Microstructure and Phase Formation of Novel Al80Mg5Sn5Zn5X5 Light-Weight Complex Concentrated Aluminum Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermo-Physical Parameters for Phase Formation in HEAs
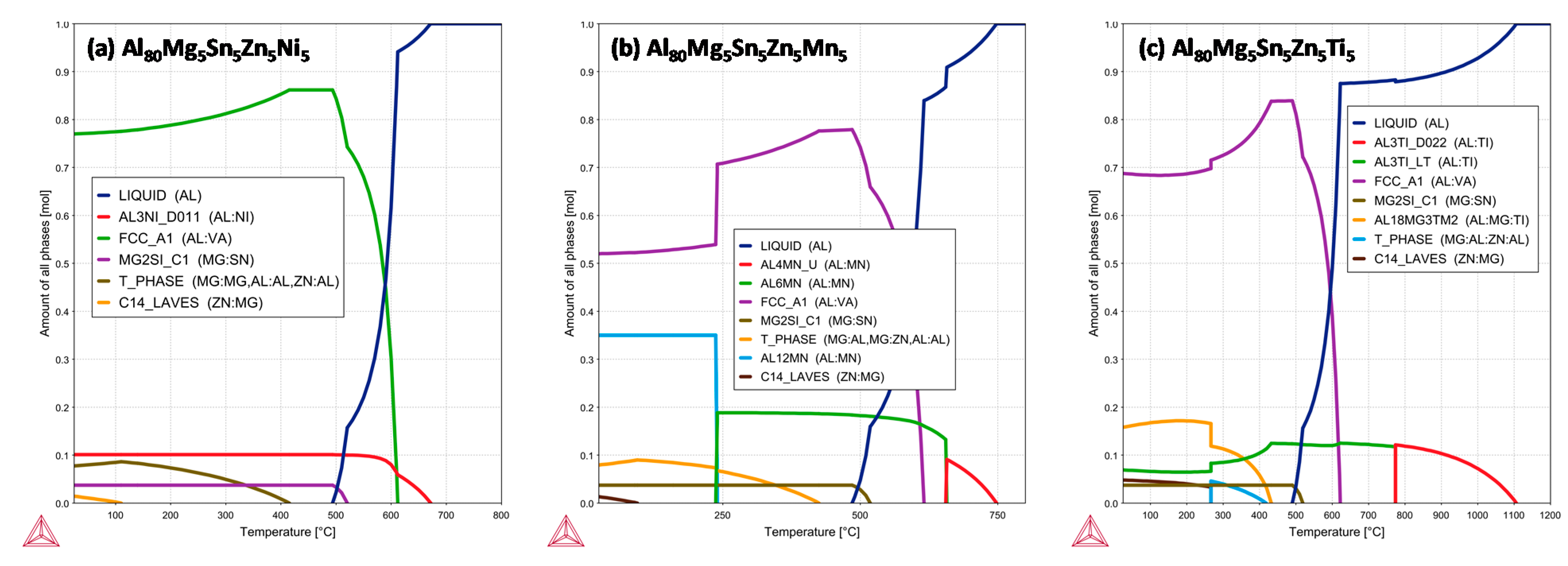
3.2. Equilibrium Phase Diagram of the Alloys
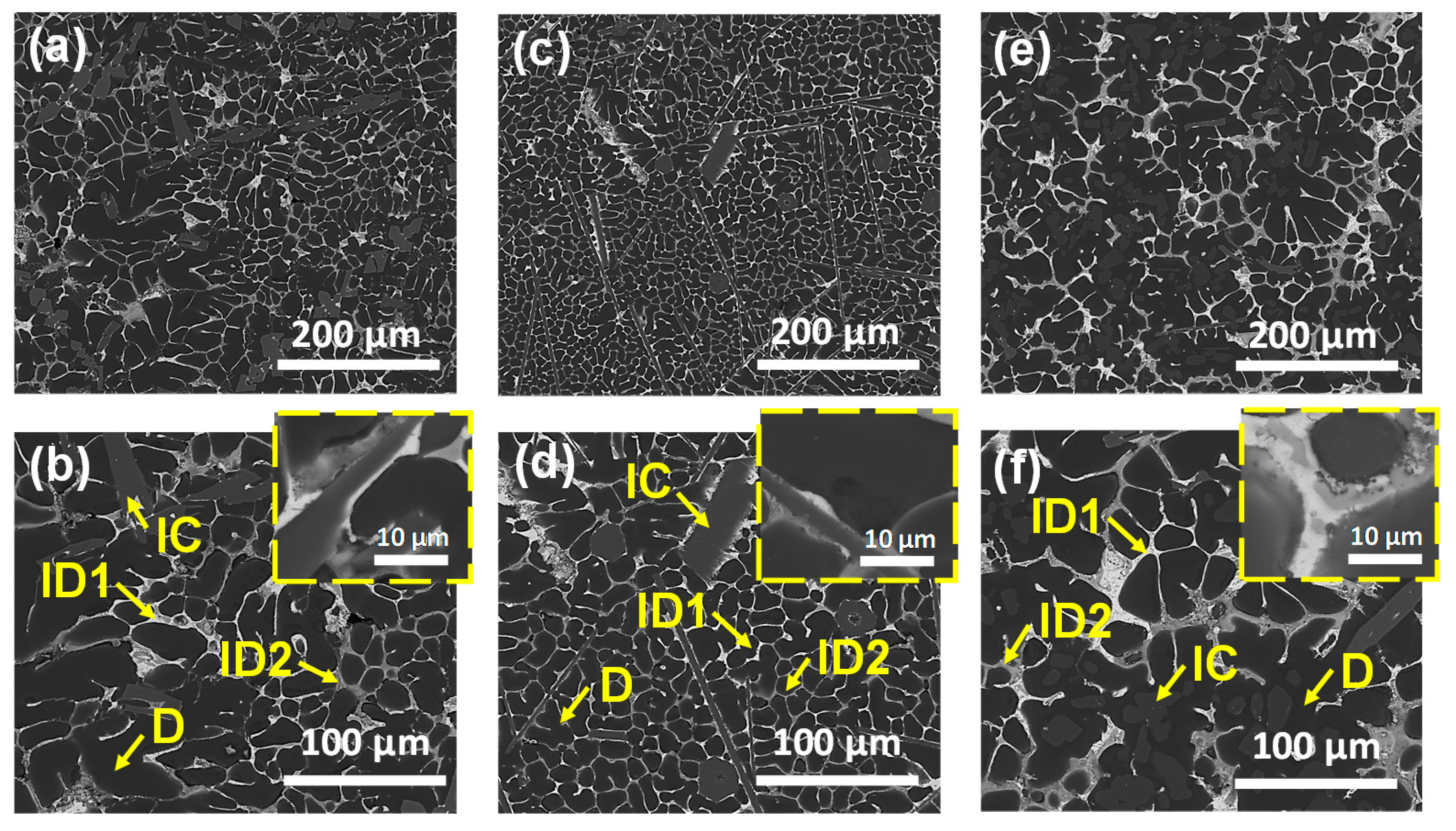
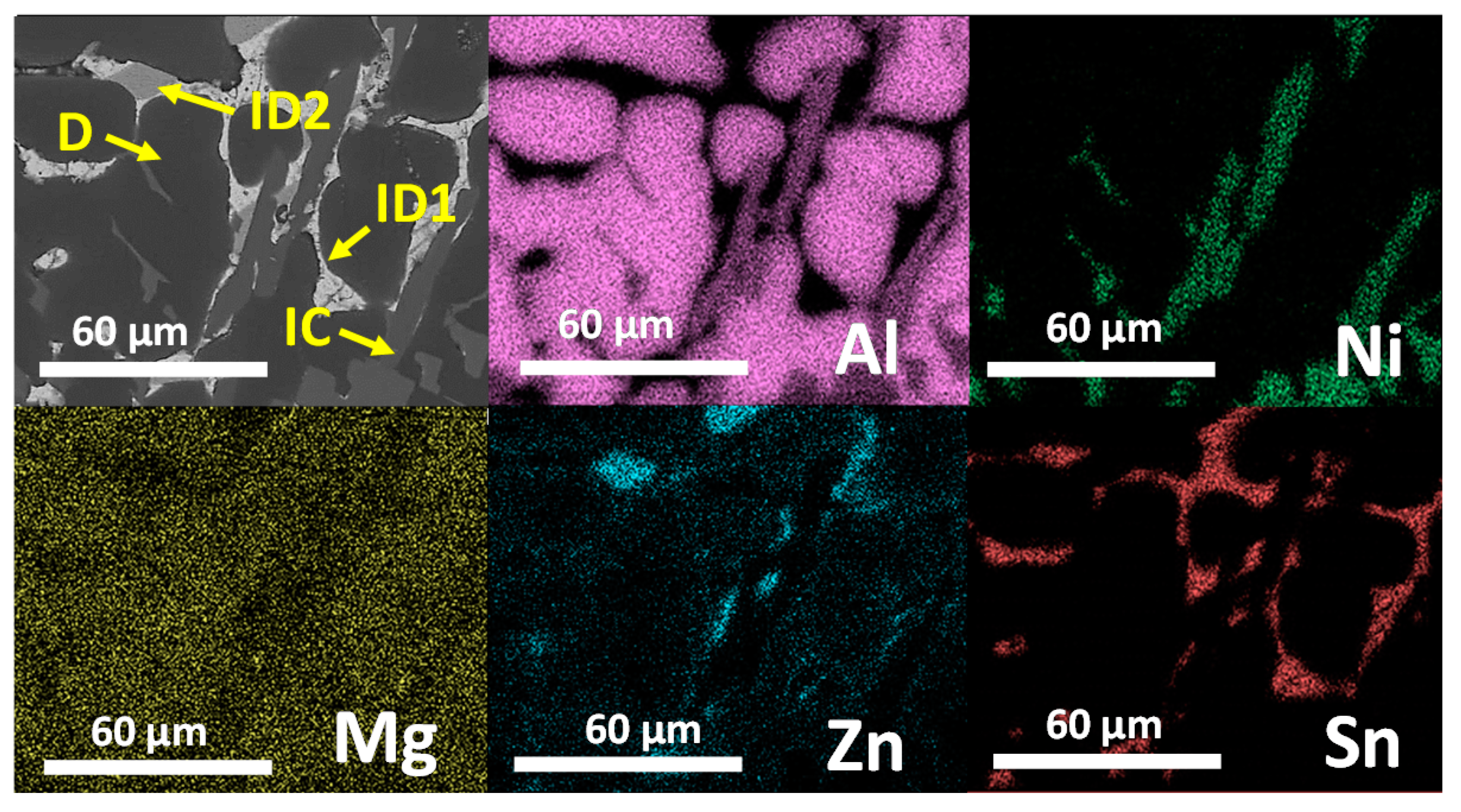
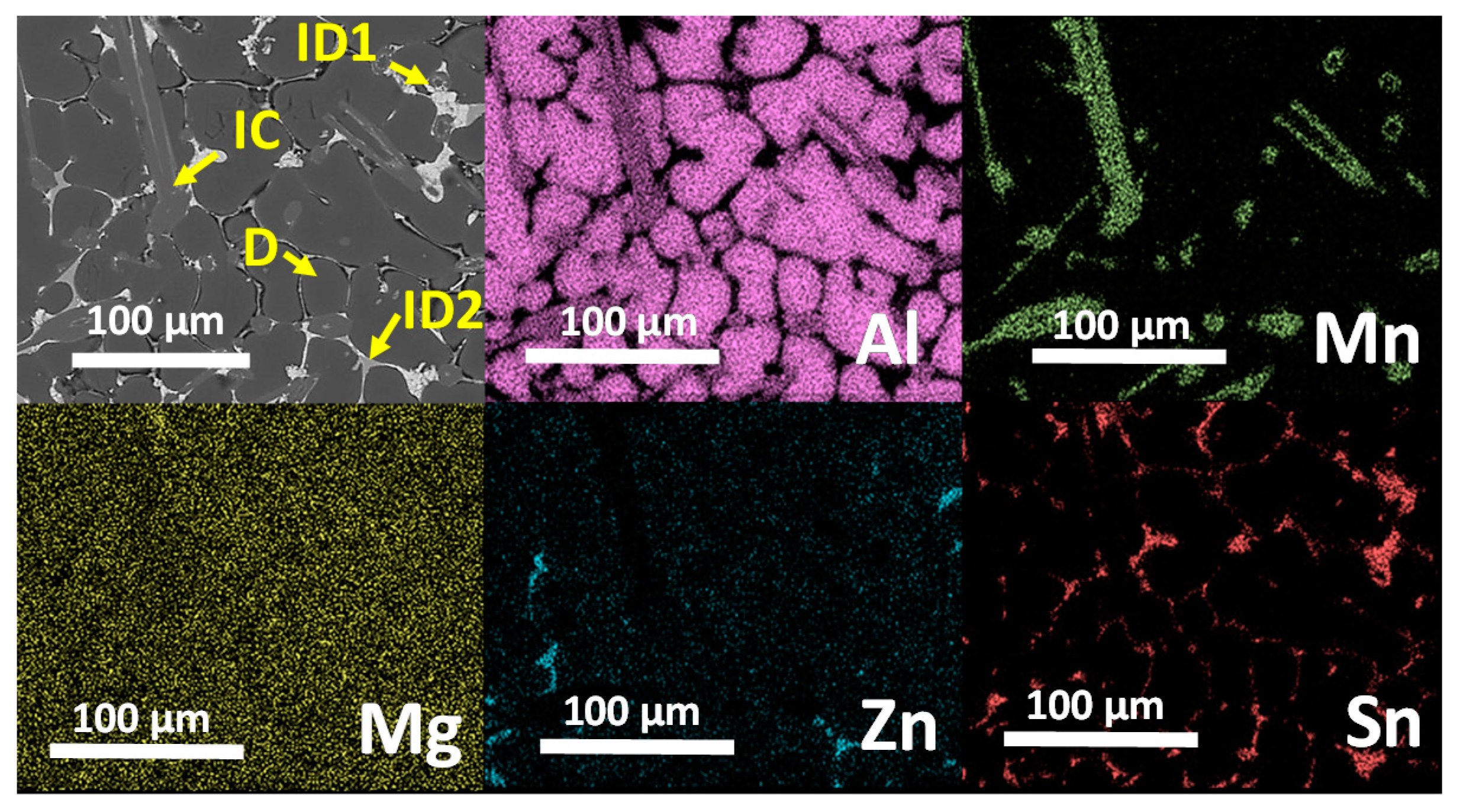
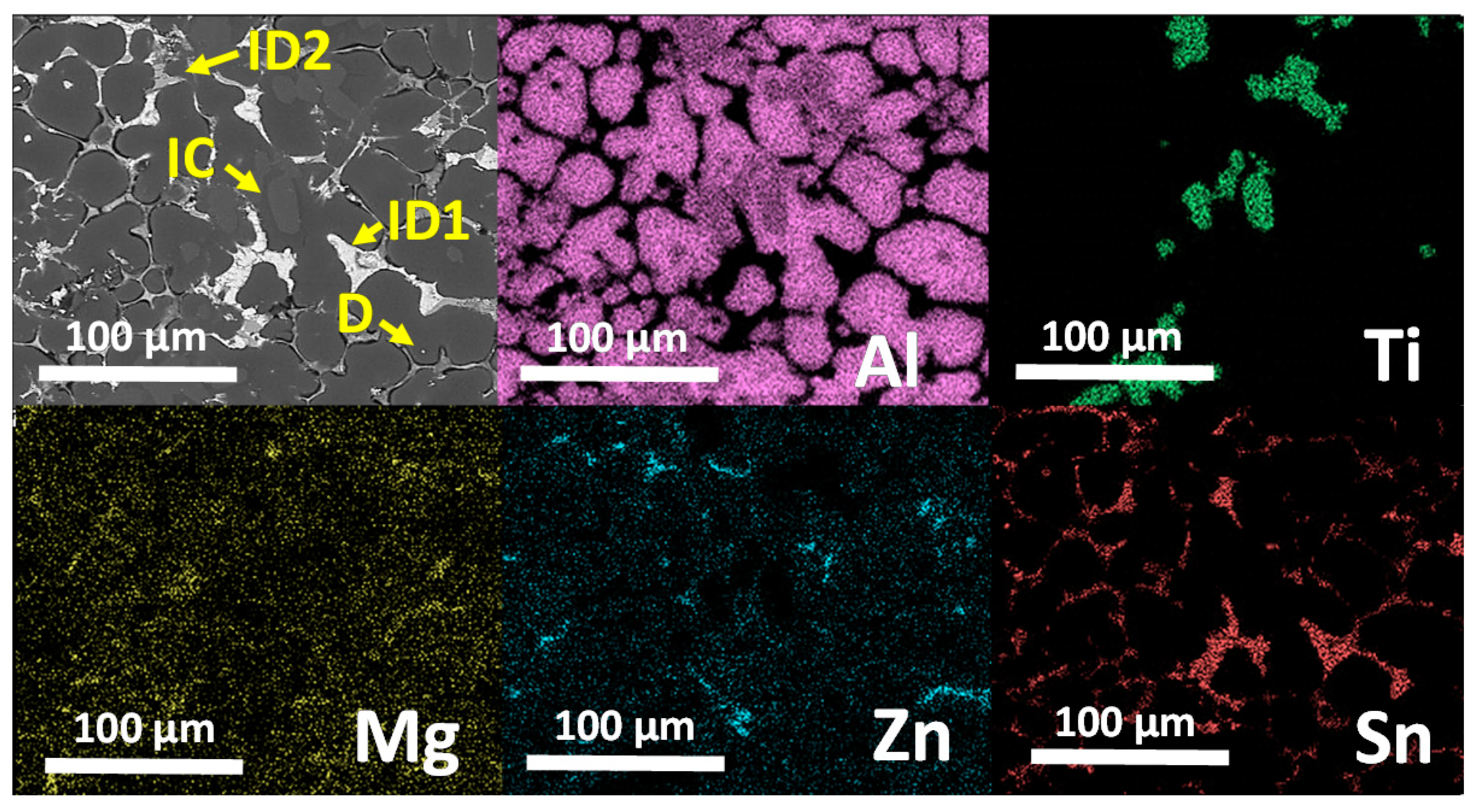
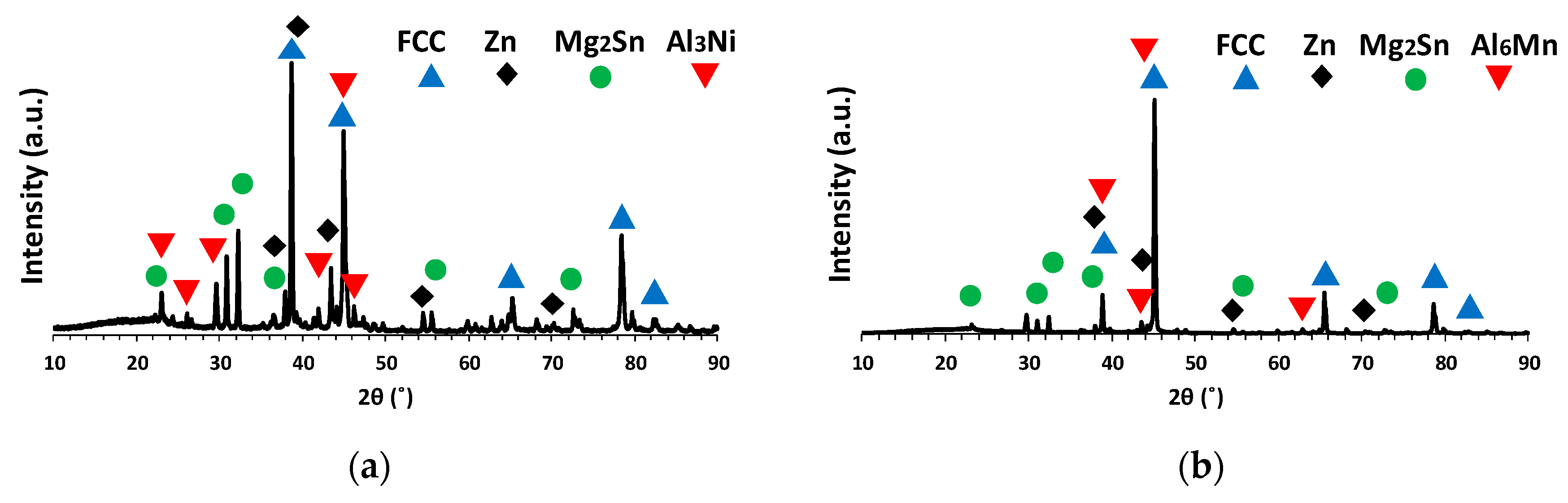
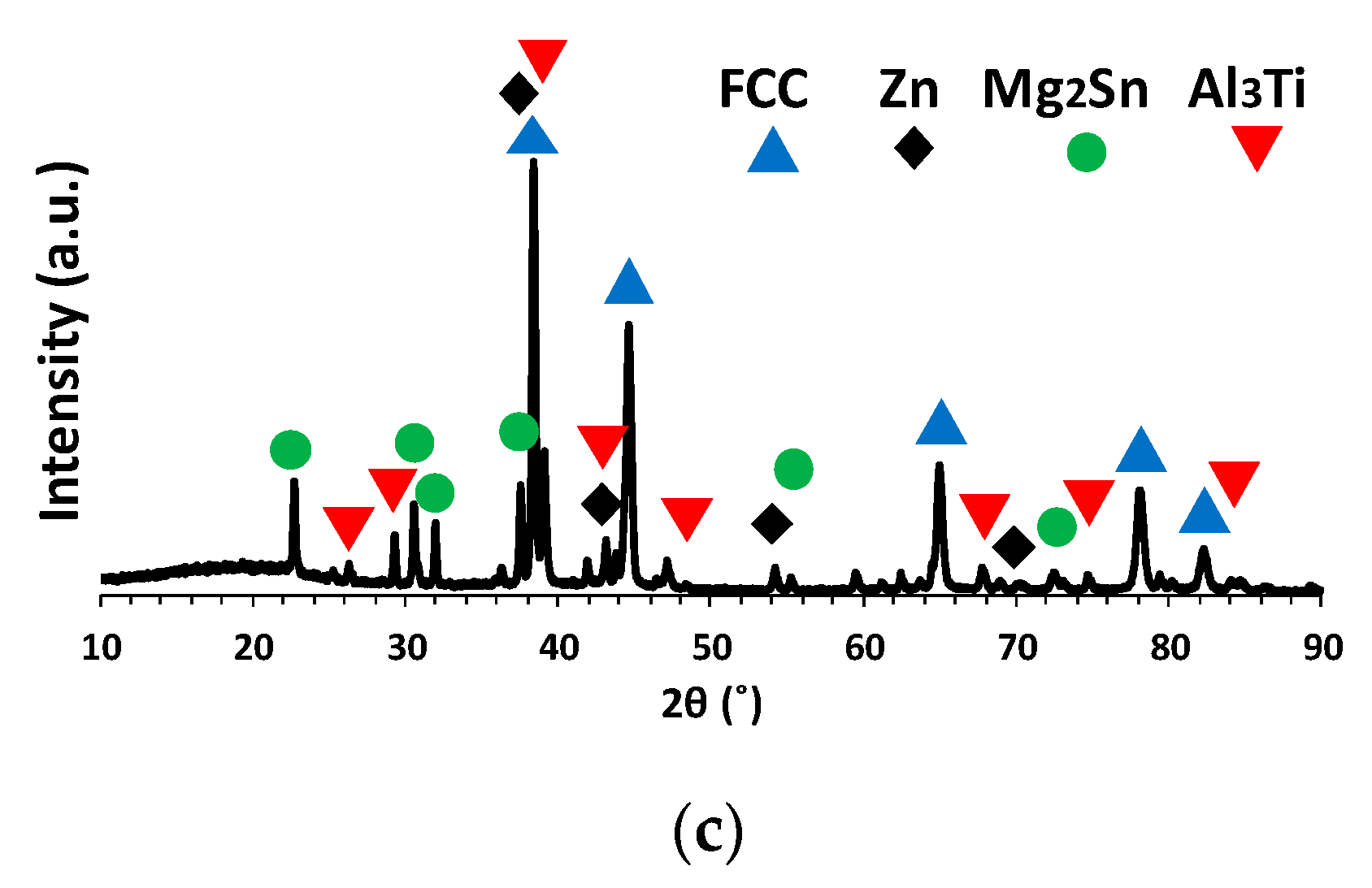
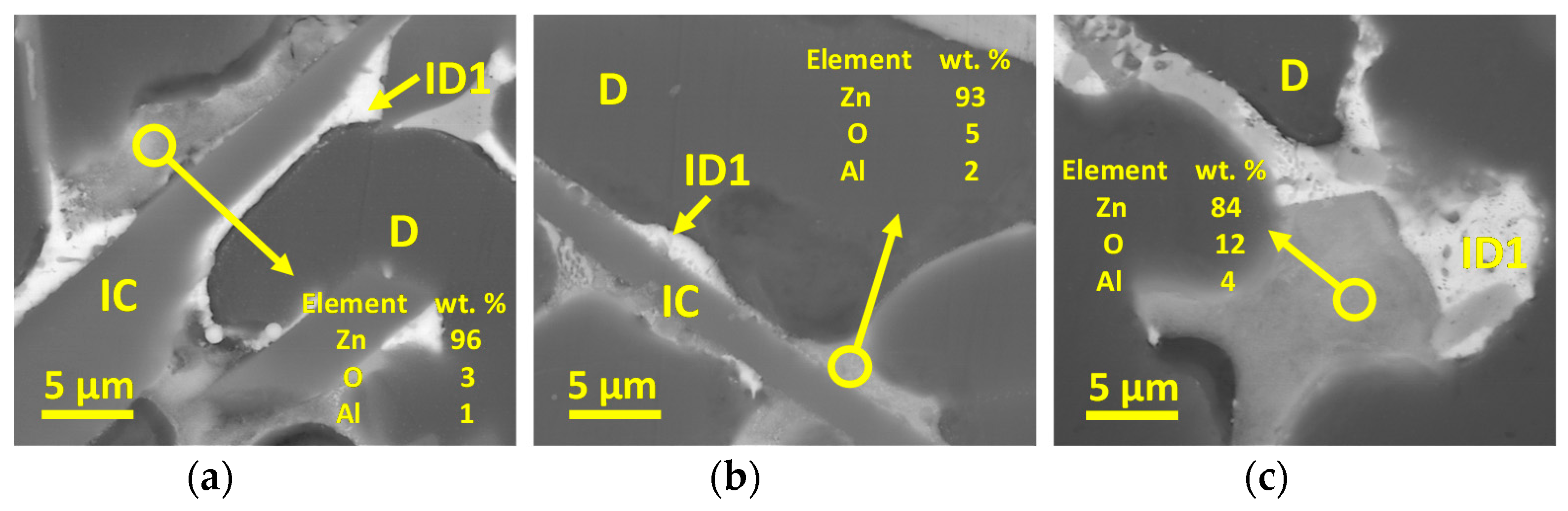
3.3. Microstructural Characterization
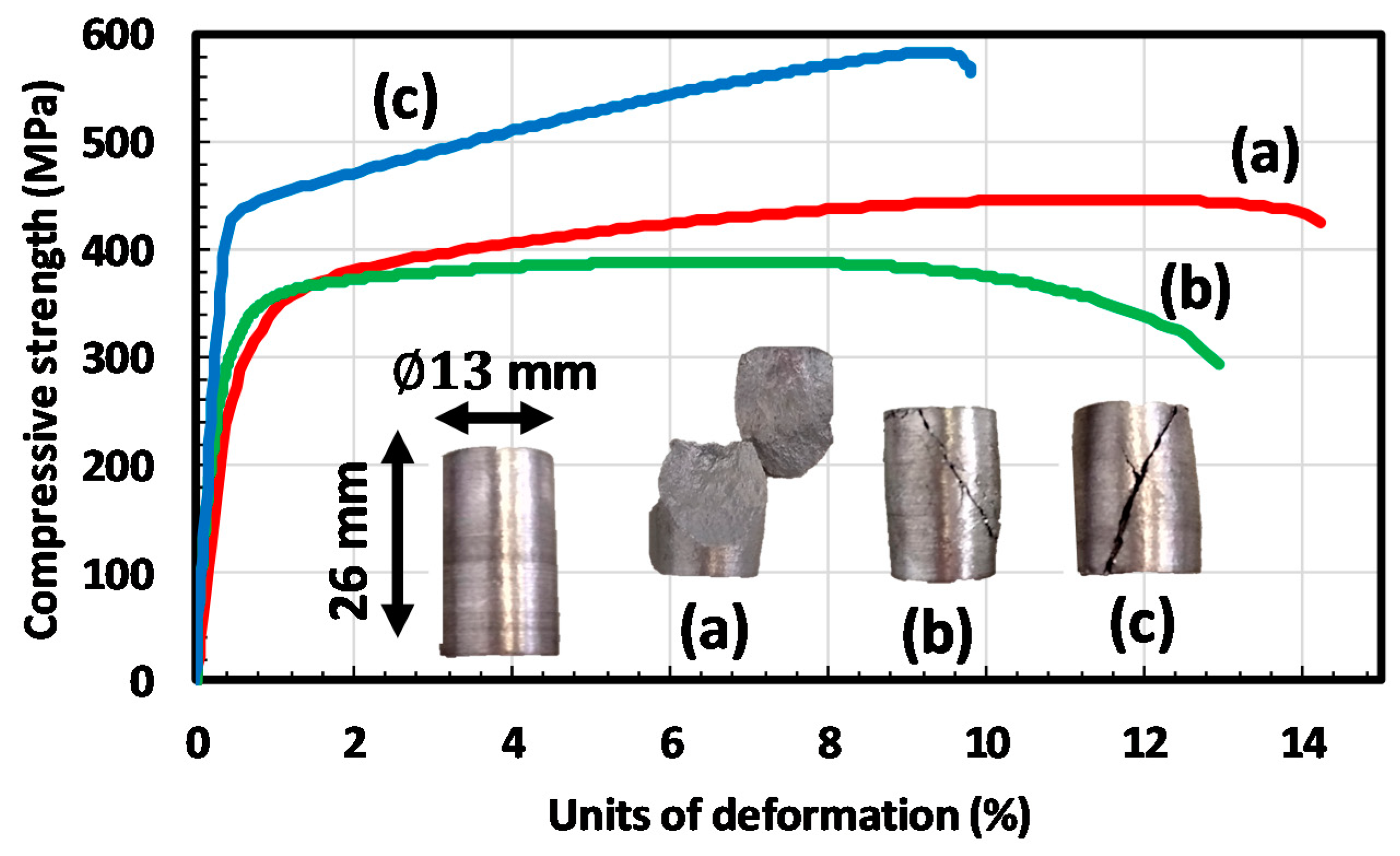
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, M.C.; Liaw, P.K.; Yeh, J.-W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319270135. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Grewal, H.; Arora, H.; Mukherjee, S. Corrosion, Erosion and Wear Behavior of Complex Concentrated Alloys: A Review. Metals 2018, 8, 603. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Z.; Fan, X.; Lee, C.; Wang, S.Y.; Feng, R.; Liaw, P.K. Fundamental understanding of mechanical behavior of high-entropy alloys at low temperatures: A review. J. Mater. Res. 2018, 33, 2998–3010. [Google Scholar] [CrossRef] [Green Version]
- Gorsse, S.; Miracle, D.B.; Senkov, O.N. Mapping the world of complex concentrated alloys. Acta Mater. 2017, 135, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. Applications and future directions. In High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–257. ISBN 9780128160671. [Google Scholar]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S. Phase selection rules for cast high entropy alloys: An overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Gao, M.C.; Zhang, C.; Gao, P.; Zhang, F.; Ouyang, L.Z.; Widom, M.; Hawk, J.A. Thermodynamics of concentrated solid solution alloys. Curr. Opin. Solid State Mater. Sci. 2017, 21, 238–251. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Koval, N.; Garcia, J. Compound Formation and Microstructure of As-Cast High Entropy Aluminums. Metals 2018, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Garcia, J.C. Phase prediction, microstructure and high hardness of novel light-weight high entropy alloys. J. Mater. Res. Technol. 2018, 8, 795–803. [Google Scholar] [CrossRef]
- Feng, R.; Gao, M.; Lee, C.; Mathes, M.; Zuo, T.; Chen, S.; Hawk, J.; Zhang, Y.; Liaw, P. Design of Light-Weight High-Entropy Alloys. Entropy 2016, 18, 333. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase Stability of Low-Density, Multiprincipal Component Alloys Containing Aluminum, Magnesium, and Lithium. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Sun, W.; Huang, X.; Luo, A.A. Phase formations in low density high entropy alloys. Calphad 2017, 56, 19–28. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M. An Insight into Evolution of Light Weight High Entropy Alloys: A Review. Metals 2016, 6, 199. [Google Scholar] [CrossRef] [Green Version]
- Maulik, O.; Kumar, D.; Kumar, S.; Dewangan, S.K.; Kumar, V. Structure and properties of lightweight high entropy alloys: A brief review. Mater. Res. Express 2018, 5, 052001. [Google Scholar] [CrossRef]
- Jia, Y.; Jia, Y.; Wu, S.; Ma, X.; Wang, G. Novel ultralight-weight complex concentrated alloys with high strength. Materials 2019, 12, 1136. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Acuña, E.M. Design, Microstructure and Mechanical Properties of Cast Medium Entropy Aluminium Alloys. Sci. Rep. 2019, 9, 6792. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, T.; Li, L.; Zhao, Y.; Huang, J.; Liaw, P.K.; Zhang, Y. A Low-Cost Lightweight Entropic Alloy with High Strength. J. Mater. Eng. Perform. 2018, 27, 6648–6656. [Google Scholar] [CrossRef]
- Zhang, B.; Liaw, P.K.; Brechtl, J.; Ren, J.; Guo, X.; Zhang, Y. Effects of Cu and Zn on microstructures and mechanical behavior of the medium-entropy aluminum alloy. J. Alloys Compd. 2020, 820, 153092. [Google Scholar] [CrossRef]
- Liao, Y.C.; Li, T.H.; Tsai, P.H.; Jang, J.S.C.; Hsieh, K.C.; Chen, C.Y.; Huang, J.C.; Wu, H.J.; Lo, Y.C.; Huang, C.W.; et al. Designing novel lightweight, high-strength and high-plasticity Tix(AlCrNb)100-x medium-entropy alloys. Intermetallics 2020, 117, 106673. [Google Scholar] [CrossRef]
- Mitrica, D.; Badea, I.C.; Olaru, M.T.; Serban, B.A.; Vonica, D.; Burada, M.; Geanta, V.; Rotariu, A.N.; Stoiciu, F.; Badilita, V.; et al. Modeling and Experimental Results of Selected Lightweight Complex Concentrated Alloys, before and after Heat Treatment. Materials 2020, 13, 4330. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.; Jin, X.; Zhang, L.; Du, X.; Li, B. Design of non-equiatomic medium-entropy alloys. Sci. Rep. 2018, 8, 1236. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Wang, P.Y.; Wang, B.Y.; Wang, C.; Wang, J.G.; Ma, C.Y.; Li, J.S.; Zha, M.; Wang, H.Y. Design of multicomponent Mg–Al–Zn–Sn–Bi alloys with refined microstructure and enhanced tensile properties. Mater. Sci. Eng. A 2020, 791, 139696. [Google Scholar] [CrossRef]
- Wu, K.C.; Yeh, J.W.; Chang, S.Y. Refined microstructure and improved mechanical properties of high-ratio extruded AZ91-xSn magnesium alloy. Mater. Chem. Phys. 2015, 162, 757–763. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Cao, L. Co-precipitation of T′ and η′ phase in Al-Zn-Mg-Cu alloys. Mater. Charact. 2020, 169, 110610. [Google Scholar] [CrossRef]
- Yang, P.; Engler, O.; Klaar, H.J. Orientation relationship between Al6Mn precipitates and the Al matrix during continuous recrystallization in Al-1.3%Mn. J. Appl. Crystallogr. 1999, 32, 1105–1118. [Google Scholar] [CrossRef]
| Alloy | ∆Hmix (kJ/mol) | (%) | ∆χ | Ω | ρt (g/cm3) |
|---|---|---|---|---|---|
| AlMgSnZnNi | −7.7 | 8.6 | 0.234 | 1.7 | 5.20 |
| Al35Mg35Sn5Zn20Ni5 | −4.3 | 6.9 | 0.196 | 2.3 | 3.49 |
| Al80Mg5Sn5Zn5Ni5 | −3.3 | 4.3 | 0.122 | 1.8 | 3.37 |
| AlMgSnZnMn | −4.9 | 8.3 | 0.209 | 2.5 | 5.12 |
| Al35Mg35Sn5Zn20Mn5 | −3.1 | 6.8 | 0.177 | 3.3 | 3.48 |
| Al80Mg5Sn5Zn5Mn5 | −2.7 | 4.2 | 0.104 | 2.2 | 3.36 |
| AlMgSnZnTi | −5.8 | 5.2 | 0.209 | 2.3 | 4.68 |
| Al35Mg35Sn5Zn20Ti5 | −3.7 | 5.9 | 0.177 | 2.8 | 3.40 |
| Al80Mg5Sn5Zn5Ti5 | −4.4 | 3.2 | 0.105 | 1.4 | 3.27 |
| Alloy | Al | Mg | Sn | Zn | Ni | Mn | Ti | Other/s |
|---|---|---|---|---|---|---|---|---|
| Al80Mg5Sn5Zn5Ni5 | 77 | 3 | 5 | 7 | 5 | - | - | ~2 |
| Al80Mg5Sn5Zn5Mn5 | 76 | 4 | 6 | 8 | - | 4 | - | ~2 |
| Al80Mg5Sn5Zn5Ti5 | 75 | 4 | 6 | 8 | - | - | 5 | ~2 |
| Alloy | ρ (g/cm3) | HV0.1 | E (GPa) | σC0.2 (MPa) | σCU (MPa) | ε (%) |
|---|---|---|---|---|---|---|
| Al80Mg5Sn5Zn5Ni5 | 3.33 | 128 ± 12 | 62 ± 1 | 317 ± 3 | 447 ± 32 | 15 ± 2 |
| Al80Mg5Sn5Zn5Mn5 | 3.15 | 130 ± 14 | 80 ± 3 | 336 ± 8 | 368 ± 15 | 14 ± 3 |
| Al80Mg5Sn5Zn5Ti5 | 3.28 | 159 ± 11 | 98 ± 19 | 420 ± 57 | 563 ± 30 | 12 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, J.M.; Pascual, A.; Vicario, I.; Albizuri, J.; Guraya, T.; Galarraga, H. Microstructure and Phase Formation of Novel Al80Mg5Sn5Zn5X5 Light-Weight Complex Concentrated Aluminum Alloys. Metals 2021, 11, 1944. https://doi.org/10.3390/met11121944
Sanchez JM, Pascual A, Vicario I, Albizuri J, Guraya T, Galarraga H. Microstructure and Phase Formation of Novel Al80Mg5Sn5Zn5X5 Light-Weight Complex Concentrated Aluminum Alloys. Metals. 2021; 11(12):1944. https://doi.org/10.3390/met11121944
Chicago/Turabian StyleSanchez, Jon Mikel, Alejandro Pascual, Iban Vicario, Joseba Albizuri, Teresa Guraya, and Haize Galarraga. 2021. "Microstructure and Phase Formation of Novel Al80Mg5Sn5Zn5X5 Light-Weight Complex Concentrated Aluminum Alloys" Metals 11, no. 12: 1944. https://doi.org/10.3390/met11121944
APA StyleSanchez, J. M., Pascual, A., Vicario, I., Albizuri, J., Guraya, T., & Galarraga, H. (2021). Microstructure and Phase Formation of Novel Al80Mg5Sn5Zn5X5 Light-Weight Complex Concentrated Aluminum Alloys. Metals, 11(12), 1944. https://doi.org/10.3390/met11121944







