Formation of Complex Inclusions in Gear Steels for Modification of Manganese Sulphide
Abstract
:1. Introduction
2. Materials and Methods
Analysis of Steel
3. Results and Discussion
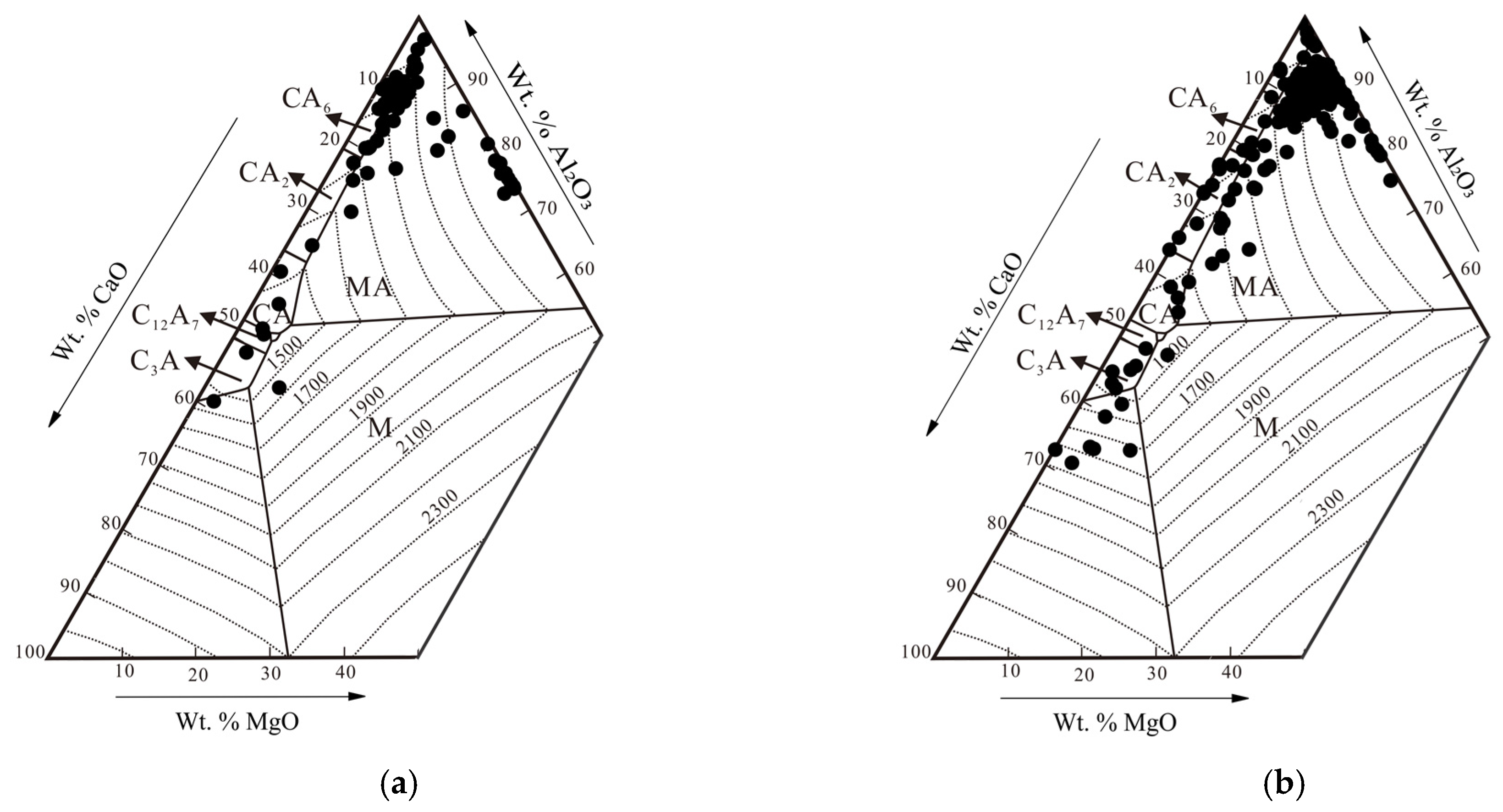
3.1. Morphology and Composition of Inclusions
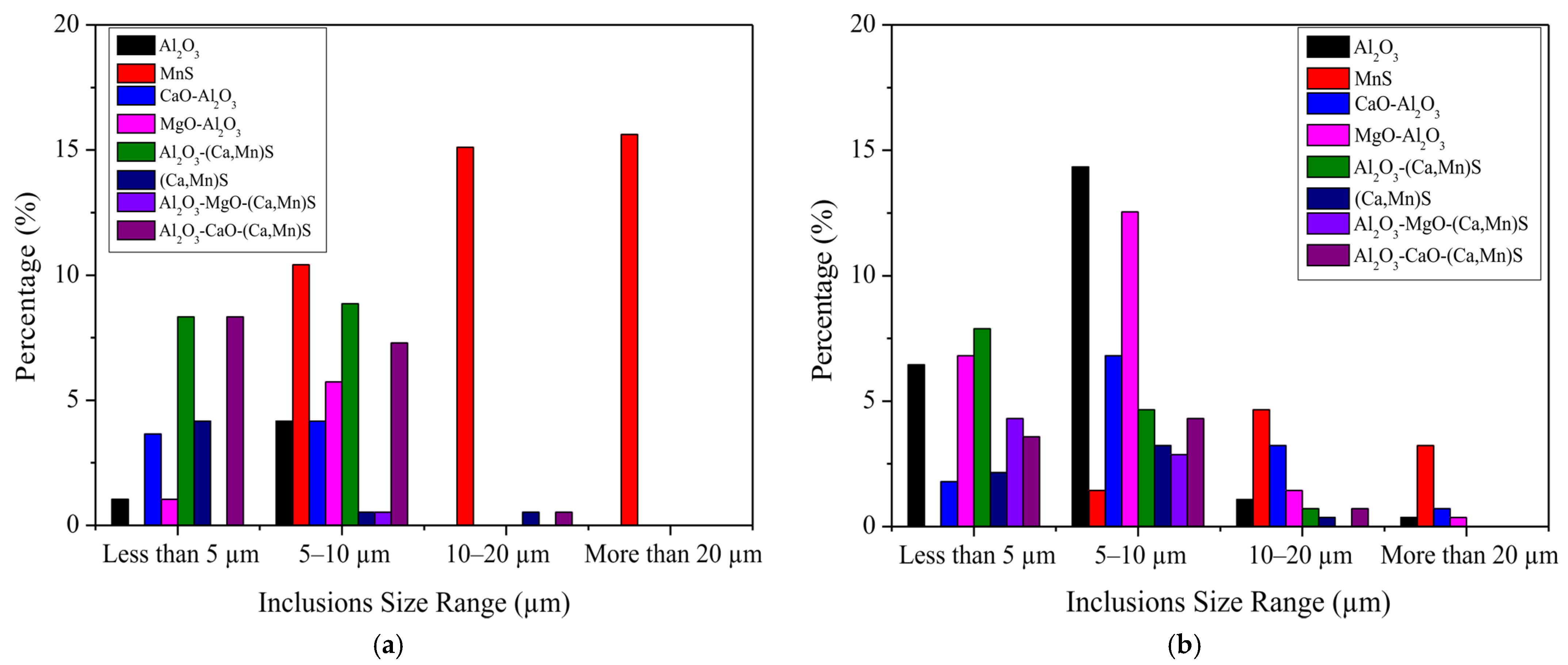
3.2. Number Fraction and Size Range of Inclusions
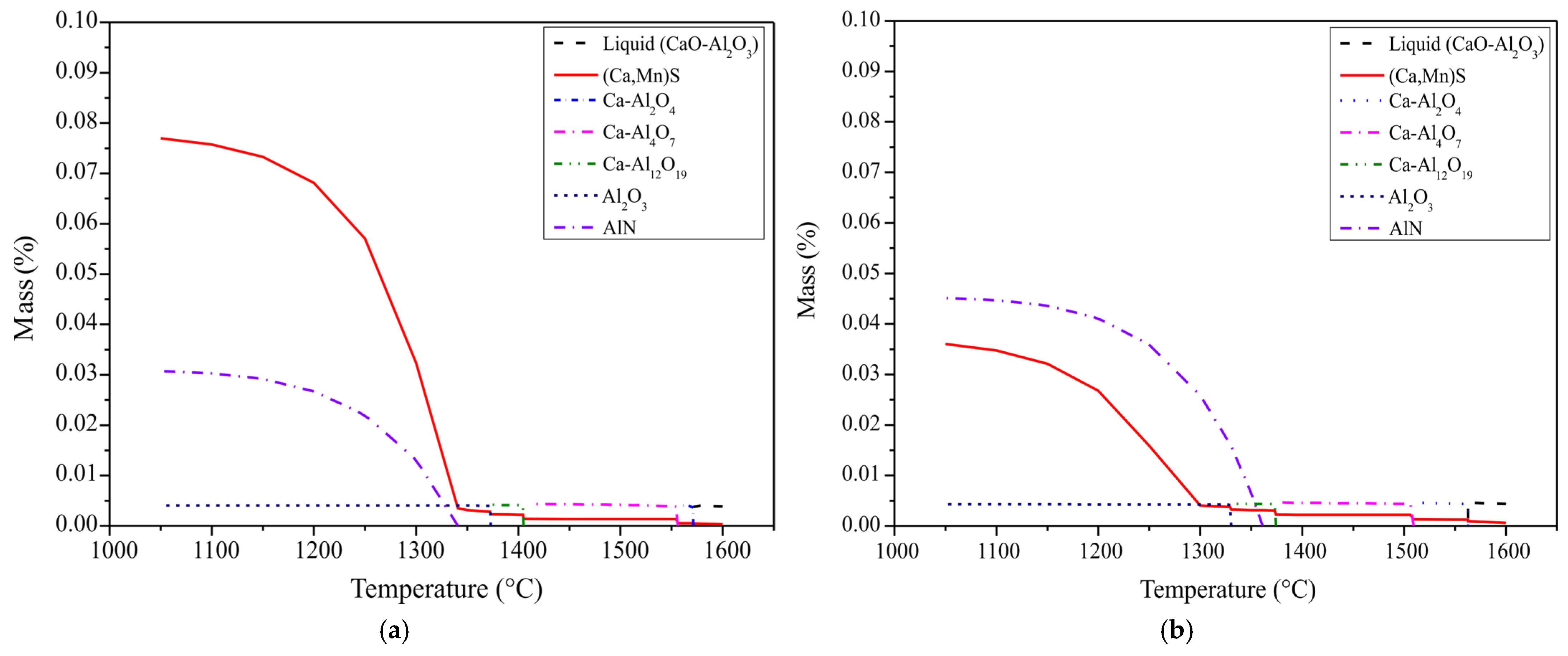
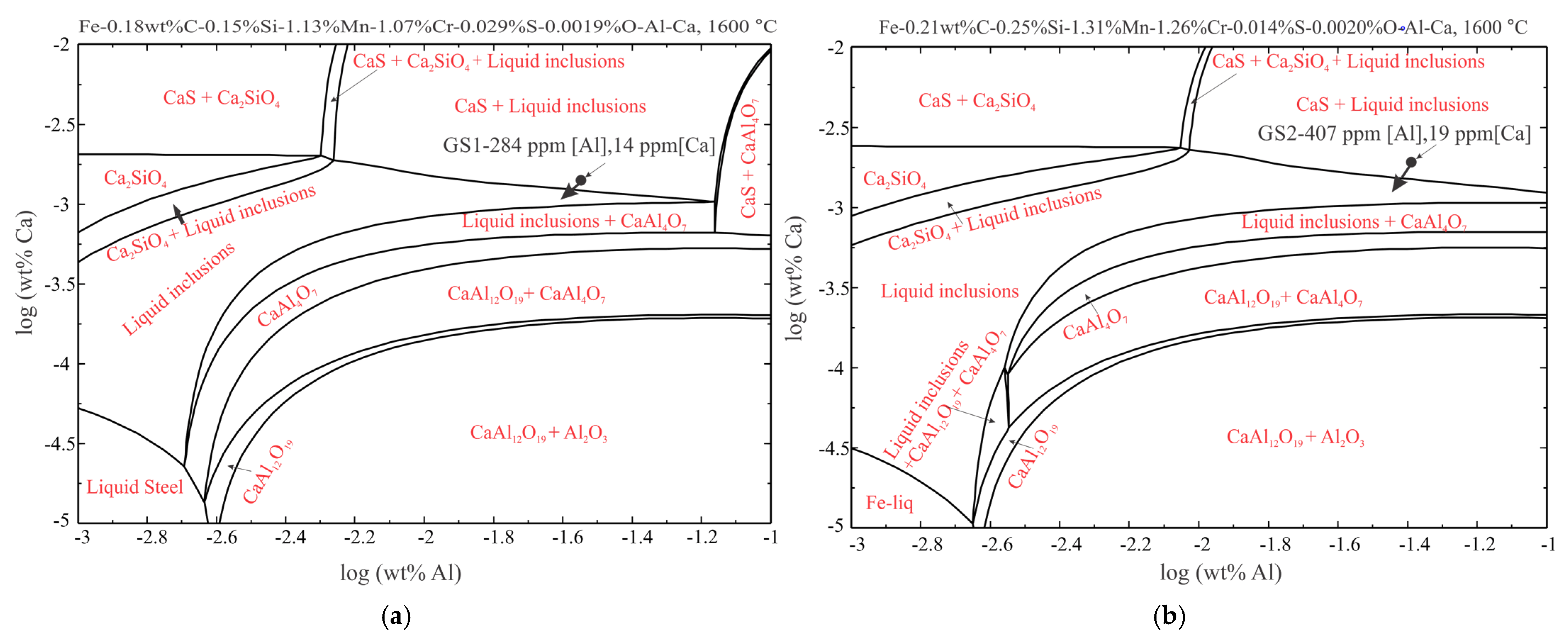
4. Thermodynamics of Inclusions Formation
5. Formation Mechanism of Inclusions
- 1.
- Ca injection into the ladle, which immediately melts the Ca, because its melting point is much lower than the melting temperature of steel, followed by the dissolution of calcium from the gas bubbles-steel interface to the bulk of the steel and finally transfer of the dissolved calcium from the bulk steel to the steel-inclusion interface:
- 2.
- Diffusion of calcium from the steel-inclusion interface into the alumina core followed by chemical reaction of the calcium with alumina:
- 3.
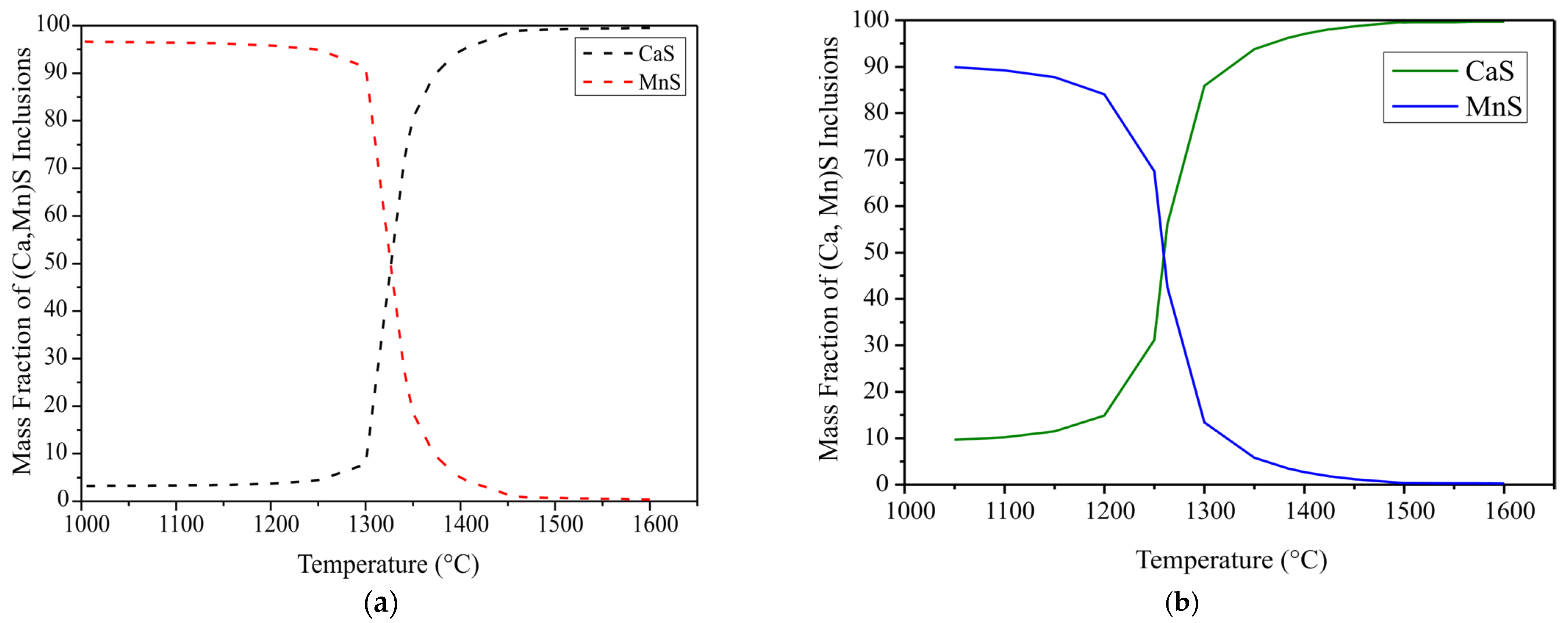
- The sulphur content varies in both samples, consequently the extent of CaS formation changes. Ca can react with sulphur in two ways:
6. Conclusions
- A significant number of pure MnS inclusions were observed in high sulphur steel as compared to low sulphur steel. Duplex inclusions and some spinel cores encapsulated by sulphides were also observed. (Ca,Mn)S encapsulated most of the oxide inclusions in both samples and no transient (CaO-Al2O3-CaS) inclusions were precipitated in the solidified steel despite it having a high sulphur content, i.e., 290 ppm and 140 ppm, respectively.
- Calcium aluminates with a low melting temperature were formed in low sulphur steel, which is considered desirable for continuous casting. The main factors that influence the size, morphology and distribution of inclusions are the Al, S, O and Ca contents in the steel and its temperature.
- The thermodynamic stability diagram of inclusions in Fe-C-Si-Mn-Cr-S-O-Al-Ca systems at 1600 °C agrees well with the inclusions of both sulphur-level Ca-treated samples, which shows inclusions can be completely modified into liquid ones by decreasing the content of Ca up to 10 ppm. Further decreasing the Ca content will result in incomplete modification of oxides and sulphides, and the formation of (high temperature stable) aluminates (CA2) will occur.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tönshoff, H.; Stanske, C. High productivity machining: Materials and processes. In Proceedings of International Conference on High Productivity Machining, Materials and Processing, New Orleans, LA, USA, 7–9 May 1985; pp. 7–9. [Google Scholar]
- Kirsch-Racine, A.; Bomont-Arzur, A.; Confente, M. Calcium treatment of medium carbon steel grades for machinability enhancement: From the theory to industrial practice. Metall. Res. Technol. 2007, 104, 591–597. [Google Scholar] [CrossRef]
- Vasconcellos da Costa e Silva, A.L. The effects of non-metallic inclusions on properties relevant to the performance of steel in structural and mechanical applications. J. Mater. Res. Technol. 2019, 8, 2408–2422. [Google Scholar] [CrossRef]
- Maciejewski, J. The Effects of Sulfide Inclusions on Mechanical Properties and Failures of Steel Components. J. Fail. Anal. Prev. 2015, 15, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Cyril, N.; Fatemi, A. Experimental evaluation and modeling of sulfur content and anisotropy of sulfide inclusions on fatigue behavior of steels. Int. J. Fatigue 2009, 31, 526–537. [Google Scholar] [CrossRef]
- Murty, Y.; Morral, J.; Kattamis, T.; Mehrabian, R. Initial coarsening of manganese sulfide inclusions in rolled steel during homogenization. Metall. Trans. A 1975, 6, 2031–2035. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The Effect of Different Non-Metallic Inclusions on the Machinability of Steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef]
- Li, M.-L.; Wang, F.-M.; Li, C.-R.; Yang, Z.-B.; Meng, Q.-Y.; Tao, S.-F. Effects of cooling rate and Al on MnS formation in me-dium-carbon non-quenched and tempered steels. Int. J. Miner. Metall. Mater. 2015, 22, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Su, Y.F.; Kuo, J.-C. Microstructure and Deformation Characteristics of MnS in 1215MS Steel at 1050 °C. Int. Conf. Min. Mater. Metall. Eng. 2017, 191, 1333–1345. [Google Scholar]
- Liu, Y.; Zhang, L. Relationship between Dissolved Calcium and Total Calcium in Al-Killed Steels after Calcium Treatment. Met. Mater. Trans. A 2018, 49, 1624–1631. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Lee, J.-S.; Lee, I.-S.; Park, Y.-S. Effects of Sulfur Addition on the Formation of Inclusions and the Corrosion Behavior of Super Duplex Stainless Steels in Chloride Solutions of Different pH. Mater. Trans. 2012, 53, 1617–1626. [Google Scholar] [CrossRef]
- Xin, X.; Yang, J.; Wang, Y.; Wang, R.; Wang, W.; Zheng, H.; Hu, H. Effects of Al content on non-metallic inclusion evolution in Fe-16Mn-x Al-0.6 C high Mn TWIP steel. Ironmak. Steelmak. 2016, 43, 234–242. [Google Scholar] [CrossRef]
- Guo, Y.-T.; He, S.-P.; Chen, G.-J.; Wang, Q. Thermodynamics of Complex Sulfide Inclusion Formation in Ca-Treated Al-Killed Structural Steel. Met. Mater. Trans. A 2016, 47, 2549–2557. [Google Scholar] [CrossRef]
- Diederichs, R.; Bleck, W. Modelling of Manganese Sulphide Formation during Solidification, Part I: Description of MnS Formation Parameters. Steel Res. Int. 2006, 77, 202–209. [Google Scholar] [CrossRef]
- Blais, C.; L’Espérance, G.; LeHuy, H.; Forget, C. Development of an integrated method for fully characterizing multi-phase inclusions and its application to calcium-treated steels. Mater. Charact. 1997, 38, 25–37. [Google Scholar] [CrossRef]
- Higuchi, Y.; Numata, M.; Fukagawa, S.; Shinme, K. Inclusion Modification by Calcium Treatment. ISIJ Int. 1996, 36, S151–S154. [Google Scholar] [CrossRef] [Green Version]
- Tiekink, W.; Santillana, B.; Kooter, R.; Mensonides, F.; Deo, B.; Boom, R. Calcium: Toy, tool or trouble. AIST Trans. 2008, 5, 395–403. [Google Scholar]
- Verma, N.; Pistorius, P.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S. Transient Inclusion Evolution during Modification of Alumina Inclusions by Calcium in Liquid Steel: Part, I. Background, Experimental Techniques and Analysis Methods. Met. Mater. Trans. A 2011, 42, 711–719. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S.R. Transient inclusion evolution during modification of alumina inclusions by calcium in liquid steel: Part II. Results and discussion. Metall. Mater. Trans. B 2011, 42, 720–729. [Google Scholar] [CrossRef]
- Wang, Y.; Sridhar, S.; Valdez, M. Formation of CaS on Al2O3-CaO inclusions during solidification of steels. Met. Mater. Trans. A 2002, 33, 625–632. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Formation Mechanism of Oxide-Sulfide Complex Inclusions in High-Sulfur-Containing Steel Melts. Met. Mater. Trans. A 2017, 49, 311–324. [Google Scholar] [CrossRef]
- Wakoh, M.; Sawai, T.; Mizoguchi, S. Effect of S Content on the MnS Precipitation in Steel with Oxide Nuclei. ISIJ Int. 1996, 36, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Fukaya, H.; Miki, T. Phase Equilibrium between CaO· Al2O3 Saturated Molten CaO–Al2O3–MnO and (Ca, Mn) S Solid Solution. ISIJ Int. 2011, 51, 2007–2011. [Google Scholar] [CrossRef]
- Park, J.H.; Todoroki, H. Control of MgO· Al2O3 spinel inclusions in stainless steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Harada, A.; Matsui, A.; Nabeshima, S.; Kikuchi, N.; Miki, Y. Effect of slag composition on MgO· Al2O3 spinel-type inclusions in molten steel. ISIJ Int. 2017, 57, 1546–1552. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Park, J.H. Modification of inclusions in molten steel by Mg-Ca transfer from top slag: Experimental confirma-tion of the ‘refractory-slag-metal-inclusion (ReSMI)’multiphase reaction model. Metall. Mater. Trans. B 2017, 48, 2820–2825. [Google Scholar] [CrossRef]
- Piao, R.; Lee, H.-G.; Kang, Y.-B. Experimental investigation of phase equilibria and thermodynamic modeling of the CaO-Al2O3-CaS and the CaO-SiO2-CaS oxysulfide systems. Acta Mater. 2013, 61, 683–696. [Google Scholar] [CrossRef]
- Bale, C.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Ben Mahfoud, R.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Dogan, Ö.N.; Michal, G.; Kwon, H.-W. Pinning of austenite grain boundaries by AlN precipitates and abnormal grain growth. Metall. Trans. A 1992, 23, 2121–2129. [Google Scholar] [CrossRef]
- Kor, J.; Glaws, P. The Making, Shaping and Treating of Steel. Ladle refining and vacuum degassing. Pittsburg 1998, 10, 661–692. [Google Scholar]
- Tabatabaei, Y.; Coley, K.S.; Irons, G.A.; Sun, S. Model of inclusion evolution during calcium treatment in the ladle furnace. Metall. Mater. Trans. B 2018, 49, 2022–2037. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhang, Y.; Duan, H.; Ren, Y.; Yang, W. Effect of Sulfur in Steel on Transient Evolution of Inclusions During Calcium Treatment. Met. Mater. Trans. A 2018, 49, 610–626. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, X.; Ren, Y.; Liu, X.; Shan, Q. Characteristics of Inclusions in Low Carbon Al-Killed Steel during Ladle Furnace Refining and Calcium Treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Li, H.; Bao, C.; Yang, J. Inclusion Evolution during Modification of Alumina Inclusions by Calcium in Liquid Steel and Deformation during Hot Rolling Process. ISIJ Int. 2015, 55, 2115–2124. [Google Scholar] [CrossRef] [Green Version]
- Gollapalli, V.; Rao, M.V.; Karamched, P.S.; Borra, C.R.; Roy, G.G.; Srirangam, P. Modification of oxide inclusions in calcium-treated Al-killed high sulphur steels. Ironmak. Steelmak. 2019, 46, 663–670. [Google Scholar] [CrossRef] [Green Version]





| Grade | C | Si | Mn | Ni | Cr | *N | *P | *S | *Al | *Ca | *O | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS1 | 0.18 | 0.15 | 1.13 | 0.17 | 1.07 | 106 | 81 | 290 | 284 | 14 | 19 | Bal. |
| GS2 | 0.21 | 0.25 | 1.31 | 0.028 | 1.26 | 155 | 68 | 140 | 407 | 19 | 20 | Bal. |
| Inclusion Type | Composition Range (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | MgO | SiO2 | FeO | CaS | MnS | ||
| Single Oxides | Al2O3 | >80 | <05 | <05 | <01 | <05 | <05 | <05 |
| Al2O3-CaO | >50 | >40 | <05 | <01 | <05 | <05 | <05 | |
| Al2O3-MgO | >60 | <05 | >30 | <01 | <05 | <05 | <05 | |
| Sulphides | (Ca,Mn)S | <05 | <05 | <05 | <01 | <05 | >45 | >45 |
| MnS | <05 | <05 | <05 | <01 | <05 | <05 | >90 | |
| Dual Oxy-Sulphides | Al2O3-(Ca,Mn)S | >50 | <05 | <05 | <01 | <05 | >20 | >20 |
| Al2O3-CaO-(Ca,Mn)S | >40 | >20 | <05 | <01 | <05 | >15 | >05 | |
| Al2O3-MgO-(Ca,Mn)S | >50 | <05 | >20 | <01 | <05 | >15 | >10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, H.; Zhao, B.; Lyu, S.; Huang, Z.; Xu, Y.; Zhao, S.; Ma, X. Formation of Complex Inclusions in Gear Steels for Modification of Manganese Sulphide. Metals 2021, 11, 2051. https://doi.org/10.3390/met11122051
Ahmad H, Zhao B, Lyu S, Huang Z, Xu Y, Zhao S, Ma X. Formation of Complex Inclusions in Gear Steels for Modification of Manganese Sulphide. Metals. 2021; 11(12):2051. https://doi.org/10.3390/met11122051
Chicago/Turabian StyleAhmad, Haseeb, Baojun Zhao, Sha Lyu, Zongze Huang, Yingtie Xu, Sixin Zhao, and Xiaodong Ma. 2021. "Formation of Complex Inclusions in Gear Steels for Modification of Manganese Sulphide" Metals 11, no. 12: 2051. https://doi.org/10.3390/met11122051
APA StyleAhmad, H., Zhao, B., Lyu, S., Huang, Z., Xu, Y., Zhao, S., & Ma, X. (2021). Formation of Complex Inclusions in Gear Steels for Modification of Manganese Sulphide. Metals, 11(12), 2051. https://doi.org/10.3390/met11122051








