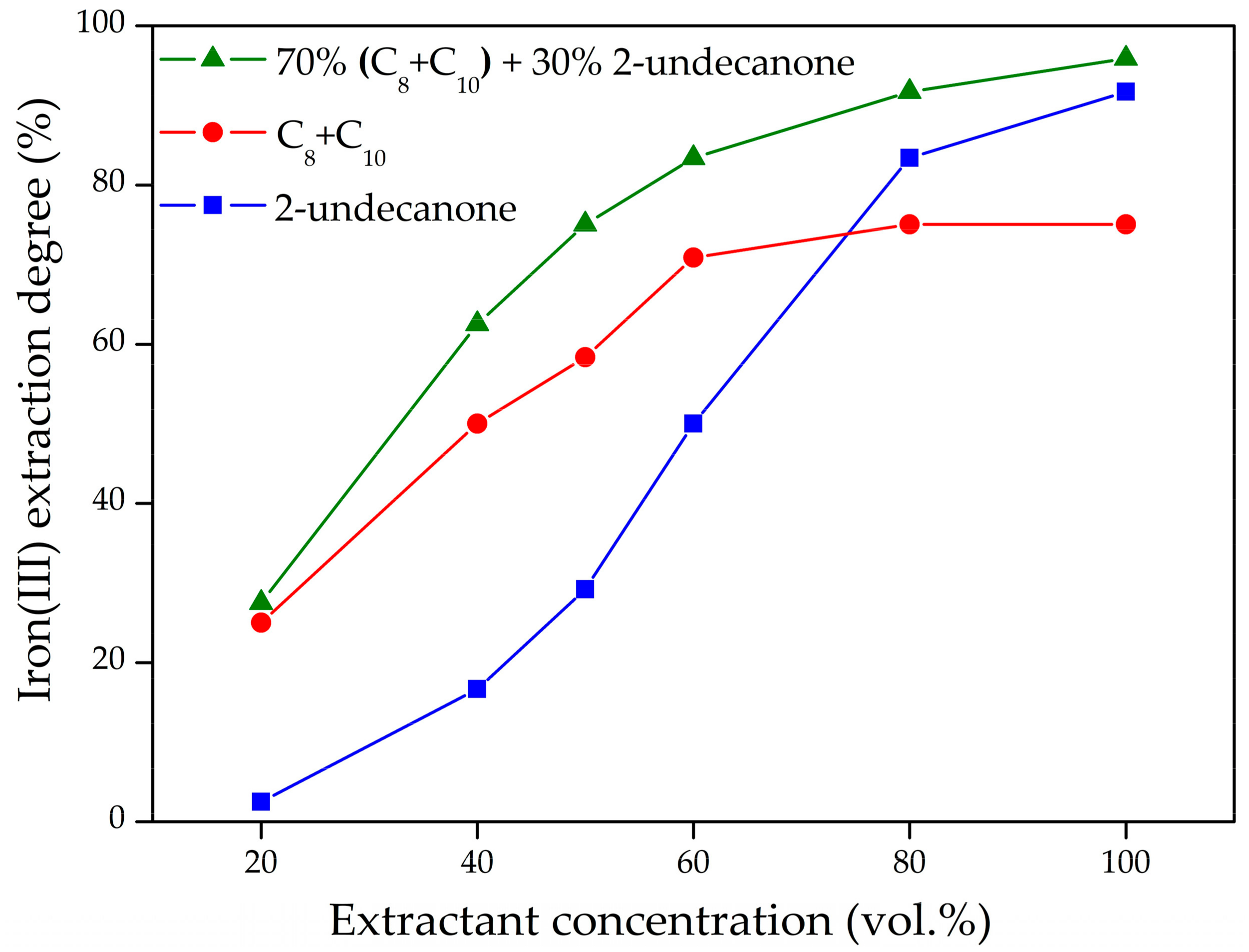
3.1.2. Effect of Dilution of Extractants on Iron(III) Extraction
3.2. Iron(III) Extraction from Al Chloride Solution of Bauxite HCl Leaching by Mixture of C8 + C10 Alcohols and 2-Undecanone
3.2.1. Iron(III) Extraction by a Mixture of Alcohols C8 + C10 (70 vol.%) and 2-Undecanone (30 vol.%)
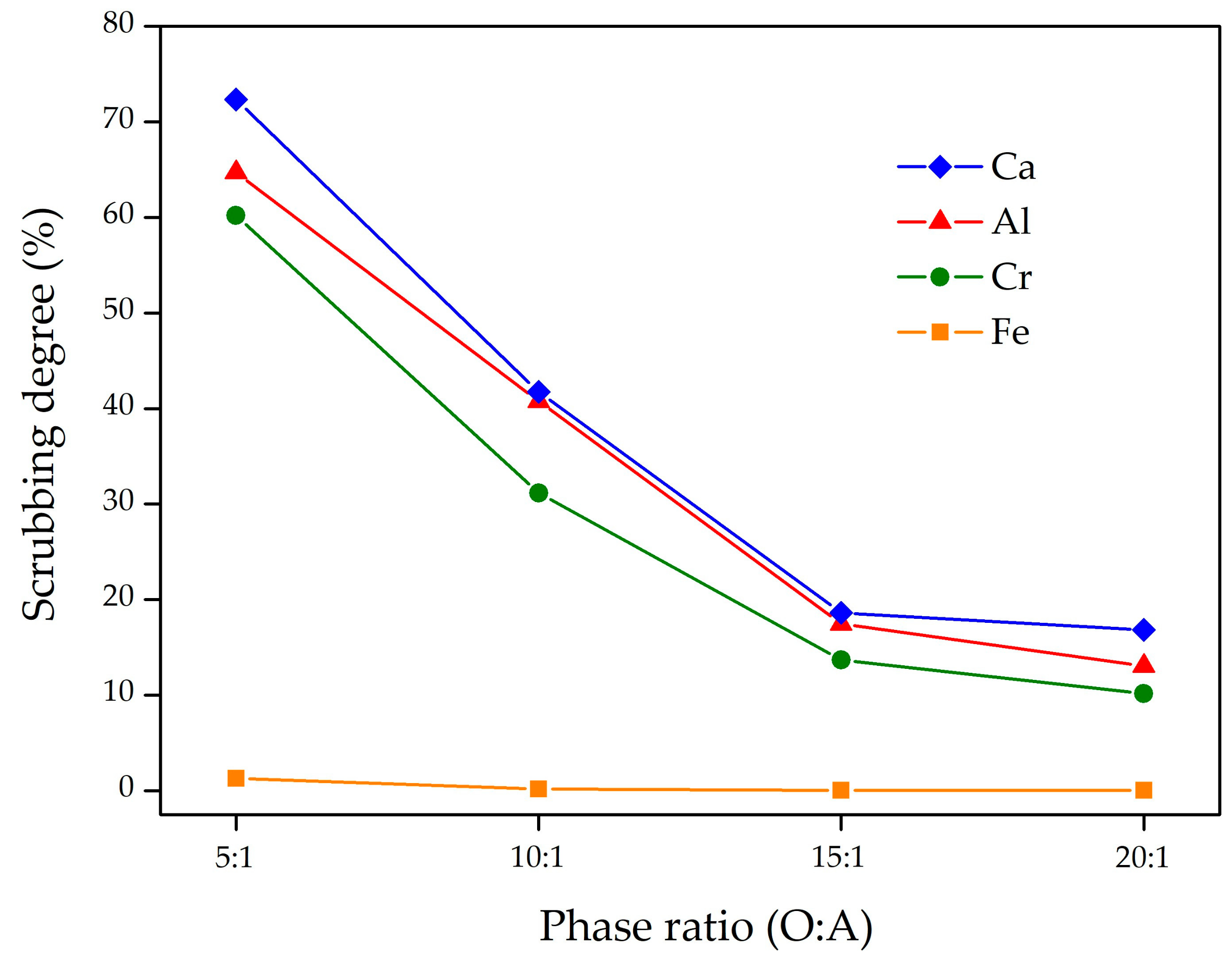
3.2.2. Scrubbing of Organic Phase from Non-Ferrous Metals
3.2.3. Iron(III) Stripping
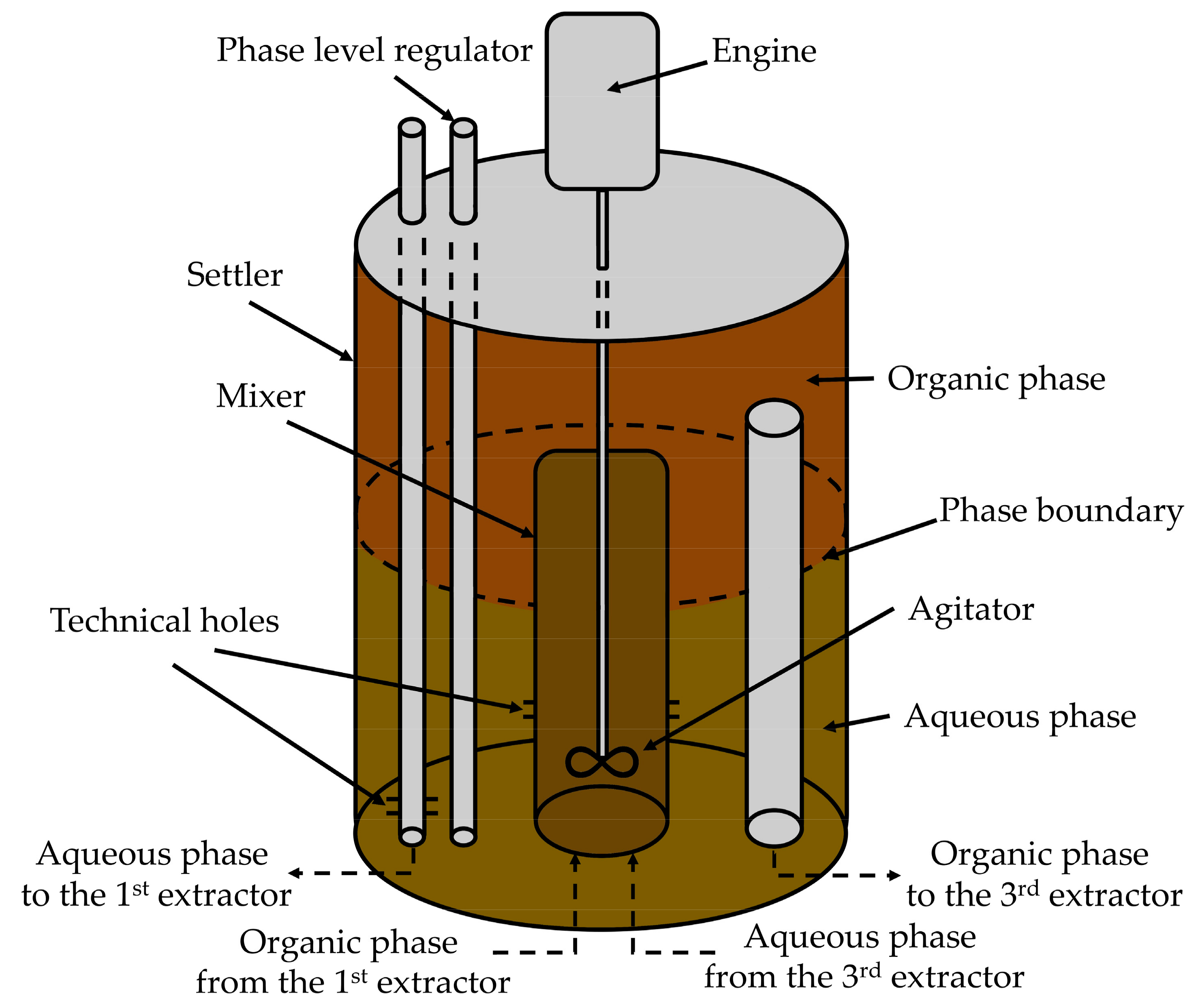
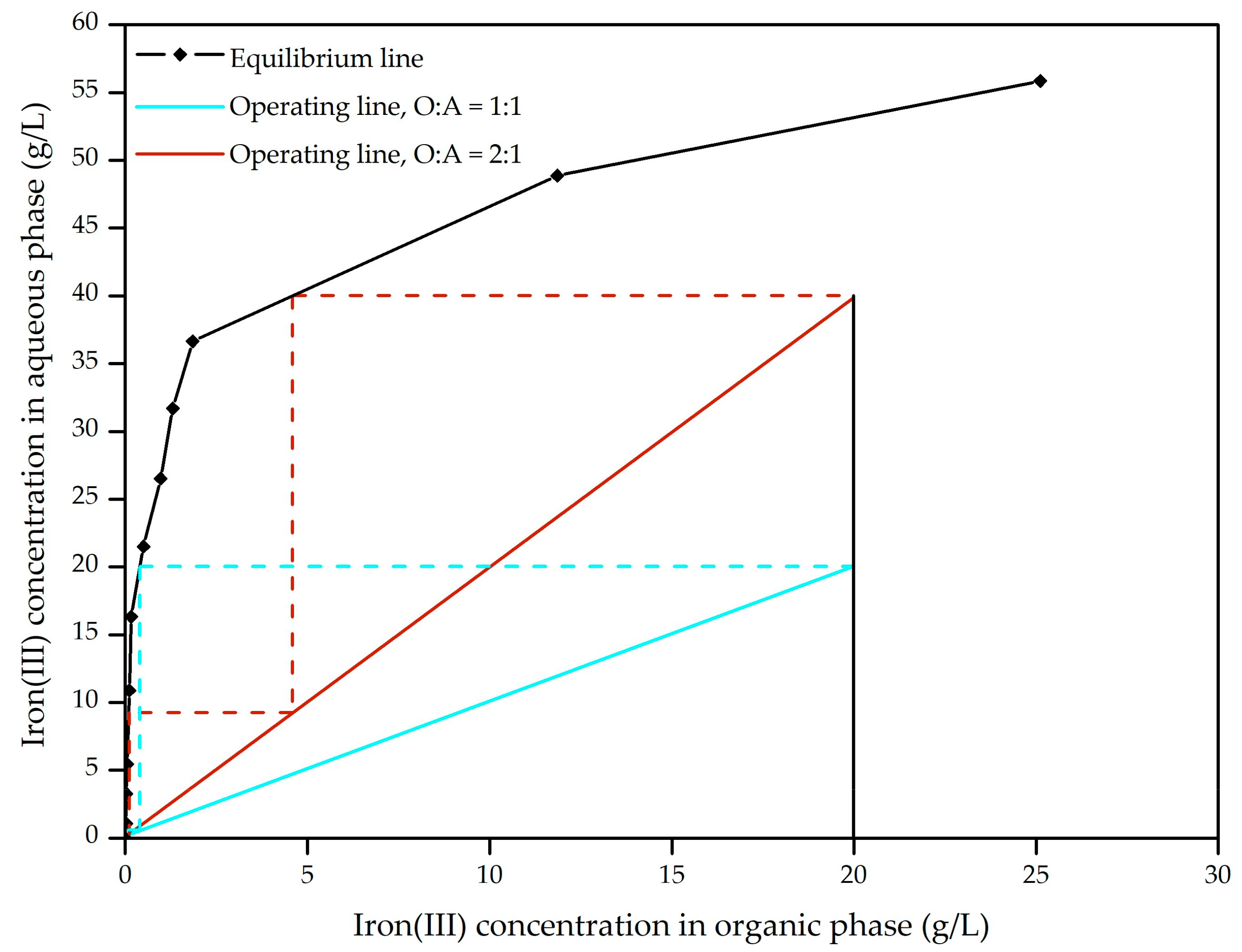
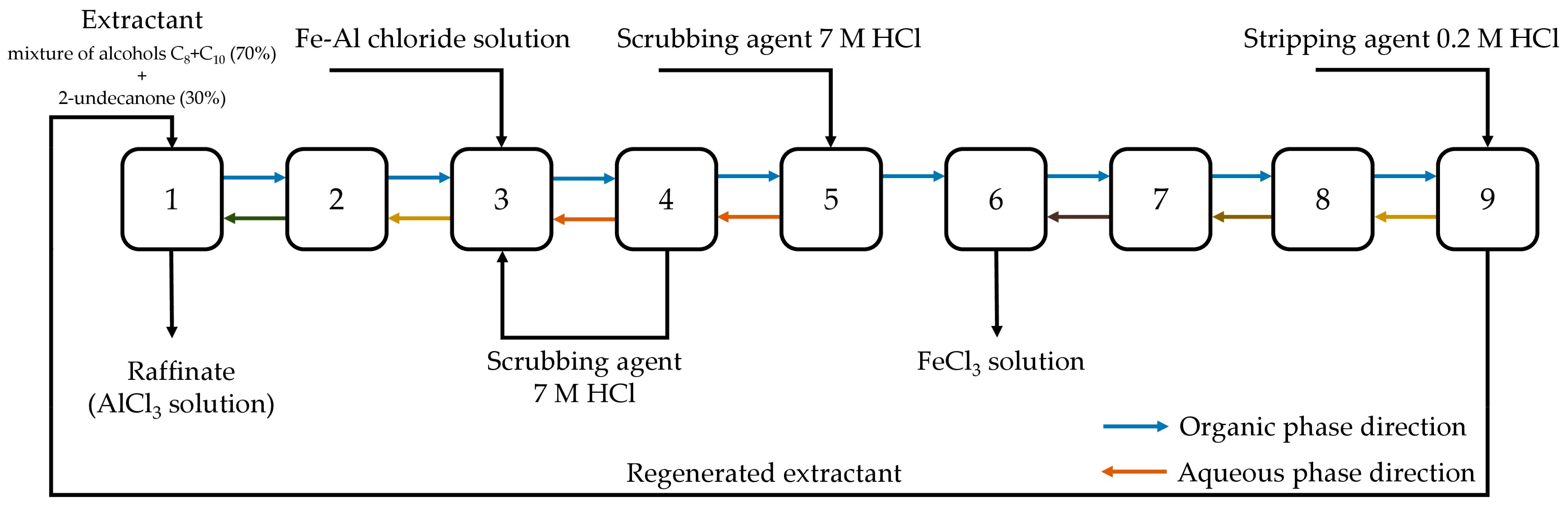
3.2.4. Iron(III) Extraction at the Cascade of Extractors Mixer-Settler Types by the Mixture of Alcohols C8 + C10 and 2-Undecanone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinoveev, D.V.; Grudinskii, P.I.; Dyubanov, V.G.; Kovalenko, L.V.; Leont’ev, L.I. Global recycling experience of red mud—A review. Part i: Pyrometallurgical methods. Izv. Ferr. Metall. 2018, 61, 843–858. [Google Scholar] [CrossRef]
- Shoppert, A.A.; Loginova, I.V.; Rogozhnikov, D.A.; Karimov, K.A.; Chaikin, L.I. Increased as Adsorption on Maghemite-Containing Red Mud Prepared by the Alkali Fusion-Leaching Method. Minerals 2019, 9, 60. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Bogdanova, E.A.; Chufarov, A.Y.; Kellerman, D.G.; Medyankina, I.S.; Yatsenko, S.P. A promising process for transformation of hematite to magnetite with simultaneous dissolution of alumina from red mud in alkaline medium. Hydrometallurgy 2020, 196, 105438. [Google Scholar] [CrossRef]
- Shoppert, A.A.; Loginova, I.V.; Napol’Skikh, J.A. Obtaining of Pigment-Quality Magnetite from Sintering Process Red Mud. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 969, p. 165180. [Google Scholar]
- Long, Q.; Li, J.; Chen, C.; Lan, Y.; Wei, G. Optimization of iron and aluminum recovery in bauxite. J. Iron Steel Res. Int. 2020, 27, 310–318. [Google Scholar] [CrossRef]
- Yuan, S.; Xiao, H.; Yu, T.; Li, Y.; Gao, P. Enhanced removal of iron minerals from high-iron bauxite with advanced roasting technology for enrichment of aluminum. Powder Technol. 2020, 372, 1–7. [Google Scholar] [CrossRef]
- Erdemoğlu, M.; Birinci, M.; Uysal, T. Thermal Behavior of Pyrophyllite Ore during Calcination for Thermal Activation for Aluminum Extraction by Acid Leaching. Clays Clay Miner. 2020, 68, 89–99. [Google Scholar] [CrossRef]
- Smirnov, A.; Kibartas, D.; Senyuta, A.; Panov, A. Miniplant Tests of HCl Technology of Alumina Production. Miner. Met. Mater. Ser. 2018, Part F4, 57–62. [Google Scholar]
- Valeev, D.V.; Lainer, Y.A.; Mikhailova, A.B.; Dorofievich, I.V.; Zheleznyi, M.V.; Gol’dberg, M.A.; Kutsev, S.V. Reaction of Bauxite with Hydrochloric Acid under Autoclave Conditions. Metallurgist 2016, 60, 204–211. [Google Scholar] [CrossRef]
- Engineering, M. The Leaching of Iron Oxides in Boehmitic Bauxite by Hydrochloric Acid. Hydrometallurgy 1989, 23, 77–90. [Google Scholar]
- Regel-Rosocka, M.; Szymanowski, J. Iron(II) transfer to the organic phase during zinc(II) extraction from spent pickling solutions with tributyl phosphate. Solvent Extr. Ion Exch. 2005, 23, 411–424. [Google Scholar] [CrossRef]
- Cui, L.; Guo, Y.; Wang, X.; Du, Z.; Cheng, F. Dissolution kinetics of aluminum and iron from coal mining waste by hydrochloric acid. Chin. J. Chem. Eng. 2015, 23, 590–596. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kumar, B.N.; Lee, M.S. Selective recovery of Fe(III), Pd(II), Pt(IV), Rh(III) and Ce(III) from simulated leach liquors of spent automobile catalyst by solvent extraction and cementation. Korean J. Chem. Eng. 2016, 33, 2684–2690. [Google Scholar] [CrossRef]
- Grzeszczyk, A.; Regel-Rosocka, M. Extraction of zinc(II), iron(II) and iron(III) from chloride media with dibutylbutylphosphonate. Hydrometallurgy 2007, 86, 72–79. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nathsarma, K.C. A comparative study on extraction of Fe(III) from chloride leach liquor using TBP, Cyanex 921 and Cyanex 923. Hydrometallurgy 2010, 104, 298–303. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Pang, M.; Zhang, L.; Zhou, H.; Dang, Q.; Sun, G. Effect of diluents on the extraction and separation of Fe(III) and Cu(II) from hydrochloric acid solutions using N,N,N′,N′-tetrabutyl succinamide. Hydrometallurgy 2015, 152, 1–6. [Google Scholar] [CrossRef]
- Cai, X.; Han, J.; Pang, M.; Cui, Y.; Li, Y.; Sun, G. Structural effect of diamide extractants on the extraction behaviour of Fe(III) from hydrochloric acid. Hydrometallurgy 2016, 164, 48–53. [Google Scholar] [CrossRef]
- Cai, X.; Wei, B.; Han, J.; Li, Y.; Cui, Y.; Sun, G. Solvent extraction of iron(III) from hydrochloric acid solution by N,N,N′,N′-tetra-2-ethylhexyldiglycolamide in different diluents. Hydrometallurgy 2016, 164, 1–6. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lee, K.-J.; Oh, Y.-J. Solvent extraction equilibria of FeCl3 from hydrochloric acid solution with Alamine336. Mater. Trans. 2004, 45, 2364–2368. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Zhou, K.; Lei, Q.; Huang, Y.; Peng, C.; Chen, W.E.I. Selective Removal of Iron from Acid Leachate of Red Mud by Aliquat 336. JOM 2019, 71, 4608–4615. [Google Scholar] [CrossRef]
- Wojciechowska, I.; Wieszczycka, K.; Wojciechowska, A.; Aksamitowski, P. Ether derivatives—Efficient Fe(III) extractants from HCl solution. Sep. Purif. Technol. 2019, 209, 756–763. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nathsarma, K.C. Solvent extraction of Fe(III) from the chloride leach liquor of low grade iron ore tailings using Aliquat 336. Hydrometallurgy 2011, 108, 93–99. [Google Scholar] [CrossRef]
- Mao, X. Solvent extraction of iron (III) from chloride acid solutions by decanol. In Proceedings of the 3rd International Conference on Material, Mechanical and Manufacturing Engineering (IC3ME 2015), Guangzhou, China, 27–28 June 2015; Atlantis Press: Beijing, China, 2015; pp. 126–132. [Google Scholar]
- Wang, X.; Liu, W.; Liang, B.; Lü, L.; Li, C. Combined oxidation and 2-octanol extraction of iron from a synthetic ilmenite hydrochloric acid leachate. Sep. Purif. Technol. 2016, 158, 96–102. [Google Scholar] [CrossRef]
- Wang, X.; Liang, B.; Lü, L.; Wu, P.; Li, C.; Ma, J. Simultaneous oxidation and extraction of iron from simulated ilmenite hydrochloric acid leachate. Hydrometallurgy 2012, 129–130, 105–110. [Google Scholar] [CrossRef]
- PubChem—A Database of Chemical Compounds. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 15 December 2020).
- Inoue, H.; Sakata, M.; Imoto, E. The oxidative coupling of alkyl aryl ketones by iron(III) chloride. ChemInform 1973, 4, 2211–2215. [Google Scholar] [CrossRef]
- Yi, X.; Huo, G.; Tang, W. Removal of Fe(III) from Ni-Co-Fe chloride solutions using solvent extraction with TBP. Hydrometallurgy 2020, 192, 105265. [Google Scholar] [CrossRef]
- Azizitorghabeh, A.; Rashchi, F.; Babakhani, A. Stoichiometry and structural studies of Fe(III) and Zn(II) solvent extraction using D2EHPA/TBP. Sep. Purif. Technol. 2016, 171, 197–205. [Google Scholar] [CrossRef]
- Das, D.; Juvekar, V.A.; Bhattacharya, R. Co-Extraction/Stripping of Mineral Acids and Iron(III) by Tri-n-Butyl Phosphate. Sep. Sci. Technol. 2015, 50, 545–553. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Wei, G.; Zhao, H.; Wang, L.; Qi, T.; Meng, F.; Meng, L. Extraction of iron (III) from chloride leaching liquor with high acidity using tri-n-butyl phosphate and synergistic extraction combined with methyl isobutyl ketone. Sep. Purif. Technol. 2015, 150, 132–138. [Google Scholar] [CrossRef]
- Sahu, K.K.; Das, R.P. Mixed solvent systems for the extraction and stripping of iron(III) from concentrated acid chloride solutions. Metall. Mater. Trans. B 2000, 31, 1169–1174. [Google Scholar] [CrossRef]
- Mao, X.H. Solvent extraction of iron(III) from acid chloride solutions by trioctylamine and decanol. Mater. Sci. Environ. Eng. 2016, 145–150. [Google Scholar]
- Voshkin, A.A.; Belova, V.V.; Zakhodyaeva, Y.A. Iron Extraction with Di(2-Ethylhexyl)dithiophosphoric Acid and a Binary Extractant Based on It. Russ. J. Inorg. Chem. 2018, 63, 387–390. [Google Scholar] [CrossRef]
- Kasikov, A.G.; Sokolov, A.Y.; Shchelokova, E.A.; Glukhovskaya, I.V. Extraction of Iron(III) from Chloride Nickel Solutions with Aliphatic Ketones. Russ. J. Appl. Chem. 2019, 92, 1107–1112. [Google Scholar] [CrossRef]
- Kasikov, A.G.; Sokolov, A.Y. Extraction of Iron (III) From Hydrochloric Acid Solution by Isomers of Octanol with Inert Diluent. Mod. High Technol. 2019, 3, 187–192. [Google Scholar] [CrossRef]
- TGSC Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 15 December 2020).
- Chao, J.-P.; Dai, M. Studies of thermodynamic properties of binary mixtures containing an alcohol: Part XV. Excess molar enthalpies of alkan-1-ol+methyl ethyl ketone and + methyl isobutyl ketone at 298.15 K11Project support by the National Natural Science Foundation of China. Thermochim. Acta 1991, 179, 257–264. [Google Scholar] [CrossRef]
- Collins, S.G.; Christensen, J.J.; Izatt, R.M.; Hanks, R.W. The excess enthalpies of 10 (n-pentane + an n-alkanol) mixtures at 298.15 K. J. Chem. Thermodyn. 1980, 12, 609–614. [Google Scholar] [CrossRef]
- Reddy, B.R.; Bhaskara Sarma, P.V.R. Extraction of iron(III) at macro-level concentrations using TBP, MIBK and their mixtures. Hydrometallurgy 1996, 43, 299–306. [Google Scholar] [CrossRef]
- Saji, J.; Reddy, M.L.P. Liquid–liquid extraction separation of iron(III) from titania wastes using TBP–MIBK mixed solvent system. Hydrometallurgy 2001, 61, 81–87. [Google Scholar] [CrossRef]
- Chiarizia, R.; Danesi, P.R. Mass transfer rate in liquid anion exchange processes—II: Kinetic of iron(III) transfer in the biphasic system trilaurylamine hydrochloride-aromatic diluent, HCl-water. J. Inorg. Nucl. Chem. 1978, 40, 1811–1816. [Google Scholar] [CrossRef]
- Van Weert, G.; Peek, E.M.L. Reagent recovery in chloride hydrometallurgy—Some missing links. Hydrometallurgy 1992, 29, 513–526. [Google Scholar] [CrossRef]
- Dry, M.J.; Harris, G.B. Hydrolysis for iron control and acid regeneration in chloride hydrometallurgy circuits. In Proceedings of the 28th International Mineral Processing Congress (IMPC 2016), Quebec City, QC, Canada, 11–15 September 2016; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2016; p. 135047. [Google Scholar]
- Hofbauer, T.; Baerhold, F.; Mitterecker, S.K. Andritz metals spray-roast acid regeneration pilot plant. In Proceedings of the 28th International Mineral Processing Congress (IMPC 2016), Quebec City, QC, Canada, 11–15 September 2016; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2016; p. 135047. [Google Scholar]






| Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | Cr2O3 | LOI | |
|---|---|---|---|---|---|---|---|
| Raw bauxite | 49.26 | 24.18 | 5.56 | 2.94 | 0.88 | 0.86 | 16.02 |
| Bauxite after HCl leaching | 55.83 | 30.89 | 0.84 | 3.10 | 0.04 | 0.24 | 11.06 |
| Ketone | Molecular Formula | Structure | Flash Point, °C | Solubility in Water, g/L (25 °C) |
|---|---|---|---|---|
| MIBK | C6H12O | 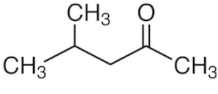 | 14–22.78 | 19 |
| 4-heptanone | C7H14O | 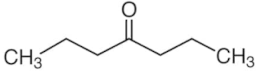 | 48.89 | 3.2 |
| 2-octanone | C8H16O | 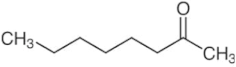 | 51.67 | 0.899 (20 °C) |
| 2-nonanone | C9H18O | 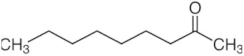 | 64.44 | 0.371 |
| 4-nonanone | C9H18O |  | 61.40 | – |
| 2-decanone | C10H20O |  | 85.00 | 0.077 |
| 2-undecanone | C11H22O | 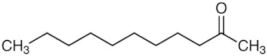 | 88.89 | 0.020 |
| Solution | Metal Concentration, g/L | |||
|---|---|---|---|---|
| Fe | Al | Ca | Cr | |
| Feed | 10.17 | 2.36 | 0.43 | 0.31 |
| Raffinate | 0.84 | 2.35 | 0.41 | 0.30 |
| Scrub solution | 4.99 | 0.034 | 0.026 | 0.018 |
| FeCl3 strip solution | 90.50 | 0.007 | 0.023 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, A.; Valeev, D.; Kasikov, A. Solvent Extraction of Iron(III) from Al Chloride Solution of Bauxite HCl Leaching by Mixture of Aliphatic Alcohol and Ketone. Metals 2021, 11, 321. https://doi.org/10.3390/met11020321
Sokolov A, Valeev D, Kasikov A. Solvent Extraction of Iron(III) from Al Chloride Solution of Bauxite HCl Leaching by Mixture of Aliphatic Alcohol and Ketone. Metals. 2021; 11(2):321. https://doi.org/10.3390/met11020321
Chicago/Turabian StyleSokolov, Artem, Dmitry Valeev, and Aleksandr Kasikov. 2021. "Solvent Extraction of Iron(III) from Al Chloride Solution of Bauxite HCl Leaching by Mixture of Aliphatic Alcohol and Ketone" Metals 11, no. 2: 321. https://doi.org/10.3390/met11020321
APA StyleSokolov, A., Valeev, D., & Kasikov, A. (2021). Solvent Extraction of Iron(III) from Al Chloride Solution of Bauxite HCl Leaching by Mixture of Aliphatic Alcohol and Ketone. Metals, 11(2), 321. https://doi.org/10.3390/met11020321







