Mechanism of Phosphorus Enrichment in Dephosphorization Slag Produced Using the Technology of Integrating Dephosphorization and Decarburization
Abstract
1. Introduction
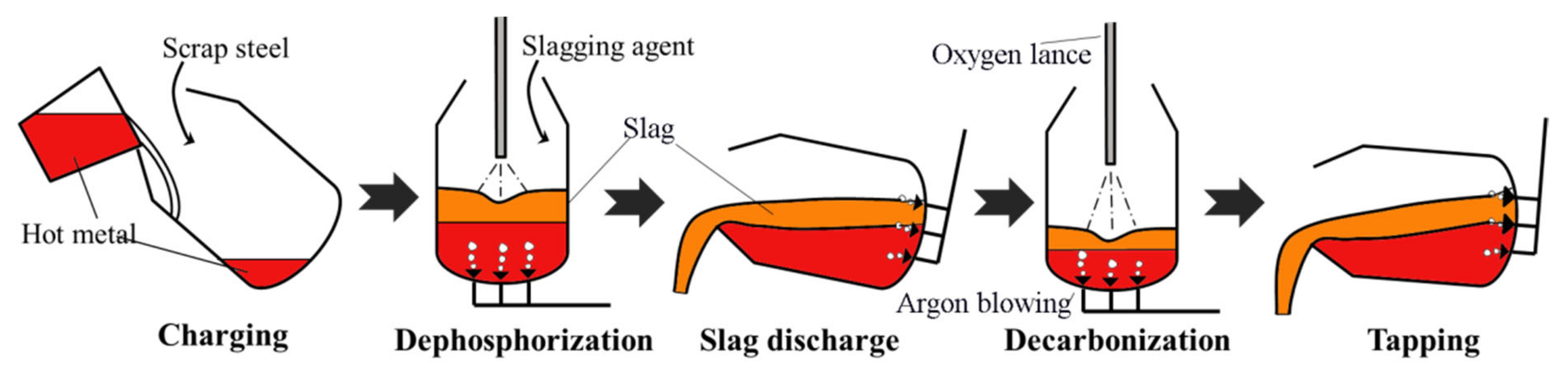
2. Technical Details for the IDDSC Process
3. Thermodynamic Calculations for Dephosphorization
4. Sampling and Analysis
5. Experimental Results
5.1. Compositions of Slag and Hot Metal
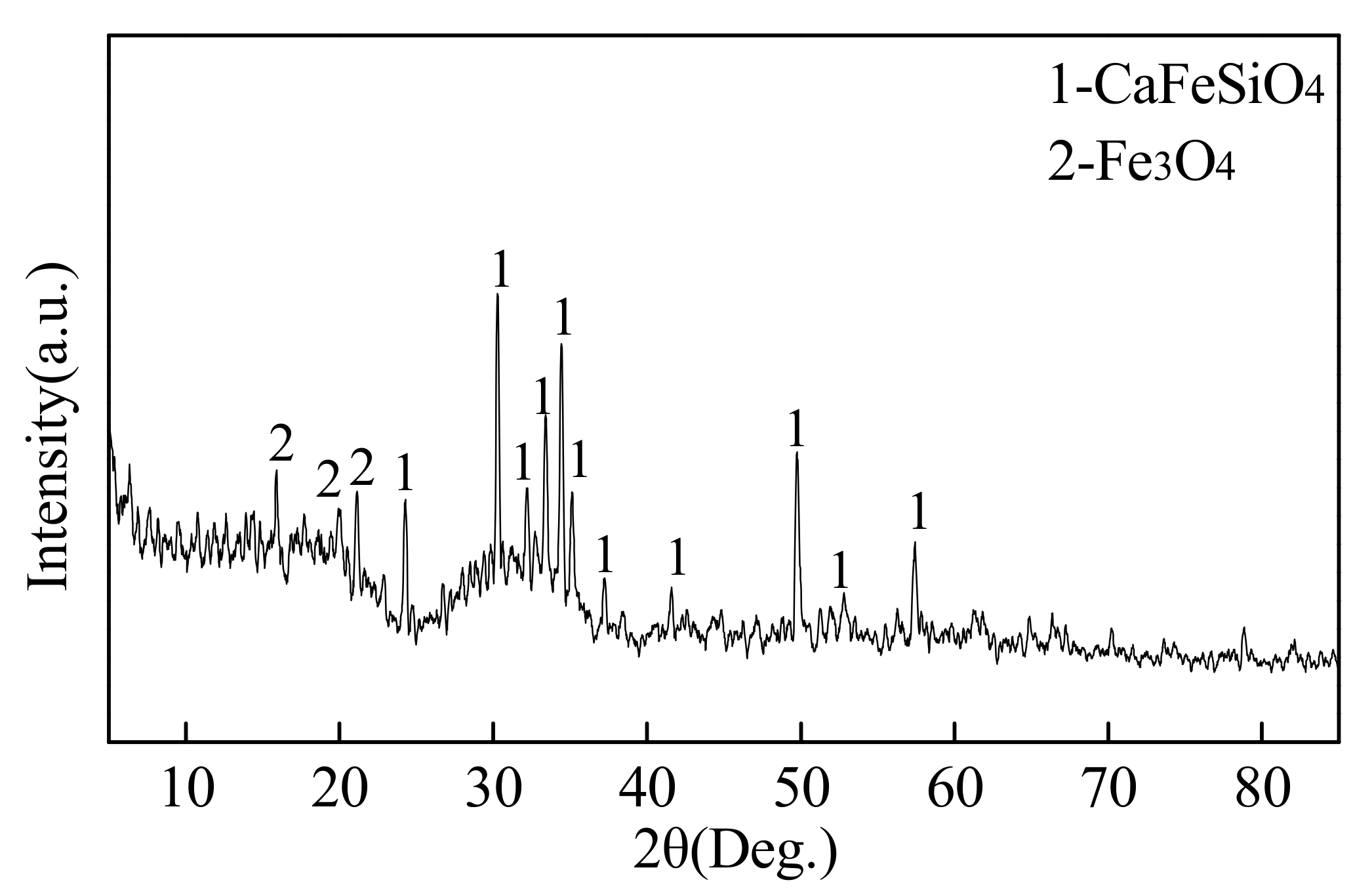
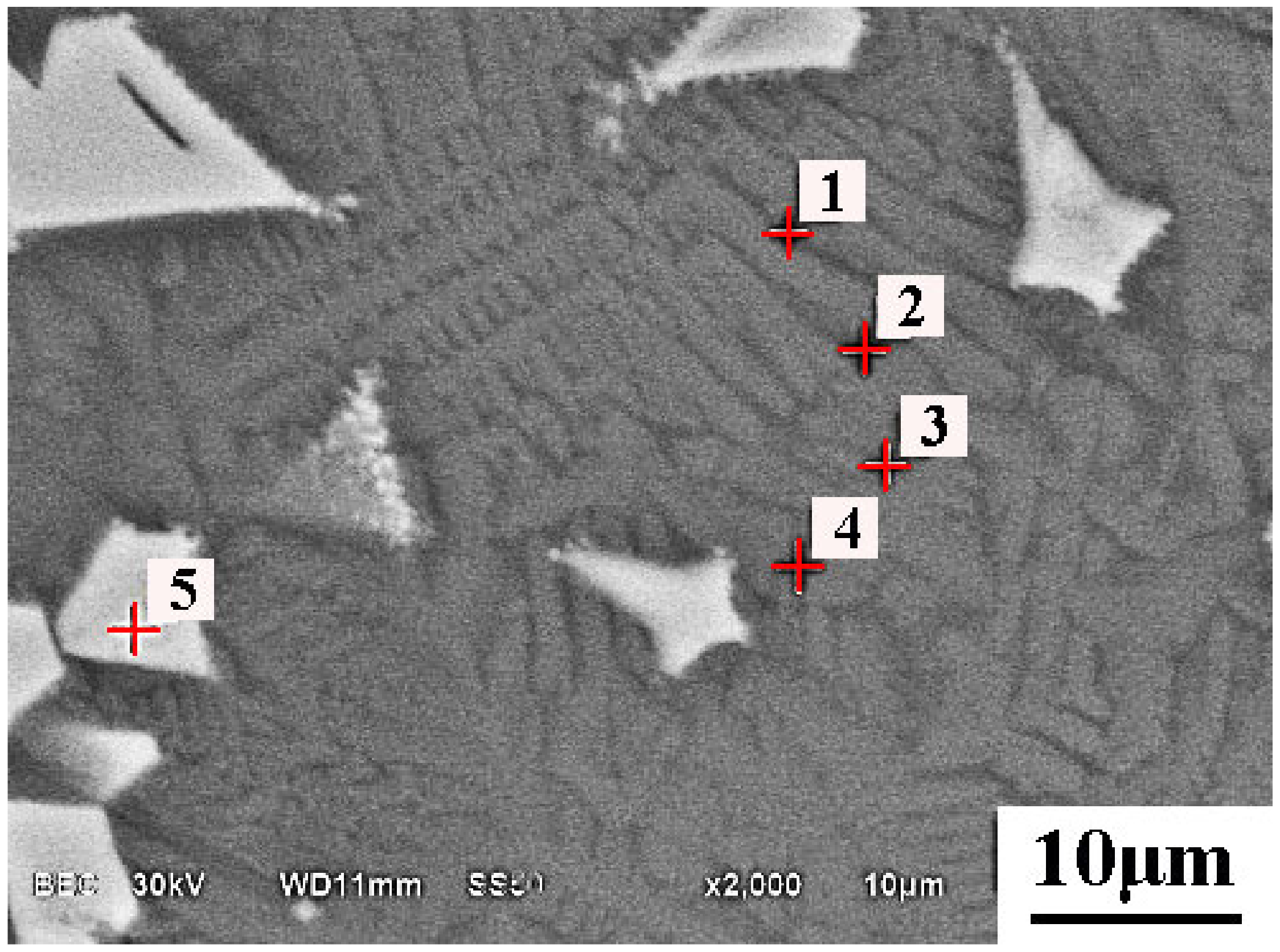
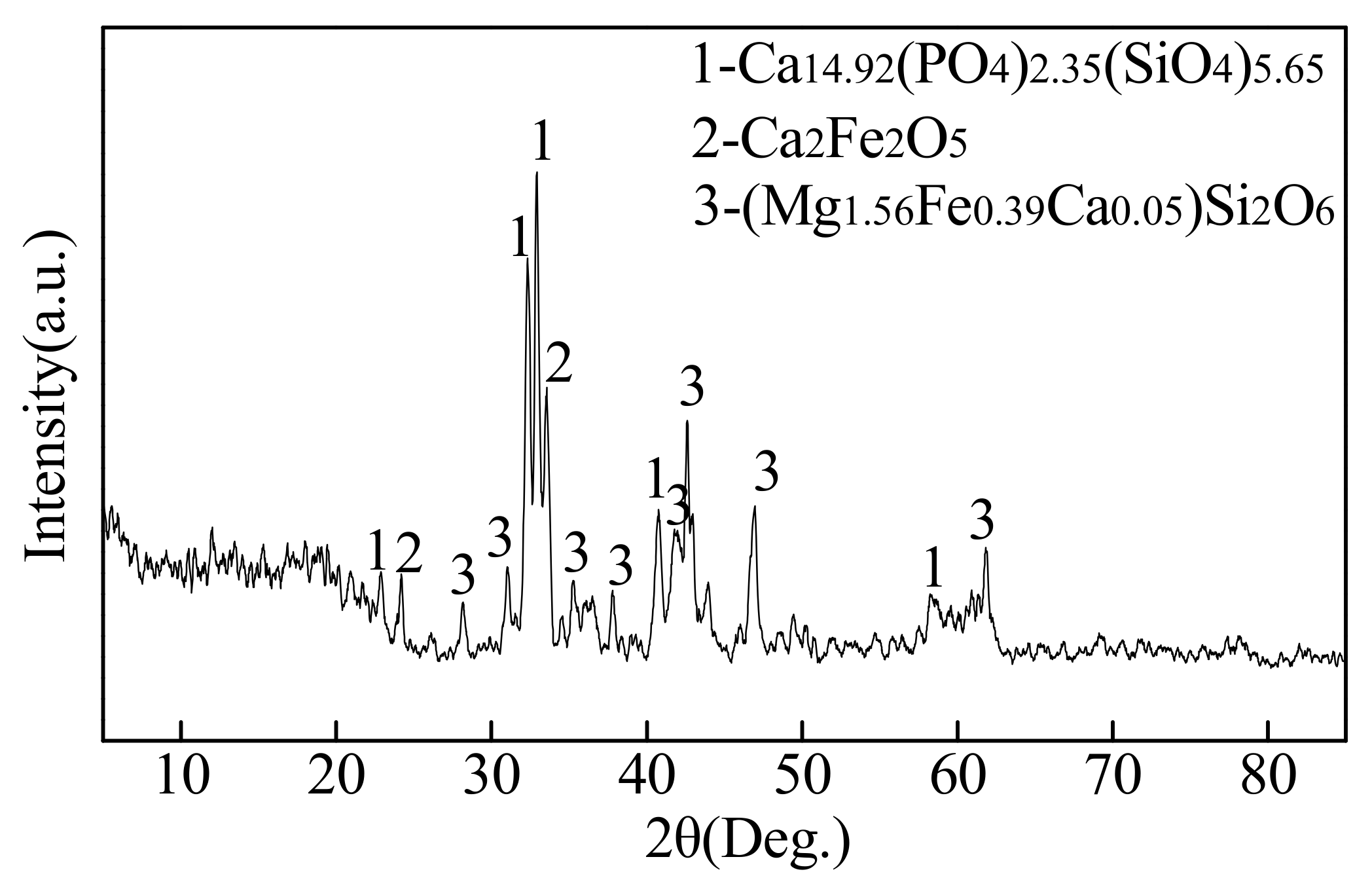
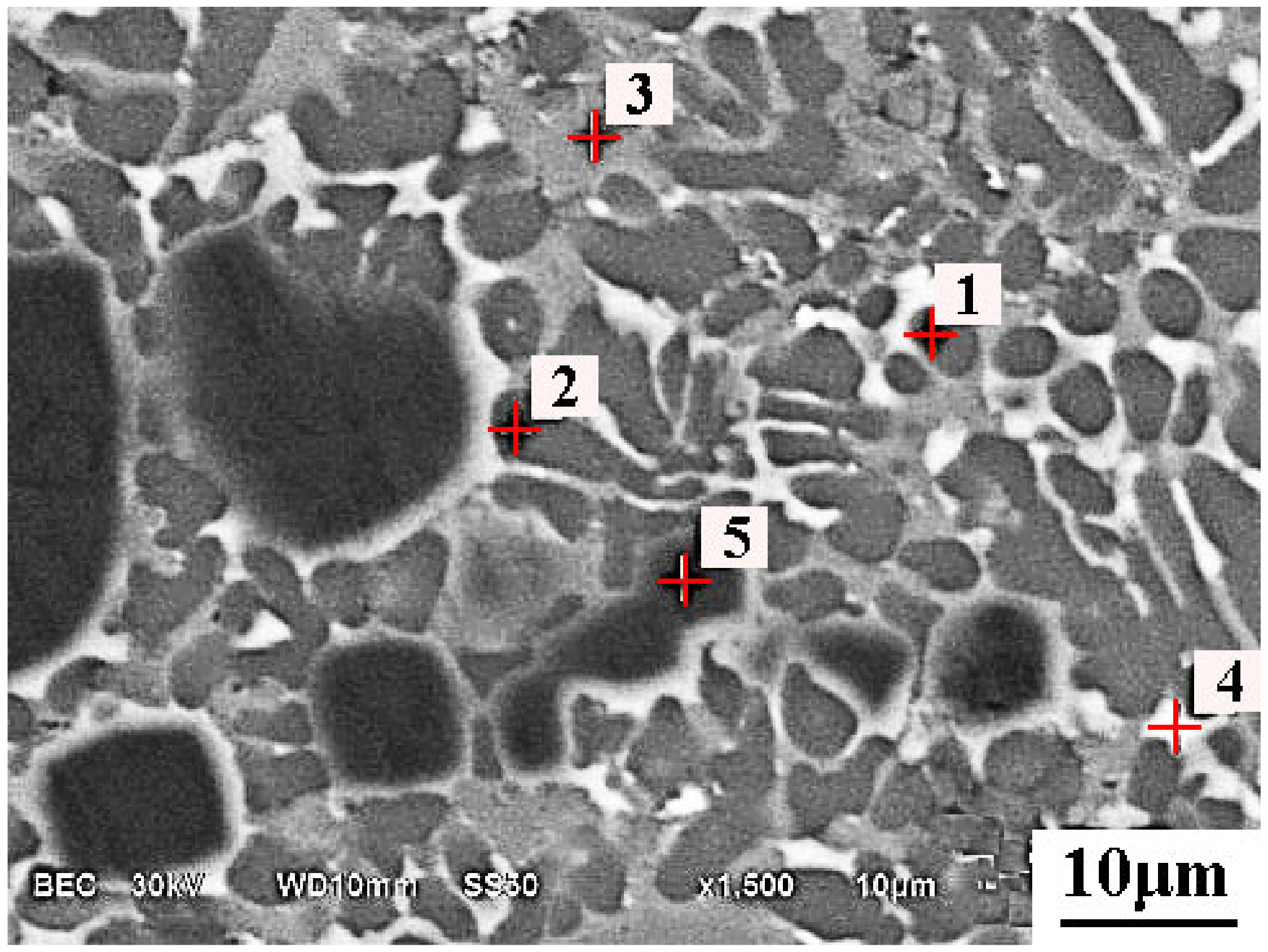
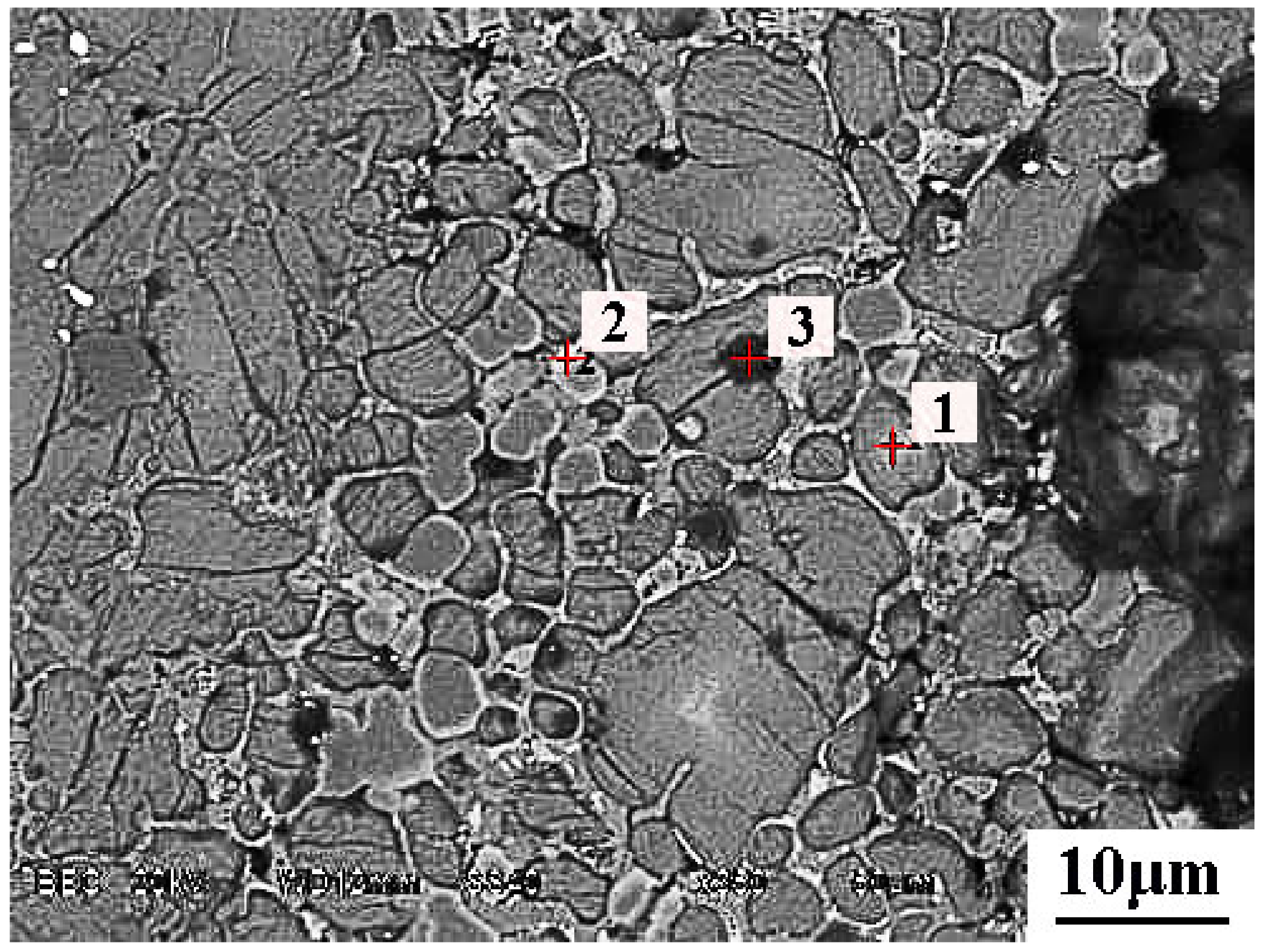
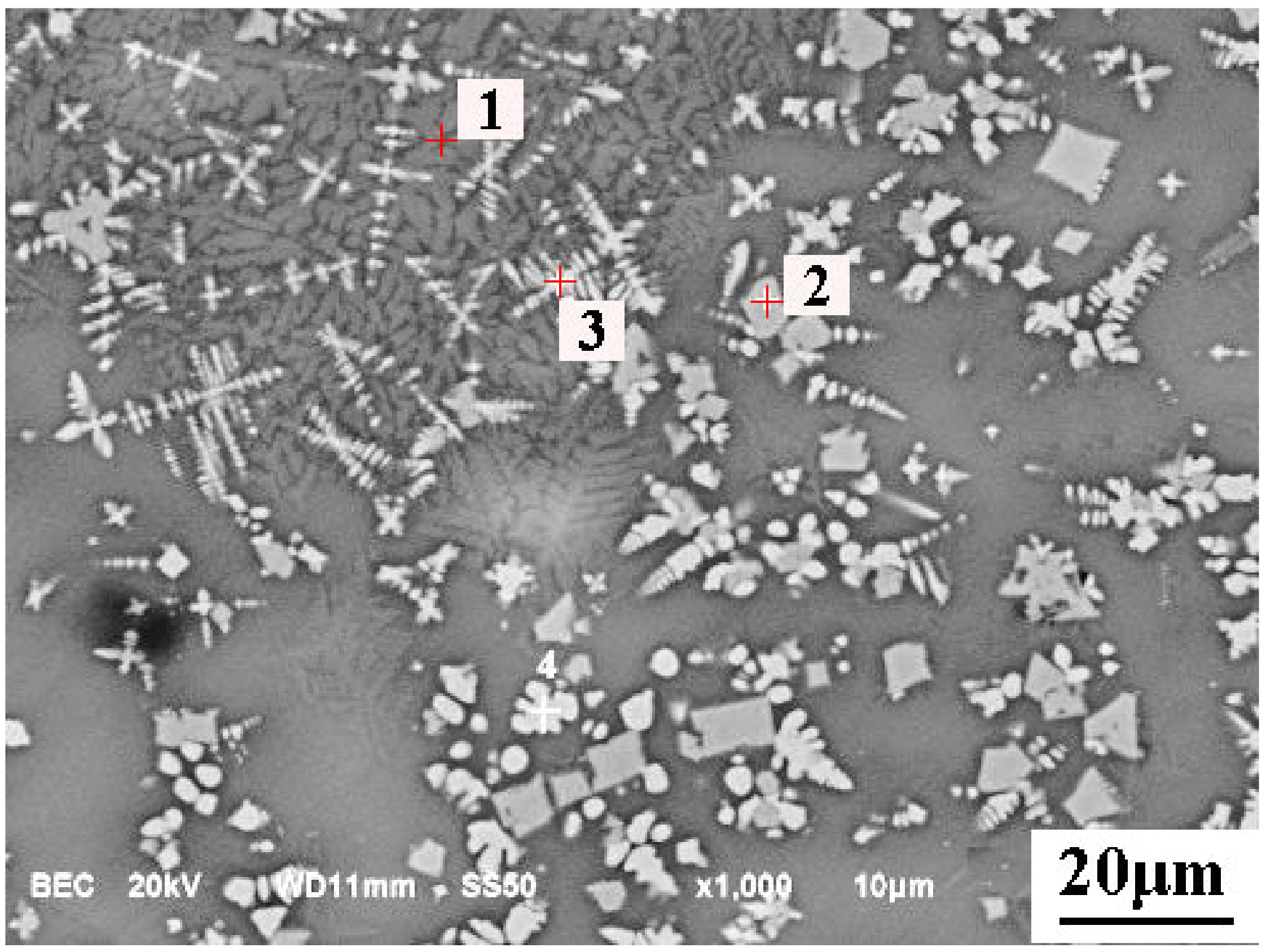
5.2. Microsturcture of Dephosphorizaiton Slag
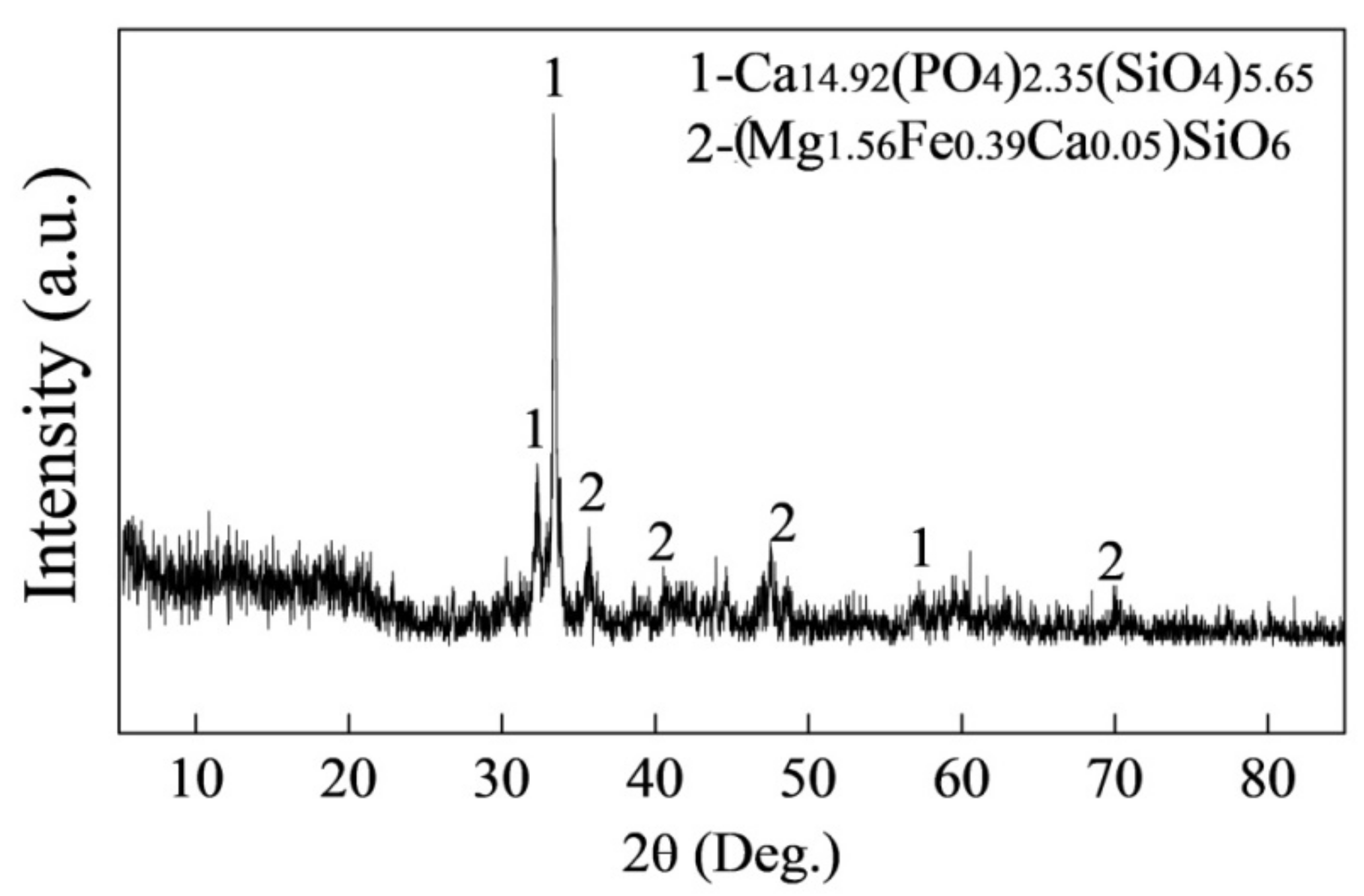
6. Discussions
7. Conclusions
- The relationship between phosphorus distribution ratio (LP), temperature (T), and slag composition can be presented as:
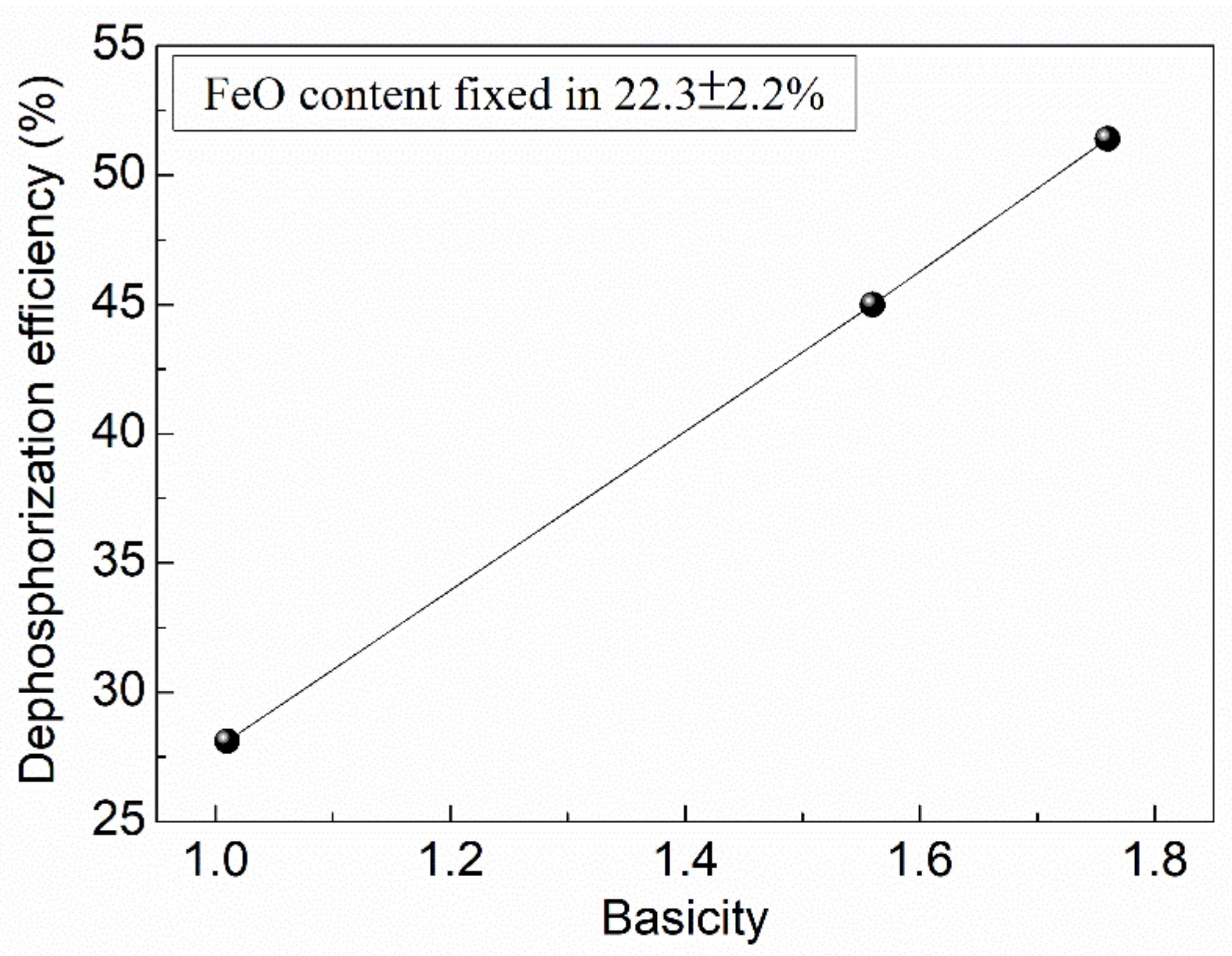
- In order to obtain optimal dephosphorization efficiency, the FeO content in slag should be limited in the range of 20–25%, the slag basicity should be elevated to a high level, while the temperature should be lowered as much as possible in the premise of ensuring required fluidity.
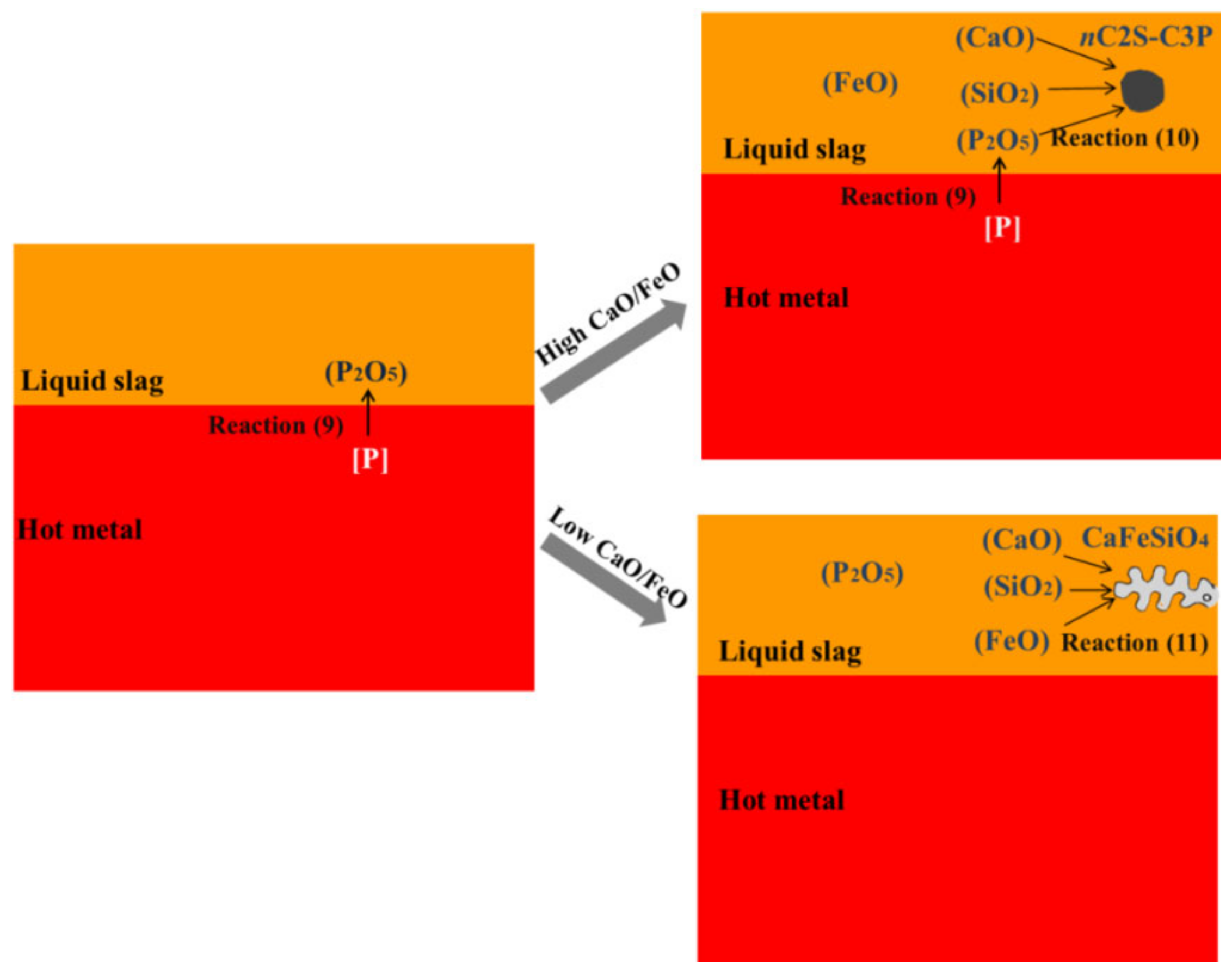
- For the dephosphorization slag containing proper FeO content and having relatively high basicity, nC2S-C3P solid solution generates and gathers phosphorus, which promotes the removal of phosphorus from the hot metal. For the dephosphorization slag having a relatively low basicity or containing a relatively high FeO, no P2O5-rich nC2S-C3P generates and, thus, the dephosphorization efficiency is low.
- There is a competition relationship between P2O5 and FeO in reacting with CaO and SiO2. When CaO/FeO is relatively high, P2O5 is in priority to combine with CaO and SiO2 to generate nC2S-C3P solid solution by [3n + 2](CaO) + 2SiO2 + n(P2O5) = n(3CaO·P2O5)-2CaO·SiO2(s), which facilitates the removal of [P] from the hot metal. When CaO/FeO is relatively low, FeO instead of P2O5 is more likely to react with CaO and SiO2 to generate CaFeSiO4 by a(CaO) + b(SiO2) + c(FeO) = aCaO·bSiO2·cFeO(s), resulting in a poor dephosphorization performance of slag.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.Y.; Yu, C.H.; Xu, J.B.; Feng, J. Experimental study on hot metal dephosphorizing pretreatment and direct steelmaking process in converter. Steelmaking 2004, 20, 40–43. [Google Scholar]
- Wang, X.B.; Feng, M.X.; Zou, Z.S.; Zhao, G.G.; Liu, Z.X. Slag forming route and dephosphorization of BOF dephosphorizing pretreatment and direct Steelmaking. Iron Steel 2009, 44, 23–26. [Google Scholar] [CrossRef]
- Li, J.; Huang, B.C.; Fang, Y.R.; Chen, B.Y.; Liu, L. Research on hot metal dephosphorizing pretreatment and direct steelmaking process in 100 t converter. Iron Steel 2010, 45, 36–39. [Google Scholar]
- Liu, L. Technological progress in converter steelmaking. Steelmaking 2000, 40, 1–5. [Google Scholar]
- Ogawa, Y.; Yano, M.; Kitamura, S.; Hirata, H. Development of the continuous dephosphorization and decarburization process using BOF. Tetsu-to-Hagane 2001, 87, 21–28. [Google Scholar] [CrossRef]
- Ogawa, Y.; Yano, M.; Kitamura, S. Development of the continuous dephosphorization and decarburization process using BOF. Steel Res. Int. 2003, 74, 70–76. [Google Scholar] [CrossRef]
- Iwasaki, M.; Matsuo, M. Change and development of steelmaking technology. Nippon Steel Tech. Rep. 2011, 391, 88–93. [Google Scholar]
- Kumakura, M. Advances in the refining technology and the future prospects. Nippon Steel Tech. Rep. 2012, 394, 4–11. [Google Scholar]
- Iwasaki, M.; Matsuo, M. Improvement of hot metal dephosphorization technique. Nippon Steel Tech. Rep. 2012, 394, 26–32. [Google Scholar]
- Hashimoto, T.; Iiboshi, H.; Kume, K. Improvement in production capacity at Oita Works. Nippon Steel Tech. Rep. 2012, 394, 84–90. [Google Scholar]
- Kobayashi, M.; Isobe, K.; Arai, M. Technical progress in steelmaking and casting for special bar and wire steel at Muroran Work. Nippon Steel Tech. Rep. 2012, 394, 119–124. [Google Scholar]
- Li, H.B.; Lu, Y.C.; Zhu, G.H.; Wang, X.H. Development of ‘Slag-Remaining+Double-Slag’ BOF steelmaking technology in Shougang Co. In Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags; Springer: Cham, Switzerland, 2016; pp. 1177–1184. [Google Scholar]
- Yang, X.; Sun, F.M.; Yang, J.L.; Liu, F.; Cheng, K.S.; Wang, J.H. Optimization of low phosphorus steel production with double slag process in BOF. J. Iron Steel Res. Int. 2013, 20, 41–47. [Google Scholar] [CrossRef]
- Fang, Y.R.; Hang, B.C. Study on dephosphorization pretreatment process in 120t converter. In Proceedings of the 17th National Steelmaking Academic Conference, Hangzhou, China, 15–17 May 2013; pp. 112–118. [Google Scholar]
- Wang, X.; Zou, Z.S. Theoretics study on demarcation point of dephosphorization pretreatment and direct steelmaking process in converter. China Metall. 2007, 17, 10–13. [Google Scholar]
- Healy, G.W. New look at phosphorus distribution. J. Iron Steel Inst. 1970, 208, 664–668. [Google Scholar]
- Tsukihashi, F.; Nakamura, M.; Orimoto, T.; Sano, N. Thermodynamics of phosphorus for the CaO-BaO-CaF2-SiO2 and CaO-Al2O3 systems. Tetsu-to-Hagané 1990, 76, 1664–1671. [Google Scholar] [CrossRef]
- Liao, P.; Hou, Z.W.; Qin, Z.; Zhang, X.Z.; Qiu, S.T. Experimental study of double slag combined blowing converter blowing dephosphorization process. Iron Steel 2013, 48, 30–36. [Google Scholar]
- Zhou, C.G.; Li, J.; Wu, H. Study on the temperature of first deslagging of double slag dephosphorization in converter. Iron Steel 2014, 49, 24–28. [Google Scholar]
- Wang, W.; Sun, K.; Liu, H.T. Effects of different aluminum sources on morphologies and properties of ceramic floor tiles from red mud. Constr. Build. Mater. 2020, 241. [Google Scholar] [CrossRef]



| [C] | [Si] | [Mn] | [P] | [S] | Temperature of Hot Metal (K) |
|---|---|---|---|---|---|
| 4.30–4.60 | 0.50–0.60 | 0.25–0.35 | 0.120–0.140 | 0.001–0.003 | 1593–1613 |
| Heat No. | Sampling Position | C | Si | Mn | P | S | Dephosphorization Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 1 | Before dephosphorization | 4.37 | 0.55 | 0.30 | 0.125 | 0.028 | 28.1% |
| After dephosphorization | 2.78 | 0.01 | 0.04 | 0.0899 | 0.024 | ||
| 2 | Before dephosphorization | 4.42 | 0.54 | 0.32 | 0.136 | 0.026 | 45% |
| After dephosphorization | 2.51 | 0.01 | 0.060 | 0.0748 | 0.020 | ||
| 3 | Before dephosphorization | 4.56 | 0.57 | 0.27 | 0.132 | 0.023 | 51.4% |
| After dephosphorization | 2.86 | 0.01 | 0.070 | 0.0641 | 0.019 | ||
| 4 | Before dephosphorization | 4.52 | 0.53 | 0.25 | 0.137 | 0.023 | 28.1% |
| After dephosphorization | 2.98 | 0.01 | 0.060 | 0.106 | 0.020 |
| Heat No. | SiO2 | CaO | FeO | MgO | MnO | P2O5 | Basicity | Dephosphorization Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 25.0 | 25.2 | 24.5 | 3.6 | 8.6 | 2.7 | 1.01 | 28.1 |
| 2 | 22.8 | 35.7 | 20.1 | 4.2 | 5.0 | 4.8 | 1.56 | 45 |
| 3 | 20.9 | 36.8 | 20.7 | 6.5 | 3.3 | 3.5 | 1.76 | 51.4 |
| 4 | 17.5 | 22.9 | 31.4 | 3.8 | 5.7 | 2.2 | 1.31 | 22.9 |
| Collected Position | Color | Ca | Si | Fe | P | Mg | Mn | O |
|---|---|---|---|---|---|---|---|---|
| Point 1 | Grey | 21.5 | 14.2 | 14.5 | 1.74 | 1.7 | 6.7 | 39.7 |
| Point 2 | Grey | 21.0 | 14.4 | 14.6 | 1.76 | 1.8 | 6.6 | 39.8 |
| Point 3 | Grey | 21.0 | 13.7 | 14.9 | 1.59 | 2.6 | 7.7 | 38.5 |
| Point 4 | Grey | 20.6 | 13.2 | 15.4 | 1.52 | 2.4 | 7.5 | 39.4 |
| Point 5 | White | 3.54 | 1.7 | 37.0 | - | 2.2 | 9.4 | 46.2 |
| Collected Position | Color | Ca | Si | Fe | P | Mg | Mn | O |
|---|---|---|---|---|---|---|---|---|
| Point 1 | Dark grey | 43.02 | 9.24 | 8.04 | 2.60 | - | 0.81 | 36.29 |
| Point 2 | Dark grey | 38.85 | 8.72 | 9.20 | 2.60 | 1.19 | 1.13 | 38.31 |
| Point 3 | Light grey | 45.18 | 0.80 | 15.50 | 0.48 | - | 4.00 | 34.04 |
| Point 4 | Light grey | 35.40 | 2.06 | 17.99 | 0.76 | 1.67 | 2.02 | 40.1 |
| Point 5 | Black | 10.21 | 2.27 | 16.76 | - | 31.88 | 3.11 | 35.77 |
| Collected Position | Color | Ca | Si | Fe | P | Mg | Mn | O |
|---|---|---|---|---|---|---|---|---|
| Point 1 | Dark grey | 38.1 | 12.3 | - | 3.8 | - | - | 45.8 |
| Point 2 | Light grey | - | 5.0 | 30.5 | - | 33.8 | 6.1 | 24.6 |
| Point 3 | Black | 56.23 | - | - | - | - | - | 27.32 |
| Collected Position | Color | Ca | Si | Fe | P | Mg | Mn | O |
|---|---|---|---|---|---|---|---|---|
| Point 1 | Light grey | 14.48 | 13.22 | 18.13 | 1.88 | 1.74 | 8.9 | 35.51 |
| Point 2 | White | 2.41 | 1.59 | 32.03 | - | 1.49 | 8.27 | 21.71 |
| Point 3 | White | 4.67 | 3.32 | 45.36 | 0.58 | 1.91 | 8.31 | 26.18 |
| Point 4 | White | 1.72 | 1.16 | 54.99 | - | 1.67 | 8.37 | 23.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Li, J.; Xia, Y.; Wan, Y.; Chen, L.; Lv, C. Mechanism of Phosphorus Enrichment in Dephosphorization Slag Produced Using the Technology of Integrating Dephosphorization and Decarburization. Metals 2021, 11, 216. https://doi.org/10.3390/met11020216
Xue H, Li J, Xia Y, Wan Y, Chen L, Lv C. Mechanism of Phosphorus Enrichment in Dephosphorization Slag Produced Using the Technology of Integrating Dephosphorization and Decarburization. Metals. 2021; 11(2):216. https://doi.org/10.3390/met11020216
Chicago/Turabian StyleXue, Haimeng, Jie Li, Yunjin Xia, Yong Wan, Liangjun Chen, and Changji Lv. 2021. "Mechanism of Phosphorus Enrichment in Dephosphorization Slag Produced Using the Technology of Integrating Dephosphorization and Decarburization" Metals 11, no. 2: 216. https://doi.org/10.3390/met11020216
APA StyleXue, H., Li, J., Xia, Y., Wan, Y., Chen, L., & Lv, C. (2021). Mechanism of Phosphorus Enrichment in Dephosphorization Slag Produced Using the Technology of Integrating Dephosphorization and Decarburization. Metals, 11(2), 216. https://doi.org/10.3390/met11020216






