The Interface and Fabrication Process of Diamond/Cu Composites with Nanocoated Diamond for Heat Sink Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Composite Preparation
2.3. Characterization
3. Results and Discussion
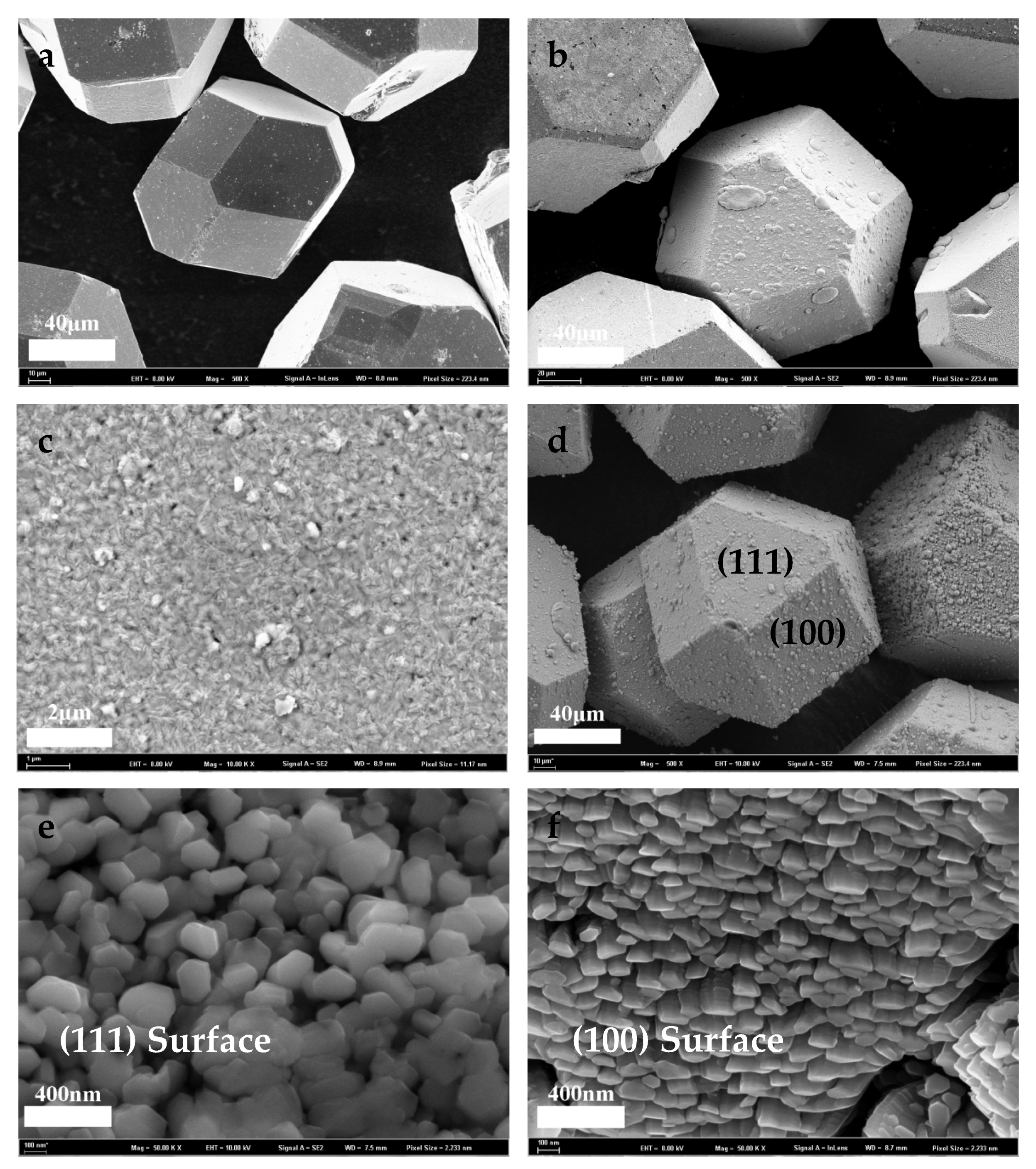
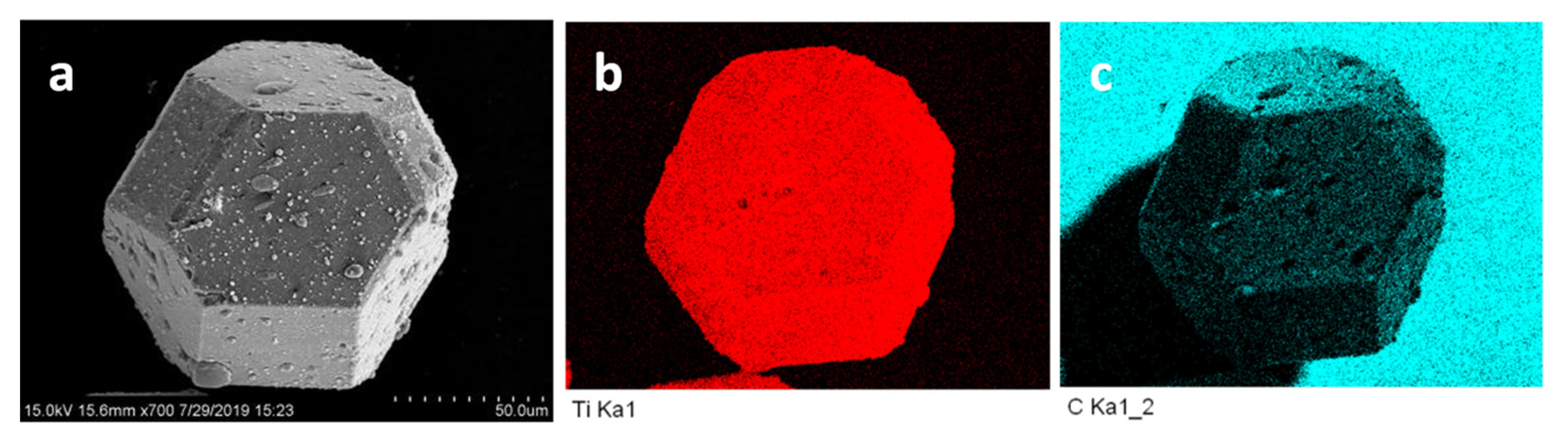
3.1. Surface Morphology of Cu-Coated and Ti-Coated Diamond Particles
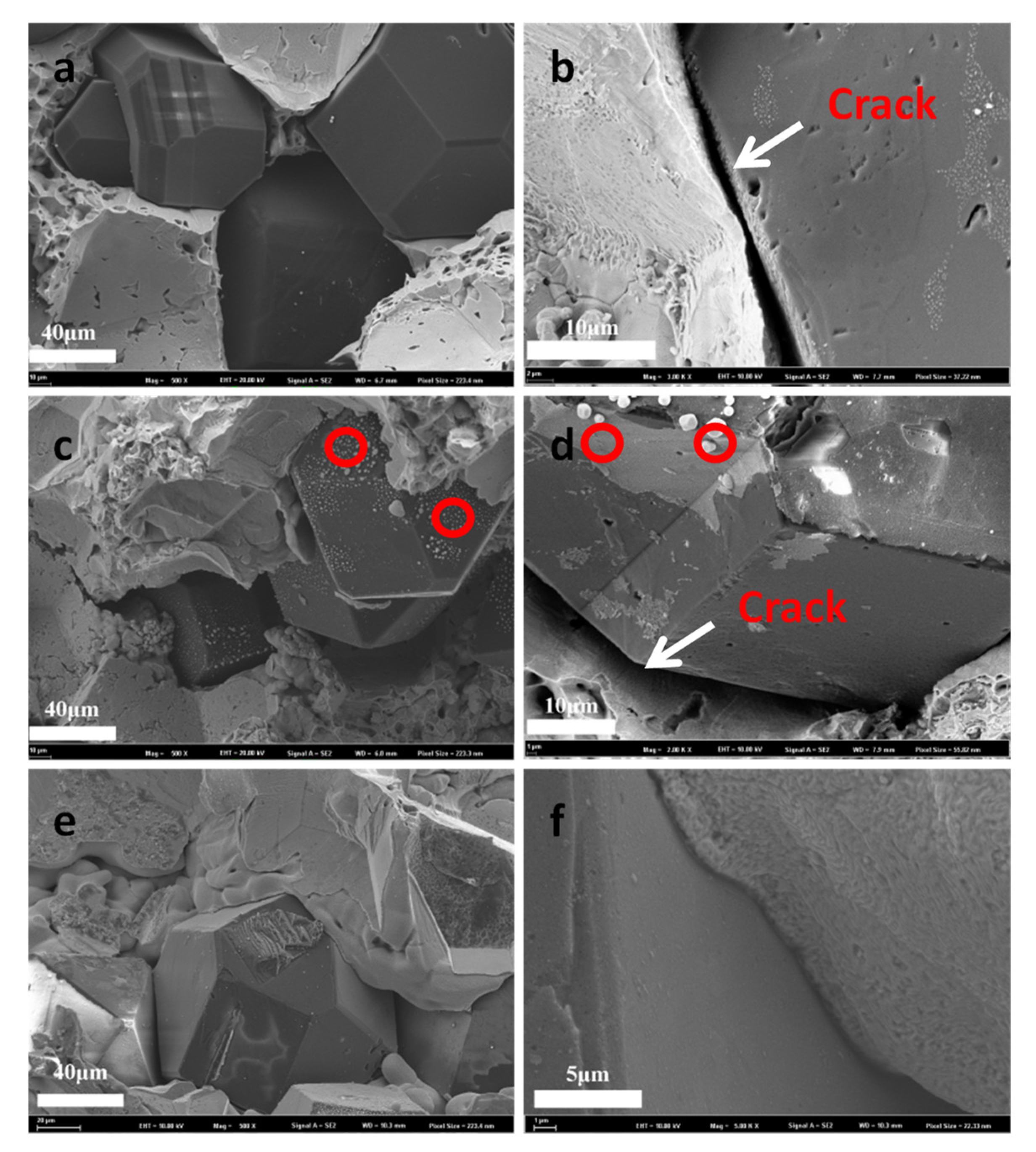
3.2. Microstructures of the Composites
3.3. Thermal Conductivities of the Diamond/Cu Composites
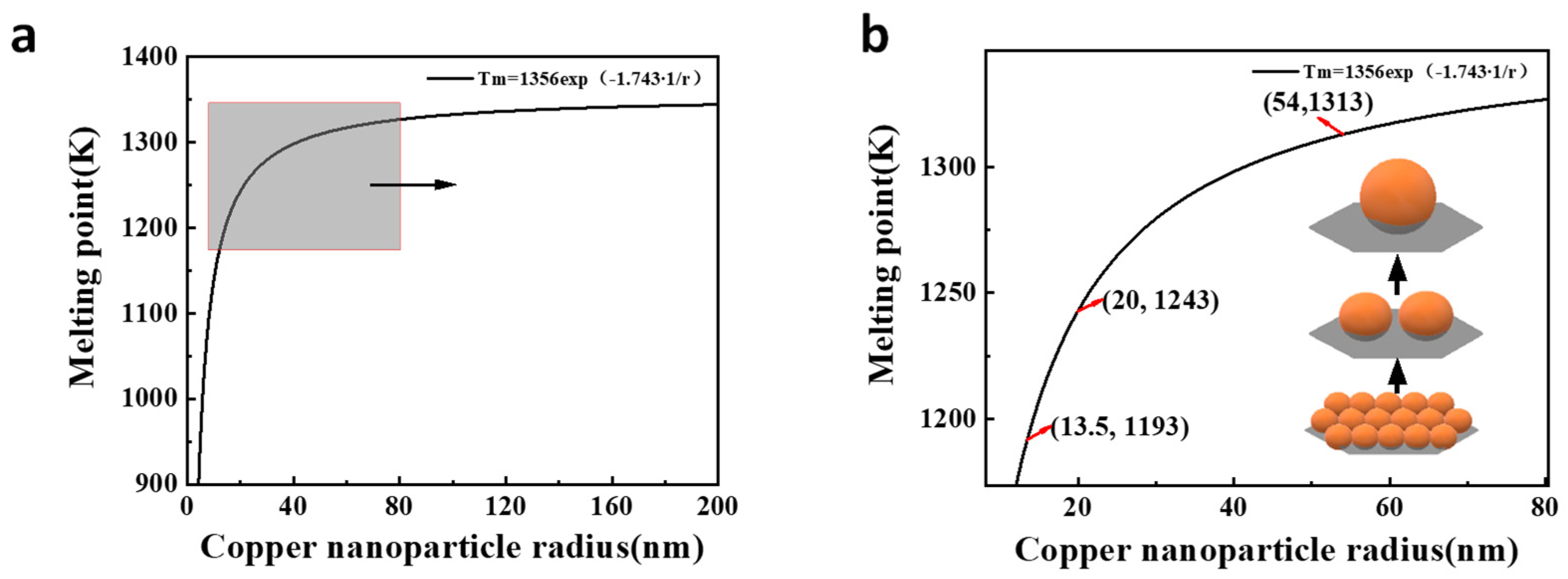
3.4. Nano Effect Mechanism of the Copper Coating
3.5. Nano Effect Mechanism of the Titanium Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.W.; Wang, X.T.; Qiao, Y.; Zhang, Y.; He, Z.B.; Zhang, H.L. High thermal conductivity through interfacial layer optimization in diamond particles dispersed Zr-alloyed Cu matrix composites. Scr. Mater. 2015, 109, 72–75. [Google Scholar] [CrossRef]
- Chu, K.; Wang, F.; Li, Y.B.; Wang, X.H.; Huang, D.J.; Geng, Z.R. Interface and mechanical/thermal properties of graphene/copper composite with Mo2C nanoparticles grown on graphene. Compos. Part A Appl. Sci. Manuf. 2018, 109, 267–279. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Liu, X.; Yin, Z.; Li, Y.Q.; Liu, C.; Liu, S.B.; Wu, C.J.; Liu, J.Y. The fabrication of functional gradient hypereutectic Al-Si composites by liquid-solid separation technology. J. Alloys Compd. 2018, 763, 49–55. [Google Scholar] [CrossRef]
- Jarzabek, D.M.; Chmielewski, M.; Wojciechowski, T. The measurement of the adhesion force between ceramic particles and metal matrix in ceramic reinforced-metal matrix composites. Compos. Part A Appl. Sci. Manuf. 2015, 76, 124–130. [Google Scholar] [CrossRef]
- Yoshida, K.; Morigami, H. Thermal properties of diamond/copper composite material. Microelectron. Reliab. 2004, 44, 303–308. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Suetin, N.V.; Popovich, A.F.; Ralchenko, V.G. Thermal conductivity of diamond composites sintered under high pressures. Diam. Relat. Mater. 2008, 17, 838–843. [Google Scholar] [CrossRef]
- Neubauer, E.; Angerer, P. Advanced composite materials with tailored thermal properties for heat sink applications. In Proceedings of the IEEE 2007 European Conference on Power Electronics and Applications, Aalborg, Denmark, 2–5 September 2007; pp. 2904–2911. [Google Scholar]
- Chen, Y.J.; Young, T.F. Thermal stress and heat transfer characteristics of a Cu/diamond/Cu heat spreading device. Diam. Relat. Mater. 2009, 18, 283–286. [Google Scholar] [CrossRef]
- Schubert, T.; Trindade, B.; Weissgarber, T.; Kieback, B. Interfacial design of Cu-based composites prepared by powder metallurgy for heat sink applications. Mater. Sci. Eng. A Struct. 2008, 475, 39–44. [Google Scholar] [CrossRef]
- Chen, H.; Jia, C.C.; Li, S.J. Interfacial characterization and thermal conductivity of diamond/Cu composites prepared by two HPHT techniques. J. Mater. Sci. 2012, 47, 3367–3375. [Google Scholar] [CrossRef]
- Sang, J.Q.; Yang, W.L.; Zhu, J.J.; Fu, L.C.; Li, D.Y.; Zhou, L.P. Regulating interface adhesion and enhancing thermal conductivity of diamond/copper composites by ion beam bombardment and following surface metallization pretreatment. J. Alloys Compd. 2018, 740, 1060–1066. [Google Scholar] [CrossRef]
- Ren, S.B.; Shen, X.Y.; Guo, C.Y.; Liu, N.; Zang, J.B.; He, X.B.; Qu, X.H. Effect of coating on the microstructure and thermal conductivities of diamond-Cu composites prepared by powder metallurgy. Compos. Sci. Technol. 2011, 71, 1550–1555. [Google Scholar] [CrossRef]
- Shen, X.Y.; He, X.B.; Ren, S.B.; Zhang, H.M.; Qu, X.H. Effect of molybdenum as interfacial element on the thermal conductivity of diamond/Cu composites. J. Alloys Compd. 2012, 529, 134–139. [Google Scholar] [CrossRef]
- Ciupinski, L.; Kruszewski, M.J.; Grzonka, J.; Chmielewski, M.; Zielinsk, R.; Moszczynska, D.; Michalski, A. Design of interfacial Cr3C2 carbide layer via optimization of sintering parameters used to fabricate copper/diamond composites for thermal management applications. Mater. Des. 2017, 120, 170–185. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, J.W.; Catalano, M.; Bai, G.Z.; Li, N.; Dai, J.J.; Wang, X.T.; Zhang, H.L.; Wang, J.G.; Kim, M.J. Enhanced thermal conductivity in Cu/diamond composites by tailoring the thickness of interfacial TiC layer. Compos. Part A Appl. Sci. Manuf. 2018, 113, 76–82. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, H.L.; Zhang, Y.; Che, Z.F.; Wang, X.T. Microstructure and thermal conductivity of Cu/diamond composites with Ti-coated diamond particles produced by gas pressure infiltration. J. Alloys Compd. 2015, 647, 941–946. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, R.Q.; He, X.B.; Ye, Z.G.; Qu, X.H. Fabrication and infiltration kinetics analysis of Ti-coated diamond/copper composites with near-net-shape by pressureless infiltration. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2012, 177, 1524–1530. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, Y.J.; Erb, U. Thermal conductivity of copper-diamond composite materials produced by electrodeposition and the effect of TiC coatings on diamond particles. Compos. Part B Eng. 2018, 155, 197–203. [Google Scholar] [CrossRef]
- Molina-Jorda, J.M. Thermal conductivity of metal matrix composites with coated inclusions: A new modelling approach for interface engineering design in thermal management. J. Alloys Compd. 2018, 745, 849–855. [Google Scholar] [CrossRef]
- Chang, G.; Sun, F.Y.; Duan, J.L.; Che, Z.F.; Wang, X.T.; Wang, J.G.; Kim, M.J.; Zhang, H.L. Effect of Ti interlayer on interfacial thermal conductance between Cu and diamond. Acta Mater. 2018, 160, 235–246. [Google Scholar] [CrossRef]
- Sun, F.L.; Feng, J.C.; Li, D. Bonding of CVD diamond thick films using an Ag-Cu-Ti brazing alloy. J. Mater. Process. Technol. 2001, 115, 333–337. [Google Scholar] [CrossRef]
- Lin, B.; Wang, X.; Zhang, Y.; Zhu, J.; Zhang, H. Interface characterization of a Cu-Ti-coated diamond system. Surf. Coat. Technol. 2015, 278, 163–170. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Imai, T.; Tanabe, K.; Tsuno, T.; Kumazawa, Y.; Fujimori, N. The measurement of thermal properties of diamond. Diam. Relat. Mat. 1997, 6, 1057–1061. [Google Scholar] [CrossRef]
- Qing Yun, W.; Wei Ping, S.; Ming Liang, M. Mean and instantaneous thermal expansion of uncoated and Ti coated diamond/copper composite materials. Adv. Mater. Res. 2013, 702, 202–206. [Google Scholar] [CrossRef]
- Hahn, T.A. Thermal expansion of copper from 20 to 800 K-standard reference material 736. J. Appl. Phys. 1970, 41, 5096–5101. [Google Scholar] [CrossRef]
- Raab, S.J.; Guschlbauer, R.; Lodes, M.A.; Korner, C. Thermal and electrical conductivity of 99.9% pure copper processed via selective electron beam melting. Adv. Eng. Mater. 2016, 18, 1661–1666. [Google Scholar] [CrossRef]
- Ma, D.; Jie, W.; Liu, S.; Xu, W. Solute redistribution and growth velocity response in directional solidification process. J. Cryst. Growth 1996, 169, 170–174. [Google Scholar] [CrossRef]
- Xue, Y.Q.; Zhao, Q.S.; Luan, C.H. The thermodynamic relations between the melting point and the size of crystals. J. Colloid Interface Sci. 2001, 243, 388–390. [Google Scholar] [CrossRef]
- Dorfman, S.; Fuks, D.; Suery, M. Diffusivity of carbon in copper- and silver-based composites. J. Mater. Sci. 1999, 34, 77–81. [Google Scholar] [CrossRef]
- Che, Q.L.; Chen, X.K.; Ji, Y.Q.; Li, Y.W.; Wang, L.X.; Cao, S.Z.; Jiang, Y.G.; Wang, Z. The influence of minor titanium addition on thermal properties of diamond/copper composites via in situ reactive sintering. Mater. Sci. Semicond. Process. 2015, 30, 104–111. [Google Scholar] [CrossRef]
- Ruch, P.W.; Beffort, O.; Kleiner, S.; Weber, L.; Uggowitzer, P.J. Selective interfacial bonding in Al(Si)-diamond composites and its effect on thermal conductivity. Compos. Sci. Technol. 2006, 66, 2677–2685. [Google Scholar] [CrossRef]
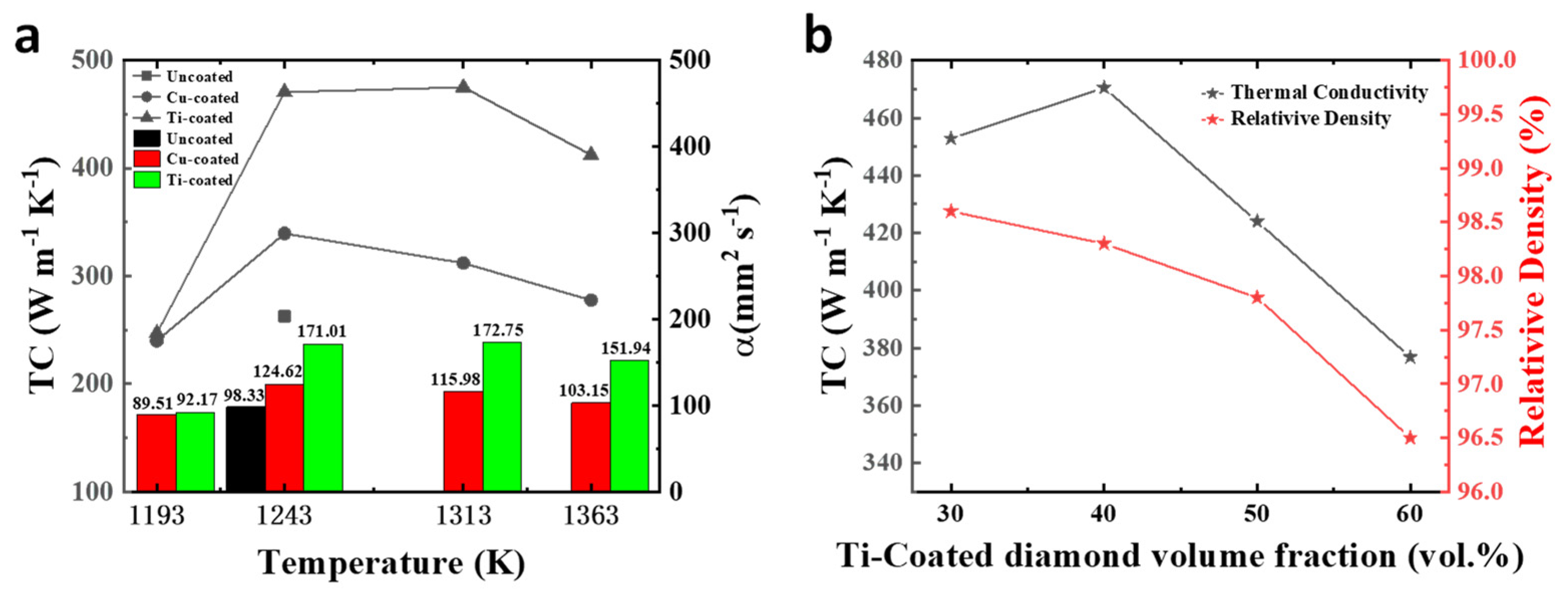

| Samples | Vp (vol.%)/ Temperature (K) | ρ (g cm−3)/ Relative Density (%) | Cp (J g−1 k−1) | a (mm2 s−1) (Standard Error) | TC (W m−1 K−1) (Standard Error) | CTE (10−6 k−1) (Standard Error) |
|---|---|---|---|---|---|---|
| Raw diamond | 40/1243 | 6.519/96.2 | 0.410 | 98.33 (1.12) | 262.82 (2.99) | 14.92 (0.67) |
| Cu-coated diamond | 40/1193 | 6.525/96.3 | 0.411 | 89.51 (0.95) | 240.05 (2.55) | 13.57 (0.40) |
| 40/1243 | 6.613/97.6 | 0.412 | 124.62 (0.75) | 339.53 (2.05) | 12.61 (0.55) | |
| 40/1313 | 6.579/97.2 | 0.409 | 115.98 (0.46) | 312.08 (1.24) | 12.94 (0.48) | |
| 40/1363 | 6.566/96.9 | 0.410 | 103.15 (0.61) | 277.69 (1.64) | 12.82 (0.64) | |
| Ti-coated diamond | 30/1243 | 7.219/98.6 | 0.399 | 157.20 (0.69) | 452.80 (1.98) | 13.22 (0.44) |
| 40/1193 | 6.552/96.7 | 0.409 | 92.17 (0.73) | 247.00 (1.96) | 13.01 (0.34) | |
| 40/1243 | 6.661/98.3 | 0.413 | 171.01 (0.95) | 470.45 (2.63) | 11.24 (0.35) | |
| 40/1313 | 6.674/98.5 | 0.412 | 172.75 (1.01) | 475.01 (2.79) | 10.93 (0.46) | |
| 40/1363 | 6.620/97.7 | 0.410 | 151.94 (0.91) | 412.40 (2.46) | 11.86 (0.36) | |
| 50/1243 | 6.093/97.8 | 0.422 | 164.89 (1.02) | 423.97 (2.63) | 10.55 (0.34) | |
| 60/1243 | 5.485/96.5 | 0.429 | 160.15 (0.81) | 376.84 (1.89) | 9.93 (0.40) |
| r/nm | 5 | 10 | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tm/°C | 684 | 866 | 970 | 1025 | 1044 | 1054 | 1060 | 1063 | 1066 | 1068 | 1070 | 1071 |
| Tm/K | 957 | 1139 | 1243 | 1298 | 1317 | 1327 | 1333 | 1336 | 1339 | 1341 | 1343 | 1344 |
| r/nm | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tm/°C | 874 | 1107 | 1241 | 1288 | 1312 | 1327 | 1337 | 1344 | 1349 | 1353 | 1357 | 1359 |
| Tm/K | 1147 | 1380 | 1514 | 1561 | 1585 | 1600 | 1610 | 1617 | 1622 | 1626 | 1630 | 1632 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, H.; Wu, C.; Yin, Z.; Liu, C.; Huang, Y.; Liu, J.; Shi, Z. The Interface and Fabrication Process of Diamond/Cu Composites with Nanocoated Diamond for Heat Sink Applications. Metals 2021, 11, 196. https://doi.org/10.3390/met11020196
Li Y, Zhou H, Wu C, Yin Z, Liu C, Huang Y, Liu J, Shi Z. The Interface and Fabrication Process of Diamond/Cu Composites with Nanocoated Diamond for Heat Sink Applications. Metals. 2021; 11(2):196. https://doi.org/10.3390/met11020196
Chicago/Turabian StyleLi, Yaqiang, Hongyu Zhou, Chunjing Wu, Zheng Yin, Chang Liu, Ying Huang, Junyou Liu, and Zhongliang Shi. 2021. "The Interface and Fabrication Process of Diamond/Cu Composites with Nanocoated Diamond for Heat Sink Applications" Metals 11, no. 2: 196. https://doi.org/10.3390/met11020196
APA StyleLi, Y., Zhou, H., Wu, C., Yin, Z., Liu, C., Huang, Y., Liu, J., & Shi, Z. (2021). The Interface and Fabrication Process of Diamond/Cu Composites with Nanocoated Diamond for Heat Sink Applications. Metals, 11(2), 196. https://doi.org/10.3390/met11020196





