Development of Rapidly-Quenched Al-Ge-Si Filler Alloys for the Joining of Stainless Steel AISI 304 and Aluminum Alloy AA6082
Abstract
:1. Introduction
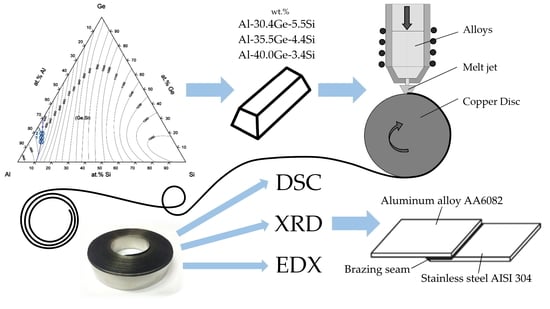
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Melting Characteristics of Filler Alloys
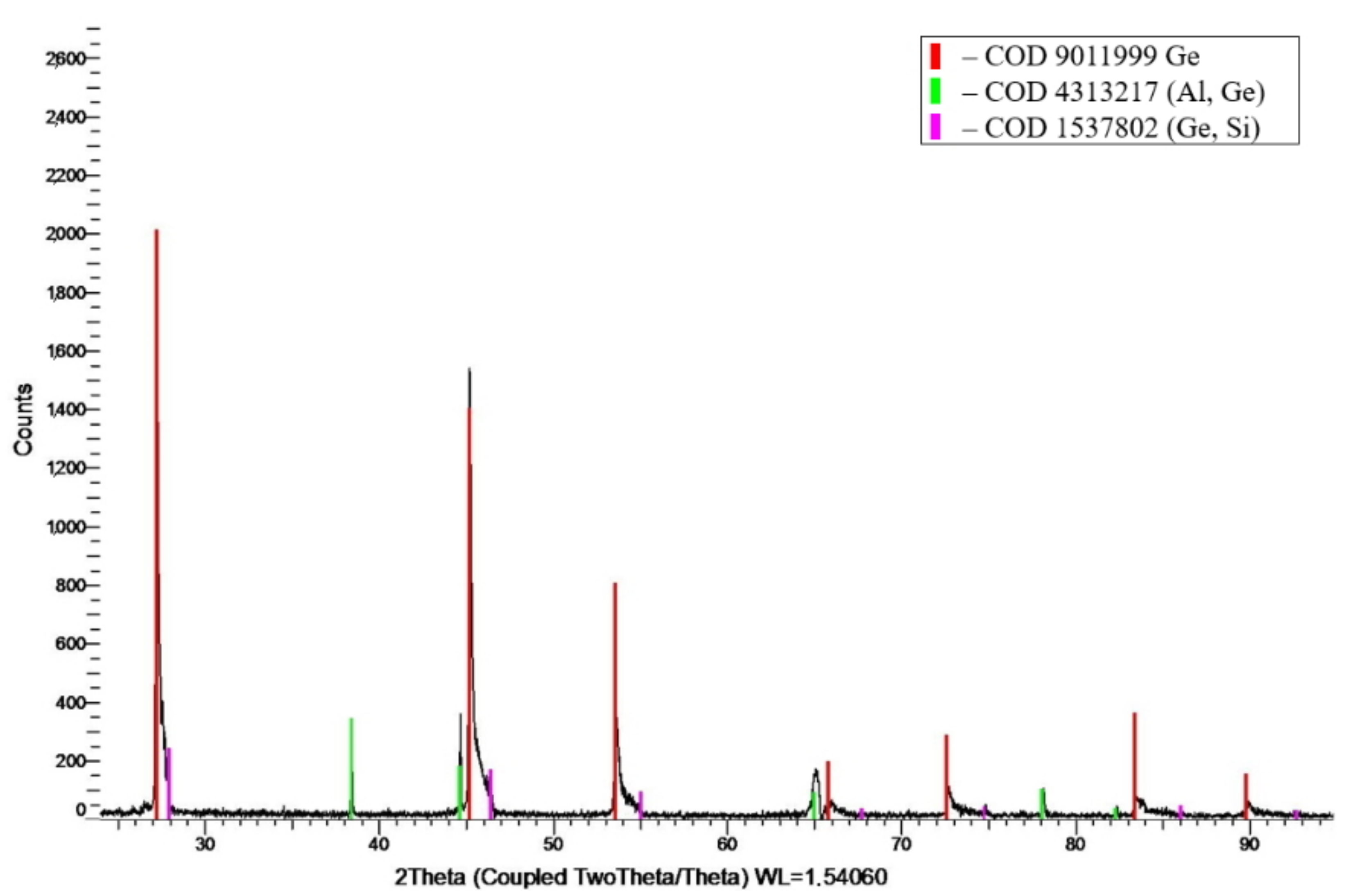
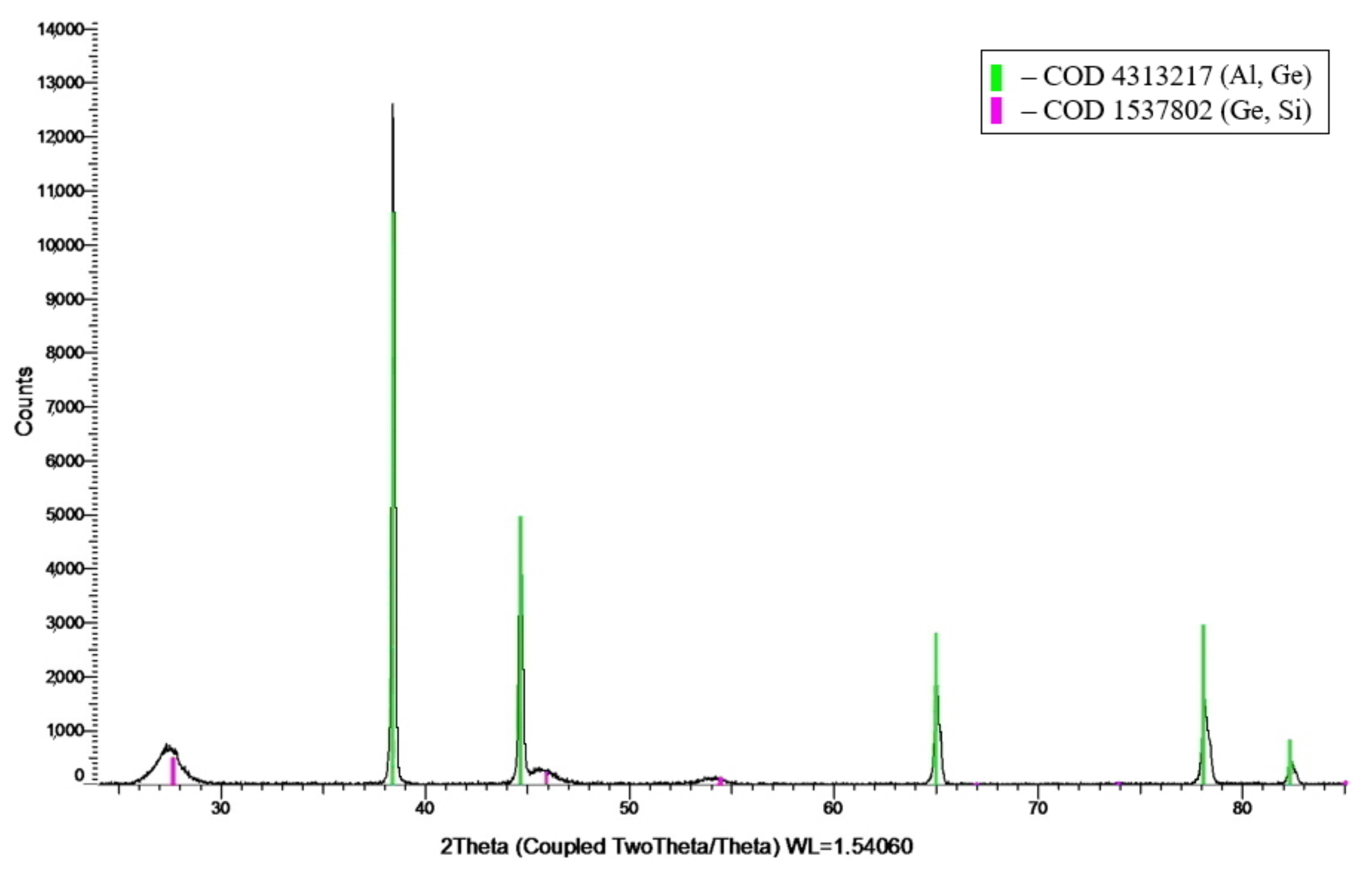
3.2. X-Ray Diffraction Analysis
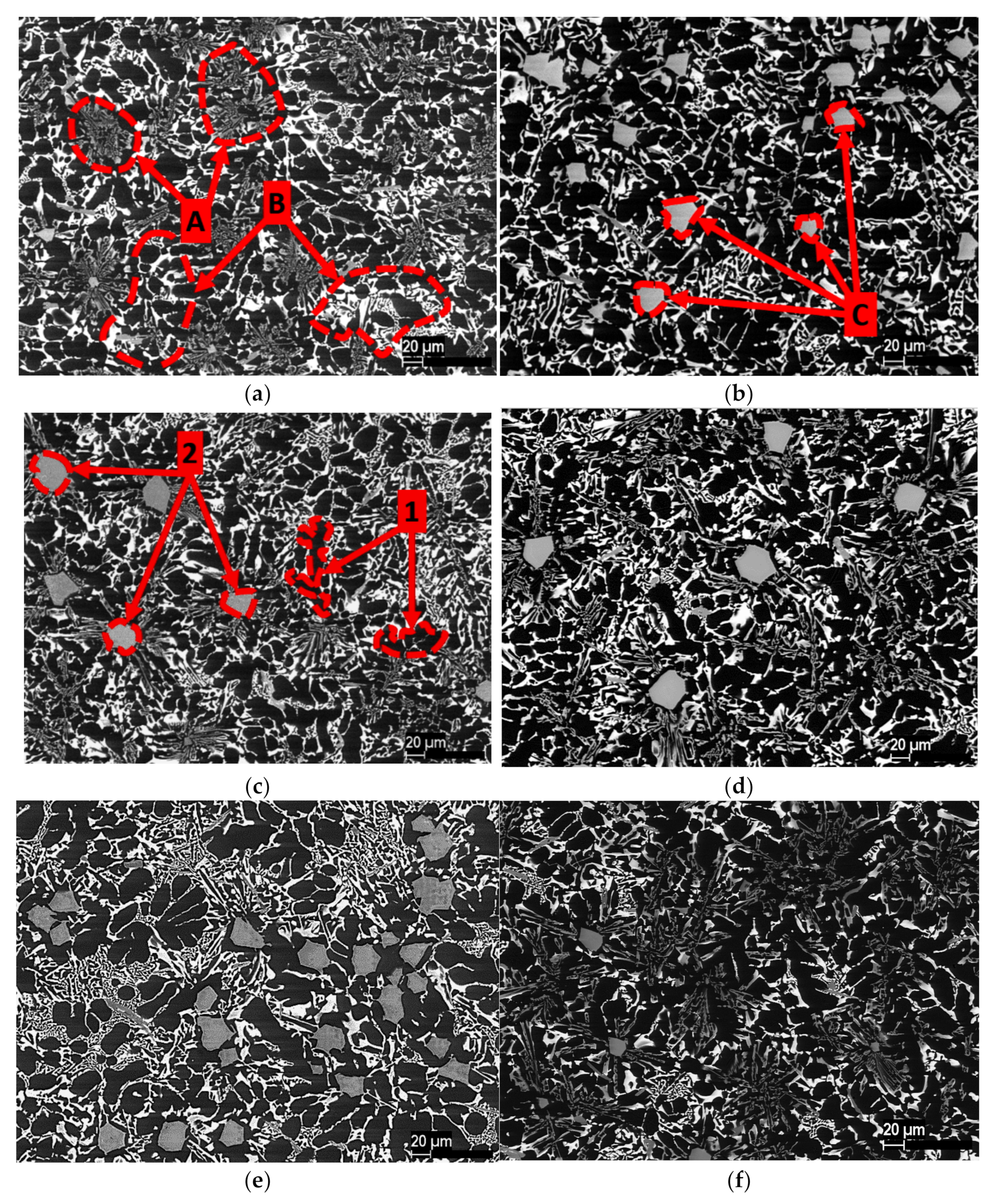
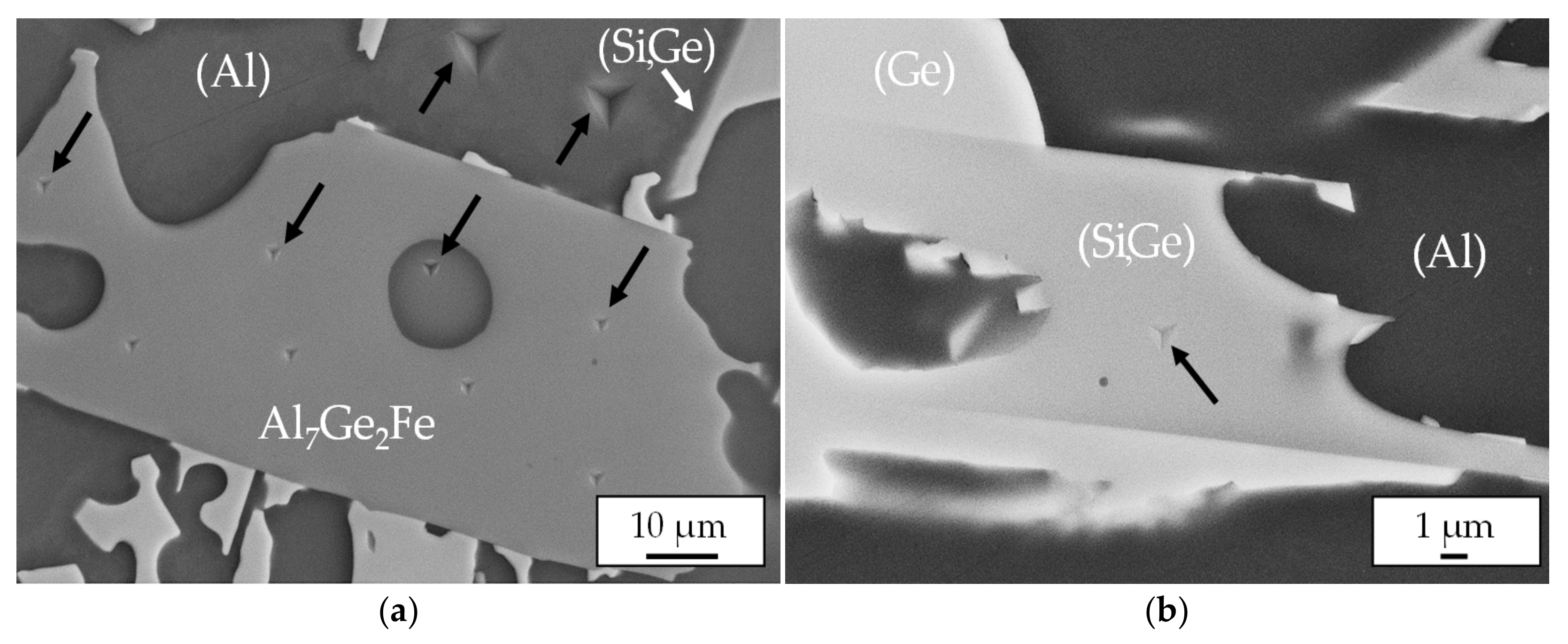
3.3. Microstructures of Al-Ge-Si Filler Alloys
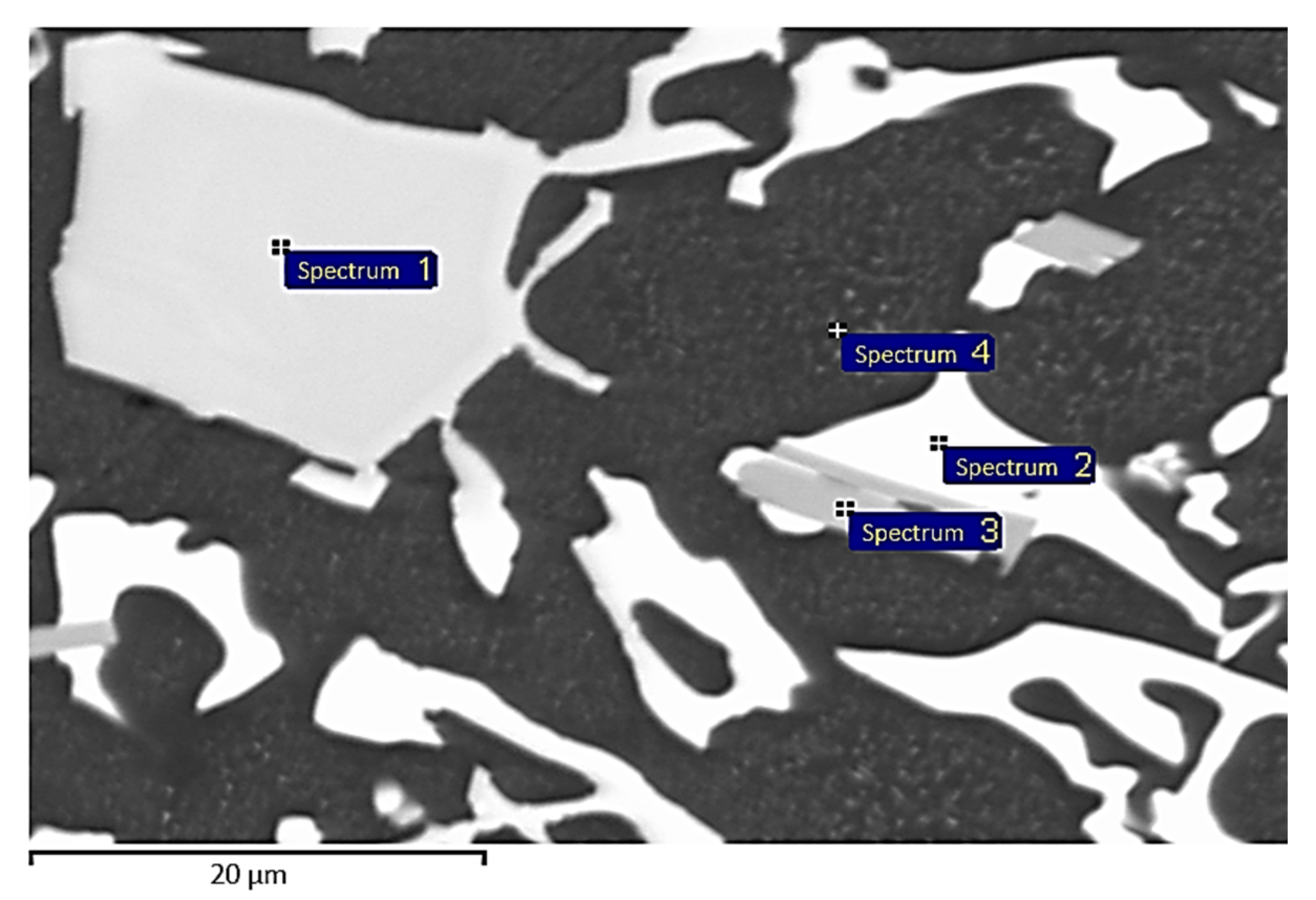
3.4. Energy-Dispersive X-Ray Analysis
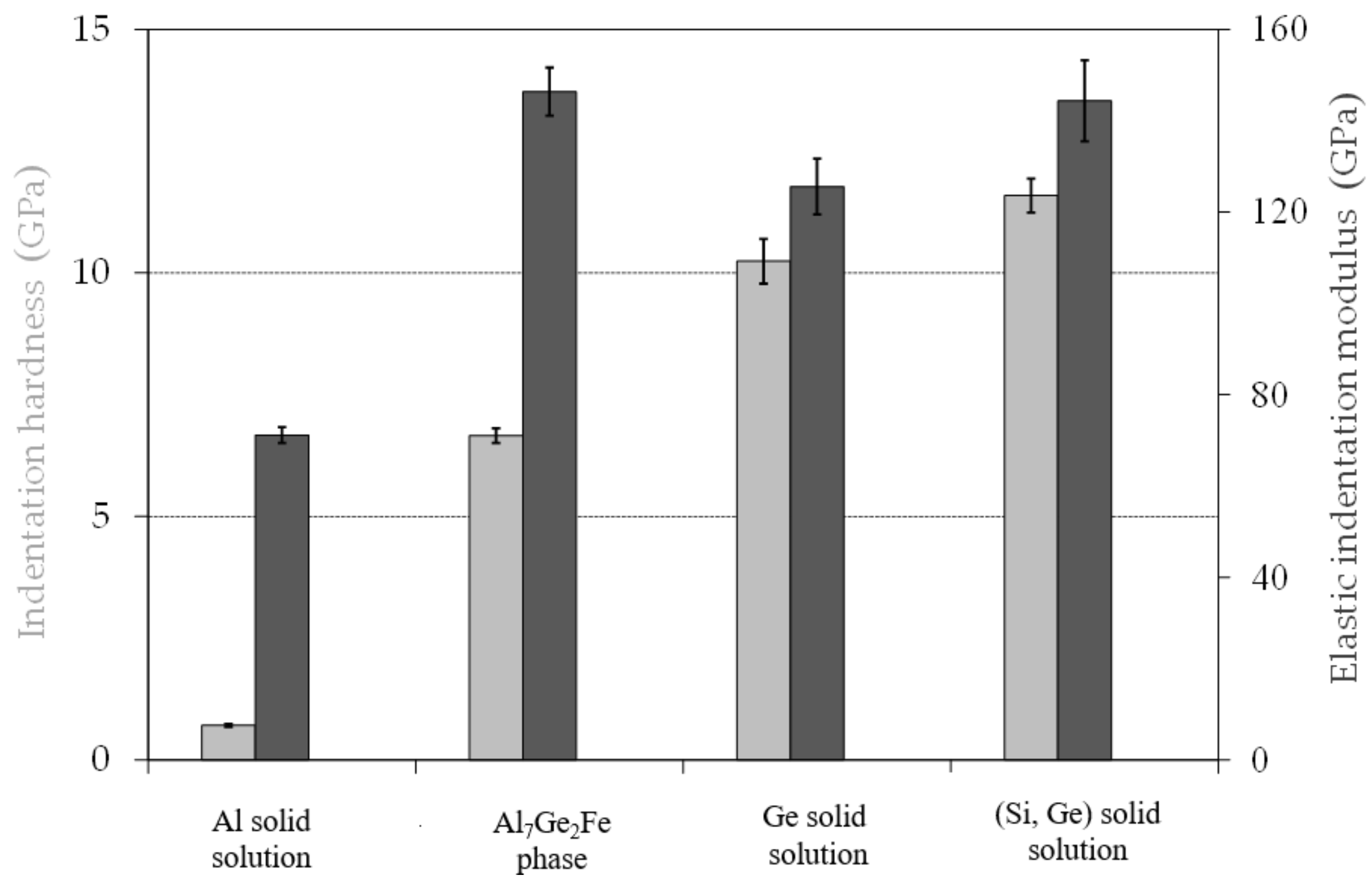
3.5. Mechanical Properties of Filler Alloy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilthey, U.; Stein, L. Multimaterial car body design: Challenge for welding and joining. Sci. Technol. Weld. Join. 2006, 11, 135–142. [Google Scholar] [CrossRef]
- Sierra, G.; Peyre, P.; Beaume, F.D.; Stuart, D.; Fras, G. Steel to aluminium braze welding by laser process with Al–12Si filler wire. Sci. Technol. Weld. Join. 2008, 13, 430–437. [Google Scholar] [CrossRef]
- Chang, S.; Tsao, L.; Li, T.; Chuang, T. Joining 6061 aluminum alloy with Al–Si–Cu filler metals. J. Alloy. Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Fedorov, V.; Uhlig, T.; Wagner, G. Influence of the Thickness of the Reaction Zone in Aluminum/Stainless Steel Brazed Joints on the Mechanical Properties. Metals 2021, 11, 217. [Google Scholar] [CrossRef]
- Wang, W.-L.; Tsai, Y.-C. Microstructural characterization and mechanical property of active soldering anodized 6061 Al alloy using Sn–3.5Ag–xTi active solders. Mater. Charact. 2012, 68, 42–48. [Google Scholar] [CrossRef]
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Niu, Z.; Huang, J.; Yang, H.; Chen, S.; Zhao, X. Preparation and Properties of a Novel Al-Si-Ge-Zn Filler Metal for Brazing Aluminum. J. Mater. Eng. Perform. 2015, 24, 2327–2334. [Google Scholar] [CrossRef]
- Das, S.K.; Kaufman, J.G. Recycling aluminum aerospace alloys. Adv. Mater. Process. 2008, 166, 34–35. [Google Scholar]
- Rajan, R.; Kah, P.; Mvola, B.; Martikainen, J. Trends in Al alloy Development and Their Joining Methods. Rev. Adv. Mater. Sci. 2016, 15, 383–397. [Google Scholar]
- Zhou, B.; Liu, B.; Zhang, S. The Advancement of 7XXX Series Aluminum Alloys for Aircraft Structures: A Review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Fedorov, V.; Uhlig, T.; Wagner, G. Investigation of fatigue damage in aluminum/stainless steel brazed joints. Weld. World 2018, 62, 609–616. [Google Scholar] [CrossRef]
- Simões, S. Diffusion Bonding and Brazing of Advanced Materials. Metals 2018, 8, 959. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, V.; Uhlig, T.; Wagner, G. Joining of aluminum and stainless steel using AlSi10 brazing filler: Microstructure and mechanical properties. AIP Conf. Proc. 2017, 1858, 030005. [Google Scholar]
- Ouyang, J.-H.; Yarrapareddy, E.; Kovacevic, R. Microstructural evolution in the friction stir welded 6061 aluminum alloy (T6-temper condition) to copper. J. Mater. Process. Technol. 2006, 172, 110–122. [Google Scholar] [CrossRef]
- Das, S.K.; Bhattacharya, D.K. Corrosion failure of Zn–Al detonator housing. Eng. Fail. Anal. 2003, 10, 639–643. [Google Scholar] [CrossRef]
- Yan, X.; Liu, S.; Long, W.; Huang, J.; Zhang, L.; Chen, Y. The effect of homogenization treatment on microstructure and properties of ZnAl15 solder. Mater. Des. 2013, 45, 440–445. [Google Scholar] [CrossRef]
- Dai, W.; Xue, S.; Lou, J.; Wang, S. Microstructure and Properties of 6061 Aluminum Alloy Brazing Joint with Al–Si–Zn Filler Metal. Mater. Trans. 2012, 53, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, V.; Uhlig, T.; Podlesak, H.; Wagner, G. Microstructural Study of Al-Ag-Cu-Si Filler Metal for Brazing High-Strength Aluminum Alloys to Stainless Steel. Metals 2020, 10, 1563. [Google Scholar] [CrossRef]
- Velu, P.K. Study of the Effect of Brazing on Mechanical Properties of Aluminum Alloys for Automotive Heat Exchanges. Master’s Thesis, Purdue University, Indianapolis, IN, USA, 2017. [Google Scholar]
- Hayes, F.H.; Longbottom, R.D.; Ahmad, E.; Chen, G. On the Al-Si, Al-Ge, and Al-Ge-Si systems and their application to brazing in high power semiconductor devices. J. Phase Equilibria Diffus. 1993, 14, 425–431. [Google Scholar] [CrossRef]
- Niu, Z.-W.; Huang, J.-H.; Chen, S.-H.; Zhao, X.-K. Effects of germanium additions on microstructures and properties of Al–Si filler metals for brazing aluminum. Trans. Nonferrous Met. Soc. China 2016, 26, 775–782. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, H.; Li, J.; Liu, R.; Wang, W. Microstructures of the eutectic and hypereutectic Al–Ge alloys solidified under different pressures. Mater. Lett. 2006, 60, 783–785. [Google Scholar] [CrossRef]
- Stepanov, V.V. Development of Al-Si-Ge System Solders for Increasing the Strength of Brazed Structures Made of Aluminum Alloys. Master’s Thesis, Moscow State Aviation Technological University, Moscow, Russia, 2006. [Google Scholar]
- Zhang, Y.; Gao, T.; Liu, X. Influence of Ge content on the microstructure, phase formation and microhardness of hypereutectic Al–Si alloys. J. Alloy. Compd. 2014, 585, 442–447. [Google Scholar] [CrossRef]
- Dai, W.; Xue, S.-B.; Lou, J.-Y.; Lou, Y.-B.; Wang, S.-Q. Torch brazing 3003 aluminum alloy with Zn—Al filler metal. Trans. Nonferrous Met. Soc. China 2012, 22, 30–35. [Google Scholar] [CrossRef]
- Lyakishev, N.P. State Diagrams of Binary Metallic Systems: A Handbook; Mechanical Engineering: Moscow, Russia, 1996. [Google Scholar]
- Schubert, T.; Loser, W.; Teresiak, A.; Mattern, N.; Bauer, H.-D. Preparation and phase transformations of melt-spun Al–Ge–Si brazing foils. J. Mater. Sci. 1997, 32, 2181–2189. [Google Scholar] [CrossRef]
- Song, H.; Hellawell, A. Solidification in the system Al-Ge-Si: The phase diagram, coring patterns, eutectic growth, and modification. Met. Mater. Trans. A 1990, 21, 733–740. [Google Scholar] [CrossRef]
- Derkachenko, L.I.; Korchunov, B.N.; Nikanorov, S.P.; Osipov, V.N.; Shpeizman, V.V. Structure, microhardness, and strength of a directionally crystallized Al-Ge alloy. Phys. Solid State 2014, 56, 527–530. [Google Scholar] [CrossRef]
- Nikanorov, S.; Burenkov, Y.A.; Volkov, M.; Gurin, V.; Derkachenko, L.; Kardashev, B.; Regel, L.; Wilcox, W. Elastic and microplastic properties of Al–Si/Ge alloys obtained from levitated melts. Mater. Sci. Eng. A 2006, 442, 449–453. [Google Scholar] [CrossRef]
- van de Walle, A.; Ceder, G. Automating first-principles phase diagram calculations. J. Phase Equilibria Diffus. 2002, 23, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.S. Precise lattice constants of germanium, aluminum, gallium arsenide, uranium, sulphur, quartz and sapphire. Acta Crystallogr. 1962, 15, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Dismukes, J.P.; Ekstrom, L.; Paff, R.J. Lattice Parameter and Density in Germanium-Silicon Alloys. J. Phys. Chem. 1964, 68, 3021–3027. [Google Scholar] [CrossRef]
- ISO 14577-1:2002 Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test method. 2015. Available online: https://www.iso.org/standard/56626.html (accessed on 2 November 2021).
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Chuang, T.H.; Yeh, M.S.; Tsao, L.C.; Tsai, T.C.; Wu, C.S. Development of a Low-Melting-Point Filler Metal for Brazing Aluminum Alloys. Metall. Mater. Trans. A. 2000, 31, 2239–2245. [Google Scholar] [CrossRef]
- Oliver, J.O. Nanoindentation-Induced Deformation Mechanisms in Germanium. PhD Thesis, The Australian National University, Canberra, Australia, 2008. [Google Scholar]
- Kiran, M.S.; Haberl, B.; Bradby, J.E.; Williams, J.S. Nanoindentation of Silicon and Germanium. III-Nitride Electron. Devices 2015, 91, 165–203. [Google Scholar] [CrossRef]
- Ge, R.; Hou, X.; Brookshire, K.; Krishnan, N.R.; Silva, D.; Bumgarner, J.; Liu, Y.; Faraone, L.; Martyniuk, M. Nanoindentation of Si1xGex thin films prepared by biased target ion beam deposition. IEEE Xplore 2014, 210–213. [Google Scholar] [CrossRef]
- Kammer, C. Aluminium Taschenbuch Band 1: Grundlagen und Werkstoffe; Aluminium-Verlag: Düsseldorf, Germany, 2009. [Google Scholar]
- El-Shennawy, M.; Abdel-Aziz, K.; Omar, A.A. Metallurgical and mechanical properties of heat treatable aluminum alloy AA6082 welds. Int. J. Appl. Eng. Res. 2017, 12, 2832–2839. [Google Scholar]
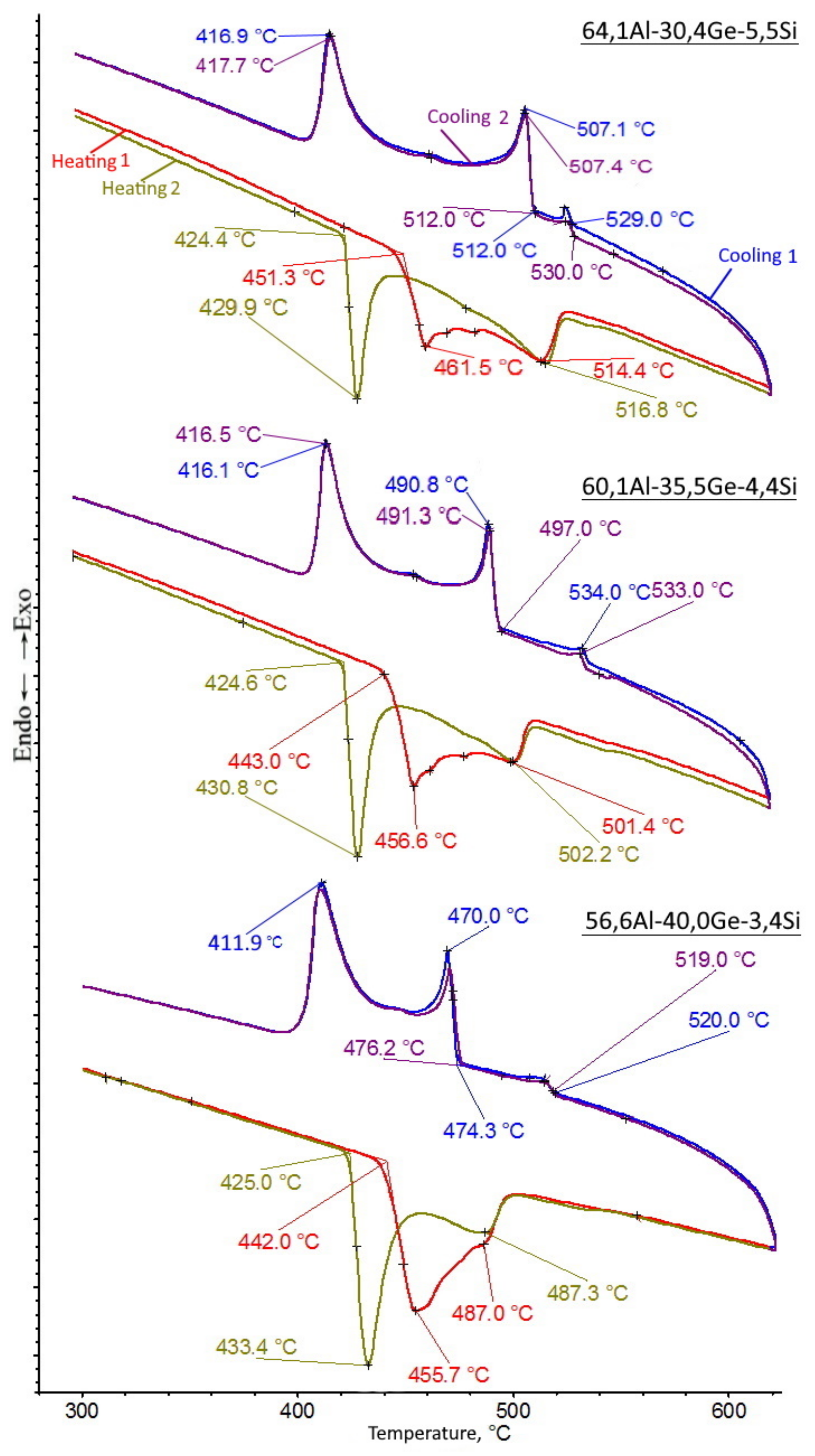




| No. | Alloy | Alloy Composition (at.%) | TL (°C) | ||
|---|---|---|---|---|---|
| Al | Si | Ge | |||
| 1 | Al-30.4Ge-5.5Si | 79.3 | 6.7 | 14.0 | 520 |
| 2 | Al-35.5Ge-4.4Si | 77.4 | 5.6 | 17.0 | 505 |
| 3 | Al-40.0Ge-3.4Si | 75.8 | 4.3 | 19.9 | 490 |
| Sample | Phase Composition | Crystal Lattice Parameters |
|---|---|---|
| 64.1Al-30.4Ge-5.5Si foil | Solid solution of (Al, Ge) | FCC (Fm-3m), а = 4.0519 Å, d = 243 nm |
| Solid solution of (Ge, Si) | FCC diamond (Fd-3m), а = 5.6006 Å, d = 18 nm | |
| 64.1Al-30.4Ge-5.5Si ingot | Solid solution of (Al, Ge) | FCC (Fm-3m), а = 4.0546 Å, d = 461 nm |
| Pure Ge | FCC diamond (Fd-3m), а = 5.6628 Å, d = 82 nm | |
| Solid solution of (Ge, Si) | FCC diamond (Fd-3m), а = 5.6073 Å, d = 29 nm |
| Measurement Point | Chemical Composition (at.%) | Phase Interpretation | |||
|---|---|---|---|---|---|
| Al | Si | Fe | Ge | ||
| Spectrum 1 | 2.0 | 15.2 | 1.2 | 81.6 | (Ge, Si)1 |
| Spectrum 2 | 3.9 | 2.4 | 0.0 | 93.7 | (Ge) |
| Spectrum 3 | 68.8 | 2.5 | 10.8 | 17.9 | Al7Ge2Fe |
| Spectra 4, 6 and 8 | 1.3 | 56.8 | 0.0 | 42.9 | (Si, Ge) |
| Spectrum 5 | 98.4 | 0.2 | 0.0 | 1.4 | (Al) |
| Spectrum 7 | 1.7 | 32.3 | 0.0 | 66.0 | (Ge, Si)2 |
| Measurement Point | Chemical Composition (at.%) | Phase Interpretation | |||
|---|---|---|---|---|---|
| Al | Si | Fe | Ge | ||
| Spectrum 1 | 1.8 | 53.6 | 0.0 | 44.6 | (Si, Ge) |
| Spectrum 2 | 2.9 | 5.0 | 0.0 | 92.1 | (Ge) |
| Spectrum 3 | 64.0 | 2.3 | 9.0 | 24.7 | Al7Ge2Fe |
| Spectrum 4 | 98.7 | 0.2 | 0.0 | 1.1 | (Al) |
| Measurement Point | Chemical Composition (at.%) | Phase Interpretation | ||
|---|---|---|---|---|
| Al | Si | Ge | ||
| Spectra 1–4 | 94.4 | 2.3 | 3.3 | (Al) |
| Spectra 5 and 6 | 60.4 | 13.4 | 26.2 | (Al, Ge, Si) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivannikov, A.; Fedorov, V.; Abramov, A.; Penyaz, M.; Bachurina, D.; Uhlig, T.; Suchkov, A.; Wagner, G.; Morokhov, P.; Sevryukov, O. Development of Rapidly-Quenched Al-Ge-Si Filler Alloys for the Joining of Stainless Steel AISI 304 and Aluminum Alloy AA6082. Metals 2021, 11, 1926. https://doi.org/10.3390/met11121926
Ivannikov A, Fedorov V, Abramov A, Penyaz M, Bachurina D, Uhlig T, Suchkov A, Wagner G, Morokhov P, Sevryukov O. Development of Rapidly-Quenched Al-Ge-Si Filler Alloys for the Joining of Stainless Steel AISI 304 and Aluminum Alloy AA6082. Metals. 2021; 11(12):1926. https://doi.org/10.3390/met11121926
Chicago/Turabian StyleIvannikov, Alexander, Vasilii Fedorov, Anton Abramov, Milena Penyaz, Diana Bachurina, Thomas Uhlig, Alexey Suchkov, Guntram Wagner, Pavel Morokhov, and Oleg Sevryukov. 2021. "Development of Rapidly-Quenched Al-Ge-Si Filler Alloys for the Joining of Stainless Steel AISI 304 and Aluminum Alloy AA6082" Metals 11, no. 12: 1926. https://doi.org/10.3390/met11121926
APA StyleIvannikov, A., Fedorov, V., Abramov, A., Penyaz, M., Bachurina, D., Uhlig, T., Suchkov, A., Wagner, G., Morokhov, P., & Sevryukov, O. (2021). Development of Rapidly-Quenched Al-Ge-Si Filler Alloys for the Joining of Stainless Steel AISI 304 and Aluminum Alloy AA6082. Metals, 11(12), 1926. https://doi.org/10.3390/met11121926