Corrosion Nature in [CoN/AlN]n Multilayers Obtained from Laser Ablation
Abstract
:1. Introduction
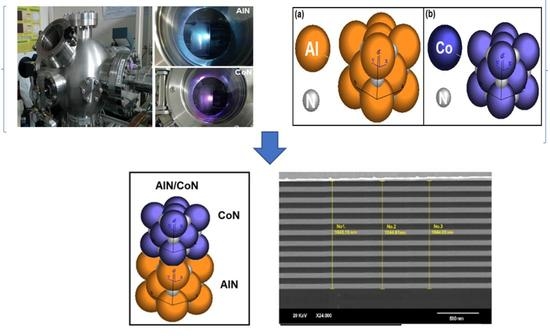
2. Materials and Methods
3. Results
3.1. X-ray Difraction (XRD)
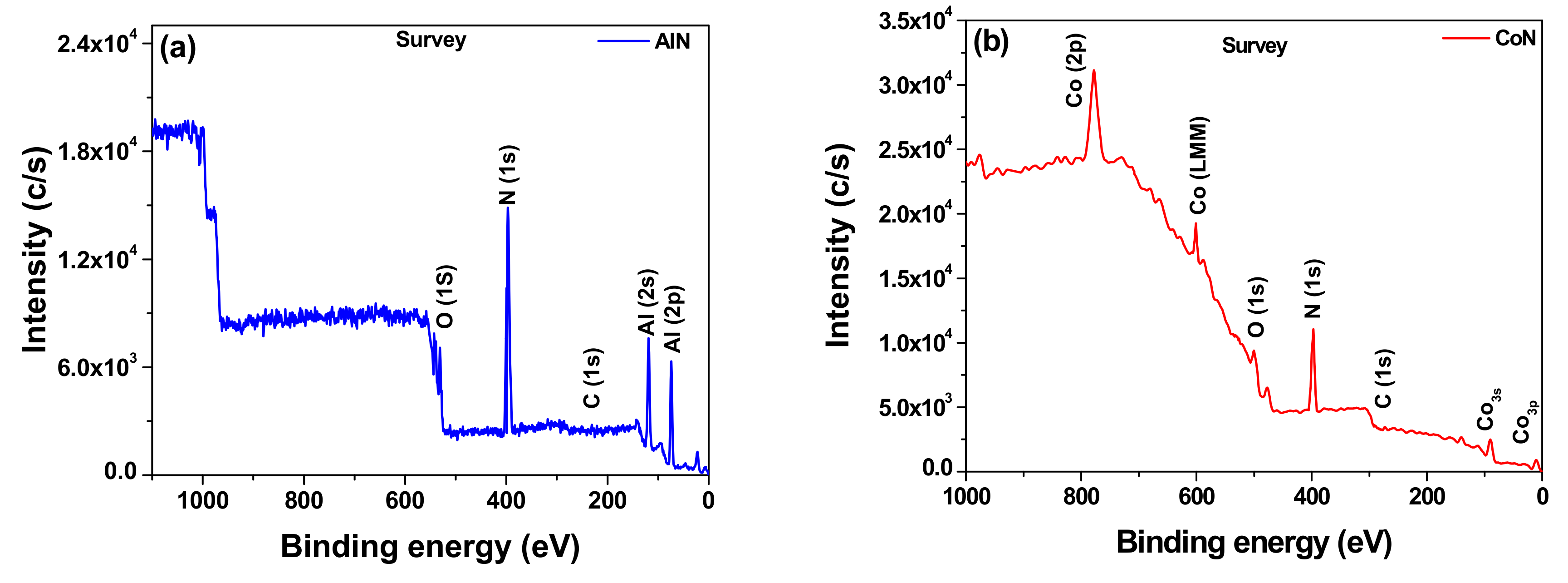
3.2. X-ray Photoelectron Spectroscopy (XPS)
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Crystallographic Simulation
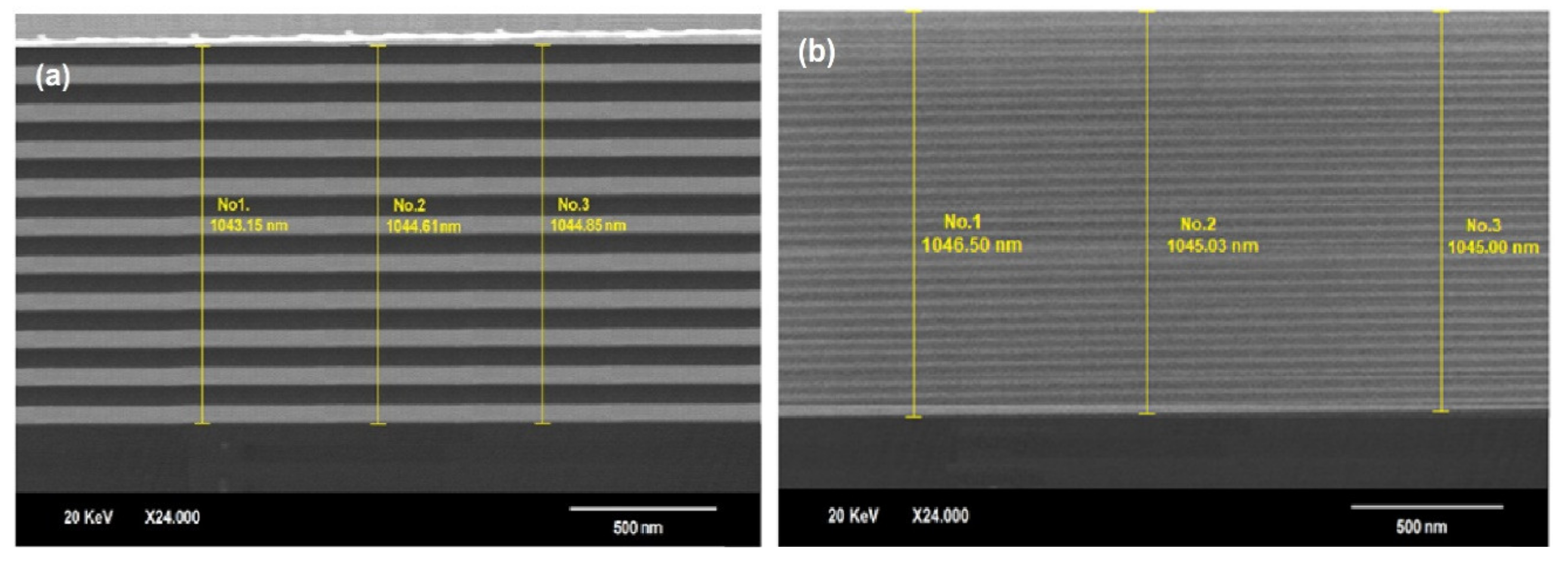
3.5. Scanning Electron Microscopy (SEM)
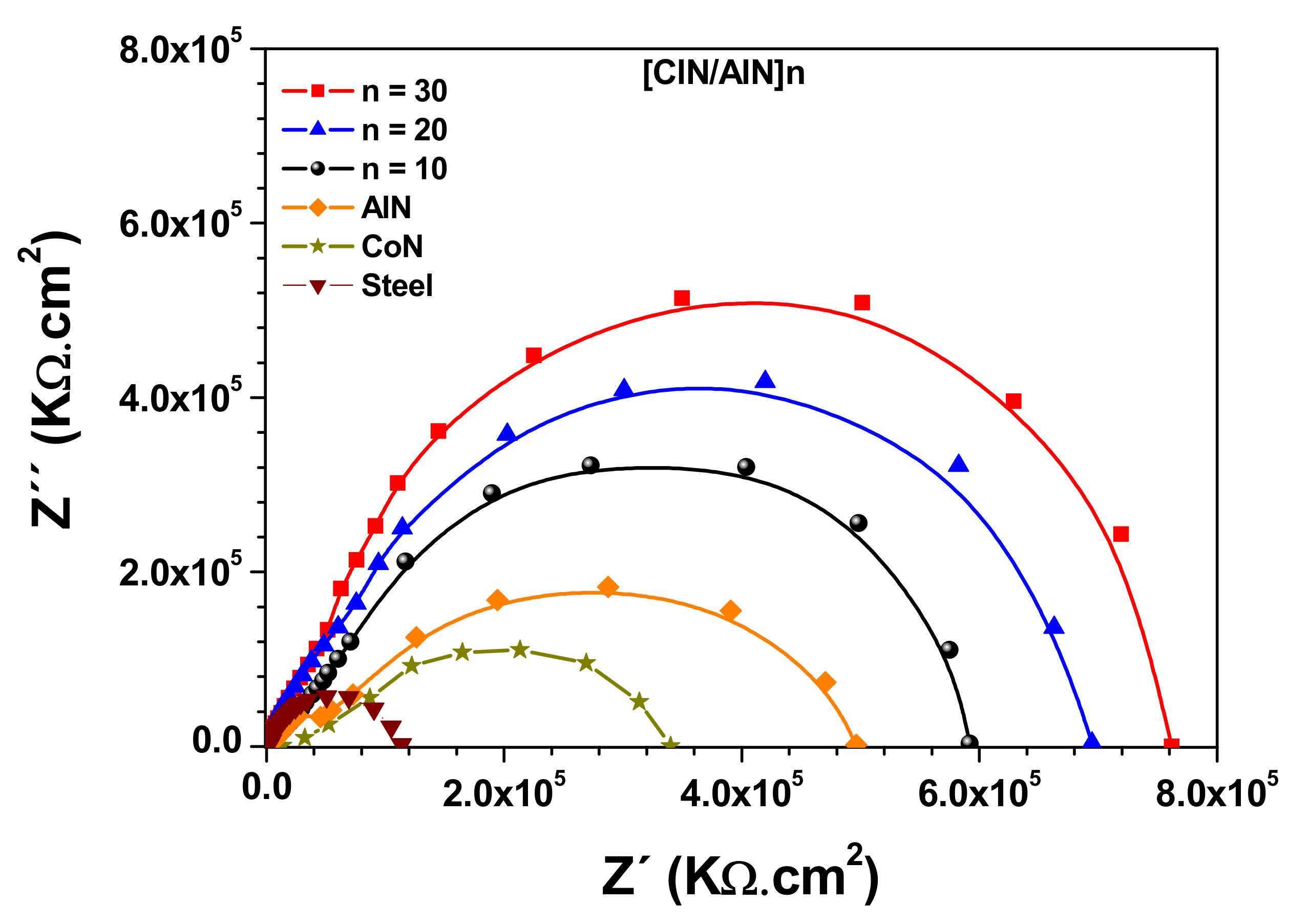
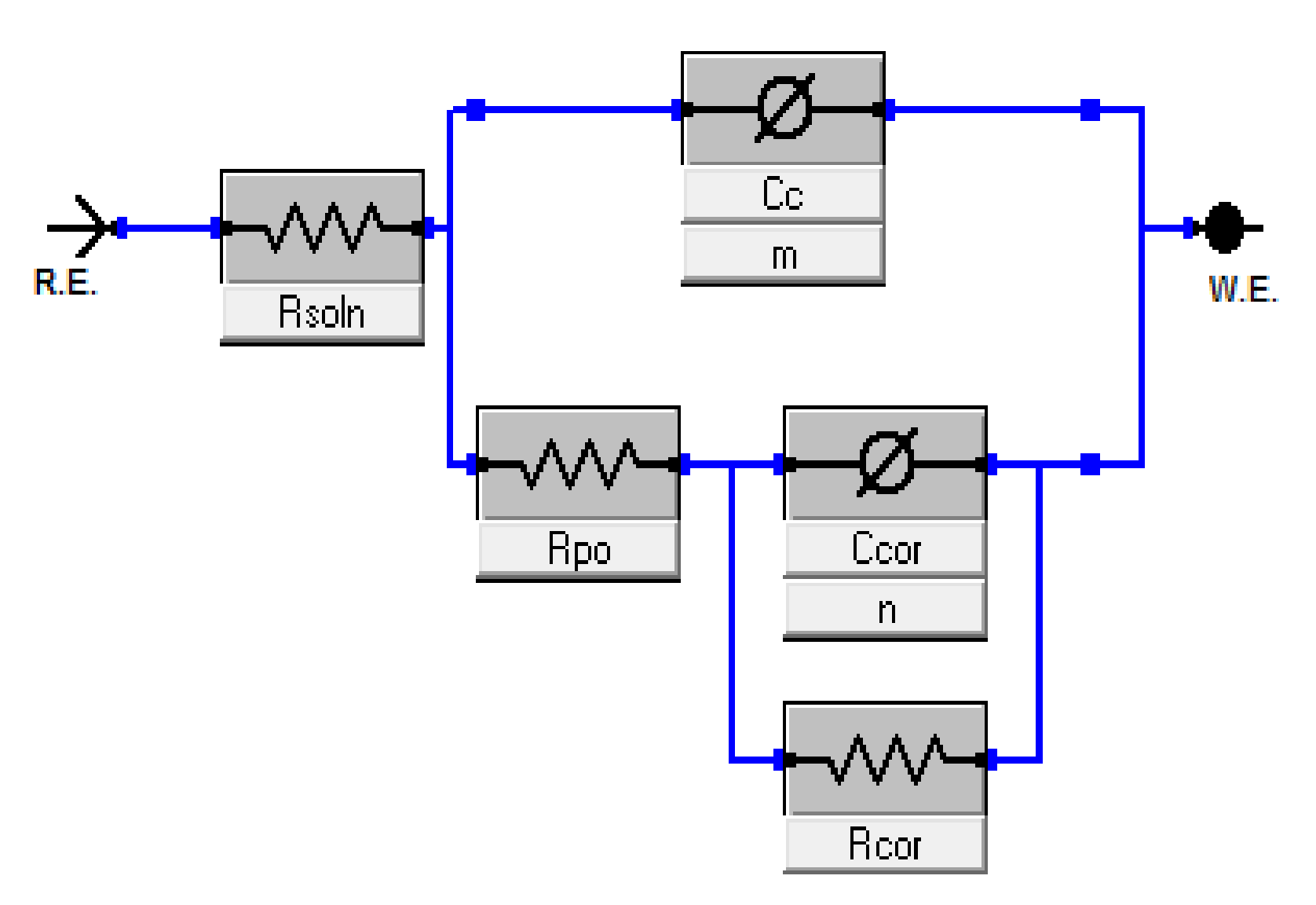
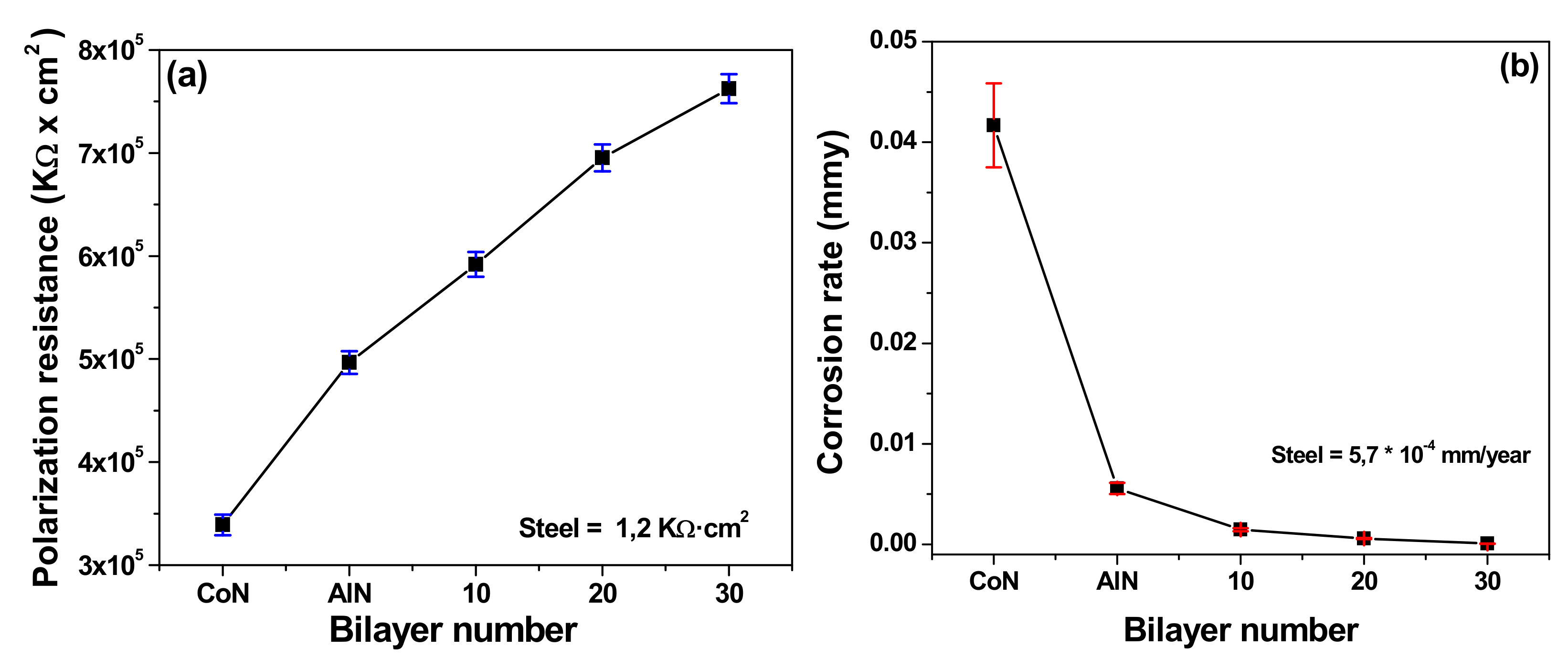
3.6. Electrochemical Characterization
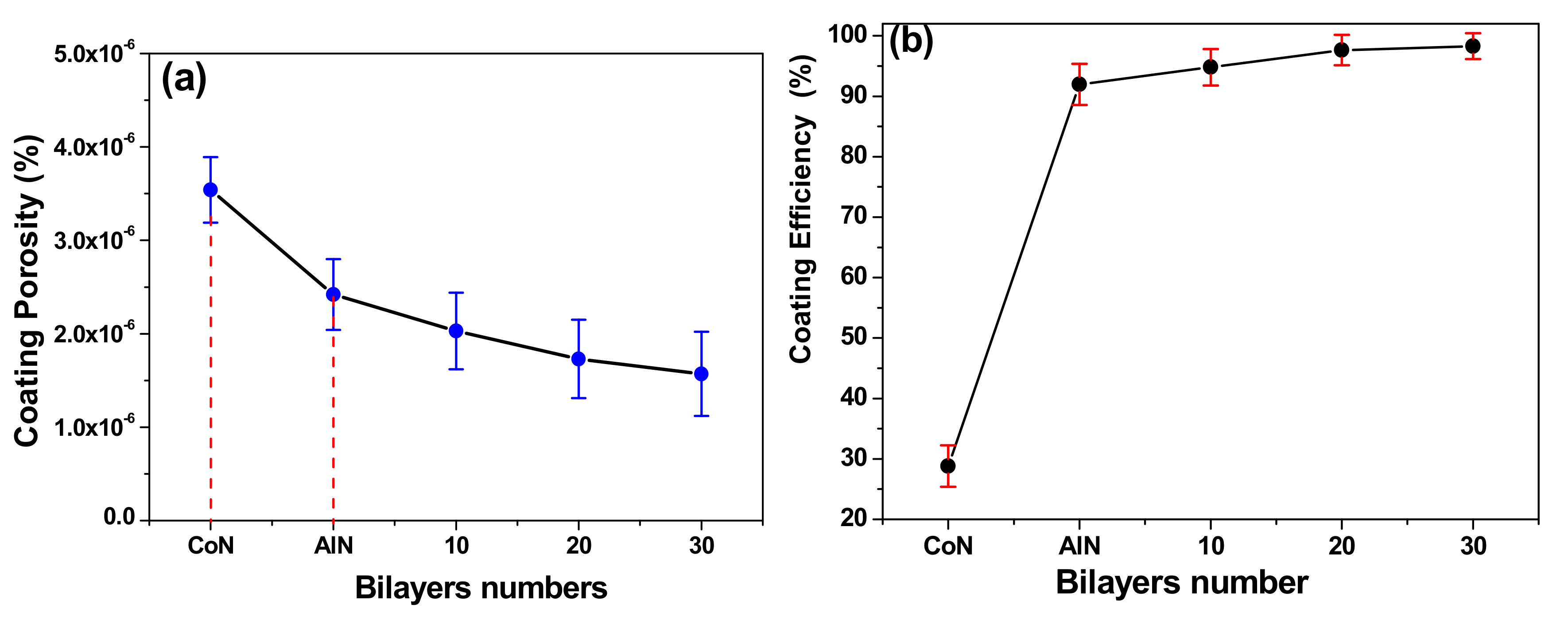
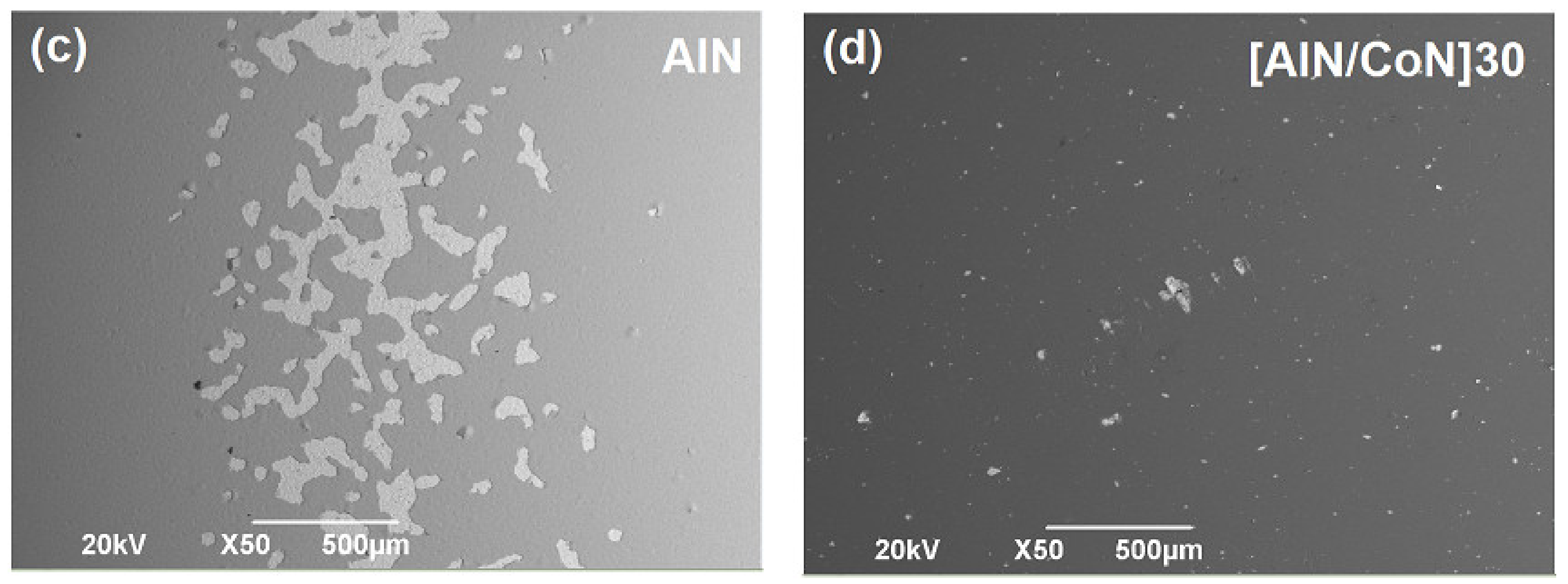
3.7. Surface Corrosion Analysis by SEM
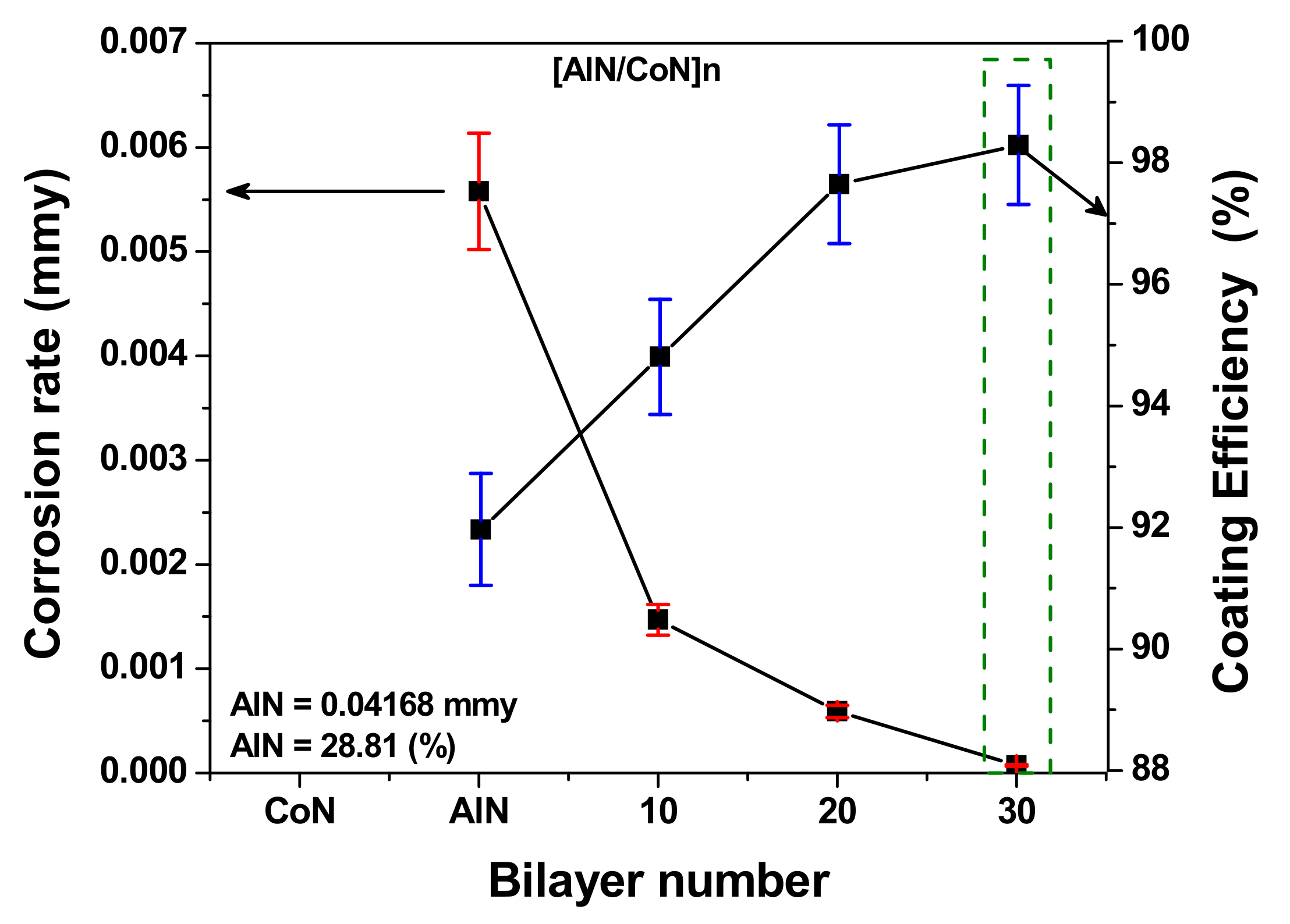
3.8. Correlation between Corrosion Rate and Coating Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hariharan, R.; Raja, R.; Nimal, R.J.G.R. Characteristic of High performance on Mild Steel/AlN Cermet Selective Surfaces Deposited by rf Magnetron Sputtering. J. Crit. Rev. 2020, 4, 319–323. [Google Scholar]
- Florence, V.; Massiani, Y. Gergaud and O. Thomas. Stress, porosity measurements and corrosion behaviour of AlN films deposited on steel substrates. Thin Solid Film. 2000, 359, 221–227. [Google Scholar]
- De la Cruz, W.; Contreras, O.; Pérez-Tijerina, E.; Soto, G. Cobalt nitride films produced by reactive pulsed laser deposition. Rev. Mex. Fis. 2006, 52, 409–412. [Google Scholar]
- Wu, Z.G.; Zhang, G.A.; Wang, M.X.; Fan, X.Y.; Yan, P.X.; Xu, T. Structure and mechanical properties of Al/AlN multilayer with different AlN layer thickness. Appl. Surf. Sci. 2006, 253, 2733–2738. [Google Scholar] [CrossRef]
- Piedrahita, W.F.; Coy, L.E.; Amaya, C.; Llarena, I.; Caicedo, J.C.; Yate, L. Influence of the negative R.F. bias voltage on the structural, mechanical and electrical properties of Hf–C–N coatings. Surf. Coat. Technol. 2016, 286, 251–255. [Google Scholar] [CrossRef]
- Zandalazini, C.I.; Oliva, M.I.; Ferrero, J.C. Pulsed Laser Deposition: Development and implementation of the technique of grown of multilayers. An. AFA 2016, 27, 40–46. [Google Scholar] [CrossRef]
- Van Straaten, G.; Deckers, R.; Vos, M.F.J.; Kessels, W.M.M.; Creatore, M. Plasma-Enhanced Atomic Layer Deposition of Cobalt and Cobalt Nitride: What Controls the Incorporation of Nitrogen? J. Phys. Chem. C 2020, 124, 22046–22054. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Riaz, S.; Naseem, S.H. Structural and Magnetic Properties of The Thin Film of Cobalt Nitride. Surf. Rev. Lett. 2014, 21, 1450081. [Google Scholar] [CrossRef]
- Kolaklieva, L.; Chitanov, V.; Szekeres, A.; Antonova, K.; Terziyska, P.; Fogarassy, Z.; Petrik, P.; Mihailescu, I.N.; Duta, L. Pulsed Laser Deposition of Aluminum Nitride Films: Correlation between Mechanical, Optical, and Structural Properties. Coatings 2019, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, W.; Yang, W.; Zhou, S.; Lin, Z.; Li, G. Growth evolution of AlN films on silicon (111) substrates by pulsed laser deposition. J. Appl. Phys. 2015, 117, 185303. [Google Scholar] [CrossRef]
- Svedberg, L.M.; Arndt, K.C.; Cima, M.J. Corrosion of Aluminum Nitride (AlN) in Aqueous Cleaning Solutions. J. Am. Ceram. Soc. 2000, 83, 41–46. [Google Scholar] [CrossRef]
- Taborda, J.A.P.; Caicedo, J.C.; Grisales, M.; Saldarriaga, W.; Riascos, H. Deposition pressure effect on chemical, morphological and optical properties of binary Al-nitrides. Opt. Laser Technol. 2015, 69, 92–103. [Google Scholar] [CrossRef]
- Gupta, R.; Pandey, N.; Tayal, A.; Gupta, M. Phase formation, thermal stability and magnetic moment of cobalt nitride thin films. AIP Adv. 2015, 5, 097131. [Google Scholar] [CrossRef]
- Iqbal, A.; Mohd-Yasin, F. Reactive Sputtering of Aluminum Nitride (002) Thin Films for Piezoelectric Applications: A Review. Sensors 2018, 18, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smecca, E.; Maita, F.; Pellegrino, G.; Vinciguerra, V. AlN texturing and piezoelectricity on flexible substrates for sensor applications. Appl. Phys. Lett. 2015, 106, 232903. [Google Scholar] [CrossRef]
- Helmersson, U.; Todorova, S.; Barnett, S.A.; Sundgren, J.-E.; Markert, L.C.; Greene, J.E. Growth of single-crystal TiN/VN strained-layer superlattices with extremely high mechanical hardness. Appl. Phys. 1987, 62, 481. [Google Scholar] [CrossRef]
- Rosenberger, L.; Baird, R.; McCullen, E.; Auner, G.; Shreve, G. XPS analysis of aluminum nitride films deposited by plasma source molecular beam epitaxy. Surf. Interface Anal. 2008, 40, 1254–1261. [Google Scholar] [CrossRef]
- Motamedi, P.; Cadien, K. XPS analysis of AlN thin films deposited by plasma enhanced atomic layer deposition. Appl. Surf. Sci. 2014, 315, 104–109. [Google Scholar] [CrossRef]
- Li1, H.; Cai, G.M.; Wang, W.J. Room temperature luminescence and ferromagnetism of AlN:Fe. AIP Adv. 2016, 6, 065025. [Google Scholar]
- Dallaev, R.; Sobola, D.; Tofel, P.; Škvarenina, L.; Sedlák, P. Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum. Coatings 2020, 10, 954. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, S.R.; Ho, J.; Ahn, B.T. Epitaxial Growth of CoSi 2 Layer on a Si 100 Substrate Using a CoN x Interlayer Deposited by Reactive Sputtering. Korean, J. Mater. Res. 2006, 16, 16–21. [Google Scholar] [CrossRef]
- Parnell, C.M.; Chhetri, B.; Mitchell, T.; Watanabe, F. Simultaneous Electrochemical Deposition of Cobalt Complex and Poly(pyrrole) Thin Films for Supercapacitor Electrodes. Sci. Rep. 2019, 9, 5650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, G.; Tan, H.; Wang, Z.; Zhang, Z.; Gao, W.; Rawat, R.S.; Fan, H.J. Enhancing bifunctionality of CoN nanowires by Mn doping for long-lasting Zn-air batteries. Sci. China-Chem. 2020, 63, 890–896. [Google Scholar] [CrossRef]
- Antsiferov, V.; Gilyov, V.; Karmanov, V. IR-Spectra and Phases Structure of Sialons. Vib. Spectrosc. 2002, 30, 169–173. [Google Scholar] [CrossRef]
- Andrews, L.; Zhou, M.; Chertihin, G.V.; Bare, W.D.; Hannachi, Y. Reactions of Laser-Ablated Aluminum Atoms with Nitrogen Atoms and Molecules. Infrared Spectra and Density Functional Calculations for the AlN. J. Phys. Chem. A 2000, 104, 1656–1661. [Google Scholar]
- Thompson, W.E.; Jacox, M.E. The vibrational spectra of molecular ions isolated in solid neon. III. N+4. J. Chem. Phys. 1990, 93, 3856–3862. [Google Scholar] [CrossRef]
- Dallaev, R.; Papež, N.; Sobola, D.; Ramazanov, S. Investigation of structure of AlN thin films using Fourier-transform infrared spectroscopy. Procedia Struct. Integr. 2019, 23, 601–606. [Google Scholar] [CrossRef]
- Andrews, L.; Citra, A.; Chertihin, G.V.; Bare, W.D.; Neurock, M. Reactions of Laser-Ablated Co and Ni Atoms with Nitrogen Atoms and Molecules. Infrared Spectra and DFT Calculations of Metal Nitride Molecular Species and Complexes. J. Phys. Chem. A 1998, 102, 2561–2571. [Google Scholar] [CrossRef]
- Thompson, W.E.; Jacox, M.E. The vibrational spectra of molecular ions isolated in solid neon. XI. NO+2, NO−2, and NO−3. J. Phys. Chem. 1993, 99, 7399. [Google Scholar] [CrossRef]
- Ituen, E.; James, A.; Akaranta, O. Fluvoxamine-based corrosion inhibitors for J55 steel in aggressive oil and gas well treatment fluids. Egypt. J. Pet. 2017, 26, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Diard, J.; le Gorrec, B.; Montella, C. Handbook of Electrochemical Impedance Spectroscopy; Diffusion Impedances; Technical Report; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Randles, J.E.B.; Schiffrin, D.J. Surface tension of dilute acid solutions. Trans. Faraday Soc. 1966, 62, 2403–2408. [Google Scholar] [CrossRef]
- Schäfer, H.; Stock, H.-R. Improving the corrosion protection of aluminium alloys using reactive magnetron sputtering. Corros. Sci. 2005, 47, 953–964. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Jehn, H.A.; Kappl, H.; Lee, J.J.; Lee, K.H.; Park, H.S. Improvement of the Corrosion Resistance of Hard Wear Resistant Coatings by Intermediate Plasma Etching or Multilayered Structure. Surf. Coat. Technol. 2002, 150, 101–106. [Google Scholar] [CrossRef]
- Caicedo, J.; Zambrano, G.; Aperador, W.; Escobar-Alarcon, L.; Camps, E. Mechanical and electrochemical characterization of vanadium nitride (VN) thin films. Appl. Surf. Sci. 2011, 258, 312–320. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Yate, L.; Cabrera, G.; Aperador, W.; Zambrano, G.; Prieto, P. Effect of negative bias voltage on mechanical and electrochemical nature in Ti–W–N coatings. J. Mater. Sci. 2011, 46, 1244–1252. [Google Scholar] [CrossRef]
- Gu, Z.W.; Han, Y.; Pan, F.S.; Wang, X.; Weng, D.; Zhou, S. Investigation on Wear- and Corrosion Resistance of TiN Films by CVD. Mater. Sci. Forum 2009, 610, 554–558. [Google Scholar]
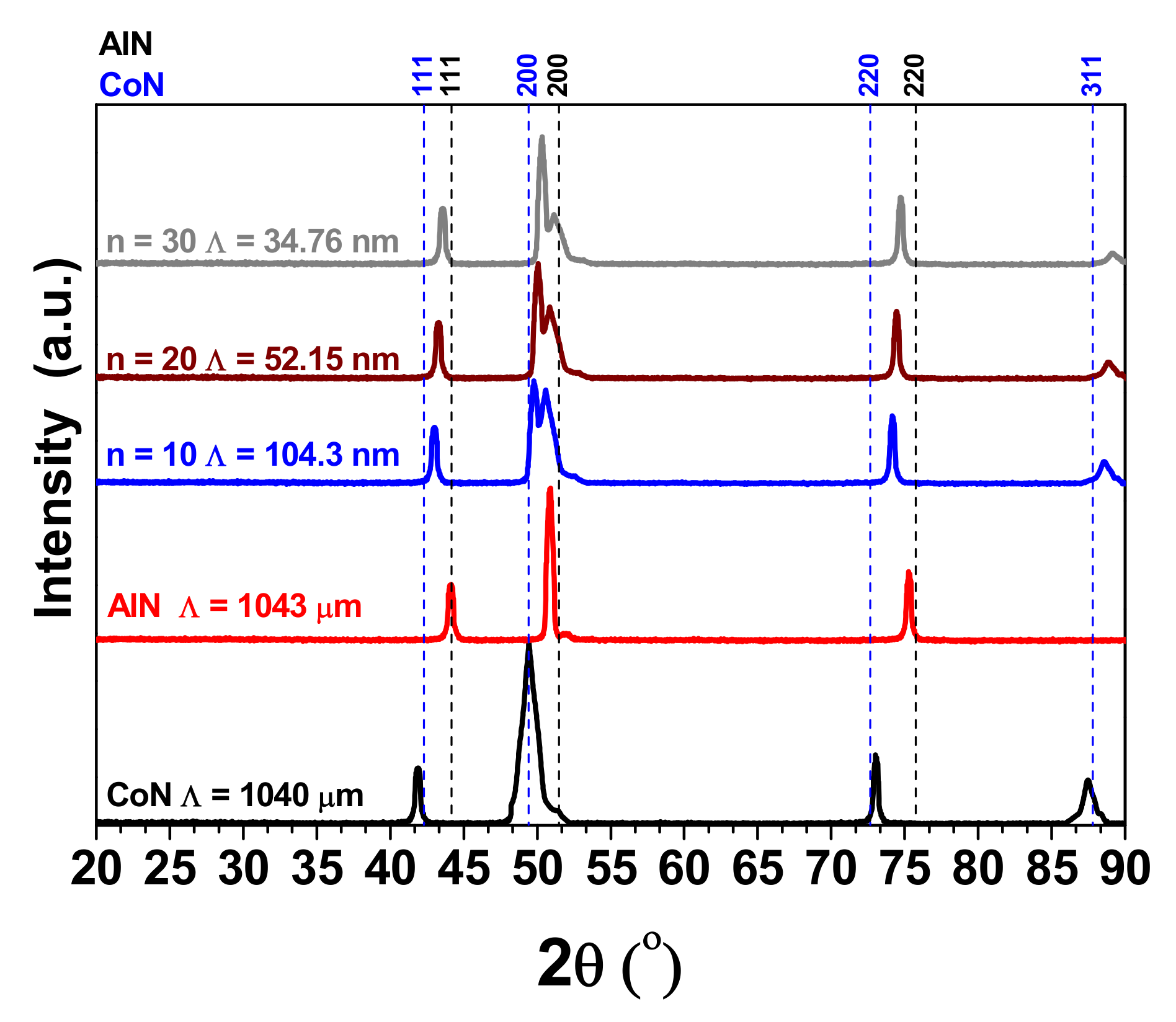
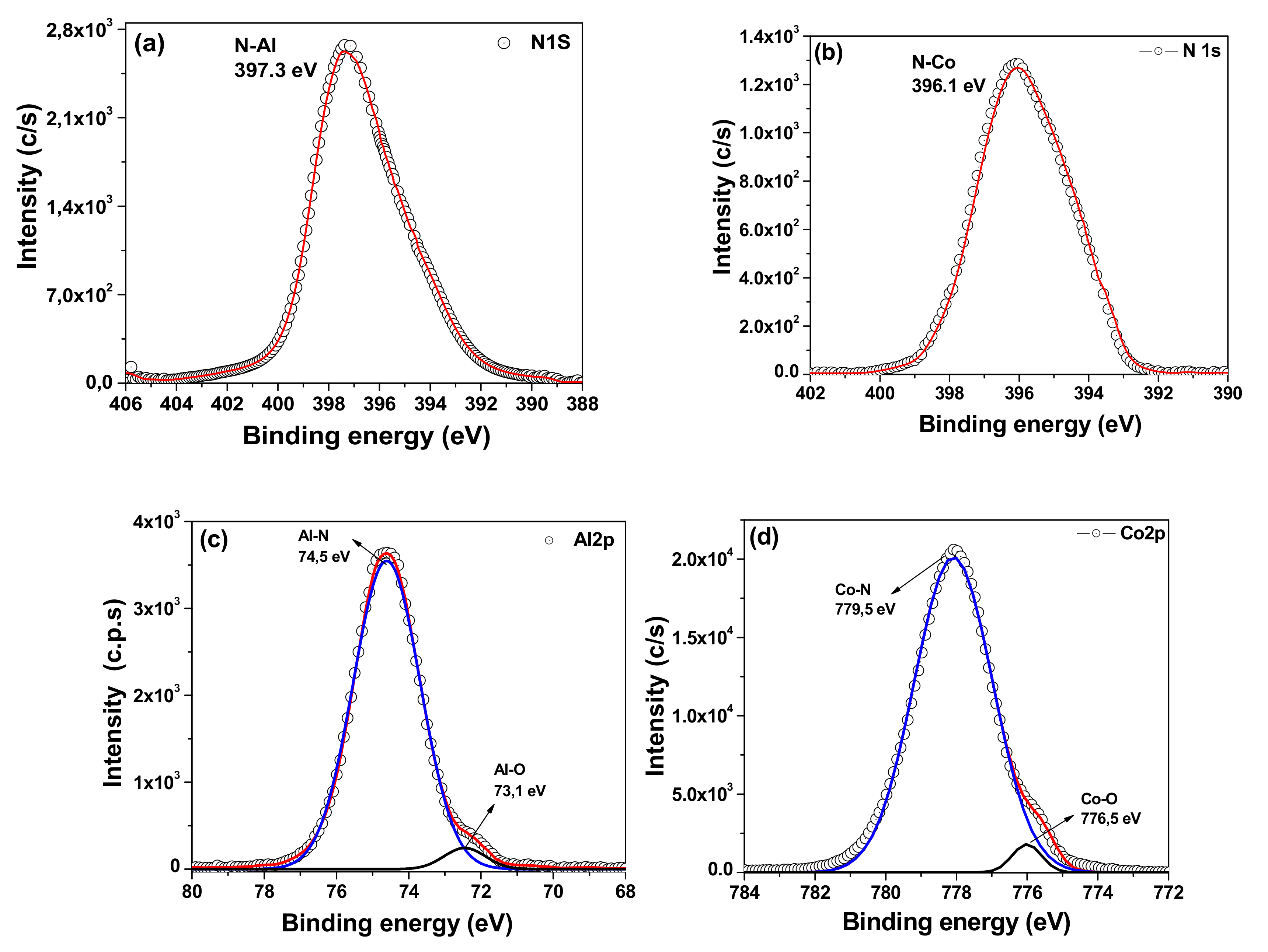
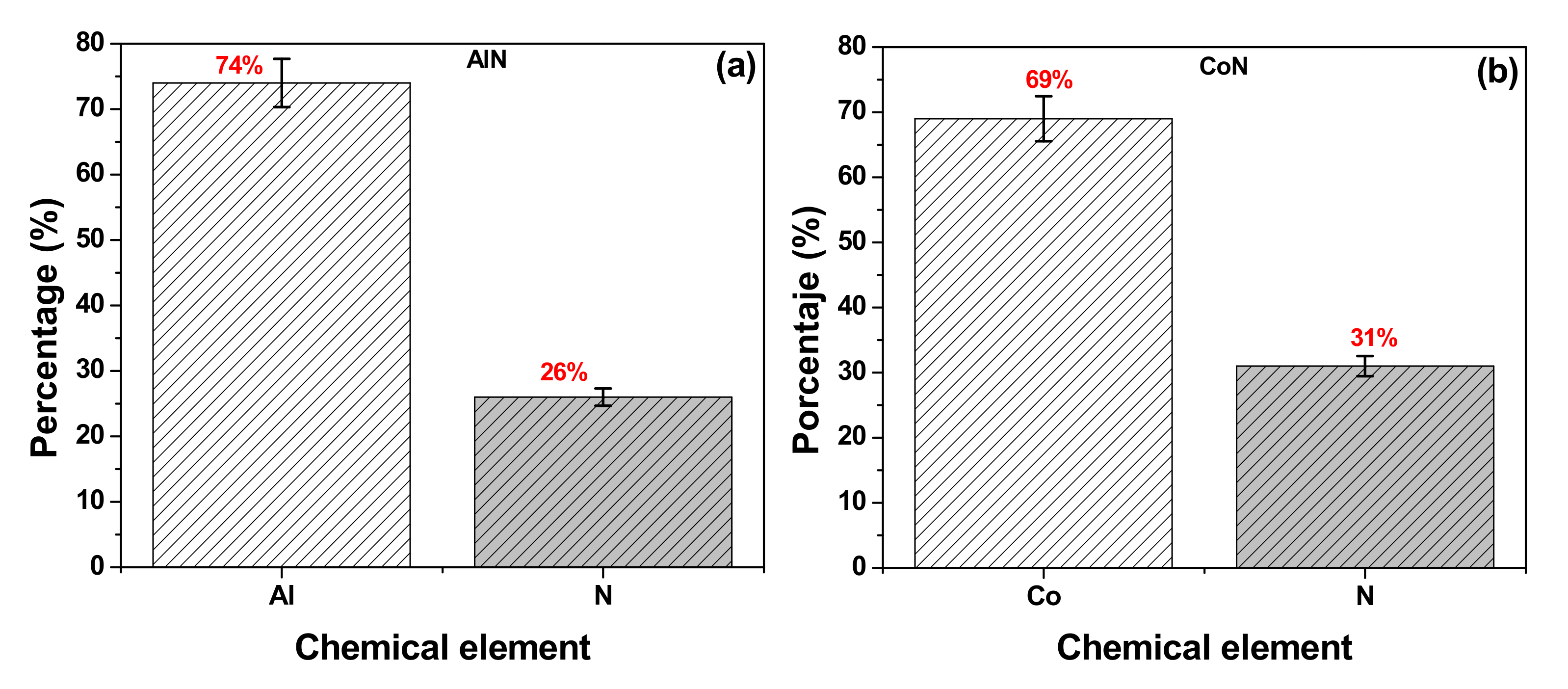
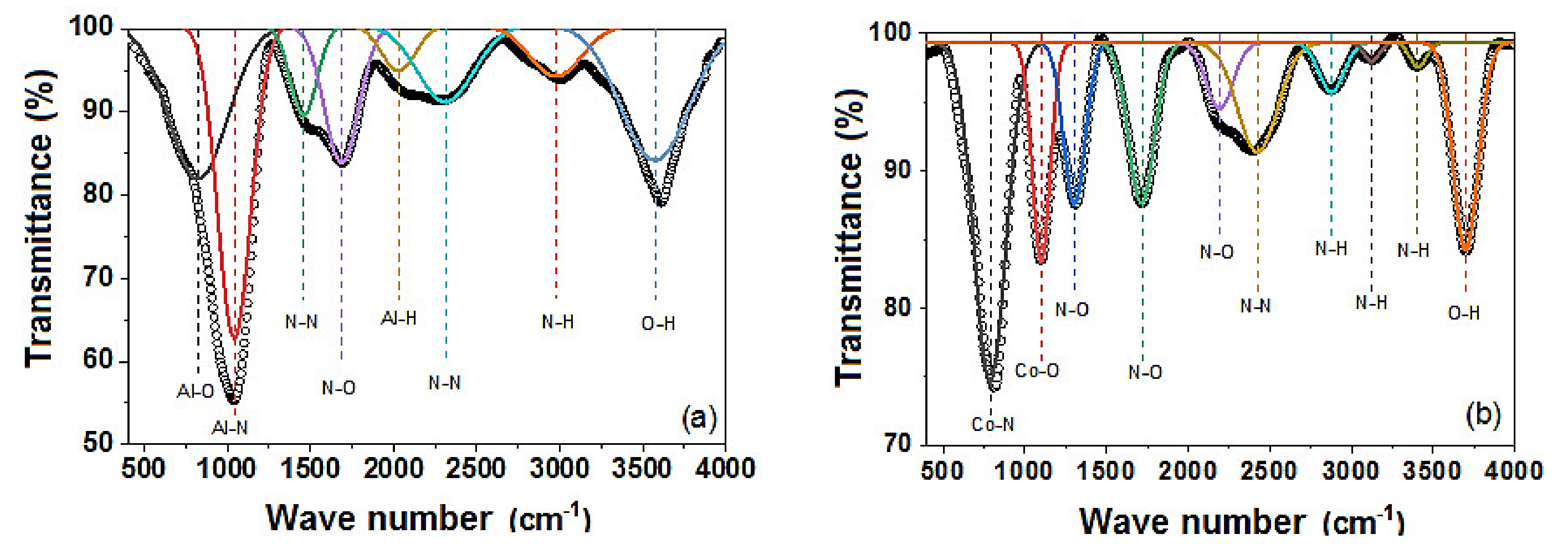
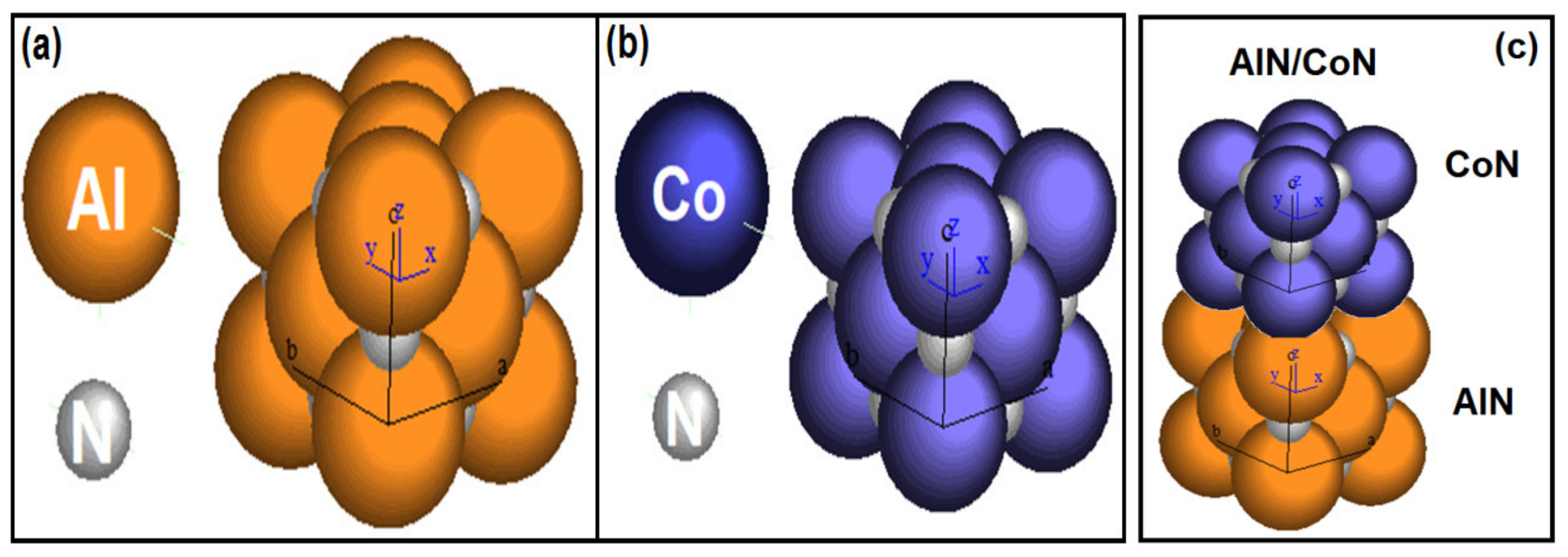
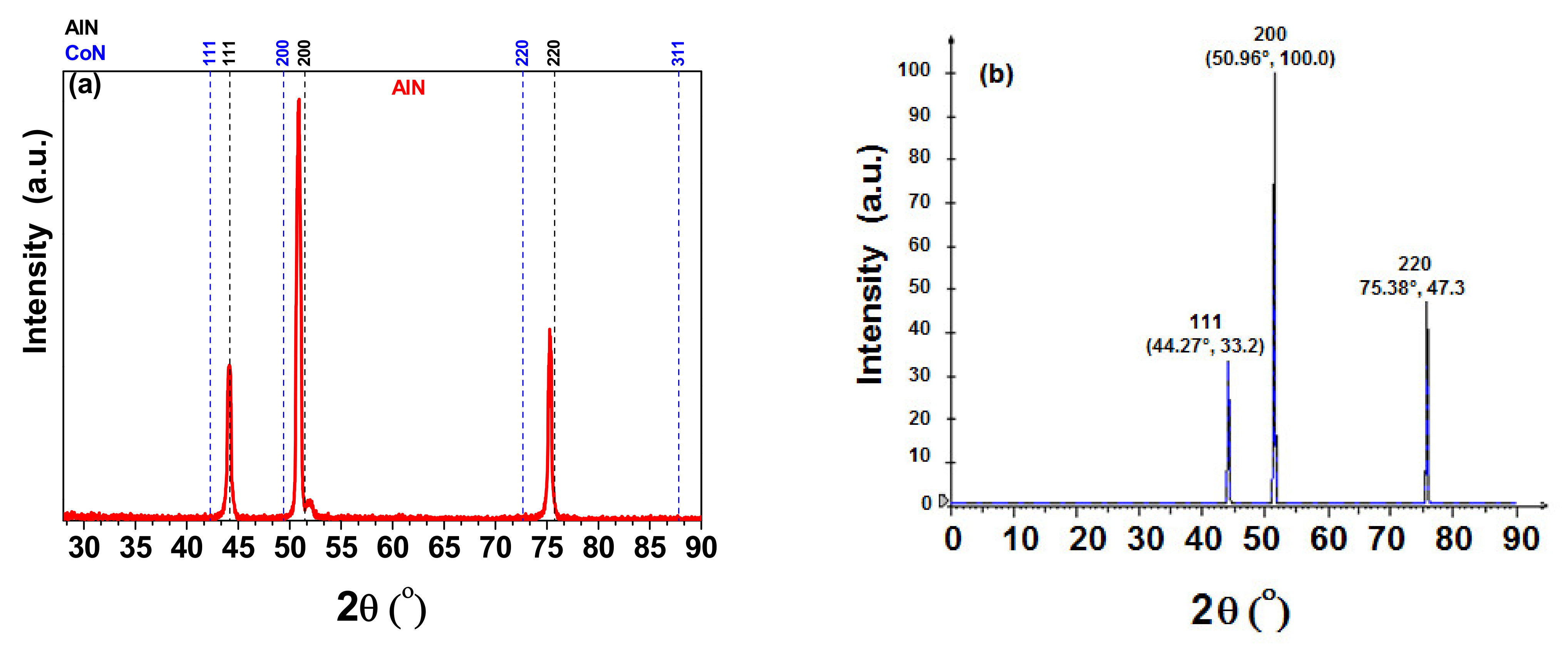
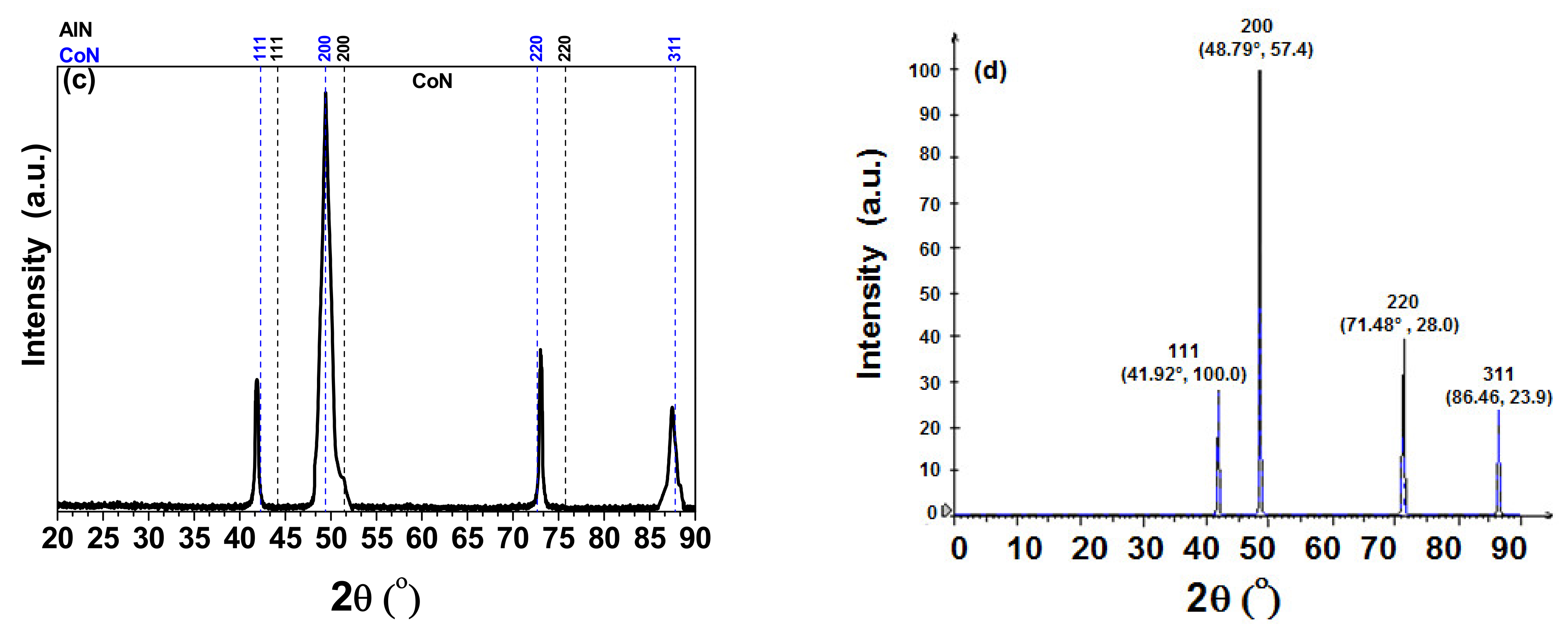


| Coatings | Lateral Crystallite Size (nm) | Bragg Plane | Preferential Direction |
|---|---|---|---|
| cAlN | 53 | (200) | - |
| CoN | 47 | (200) | - |
| [AlN/CoN]10 | 44 | - | (200) |
| [AlN/CoN]20 | 40 | - | (200) |
| [AlN/CoN]30 | 37 | - | (200) |
| Chemicals Elements (%) | |||||
|---|---|---|---|---|---|
| Coatings | Al | Co | N | N/Al | N/Co |
| AlN | 74 | -- | 26 | 0.35 | -- |
| CoN | -- | 69 | 31 | -- | 0.45 |
| Crystallographic Simulation Parameters | ||||
|---|---|---|---|---|
| Coatings | Crystal Structure | Space Group | Lattice Parameter (a0) (Å) | Stoichiometric Ratio |
| AlN | FCC | Fm3m | 3.55 | Al74N26 |
| CoN | FCC | F43m | 3.73 | Co69N31 |
| Systems | Ecorr (mV) | Icorr (nA) |
|---|---|---|
| Single layer CoN | −68.7 | 182.40 |
| Single layer AlN | −525.0 | 8.45 |
| [AlN/CoN]10 | −360.0 | 6.98 |
| [AlN/CoN]20 | −189.0 | 4.41 |
| [AlN/CoN]30 | −92.0 | 1.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, J.; Bonilla, N.; Aperador, W. Corrosion Nature in [CoN/AlN]n Multilayers Obtained from Laser Ablation. Metals 2021, 11, 2049. https://doi.org/10.3390/met11122049
Caicedo J, Bonilla N, Aperador W. Corrosion Nature in [CoN/AlN]n Multilayers Obtained from Laser Ablation. Metals. 2021; 11(12):2049. https://doi.org/10.3390/met11122049
Chicago/Turabian StyleCaicedo, Julio, Neufer Bonilla, and Willian Aperador. 2021. "Corrosion Nature in [CoN/AlN]n Multilayers Obtained from Laser Ablation" Metals 11, no. 12: 2049. https://doi.org/10.3390/met11122049
APA StyleCaicedo, J., Bonilla, N., & Aperador, W. (2021). Corrosion Nature in [CoN/AlN]n Multilayers Obtained from Laser Ablation. Metals, 11(12), 2049. https://doi.org/10.3390/met11122049