Abstract
Alumina-forming austenitic stainless steels are known for their superior high-temperature oxidation resistance. Following our previous work that solved the matching of major alloying elements in their specific 16-atom cluster formula, we here focus on the 800 °C air-oxidation resistance of 0.08 wt. % C alloy series satisfying cluster formula [(Al0.89Si0.05NbxTa0.06−x)-(Fe11.7−yNiyMn0.3)]Cr3.0−z(Mo,W)z, x = 0.03 or 0.06, y = 3.0 or 3.2, z = 0.07 or 0.2, to explore the effect of minor alloying elements Mo, Nb, Ta and W. This cluster formula is established particularly based on alloys which were originally developed by Oak Ridge National Laboratory. All samples are graded as complete oxidation resistance level according to Chinese standard HB 5258-2000, as their oxidation rate and oxidation-peeling mass are generally below 0.1 g/m2 × h and 1.0 g/m2, respectively. In alloys without Ta and W, a Cr2O3-type oxide layer is formed on the surface and Al2O3 particles of sizes up to 4 μm are distributed beneath it. In contrast, in Ta/W-containing alloys, a continuous protective Al2O3 layer is formed beneath the outer Cr2O3 layer, which prevents internal oxidation and provides the lowest weight gain. Instead of internal Al2O3 particles, AlN is formed in Ta/W-containing alloys. The W-containing alloy possesses the thinnest internal nitride zone, indicating the good inhibition effect of W on nitrogen diffusion.
1. Introduction
Alumina-forming austenitic (AFA) stainless steel is a type of heat-resistant stainless steel which can form a dense and stable aluminum oxide layer to protect it from high-temperature environments. Compared to Cr2O3 film, which is formed in traditional austenitic stainless steels, Al2O3 film has a lower growth rate (1–2 orders of magnitude slower), better thermal stability, and is more stable in water vapor, even in combustion and chemical reactions with carbon and sulfur [1,2,3]. Since the 1970s, Al with weight percentages of 4–5 has been added to austenitic stainless steel in order to improve its high-temperature oxidation resistance [4,5,6,7,8,9]. However, they can only be used as a protective coating or in low load environments because of their low creep resistance, which is caused by their ferrite-austenite dual phase structure. Until the beginning of this century, Oak Ridge National Laboratory (ORNL) in the United States found that 2.4 wt. % Al was sufficient to form a continuous, stable and dense Al2O3 film in water vapor under 650~800 °C. A series of AFA stainless steels with excellent creep resistance and oxidation resistance at high temperature [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] were thereafter developed.
The priority when designing the composition of AFA stainless steels is to guarantee the formation of stable and highly uniform austenitic state, avoiding a ferrite-austenite dual phase structure. Although Al is the essential element to form Al2O3 film, it is also a strong ferritic stabilizer, with its α stability being about 2.55–5.5 times that of Cr [25,26,27,28]. Proper amounts of austenitic stabilizers such as Ni should be added within the framework of the cluster formula [(Al,Si,Nb)1-(Fe,Ni,Mn)12](Cr,Mo,W)3 [29].
In addition to the main alloying elements Ni, Cr and Al, minor alloying elements like Mo, Nb, Ti, W and V etc. are often added to AFA stainless steels for enhancing good pitting corrosion resistance and creep resistance. Besides its well-known contribution to the improvement of pitting resistance, Mo can also form Fe2Mo-Laves phase. Nb can also form Fe2Nb-Laves phase, which increases the high-temperature creep resistance. What’s more, the formation of MC-type carbides via alloying with Ti, V and W consumes part of C so that intergranular corrosion tendency is minimized. However, the matching of all these minor and major alloying elements is a challenge in such a complex alloy system.
In fact, it is well known that solid solution alloys maintain a lattice structure of solvent atoms across long distances, and although the distribution of solute atoms in long range is uncertain, solid solutions still have short-range order structure. In 1960, Cowley [30] proposed a short-range order parameter for binary systems, which describes the distribution of atoms (i.e., a center atom surrounded by similar or different atoms). However, this short-range order parameter can only describe binary systems, but not the short-range order in ternary or multi-component alloy systems (such as stainless steels). In additional, this short-range order parameter is based on statistical methods. It only shows the average result of a large number of experiments. In order to establish the relationship between the structure and composition of alloys, it is necessary to develop a new structural model to characterize the chemical short-range order in multi-component alloys.
Our team has previously proposed a cluster-plus-glue-atom model to characterize the chemical short-range order in multi-component solid solution alloys [31,32]. This structural model describes the average composition of solid solution alloys in terms of a cluster unit covering a nearest-neighbor cluster plus a few next-neighbor glue atoms. Such a cluster formula approach has been successfully applied in optimizing a variety of industrial alloys with high efficiency [33,34,35,36,37,38].
This paper addresses the matching of minor alloying elements within the framework of the previously established cluster formula for AFA stainless steels. Alloy series conforming to the cluster formula [(Al0.89Si0.05NbxTa0.06−x)-(Fe11.7−yNiyMn0.3)]Cr3.0−z(Mo,W)z are designed, where x = 0.03 or 0.06, y = 3.0 or 3.2, z = 0.07 or 0.2. In comparison to our previous work [29], which focuses on the matching of Ni and Al to reach stable austenitic state, the present objective is to investigate high-temperature austenite stability as influenced by Ni and high-temperature oxidation resistance after minor alloying with refractory metals such as Mo, Nb, Ta, and W.
2. Principles of Alloy Design
This article analyzes the composition of AFA stainless steels by our cluster-plus-glue-atom model. The number of atoms in a chemical structural unit of pure FCC structure has been calculated previously by atomic radius [32]. The closest integer is 16, which means the most stable FCC structure should be a CN12 cubic octahedral cluster plus three glue atoms. For solid solution alloys, the occupation of atoms is decided by the interaction between solute atoms and solvent atoms, which can be evaluated by the mixing enthalpy ΔH in binary systems. The general procedure to determine the ideal structural unit of a solid solution alloy is as follows: (1). Classify the solute atoms according to their mixing enthalpy ΔH with solvent atoms, so that their occupations (as center, shell or glue atoms) are preliminarily determined. (2). Set up a criterion: set either the number of shell atoms or total atoms in the cluster, then calculate the number of atoms in the cluster of each element. (3). Summarize the number of atoms in each occupation (center, shell and glue atoms) of typical compositions, and get the ideal structural unit of this kind of solid solution alloy, namely the general cluster formula, and afterwards design compositions according to this ideal cluster formula and verify its practicable by experiments.
To determine the cluster formula of multi-component AFA stainless steels, the occupation of atoms in the cluster should be decided according to the mixing enthalpy ΔH between solute atoms and solvent atoms, as listed in Table 1. On that basis, the alloying elements are classified into three groups: 1, Al, Si, Ti, V, Nb and Ta have negative and large value ΔH with Fe (−7–−35 kJ/mol), which means strong interaction. These Al-like atoms are therefore classified as center atoms; 2, Cr, Mo and W, which have weak interaction with Fe (−2–0 kJ/mol), are treated as glue atoms; 3, Ni and Mn also have weak interaction with Fe (ΔHFe-Ni = −2 kJ/mol, ΔHFe-Mn = 0 kJ/mol). Their interaction with the center Al-like atoms is much stronger (i.e., ΔHNi-Al = −22 kJ/mol, ΔHMn-Al = −19 kJ/mol), so that they are treated as Fe-like atoms and classified as shell atoms together with Fe.

Table 1.
Mixing enthalpy ΔH between center atoms, glue atoms and shell atoms.
On the basis of occupation of each element, as many as 190 published compositions of AFA stainless steels [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,39,40,41,42,43,44] were analyzed under three criterions: (1) Set the number of shell atoms as 12; (2) Set the total number of atoms as 16; (3) Set the total number of atoms as 19. By comparison, the interaction between the atoms can be explained best when the number of shell atoms is set as 12, so that the first criterion is applied for AFA stainless steels. The number of atoms of each element are calculated based on (Fe + Ni + Mn) = 12 in 16 atom cluster, and the cluster formula of each reported composition can therefore be obtained. For example, a typical composition of ORNL: AFA2-1 (Fe-14.30Cr-20.00Ni-2.50Al-0.90Nb-2.50Mo-0.15Si-2.00Mn-0.08C) can be written as [Al0.79Si0.04Nb0.08-Fe8.78Ni2.90Mn0.31]-Cr2.34Mo0.22, where the center, shell and glue atom can all be regarded as the average atom composed of various elements. For instance, the center atom can be regarded as the average atom composed of (Al,Si,Nb), and the glue atoms are average atoms made up by (Cr,Mo). The cluster formula of as many as 190 AFA stainless steels are calculated and summarized under the criterion that the number of shell atoms is 12, and it is found that mostly their cluster formulas are close to 1:3 model. This result is consistent with the previously calculated optimal chemical structural unit of an FCC solid solution, which is a CN12 cubic octahedron cluster plus three glue atoms [32].
Based on that, series-1 AFA stainless steels are designed to investigate the compromise of Ni and Al, in order to guarantee their single austenitic structure. After microstructural characterization, a general cluster formula for AFA stainless steels is determined as [(Al,Si,Nb)1-(Fe,Ni,Mn)12](Cr,Mo,W)3 [29]. On the fundamentals of this cluster formula, in this paper series-2 alloys are designed to further verify the guiding function of the cluster formula to design AFA stainless steels with single austenitic structure, and in addition to study the effect of minor elements such as Mo, Nb, Ta, W on its mechanical properties and oxidation resistance. The composition of both two series of designed AFA stainless steels are listed in Table 2 and the composition design flowchart of these two series is drawn as Figure 1.

Table 2.
The composition formula, mark, weight percentage, hardness (both solutionizing and aging states), Cr and Ni equivalents (calculated by Uggowitzer’s equivalent equations [28]) of designed alloys.
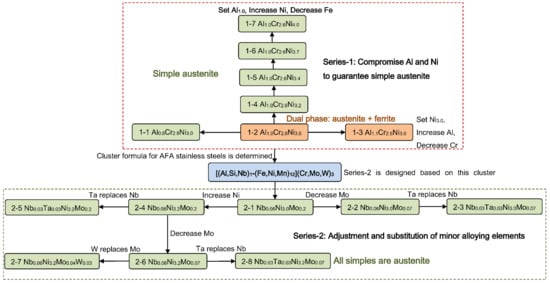
Figure 1.
The composition design flowchart of alumina-forming austenitic (AFA) stainless steels based on the cluster-plus-glue-atom model.
Based on the general cluster formula of AFA stainless steels, our series-2 alloys decrease the content of Nb from Nb0.15 to Nb0.06 (in 16 atom cluster), to achieve Nb:C = 1:1 (in at. %), expecting that Nb can be fully precipitated as NbC. The content of Si is kept as Si0.05. Combined with the ideal number of center atom Al + Nb + Si = 1, the center of the cluster formula is decided as Al0.89Nb0.06Si0.05, so that the basic composition of series-2 is designed as Al0.89Si0.05Nb0.06-Fe8.7Ni3.0Mn0.3-Cr2.8Mo0.2 (2-1). Afterwards, series-2 alloys are designed by adjusting their alloying elements through the method of equal-proportion replacement. This method here refers to the design principle of high-entropy alloys and has been an effective alloy design method at present and appears in many systems. As shown in Figure 1, by decreasing the content of Mo in alloy 2-1 from Mo0.2 to Mo0.07, Al0.89Si0.05Nb0.06-Fe8.7Ni3.0Mn0.3-Cr2.93Mo0.07 (2-2) is obtained. By replacing half of Nb in alloy 2-2 by Ta, Al0.89Si0.05Nb0.03Ta0.03-Fe8.7Ni3.0Mn0.3-Cr2.93Mo0.07 (2-3) is obtained. Increasing the content of Ni in alloy 2-1, from Ni3.0 to Ni3.2, Al0.89Si0.05Nb0.06-Fe8.5Ni3.2Mn0.3-Cr2.8Mo0.2 (2-4) is designed. On the basis of alloy 2-4, Al0.89Si0.05Nb0.03Ta0.03-Fe8.5Ni3.2Mn0.3-Cr2.8Mo0.2 (2-5) is obtained by replacing half of its Nb by Ta, while Al0.89Si0.05Nb0.06-Fe8.5Ni3.2Mn0.3-Cr2.93Mo0.07 (2-6) is designed by decreasing its Mo content from Mo0.2 to Mo0.07. Similarly, replacing Mo in alloy 2-6 by W get Al0.89Si0.05Nb0.06-Fe8.5Ni3.2Mn0.3-Cr2.93Mo0.04W0.03 (2-7), while replacing Nb in alloy 2-6 by Ta get Al0.89Si0.05Nb0.03Ta0.03-Fe8.5Ni3.2Mn0.3-Cr2.93Mo0.07 (2-8). The alloy compositions in weight percent (wt. %) of this [(Al0.89Si0.05NbxTa0.06−x)-(Fe11.7−yNiyMn0.3)]Cr3.0−z(Mo,W)z series are listed in Table 2, wherein a 16-atom cluster x = 0.03 or 0.06, y = 3.0 or 3.2, z = 0.07 or 0.2, and all alloys are marked with NbxTa0.06−xNiy(Mo,W)z hereafter.
3. Materials and Methods
These eight series-2 alloy ingots with a weight of about 13 g were prepared by non-consumable vacuum arc-melting furnace (WK model manufactured by Beijing Physcience Opto-electronics Co. Ltd., Beijing, China). The purities of the raw metals are 99.99 wt. % for Fe, Ni, C and Si, 99.5 wt. % for Cr, Mo, Nb, Ta and W, and 99.999% for Al, respectively. Before melting, the vacuum of the furnace was controlled below 6 × 10−3 Pa, and the melting process were protected by the argon atmosphere with a purity of 99.999%. These alloy ingots were melted repeatedly at least five times for composition homogeneity, in which the mass loss was controlled below 0.1 wt. %. The alloy ingots were then prepared into alloy bars with a diameter of 6 mm by using vacuum copper mold suction followed by fast cooling. These alloy bars were solutionized at 1250 °C for 1.5 h plus water quenching, and then aged at 800 °C for 24 h plus furnace cooling. The high-temperature oxidation experiment was conducted in a muffle furnace (KSL-1400X-A2, Hefei Kejing Material Technology Co. Ltd., Hefei, China) at 800 °C in air. The sample size for oxidation is Φ6 × 12 mm. The samples were weighted after 0, 25, 50, 75, 100, 150 and 200 h, respectively.
Structural identification of alloy samples with different heat treatments was carried out by means of a BRUKER X-ray diffractometer (XRD) (Billerica, MA, USA) with a Cu Kα radiation (λ = 0.15406 nm). The microstructure was observed using OLYMPUS light microscopy (LM) (Olympus Tokyo, Japan) and Zeiss Supra55 scanning electron microscopy (SEM) (Carl Zeiss AG, Oberkochen, Germany) with an etching solution of 20% HF + 10% HNO3 + 70% H2O (volume fraction). The microhardness was tested with a HVS-1000 Vickers hardness tester with a load of 500 g and loading time of 20 s. The average value was calculated after each sample was measured 10 times.
4. Results and Discussion
4.1. Microstructural Characterization
XRD results of solutionized and aged alloys are shown in Figure 2a,b, respectively. The series-2 alloys, NbxTa0.06−xNiy(Mo,W)z, exhibit a single face-centered cubic γ austenitic structure after being solutionized at 1250 °C for 1.5 h and aged at 800 °C for 24 h. No diffraction peak of ferritic structure is observed in either of the states, though tiny amounts of NiAl-B2 phase should form in aged alloys. These results indicate that the general cluster formula of AFA stainless steels [(Al,Si,Nb)1-(Fe,Ni,Mn)12](Cr,Mo,W)3 guarantees a stable austenitic state.
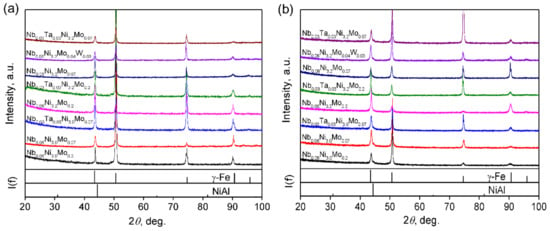
Figure 2.
The XRD patterns of series-2 designed alloys after 1250 °C/1.5 h solutionizing (a) and 800 °C/24 h aging (b).
The optical microstructures of solutionized and aged series-2 alloys are shown in Figure 3. The suction-cast ingots show typical casting microstructure, with uniform equiaxial grains in the central region and columnar grains at the outer region. These graphs of the eight NbxTa0.06−xNiy(Mo,W)z series-2 alloys do not show any ferritic structure, which is consistent with the XRD results. Again, the austenite stability is reached within the framework of the general cluster formula for AFA stainless steels.
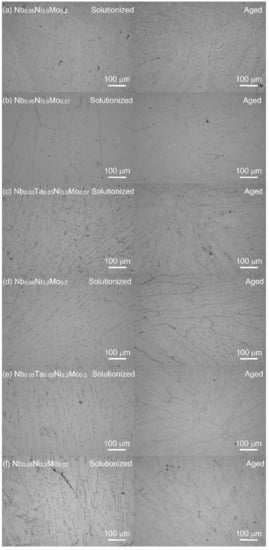
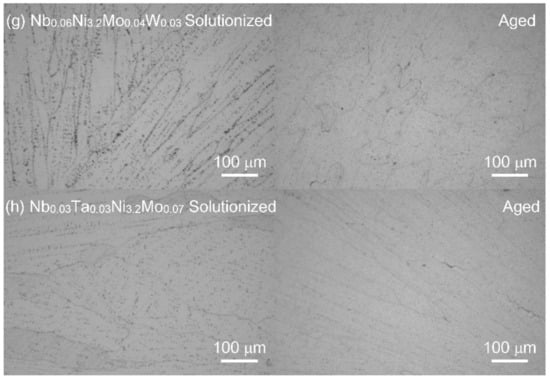
Figure 3.
The optical microstructures of series-2 alloys after being solutionized at 1250 °C for 1.5 h (left) and aged at 800 °C for 24 h (right). (a): Nb0.06Ni3.0Mo0.2, (b): Nb0.06Ni3.0Mo0.07, (c): Nb0.03Ta0.03Ni3.0Mo0.07, (d): Nb0.06Ni3.2Mo0.2, (e): Nb0.03Ta0.03Ni3.2Mo0.2, (f): Nb0.06Ni3.2Mo0.07, (g): Nb0.06Ni3.2Mo0.04W0.03, (h): Nb0.03Ta0.03Ni3.2Mo0.07.
The SEM images of five typical compositions of series-2 alloys, Nb0.06Ni3.0Mo0.2 (2-1), Nb0.06Ni3.0Mo0.07 (2-2), Nb0.03Ta0.03Ni3.2Mo0.2 (2-5), Nb0.06Ni3.2Mo0.04W0.03 (2-7) and Nb0.03Ta0.03Ni3.2Mo0.07 (2-8), are shown in Figure 4. The secondary electron morphologies after aging at 800 °C/24 h and the backscattered images after aging at 800 °C/200 h are placed on the left and right, respectively. No ferritic structure is observed that confirms the XRD and OM results. In addition, bulky NbC is not observed from the secondary electron morphologies, due to the decrease in Nb content, from Nb0.15 (in series-1) to Nb0.06 (in series-2). As shown in the backscattered images of 800 °C/200 h aged alloys, the NiAl-B2 (brighter) and Fe2Nb/Fe2Mo-Laves (darker) phases are uniformly distributed. After the 200 h aging, a bulky σ phase is formed as identified according to reference [11], showing a brightness between B2 phase and Laves phase and lengths up to 4 μm at the grain boundaries. By examining Figure 4, Nb0.06Ni3.0Mo0.2 and Nb0.03Ta0.03Ni3.2Mo0.2 with higher Mo contents have more Laves phases and σ phases than Nb0.06Ni3.0Mo0.07 and Nb0.03Ta0.03Ni3.2Mo0.07 with lower Mo contents, indicating that the decrease in Mo effectively decreases the precipitation of the Laves phase and the σ phase. The effect of decreasing Mo can be observed even more clearly by comparing the secondary electron morphologies of 2-4 Nb0.06Ni3.2Mo0.2 (high Mo) and 2-6 Nb0.06Ni3.2Mo0.07 (low Mo) after aging at 800 °C/200 h, as shown in Figure 5.
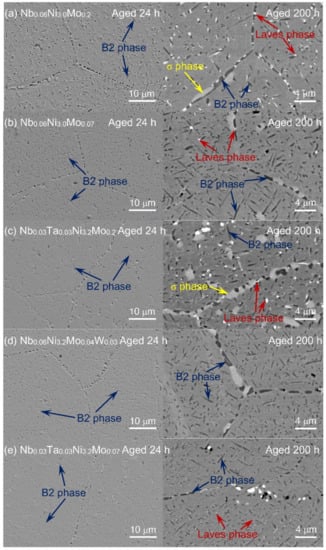
Figure 4.
The SEM morphologies of the designed alloys: secondary electron images after aging at 800 °C for 24 h (left) and backscattered images after aging at 800 °C for 200 h (right). (a): Nb0.06Ni3.0Mo0.2, (b): Nb0.06Ni3.0Mo0.07, (c): Nb0.03Ta0.03Ni3.2Mo0.2, (d): Nb0.06Ni3.2Mo0.04W0.03, (e): Nb0.03Ta0.03Ni3.2Mo0.07.
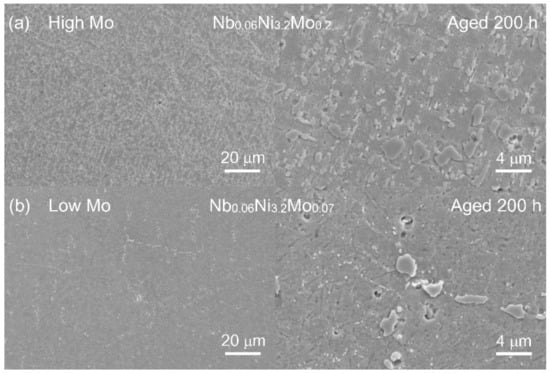
Figure 5.
The secondary electron images in different magnification of (a) 2-4 Nb0.06Ni3.2Mo0.2 (higher Mo content) and (b) 2-6 Nb0.06Ni3.2Mo0.07 (lower Mo content) after being aged at 800 °C for 200 h.
Figure 6 gives the composition distribution in terms of Creq and Nieq on a Schaeffler constitution diagram of the two alloy series, designed according to the cluster formula as well as typical Al-modified austenitic stainless steels for high-temperature oxidation purpose reported in the literature [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,39,40,41,42,43,44]. Uggowitzer’s equivalent equations are used to calculate their Cr equivalent and Ni equivalent. It can be seen that this kind of Al-contained stainless steel mostly falls in the pure austenite region and the austenite plus 5 vol.% ferrite dual-phase region. The most concentrated region is the area between 20–30 Creq and 22–32 Nieq, shown as the dashed rectangle. Within this concentrated region, relatively stable austenite can be achieved. The designed alloy series also fall within this region.
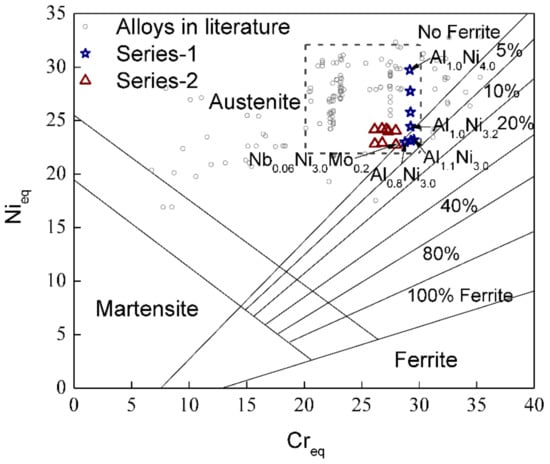
Figure 6.
The distribution of the designed alloy series and as many as 190 Al-modified austenitic stainless steels in literature [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,39,40,41,42,43,44] on a Schaeffler constitution diagram. The dashed rectangle shows the most concentrated region of these compositions. Ni and Cr equivalent are calculated according to Uggowitzer’s equations [28]: Nieq = % Ni + % Co + 0.1% Mn − 0.01 Mn2 + 18% N + 30% C; Creq = % Cr + 1.5% Mo + 1.5% W + 0.48% Si + 2.3% V + 1.75 Nb + 2.5% Al.
The austenite stability of our designed alloys is consistent with the prediction by the Schaeffler constitution diagram combined with Uggowitzer’s equivalent equations, as shown in Figure 6. The reference composition Al0.8Ni3.0 (1-1) from ONRL, which is located on the boundary of austenite and ferrite regions, maintains a single austenitic structure. With the increase in Al, Al1.0Ni3.0 (1-2) and Al1.1Ni3.0 (1-3), which have similar Nieq to Al0.8Ni3.0 (1-1) but gradually higher Creq, fall in the dual-phase region containing 5 vol.% ferrite. By increasing the amount of Ni, Al1.0Ni3.2–4.0 (1-4–1-7) return to the pure austenite region. The microstructural characterization indicated that ferrite was only observed in Al1.0Ni3.0 (1-2) and Al1.1Ni3.0 (1-3), which agreed with their locations on the Schaeffler constitution diagram. A slight increase in Ni as Al1.0Ni3.2 (1-4) is enough for austenite stability, which also means that alloys such as Al1.0Ni3.4–4.0 (1-6–1-7) contain excessive Ni contents. This is why NbxTa0.06−xNi3.0(Mo,W)z (2-1–2-3) with similar Nieq but lower Creq than Al0.8Ni3.0 (1-1) and NbxTa0.06−xNi3.2(Mo,W)z (2-5–2-8) with similar Nieq but lower Creq than Al1.0Ni3.2 (1-4) all fall into the pure austenite region. These results confirm the sufficient austenite stability of the series-2 alloys satisfying the cluster formula.
4.2. Hardness
The hardness of designed alloys versus Nieq/Creq and (Nieq2 + Creq2)1/2 are plotted in Figure 7a,b, respectively. The equivalent ratio Nieq/Creq reflects the stability of austenite relative to ferrite. The larger the ratio, the higher the relative austenite stability of an alloy is. It is noticed from Figure 7a that alloys with Nieq/Creq ≥ 0.8 all have single austenitic structure, while Al1.0Ni3.0 (1-2) and Al1.1Ni3.0 (1-3) with Nieq/Creq < 0.8 possess austenite-ferrite dual phase structure. (Nieq2 + Creq2)1/2 is the distance from point zero to the composition point on Schaeffler constitution diagram, which reflects the amount of alloying elements in the corresponding compositions.
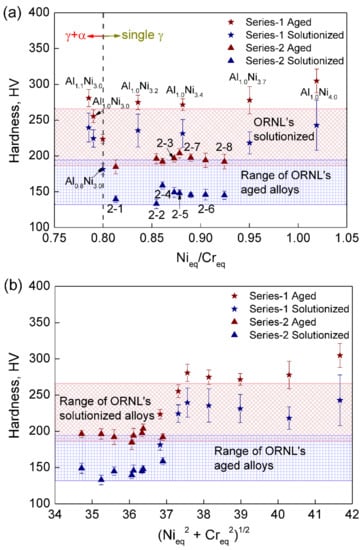
Figure 7.
Variations of microhardness HV vs. (a) relative austenite stability Nieq/Creq and (b) (Nieq2 + Creq2)1/2 which reflects the amount of alloying elements. The shaded regions show the ranges of estimated hardness of Oak Ridge National Laboratory’s (ORNL) alloys calculated based on their tensile results [13,18,23] according to the conversion equations between the hardness and tensile strength of austenitic stainless steels [45].
All alloys in series-2 have similar hardness, approximately 200 HV after being aged at 800 °C/24 h and 150 HV after being solutionized at 1250 °C/1.5 h. This is because their composition difference is quite small. It is noticed that the series-2 alloys, which possess smaller (Nieq2 + Creq2)1/2 and henceforth less alloying elements than series-1, generally have lower hardness than those in series-1. This is because the decreases of C (from 0.1 wt. % to 0.08 wt. %), Nb (from 1.6 wt. % to 0.64/0.32 wt. %) and even Al (from as high as 3.4 wt. % to 2.75 wt. %) in series-2 lead to the decrease in the amounts of strengthening phases such as NbC, NiAl, and Fe2Nb. In addition, the strengthening effect of NiAl-B2, Fe2Nb/Fe2Mo-Laves and also MC (mainly NbC) is reflected by the increase of 30–70 HV of hardness after aging in comparison with samples in the solutionizing state.
According to the tensile test results of ORNL at room temperature, the yield and ultimate strength of the solutionized AFA4-1 (4Al/0.6Nb/0.1Ti) are, respectively, 270 MPa and 600 MPa [13]; those of solutionized B-1.0 (Fe-2.87Al-0.14Si-1.01Nb-20.11Ni-1.93Mn-14.24Cr-2.00Mo-0.99W-0.47Cu-0.10C) are, respectively, 261 MPa and 613 MPa [18]; those of 20Ni-(3-4)Al-(0.6-1)Nb based AFA stainless steels are 237–282 MPa (yield strength/solutionizing state), 568–660 MPa (ultimate strength/solutionizing state), 422–434 MPa (yield strength/aging state) and 744–811 MPa (ultimate strength/aging state) [23]. For easy comparison, the tensile results of ORNL are converted to hardness, referring to the study on the conversion relationship between the hardness and tensile strength of austenitic stainless steels by Chen et al. [45], yield strength RP0.2 = 3.4 × HV − 212.90 and ultimate strength Rm = 2.1 × HV + 252.46. The estimated hardness of ORNL’s AFA stainless steels are therefore calculated as 132.32–194.07 HV under solutionizing state and 186.74–265.97 HV under aging state (shown as the shaded regions in Figure 7). It should be noted that both the ONRL’s and our designed series-1 alloys have a higher content of C (0.1 wt. % C) than the 0.08 wt. % C in series-2. Comparing the hardness of both solutionizing and aging state, the hardness of our series-1 alloys is generally higher than that of the ORNL, while the series-2 alloys with lower C/Nb/Al and thus fewer strengthening precipitates (such as MC, NiAl-B2 phase and Fe2Nb/Fe2Mo-Laves phase) still have hardness within the estimated range of the ORNL.
4.3. High-Temperature Oxidation Resistance
High-temperature oxidation resistance tests were performed on all bar samples of series-2 NbxTa0.06-xNiy(Mo,W)z (x = 0.03 or 0.06, y = 3.0 or 3.2, z = 0.07 or 0.2 in 16 atoms cluster) alloys in air at 800 °C. Their mass changes per unit area after 0, 25, 50, 75, 100, 150 and 200 h are plotted in Figure 8. From a general view, except Nb0.06Ni3.0Mo0.07 (2-2), the oxidation weight gains are relatively smooth and are less than 0.5 mg/cm2 within 200 h. However, Nb0.06Ni3.0Mo0.07 (2-2), which has the most obvious oxidation weight gain in the first 50 h, experiences continuous and severe weight losses after that. Considering the exfoliation of the oxide layer observed during the weighting procedure of Nb0.06Ni3.0Mo0.07 (2-2) bar, it can be inferred that the abnormal weight loss is highly likely to be caused by the splashing of oxide exfoliation out of the crucible.
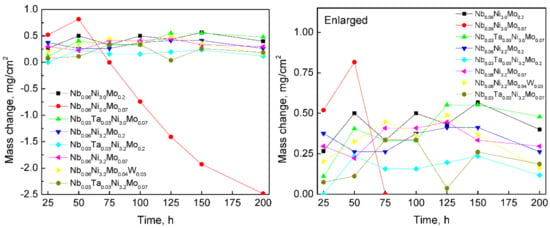
Figure 8.
The mass change of designed series-2 alloys after being oxidized in air at 800 °C for up to 200 h.
The vertical range between 0 and 1.0 mg/cm2 is magnified for a close-up view of the alloys’ mass change. It can be observed that the smallest weight gain is possessed by Nb0.03Ta0.03Ni3.2Mo0.2 (2-5) and Nb0.03Ta0.03Ni3.2Mo0.07 (2-8) alloys, which are the alloys with high Ni content and containing Ta, followed by Nb0.06Ni3.2Mo0.04W0.03 (2-7), which also has high Ni and contains W. This indicates that the addition of Ta and W has a positive effect on improving the high-temperature oxidation resistance of AFA stainless steels. The effect of Ta on the oxidation resistance of AFA stainless steels at 800 °C is consistent with our team’s previous study [46].
According to the Chinese aircraft industry’s standard testing method of oxidation resistance for steels and superalloys (HB 5258-2000) [47], the level of oxidation resistance is determined by calculating the average oxidation rate per surface area of a sample, together with its average oxidation-peeling mass per surface area. The average oxidation rate and average oxidation-peeling mass of series-2 alloys, during 200 h oxidation, are calculated as listed in Table 3. The standard classifies our designed AFA stainless steels, with average oxidation rate <0.1 g/m2 × h and average oxidation-peeling mass <1.0 g/m2, to a complete oxidation resistance level, except Nb0.06Ni3.0Mo0.07 (2-2) which has an abnormal weight loss.

Table 3.
The average oxidation rate and oxidation-peeling mass of designed series-2 alloys.
Figure 9 gives the back-scattered electron images of Nb0.06Ni3.0Mo0.2 (2-1), Nb0.06Ni3.0Mo0.07 (2-2), Nb0.03Ta0.03Ni3.2Mo0.2 (2-5), Nb0.06Ni3.2Mo0.04W0.03 (2-7), Nb0.03Ta0.03Ni3.2Mo0.07 (2-8) after oxidizing in air at 800 °C for 200 h, to show the cross section of their oxide layers. It can be seen that not all their oxide layers are uniform. Some oxide layers have an Fe-rich oxide nodule according to the elemental analysis by SEM. Nb0.06Ni3.2Mo0.04W0.03 (2-7) has the most even and uniform oxide layer among these alloys, without any nodules. In addition, a large number of black particles appeared below the oxide layer, along with the needle-like NiAl-B2 phase. Among them, Nb0.06Ni3.2Mo0.04W0.03 (2-7) has the least number of black particles with a thickness of approximately 70 μm, while the others possess thickness more than 250 μm. It indicates that Nb0.06Ni3.2Mo0.04W0.03 (2-7) has the most uniform oxide layer and the least internal particles.
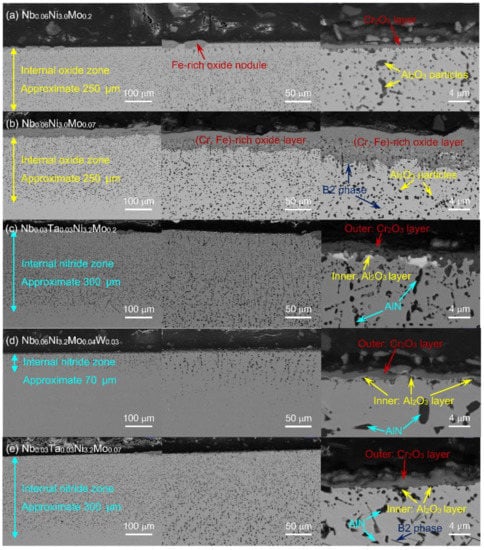
Figure 9.
SEM back-scattered electron cross-section images of oxide layers after 200 h oxidation at 800 °C of series-2 alloys. (a): Nb0.06Ni3.0Mo0.2, (b): Nb0.06Ni3.0Mo0.07, (c): Nb0.03Ta0.03Ni3.2Mo0.2, (d): Nb0.06Ni3.2Mo0.04W0.03, (e): Nb0.03Ta0.03Ni3.2Mo0.07.
Figure 10 is the SEM-EDS cross-section elemental mapping of Nb0.06Ni3.2Mo0.04W0.03 (2-7), Nb0.03Ta0.03Ni3.2Mo0.07 (2-8) and Nb0.06Ni3.0Mo0.2 (2-1), which, respectively, represent W-containing, Ta-containing, and (Ta, W)-free designed alloys. Composition mapping analysis of other alloys with relevant elements shows quite similar tendencies. It is observed that the distribution of elements is quite different between the Ta/W-containing and (Ta, W)-free alloys. In both of the Ta/W-containing alloys, the internal particles observed in SEM images are enriched with the elements Al and N, presumably AlN. More importantly, a continuous Al2O3 layer is formed under the outer (Cr, Fe)-rich oxide layer. No oxygen concentration is observed in the matrix below the oxide layer of Ta/W-containing alloys, as shown in Figure 10a,b. In contrast, the oxide layer of the (Ta, W)-free alloy is enriched with Cr and O, without Al, while the internal particles are composed of Al and O, as shown in Figure 10c.
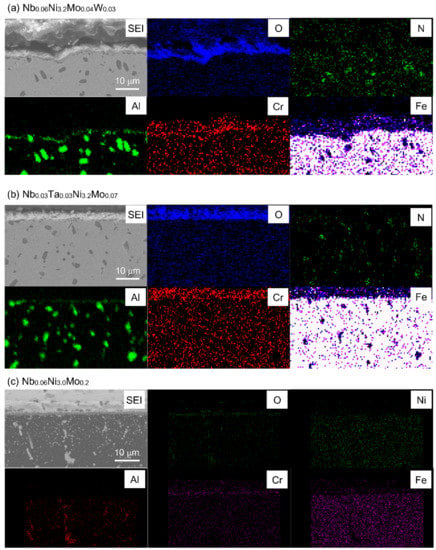
Figure 10.
SEM-EDS cross-section elemental mapping of typical W-containing, Ta-containing and (Ta, W)-free alloys. (a): Nb0.06Ni3.2Mo0.04W0.03, (b): Nb0.03Ta0.03Ni3.2Mo0.07, (c): Nb0.06Ni3.0Mo0.2.
By combining the SEM cross-section images and the corresponding elemental mapping results, we can see that Cr2O3-type layers with internal Al2O3 particles are formed in (Ta, W)-free alloys, as shown in Figure 9a,b. Different from the above, the Ta/W-containing alloys have continuous protective Al2O3 layers that inhibit oxygen from further diffusion inwards, so that internal AlN particles are formed instead of Al2O3. The internal nitride zone of Nb0.06Ni3.2Mo0.04W0.03 (2-7) is much thinner than those of Nb0.03Ta0.03Ni3.2Mo0.2 (2-5) and Nb0.03Ta0.03Ni3.2Mo0.07 (2-8), as shown in Figure 9c–e. It seems to indicate that W has a much stronger inhibiting effect on nitrogen diffusion, compared to Ta. Considering the relatively high average oxidation-peeling mass of Nb0.06Ni3.2Mo0.04W0.03, W additionally seems to promote the peeling of the outer Cr2O3-type layer. In conclusion, the addition of Ta/W is beneficial for improving the high-temperature oxidation resistance of AFA stainless steels. The addition of both Ta and W will be considered to investigate their co-effect on the oxidation behavior of AFA stainless steels in the further work.
5. Conclusions
Based on a 16-atom cluster formula, which was obtained by studying as many as 190 alumina-forming austenitic (AFA) stainless steels, a series of AFA stainless steels alloys, with 0.08 wt. % C, [(Al0.89Si0.05NbxTa0.06−x)-(Fe11.7−yNiyMn0.3)]Cr3.0−z(Mo,W)z, x = 0.03 or 0.06, y = 3.0 or 3.2, z = 0.07 or 0.2, were designed to verify the guiding function of the cluster formula to achieve austenitic structure and further to investigate the effect of minor alloying elements Nb, Ta, Mo, W on the high-temperature oxidation resistance. It is found that:
- All samples exhibit single austenitic structure both after solutionizing at 1250 °C/1.5 h and aging at 800 °C/24 h.
- The hardness, under a load of 500 g, is approximately 150 HV at solutionizing state and 200 HV at aging state, which falls in the estimated range of Oak Ridge National Laboratory.
- After being air oxidized at 800 °C for up to 200 h, most samples can be classified to complete oxidation resistance level for their low oxidation rate, below 0.1 g/m2 × h, together with low oxidation-peeling mass, below 1.0 g/m2. Among them, Nb0.03Ta0.03Ni3.2Mo0.2, Nb0.03Ta0.03Ni3.2Mo0.07 and Nb0.06Ni3.2Mo0.04W0.03, which possess continuous Al2O3 layers with internal AlN particles, possess the lowest weight gain and thus the best high-temperature oxidation resistance. In contrast, the alloys without Ta and W have Cr2O3-type layers and internal Al2O3 particles.
- The addition of Ta or W promotes the formation of a continuous protective Al2O3 layer that inhibits oxygen from further diffusion inwards. In addition, W seems to inhibit nitrogen diffusion and additionally promote oxidation peeling, which is inferred from the thinnest internal nitride zone and highest oxidation peeling mass of Nb0.06Ni3.2Mo0.04W0.03, among all Ta/W containing alloys.
Author Contributions
C.D. conceived and designed the experiments; S.Z. performed the experiments, analyzed the data, and wrote the paper; D.D., Q.W. and R.Y. discussed the results and modified the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (51801017), the State Key Lab of Advanced Metals and Materials (2018-Z03), the National Natural Science Foundation of China (No. 11674045) and the Science Challenge Project (TZ2016004).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due the data also forms part of an ongoing study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nie, S.H.; Chen, Y.; Ren, X.; Sridharan, K.; Allen, T.R. Corrosion of alumina-forming austenitic steel Fe–20Ni–14Cr–3Al–0.6Nb–0.1Ti in supercritical water. J. Nucl. Mater. 2010, 399, 231–235. [Google Scholar] [CrossRef]
- Kondo, K.; Miwa, Y.; Okubo, N.; Kaji, Y.; Tsukada, T. Development of corrosion-resistant improved Al-doped austenitic stainless steel. J. Nucl. Mater. 2011, 417, 892–895. [Google Scholar] [CrossRef]
- Xing, L.L.; Zheng, Y.J.; Yang, W.W.; Shao, M.Z.; Cui, L.S.; Lu, G.W. Effect of aluminum on high temperature oxidation resistance of alloy HK40. Corros. Sci. Prot. Technol. 2012, 24, 20–24. [Google Scholar]
- Fujioka, T.; Kinugasa, M.; Iizumi, S.; Teshima, S.; Shimizu, I. Oxidation-resisting austenitic stainless steel. U.S. Patent 4,108,641, 22 August 1978. [Google Scholar]
- Pivin, J.C.; Delaunay, D.; Roques-Carmes, C.; Huntz, A.M.; Lacombe, P. Oxidation mechanism of Fe-Ni-20-25Cr-5Al alloys-influence of small amounts of yttrium on oxidation kinetics and oxide adherence. Corros. Sci. 1980, 20, 351–373. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Mcgurty, J.A.; Jayaraman, N. Oxidation of high-aluminum austenitic stainless steels. Oxid. Met. 1988, 30, 185–200. [Google Scholar] [CrossRef]
- Satyanarayana, D.V.V.; Malakondaiah, G.; Sarma, D.S. Steady state creep behaviour of NiAl hardened austenitic steel. Mater. Sci. Eng. A 2002, 323, 119–128. [Google Scholar] [CrossRef]
- Pint, B.A.; Peraldi, R.; Maziasz, P.J. The use of model alloys to develop corrosion-resistant stainless steels. Mater. Sci. Forum 2004, 461, 815–822. [Google Scholar] [CrossRef]
- Adams, T.M.; Korinko, P.; Duncan, A. Evaluation of oxidation and hydrogen permeation in Al-containing stainless steel alloys. Mater. Sci. Eng. A 2006, 424, 33–39. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Pint, B.A. Effects of minor alloy additions and oxidation temperature on protective alumina scale formation in creep-resistant austenitic stainless steels. Scripta Mater. 2007, 57, 1117–1120. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Liu, C.T.; Takeyama, M.; Maziasz, P.J.; Pint, B.A. Alumina-forming austenitic stainless steels strengthened by Laves phase and MC carbide precipitates. Metall. Mater. Trans. A 2007, 38, 2737–2746. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Pint, B.A.; Liu, C.T.; Lu, Z.P.; Bei, H. The development of alumina-forming austenitic stainless steels for high-temperature structural use. JOM 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Development of alumina-forming austenitic stainless steels. In Proceedings of the 22nd Annual Conference on Fossil Energy Materials, Pittsburgh, PA, USA, 8–10 July 2008. [Google Scholar]
- Yamamoto, Y.; Takeyama, M.; Lu, Z.P.; Liu, C.T.; Evans, N.D.; Maziasz, P.J.; Brady, M.P. Alloying effects on creep and oxidation resistance of austenitic stainless steel alloys employing intermetallic precipitates. Intermetallics 2008, 16, 453–462. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Walker, L.R. Composition, microstructure, and water vapor effects on internal/external oxidation of alumina-forming austenitic stainless steels. Oxid. Met. 2009, 72, 311–333. [Google Scholar] [CrossRef]
- Pint, B.A.; Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Howe, J.Y.; Trejo, R.; Maziasz, P.J. Development of alumina-forming austenitic alloys for advanced recuperators. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea, and Air, Orlando, FL, USA, 8–12 June 2009; pp. 271–280. [Google Scholar]
- Yamamoto, Y.; Santella, M.L.; Brady, M.P.; Bei, H.; Maziasz, P.J. Effect of alloying additions on phase equilibria and creep resistance of alumina-forming austenitic stainless steels. Metall. Mater. Trans. A 2009, 40, 1868–1880. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Santella, M.L.; Liu, C.T.; Evans, N.D.; Maziasz, P.J.; Brady, M.P. Evaluation of Mn substitution for Ni in alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2009, 524, 176–185. [Google Scholar] [CrossRef]
- Bei, H.; Yamamoto, Y.; Brady, M.P.; Santella, M.L. Aging effects on the mechanical properties of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2010, 527, 2079–2086. [Google Scholar] [CrossRef]
- Brady, M.P.; Unocic, K.A.; Lance, M.J.; Santella, M.L.; Yamamoto, Y.; Walker, L.R. Increasing the upper temperature oxidation limit of alumina forming austenitic stainless steels in air with water vapor. Oxid. Met. 2011, 75, 337–357. [Google Scholar] [CrossRef]
- Pint, B.A.; Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Matthews, W.J. Evaluation of alumina-forming austenitic foil for advanced recuperators. J. Eng. Gas Turb. Power 2011, 133, 102301–102302. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Overview of strategies for high-temperature creep and oxidation resistance of alumina-forming austenitic stainless steels. Metall. Mater. Trans. A 2011, 42, 922–931. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Muralidharan, G.; Brady, M.P. Development of L12-ordered Ni3(Al,Ti)-strengthened alumina-forming austenitic stainless steel alloys. Scripta Mater. 2013, 69, 816–819. [Google Scholar] [CrossRef]
- Hull, F.C. Delta ferrite and martensite formation in stainless steels. Weld. J. 1973, 52, 193–203. [Google Scholar]
- Pickering, F.B. Physical Metallurgy and the Design of Steels; Applied Science Publishers Ltd.: Essex, UK, 1978; pp. 62–66. [Google Scholar]
- Tchizhik, A.A.; Tchizhik, T.A.; Tchizhik, A.A. Optimization of the heat treatment for steam and gas turbine parts manufactured from 9–12% Cr steels. J. Mater. Process. Technol. 1998, 77, 226–232. [Google Scholar] [CrossRef]
- Uggowitzer, P.J.; Bähre, W.-F.; Wohlfromm, H.; Speidel, M.O. Nickel-free high nitrogen austenitic stainless steels produced by metal injection moulding. Mater. Sci. Forum 1999. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Dong, D.D.; Wang, Q.; Dong, C.; Yang, R. Composition design of alumina-forming austenitic stainless steels based on cluster-plus-glue-atom model. Acta Metall. Sin. 2021. under review. [Google Scholar]
- Cowley, J.M. Short- and long-range order parameters in disordered solid solutions. Phys. Rev. 1960, 120, 1648–1657. [Google Scholar] [CrossRef]
- Dong, C.; Qiang, J.B.; Yuan, L.; Wang, Q.; Wang, Y.M. A cluster-plus-glue-atom model for composition design of complex alloys. Chin. J. Nonferrous Met. 2011, 21, 2502–2510. [Google Scholar]
- Dong, C.; Dong, D.D.; Wang, Q. Chemical units in solid solutions and alloy composition design. Acta Metall. Sin. 2018, 54, 293–300. [Google Scholar]
- Wang, Q.; Zha, Q.F.; Liu, E.X.; Dong, C.; Chun, J.I. Composition design of high-strength martensitic precipitation hardening stainless steels based on a cluster model. Acta Metall. Sin. 2012, 48, 1201. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.J.; Wang, Y.M.; Qiang, J.B.; Dong, C. β-Ti alloys with low Young’s moduli interpreted by cluster-plus-glue-atom model. Metall. Mater. Trans. A 2013, 44, 1872–1879. [Google Scholar] [CrossRef]
- Hong, H.L.; Wang, Q.; Dong, C.; Liaw, P.K. Understanding the Cu-Zn brass alloys using a short-range-order cluster model: Significance of specific compositions of industrial alloys. Sci. Rep. 2014, 4, 7065. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Li, C.L.; Santodonato, L.J.; Feygenson, M.; Dong, C.; Liaw, P.K. Chemical short-range orders and the induced structural transition in high-entropy alloys. Scripta Mater. 2018, 144, 64–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Dong, H.G.; Dong, C.; Zhang, H.Y.; Sun, X.F. Nickel-based single-crystal superalloys (Ni,Co)-Al-(Ta,Ti)-(Cr,Mo,W) designed by cluster-plus-glue-atom model and their 1000h long-term aging behavior at 900 °C. Acta Metall. Sin. 2018, 54, 591–602. [Google Scholar]
- Jiang, B.B.; Wen, D.H.; Wang, Q.; Che, J.D.; Dong, C. Design of near-α Ti alloys via a cluster formula approach and their high-temperature oxidation resistance. J. Mater. Sci. Technol. 2019, 35, 1008–1016. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Chen, G.; Lu, Z. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, X.; Lu, Z.P. Effects of silicon additions on the oxide scale formation of an alumina-forming austenitic alloy. Corros. Sci. 2012, 65, 317–321. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, X.; Lu, Z.P. Roles of manganese in the high-temperature oxidation resistance of alumina-forming austenitic steels at above 800 °C. Oxid. Met. 2012, 78, 349–362. [Google Scholar] [CrossRef]
- Zhou, M.X. Microstructure of hot rolled high aluminum 304, 316L austenitic stainless steel and the mechanism of aluminum elements. Cailiao Rechuli Xuebao/Trans. Mater. Heat Treat. 2012, 35, 55–60. [Google Scholar]
- Sa, X.R. Properties and Mechanism of Elements of Cast High Aluminum 304, 316 L, 310S Steel; Lanzhou University of Technology: Lanzhou, China, 2013. [Google Scholar]
- Yao, L. Effect of Al on Microstructure and Properties of 17-7PH, 2205 Stainless Steels and Its Mechanism; Lanzhou University of Technology: Lanzhou, China, 2013. [Google Scholar]
- Chen, B.C.; Li, G.F.; Yang, W. Conversion relation of Leeb-hardness, vickers-hardness and strength of austenitic stainless steels. Mater. Mech. Eng. 2009, 33, 37–40. [Google Scholar] [CrossRef]
- Wen, D.H.; Li, Z.; Jiang, B.B.; Wang, Q.; Chen, G.Q.; Tang, R.; Zhang, R.Q.; Dong, C.; Liaw, P.K. Effects of Nb/Ti/V/Ta on phase precipitation and oxidation resistance at 1073 K in alumina-forming austenitic stainless steels. Mater. Charact. 2018, 144, 86–98. [Google Scholar] [CrossRef]
- Aviation Industry Corporation of China. Testing method of oxidation resistance for steels and superalloys. In Chinese Aircraft Industry Standard; No. HB 5258-2000; Commission of Science, Technology and Industry for National Defense: Beijing, China, 2000. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).