Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment
Abstract
:1. Introduction
2. Experimental
2.1. Materials
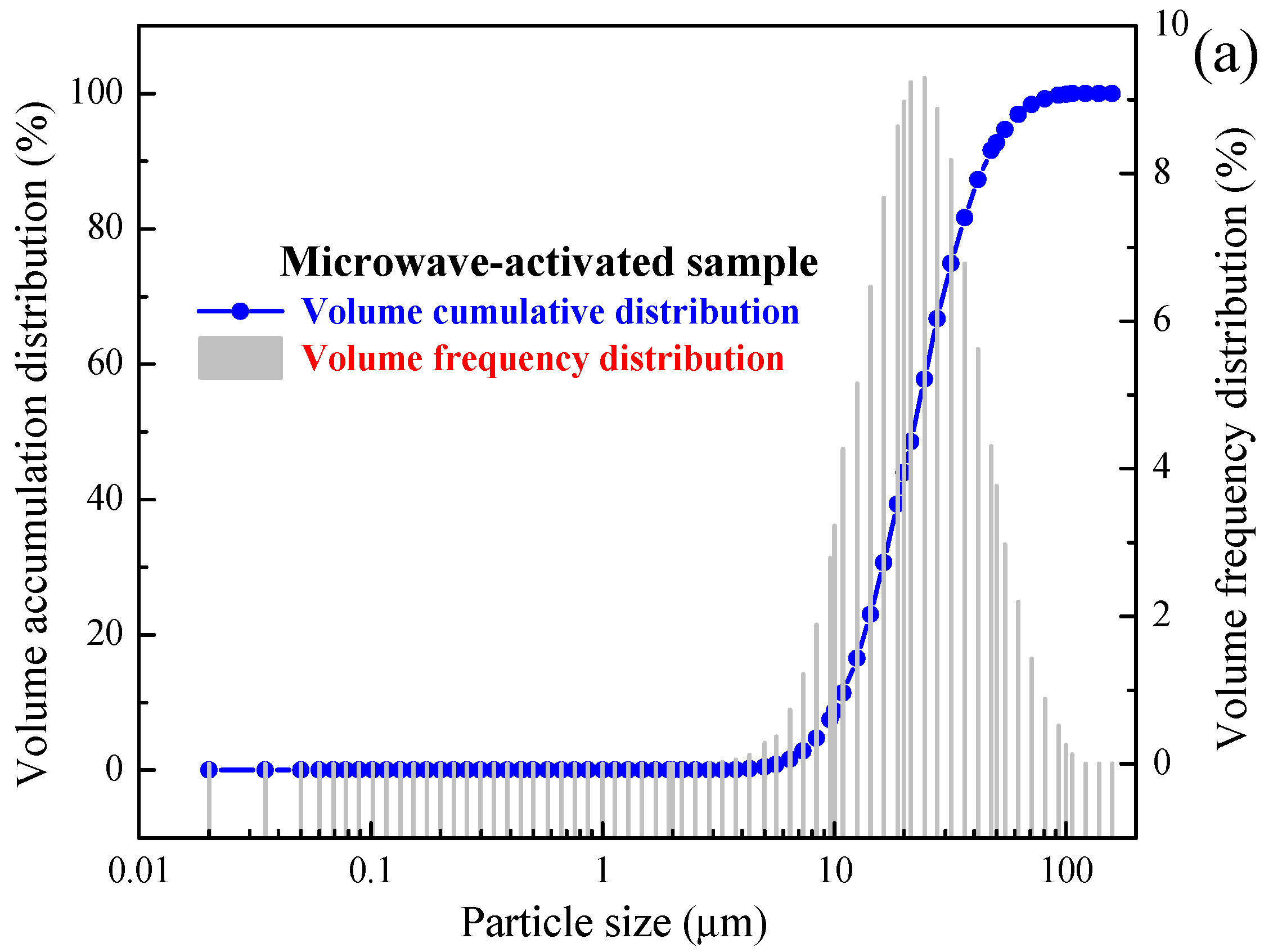
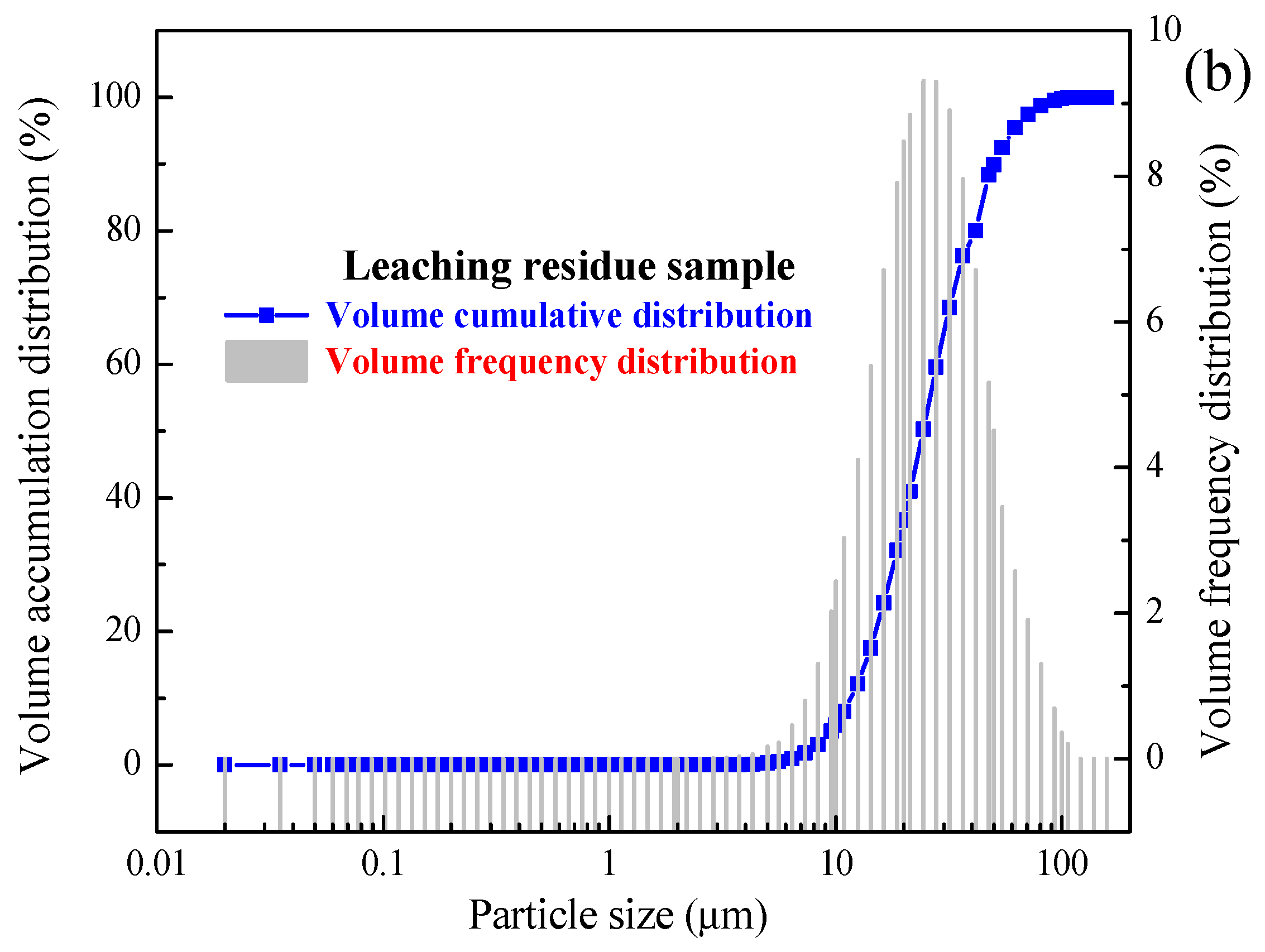
2.2. Characterisation
2.3. Procedure
3. Results and Discussion
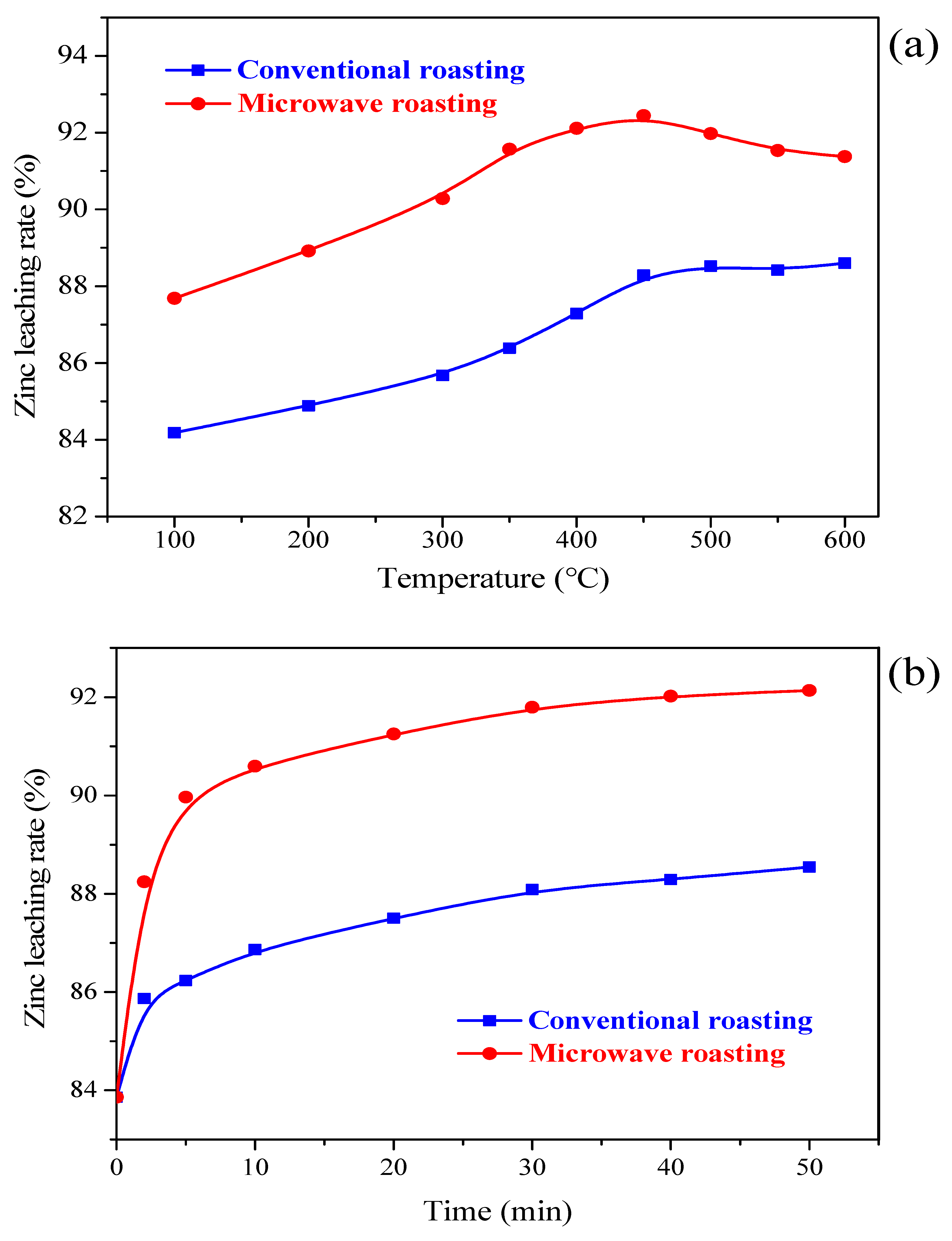
3.1. Zinc Leaching Efficiency
3.1.1. Effects of Activation Temperature
3.1.2. Effects of Activation Time
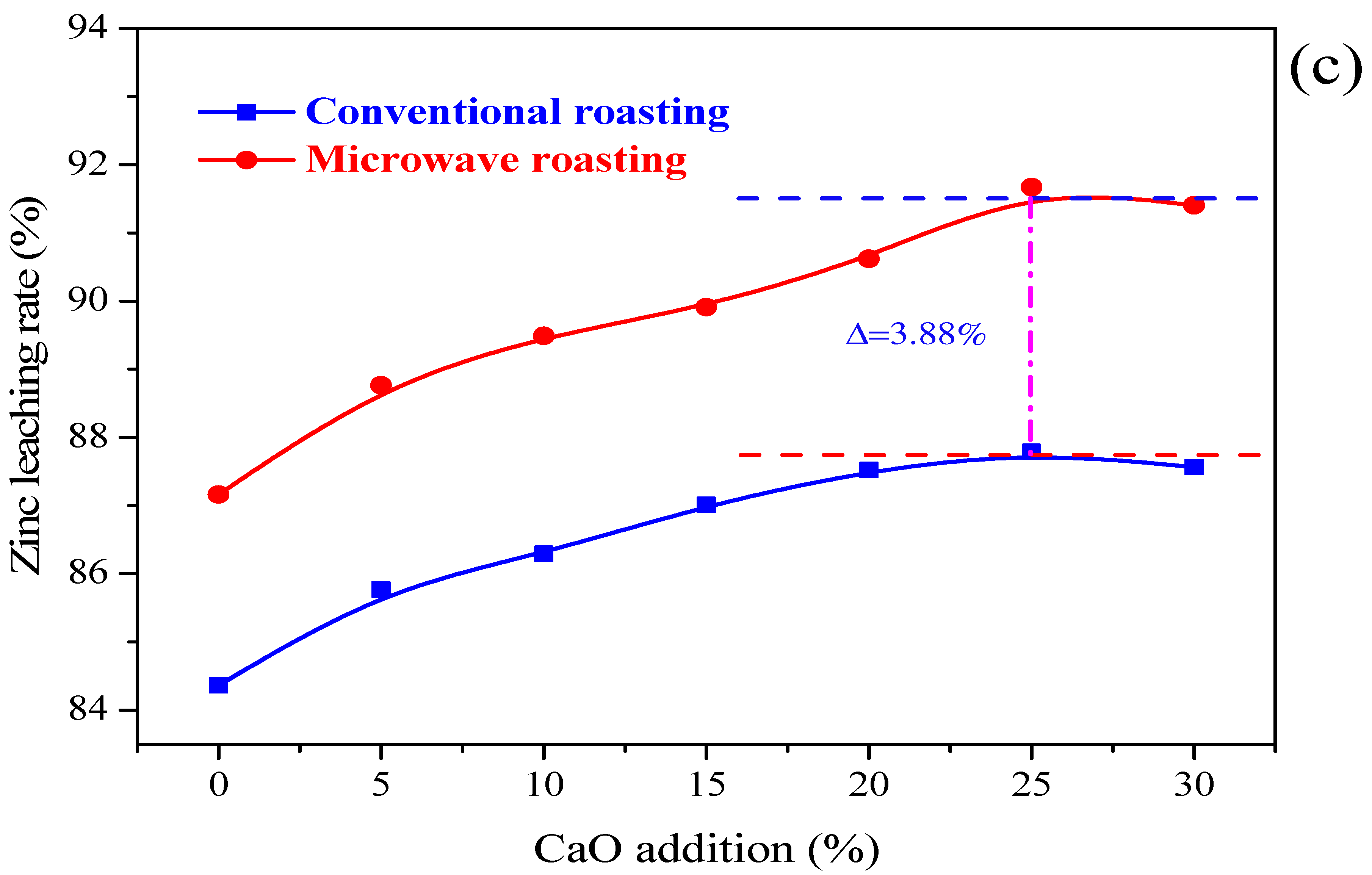
3.1.3. Effects of CaO Addition
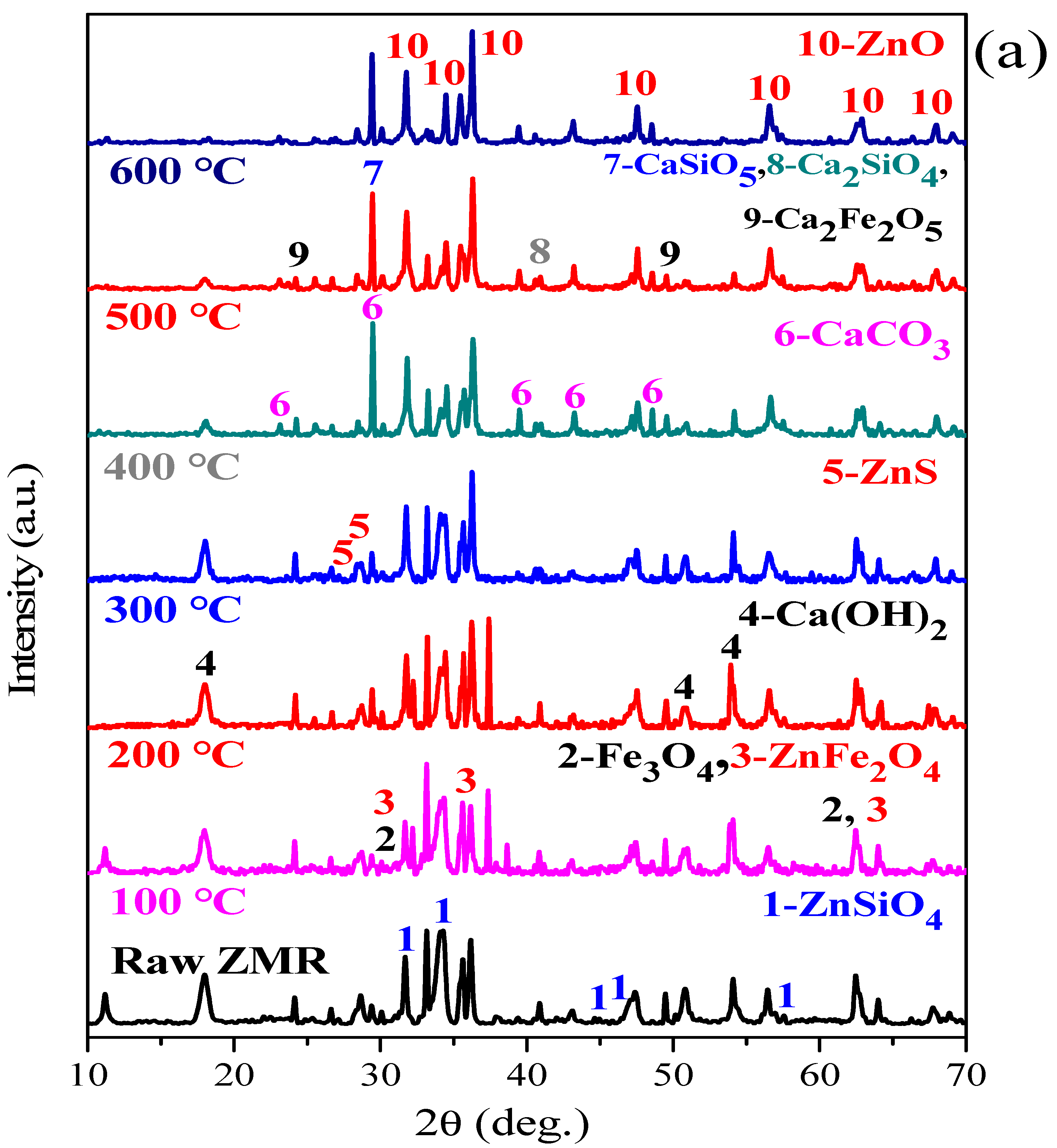
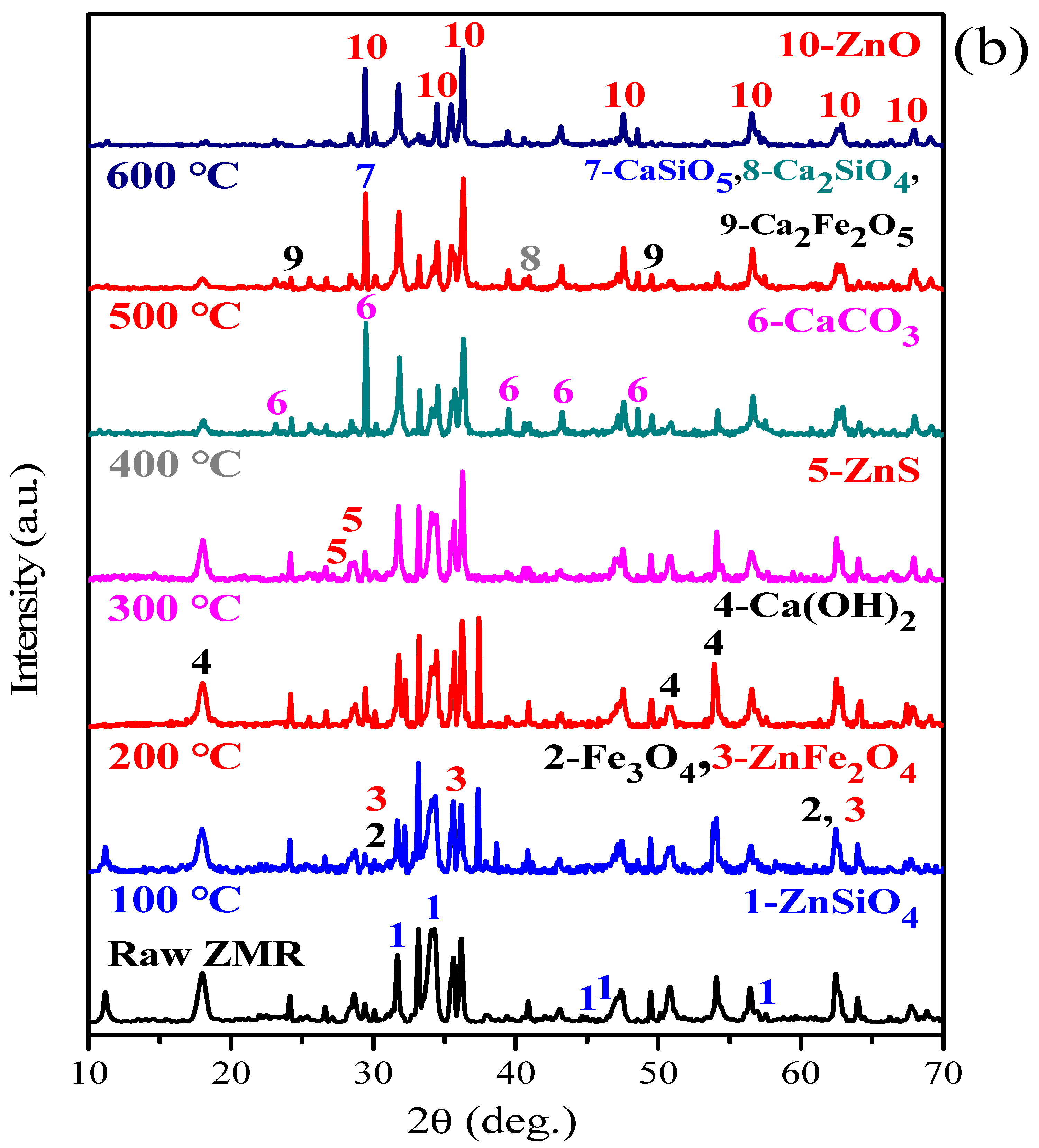
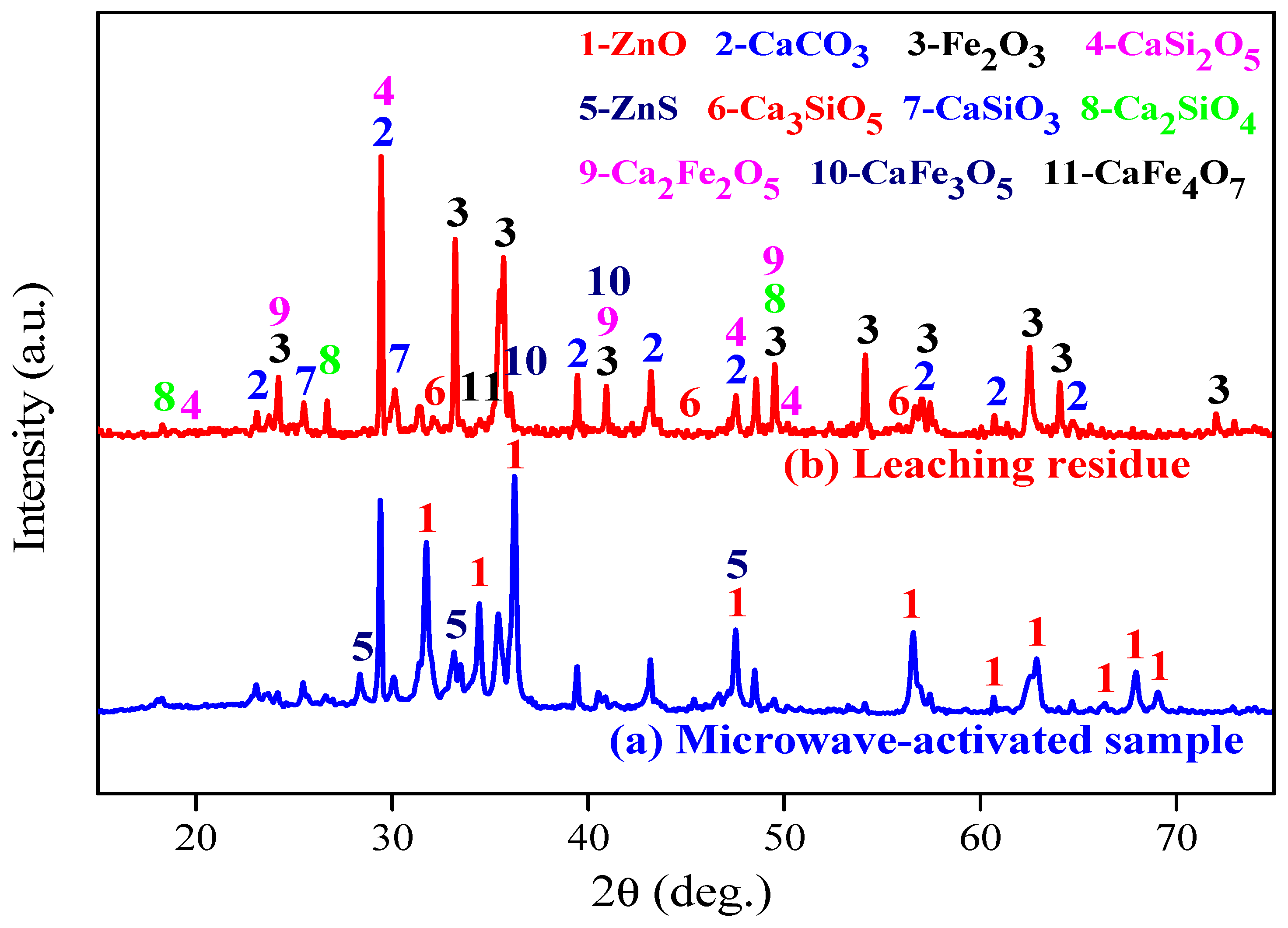
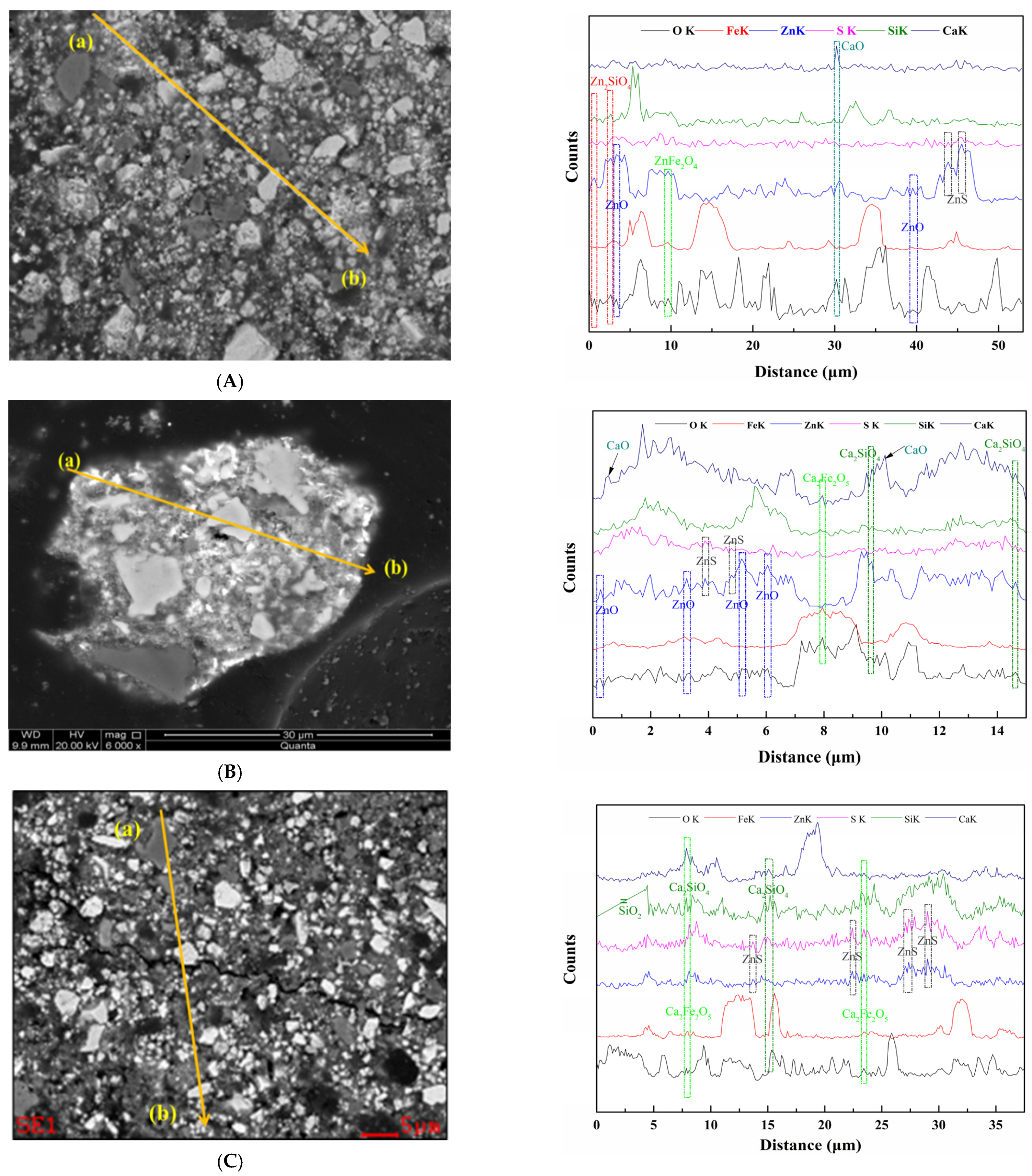
3.2. XRD Characterisation
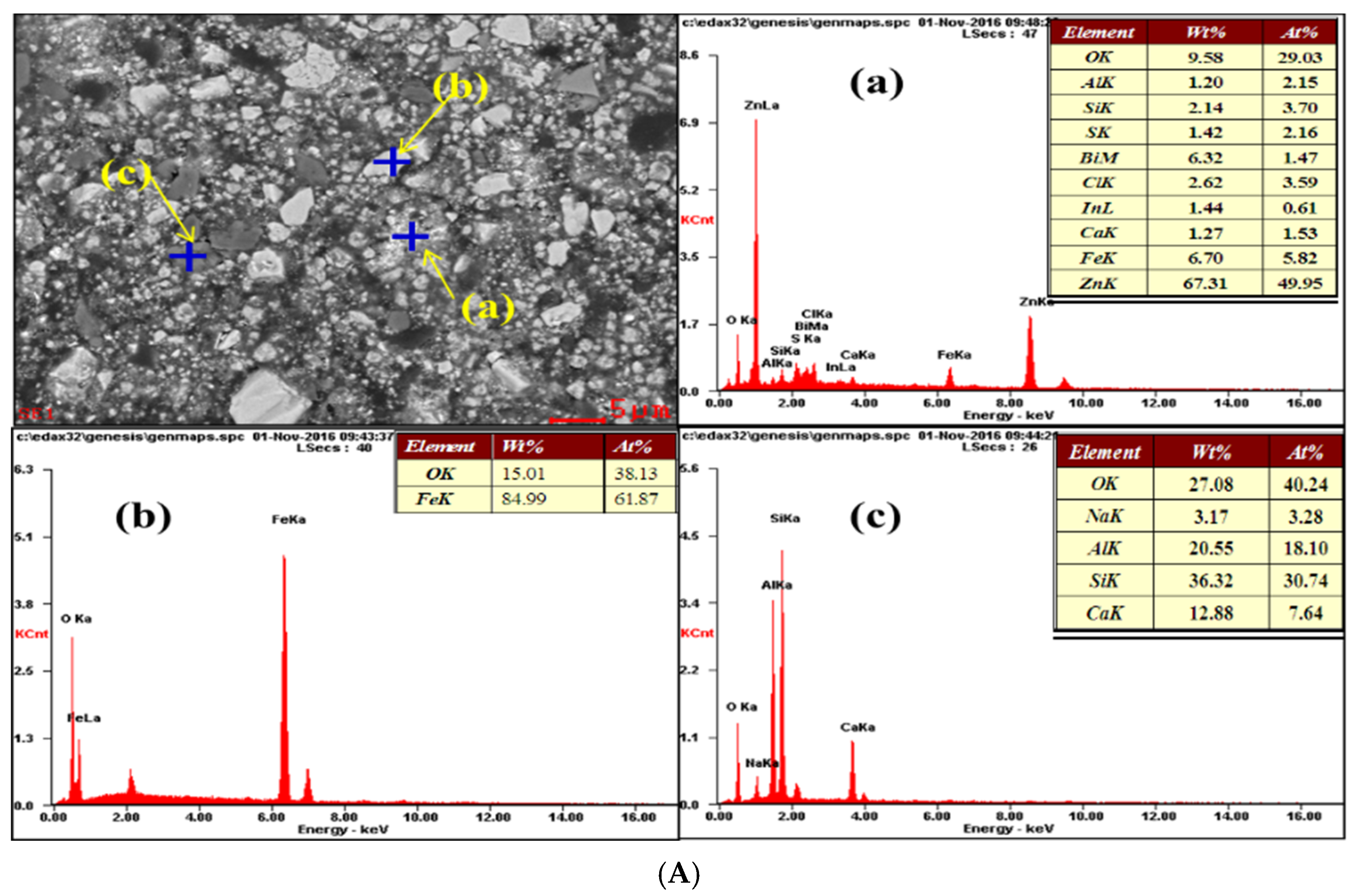
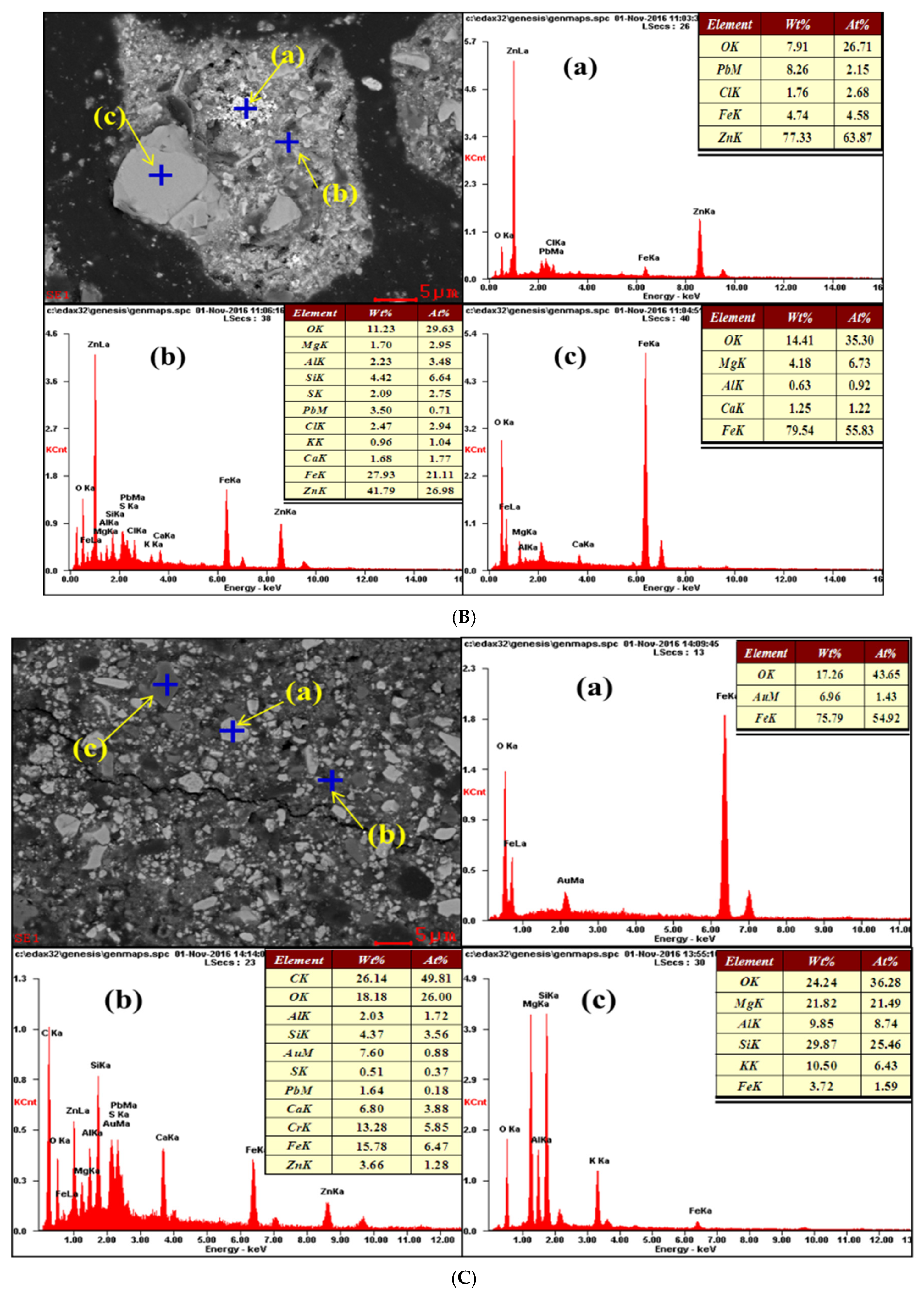
3.3. SEM-EDS Characterisation
3.3.1. SEM Characterisation
3.3.2. EDS Characterisation
3.4. Mechanism of Enhanced Leaching Zn by Microwave Calcium Activation Pretreatment
4. Conclusions
- (1)
- The influences of CaO addition, activation temperature and activation time on the Zn leaching efficiency were studied in the NH3-CH3COONH4-H2O system. The preferred microwave calcium activation pretreatment parameters were determined with a zinc leaching rate of 91.67% under a roasting temperature of 400 °C, the activation time of 20 min, and a CaO addition of 25%. Meanwhile, the Zn leaching efficiencies under microwave roasting were all higher than those by conventional roasting.
- (2)
- XRD and SEM-EDS analysis indicated that under microwave calcification pretreatment, the refractory mineral zinc silicate (Zn2SiO4) and zinc ferrite (ZnFe2O4) were converted into the easily leachable mineral phases, like zinc oxide, calcium ferrite, and calcium silicate. The diffraction peak of ZnS was weakened after microwave activation, thus increasing the content of easily leachable mineral phase ZnO and promoting the leaching of zinc. Moreover, microwave heating can shorten the process temperature, manifested by the Ca(OH)2 decomposition temperature and CaCO3 formation temperature decreased by 200 °C and 100 °C, respectively. Furthermore, the selective heating characteristics of microwave heating render the mineral inclusion particles dissociated into cracks, which increases the reaction area and the diffusion efficiency, further enhancing the leaching process of zinc from zinc-containing metallurgical residues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, X.L.; Xu, C.Y.; Tang, J.; Hua, Y.X.; Zhang, Q.B.; Liu, H.; Wang, X.; Huang, M.T. Selective recovery of zinc from zinc oxide dust using choline chloride based deep eutectic solvents. Trans. Nonferr. Met. Soc. 2019, 29, 2222–2228. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.F.; Li, T.; Xin, B.P.; Chen, S.; Guo, X.M.; Liu, C.H.; Li, Y.P. Bioleaching mechanism of Zn, Pb, In, Ag, Cd and As from Pb/Zn smelting slag by autotrophic bacteria. J. Environ. Manag. 2015, 159, 11–17. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.S.; Rashchi, F.; Naazeri, M. Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid. Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Jaafar, I.; Griffiths, A.J.; Hopkins, A.C.; Steer, J.M.; Griffiths, M.H.; Sapsford, D.J. An evaluation of chlorination for the removal of zinc from steelmaking dusts. Miner. Eng. 2011, 24, 1028–1030. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterisation and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Antrekowitsch, J.; Steinlechner, S. The recycling of heavy-metal containing wastes: Mass balances and economical estimations. JOM 2011, 63, 68–72. [Google Scholar] [CrossRef]
- Lima, L.R.P.D.A.; Bernardez, L.A. Characterisation of the lead smelter slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Yina, N.H.; Sivryb, Y.; Guyotc, F.; Lensd, P.N.L.; Hullebuscha, E.D.V. Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.P.; Deng, Q.F.; Li, C.; Xie, Y.; Dong, Z.Q.; Zhang, W. The recovery of Zn and Pb and the manufacture of lightweight bricks from zinc smelting slag and clay. J. Hazard. Mater. 2014, 271, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Peng, B.; Chai, L.Y.; Peng, N.; Yan, H.; Hou, D.K. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon. J. Hazard. Mater. 2012, 237–238, 323–330. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, X.Y.; Zhai, Y.C. Thermodynamics and kinetics of extracting zinc from zinc oxide ore by ammonium sulfate roasting method. Int. J. Miner. Metall. Mater. 2015, 22, 467–475. [Google Scholar] [CrossRef]
- Havlik, T.; Souza, B.V.; Bernardes, A.M.; Schneider, I.A.H.; Miskufova, A. Hydrometallurgical processing of carbon steel EAF dust. J. Hazard. Mater. 2006, 135, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J. Hazard. Mater. 2016, 302, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chairaksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from Electric Arc Furnace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Soria-Aguilar, M.d.J.; Davila-Pulido, G.I.; Carrillo-Pedroza, F.R.; Gonzalez-Ibarra, A.A.; Picazo-Rodriguez, N.; Lopez-Saucedo, F.d.J.; Ramos-Cano, J. Oxidative leaching of zinc and alkalis from iron blast furnace sludge. Metals 2019, 9, 1015. [Google Scholar] [CrossRef] [Green Version]
- Tsakiridis, P.E.; Oustadakis, P.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part II: Downstream processing and zinc recovery by electrowinning. J. Hazard. Mater. 2010, 179, 8–14. [Google Scholar] [CrossRef]
- Picazo-Rodríguez, N.G.; Soria-Aguilar, M.d.J.; Martínez-Luévanos, A.; Almaguer-Guzmán, I.; Chaidez-Félix, J.; Carrillo-Pedroza, F.R. Direct acid leaching of sphalerite: An approach comparative and kinetics analysis. Minerals 2020, 10, 359. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, V.; Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P. Hydrometallurgical treatment of EAF dust by direct sulphuric acid leaching at atmospheric pressure. Waste Biomass Valoriz. 2016, 7, 1531–1548. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Stanforth, R. Extraction of zinc from zinc ferrites by fusion with caustic soda. Miner. Eng. 2000, 13, 1417–1421. [Google Scholar]
- Shen, X.Y.; Shao, H.M.; Gu, H.M.; Chen, B.; Ma, P.H. Reaction mechanism of roasting Zn2SiO4 using NaOH. Trans. Nonferr. Met. Soc. 2018, 28, 1878–1886. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhang, J.X.; Liu, Z.H.; Li, Q.H. Thermodynamics of metal ion complex formation in the Zn2SiO4-NH3-(NH4)2SO4-H2O system (I): Analysis of the Zn(II) complex equilibrium Hydrometallurgy 2018, 178, 12–18. Hydrometallurgy 2018, 178, 12–18. [Google Scholar] [CrossRef]
- Yang, S.H.; Li, H.; Sun, Y.W.; Chen, Y.M.; Tang, C.B.; He, J. Leaching kinetics of zinc silicate in ammonium chloride solution. Trans. Nonferr. Met. Soc. 2016, 26, 1688–1695. [Google Scholar] [CrossRef]
- Ma, A.Y.; Zheng, X.M.; Li, S.; Wang, Y.H.; Zhu, S. Zinc recovery from metallurgical residues by coordination leaching in NH3-CH3COONH4-H2O system. R. Soc. Open Sci. 2018, 5, 180660. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Liu, Z.H.; Li, Q.H.; Cao, Z.Y.; Yang, T.Z. Dissolution behavior of willemite in the (NH4)2SO4-NH3-H2O system. Hydrometallurgy 2012, 125–126, 50–54. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Inoue, Y.; Umeda, N.; Itoh, S.; Nagasak, T. New pyrometallurgical process of EAF dust treatment with CaO addition. Int. J. Miner. Metall. Mater. 2015, 22, 788–797. [Google Scholar] [CrossRef]
- Li, K.Q.; Chen, J.; Peng, J.H.; Ruan, R.; Srinivasakannan, C.; Chen, G. Pilot-scale study on enhanced carbothermal reduction of low-grade pyrolusite using microwave heating. Powder Technol. 2020, 360, 846–854. [Google Scholar] [CrossRef]
- Li, K.Q.; Jiang, Q.; Chen, J.; Peng, J.H.; Li, X.P.; Koppala, S.; Omran, M.; Chen, G. The controlled preparation and stability mechanism of partially stabilized zirconia by microwave intensification. Ceram. Int. 2020, 46, 7523–7530. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Peng, J.H.; Zhang, L.B.; Ju, S.H.; Xia, Y.; Zheng, Q.; Wang, Y.J. Dechlorination of zinc dross by microwave roasting. J. Cent. South Univ. 2014, 21, 2627–2632. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.W.; Zhang, L.B.; Peng, J.H.; Chen, W.H.; Xie, F.; Ma, A.Y. Microwave roasting and leaching of an oxide-sulphide zinc ore. Hydrometallurgy 2016, 166, 243–251. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Heikkinen, E.-P.; Vuolio, T.; Yu, Y.; Chen, G.; Kacar, Y. Microwave catalyzed carbothermic reduction of zinc oxide and zinc ferrite: Effect of microwave energy on the reaction activation energy. RSC Adv. 2020, 10, 23959–23968. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Gao, L.; Kang, J.; Omran, M.; Chen, G. Stability optimisation of CaO-doped partially stabilised zirconia by microwave sintering. Ceram. Int. 2019, 45, 23278–23282. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Heikkinen, E.-P.; Chen, G. Dielectric properties and carbothermic reduction of zinc oxide and zinc ferrite by microwave heating. R. Soc. Open Sci. 2017, 4, 170710. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Jiang, Q.; Omran, M.; Li, K.Q.; He, A.X.; Peng, J.H. Simultaneous removal of Cr(III) and V(V) and enhanced synthesis of high-grade rutile TiO2 based on sodium carbonate decomposition. J. Hazard. Mater. 2020, 388, 12203. [Google Scholar] [CrossRef]
- Li, K.Q.; Jiang, Q.; Chen, G.; Gao, L.; Peng, J.H.; Chen, Q.; Koppala, S.; Omran, M.; Chen, J. Kinetics characteristics and microwave reduction behavior of walnut shell-pyrolusite blends. Bioresour. Technol. 2021, 319, 124172. [Google Scholar] [CrossRef]
- Li, K.Q.; Jiang, Q.; Gao, L.; Chen, J.; Peng, J.H.; Koppala, S.; Omran, M.; Chen, G. Investigations on the microwave absorption properties and thermal behavior of vanadium slag: Improvement in microwave oxidation roasting for recycling vanadium and chromium. J. Hazard. Mater. 2020, 395, 122698. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Q.; Chen, J.; Peng, J.H.; Ruan, R.; Omran, M.; Chen, G. Dielectric properties and thermal behavior of electrolytic manganese anode mud in microwave field. J. Hazard. Mater. 2020, 381, 121227. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Heikkinen, E.-P. Selective zinc removal from electric arc furnace (EAF) dust by using microwave heating. J. Sustain. Metall. 2019, 5, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, K.Q.; Omran, M.; Jiang, Q.; Li, X.P.; Peng, J.H.; Chen, J. Microstructure and enhanced volume density properties of FeMn78C8.0 alloy prepared via a cleaner microwave sintering approach. J. Clean. Prod. 2020, 262, 121364. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-assisted leaching-a review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Ling, Y.Q.; Li, Q.N.; Zheng, H.W.; Omran, M.; Gao, L.; Chen, J.; Chen, G. Optimisation on the stability of CaO-doped partially stabilised zirconia by microwave heating. Ceram. Int. 2021, 47, 8067–8080. [Google Scholar] [CrossRef]
- Kang, J.X.; Omran, M.; Zhang, M.Y.; Pu, J.; He, L.; Ruan, R.S.; Peng, J.H.; Chen, G. Synthesis of rutile TiO2 powder by microwave-enhanced roasting followed by hydrochloric acid leaching. Adv. Powder Technol. 2020, 31, 1140–1147. [Google Scholar] [CrossRef]
- Sahoo, B.K.; De, S.; Meikap, B.C. Improvement of grinding characteristics of Indian coal by microwave pre-treatment. Fuel Process. Technol. 2011, 92, 1920–1928. [Google Scholar] [CrossRef]
- Li, K.Q.; Chen, J.; Peng, J.H.; Omran, M.; Chen, G. Efficient improvement for dissociation behavior and thermal decomposition of manganese ore by microwave calcination. J. Clean. Prod. 2020, 260, 121074. [Google Scholar] [CrossRef]
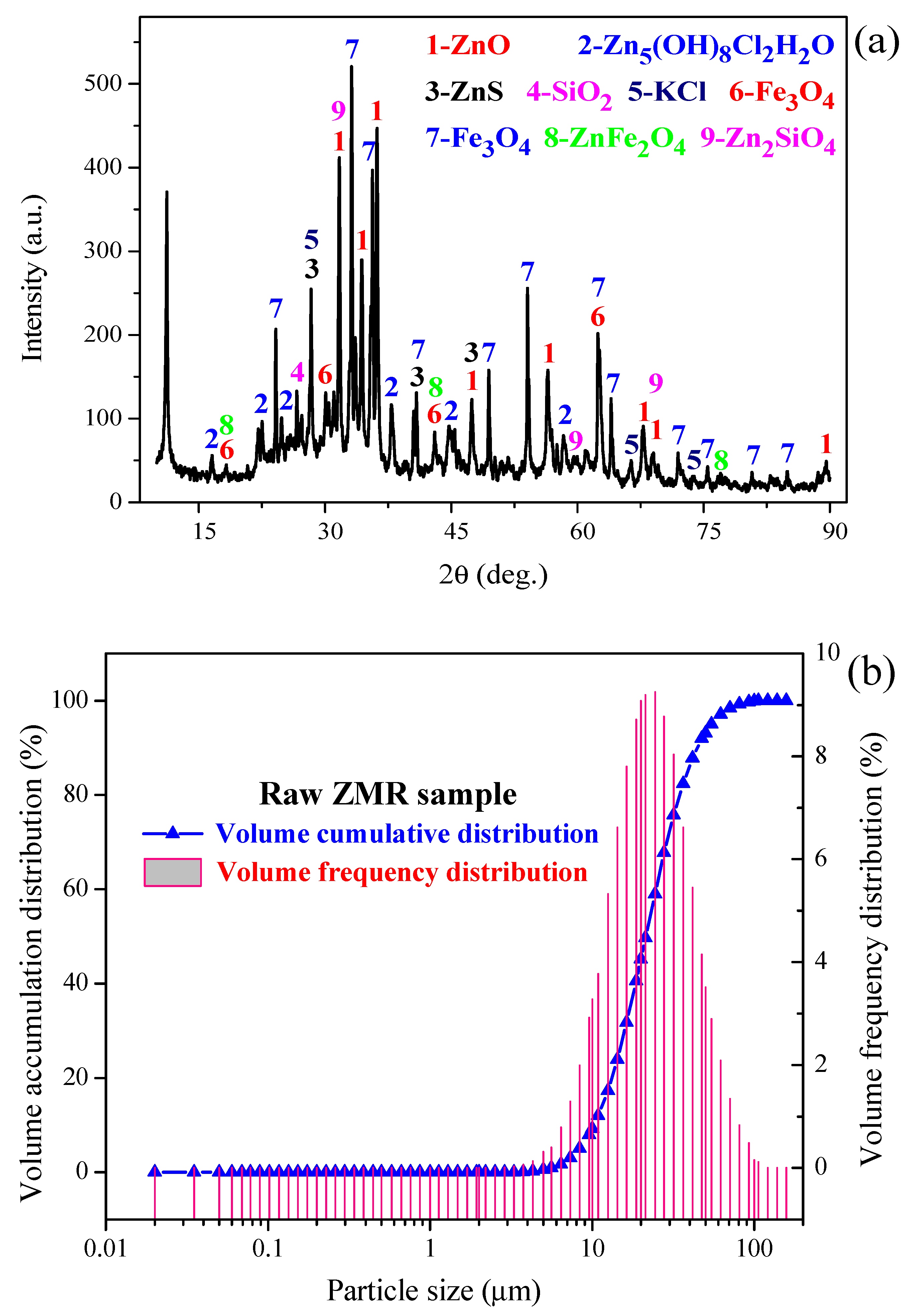
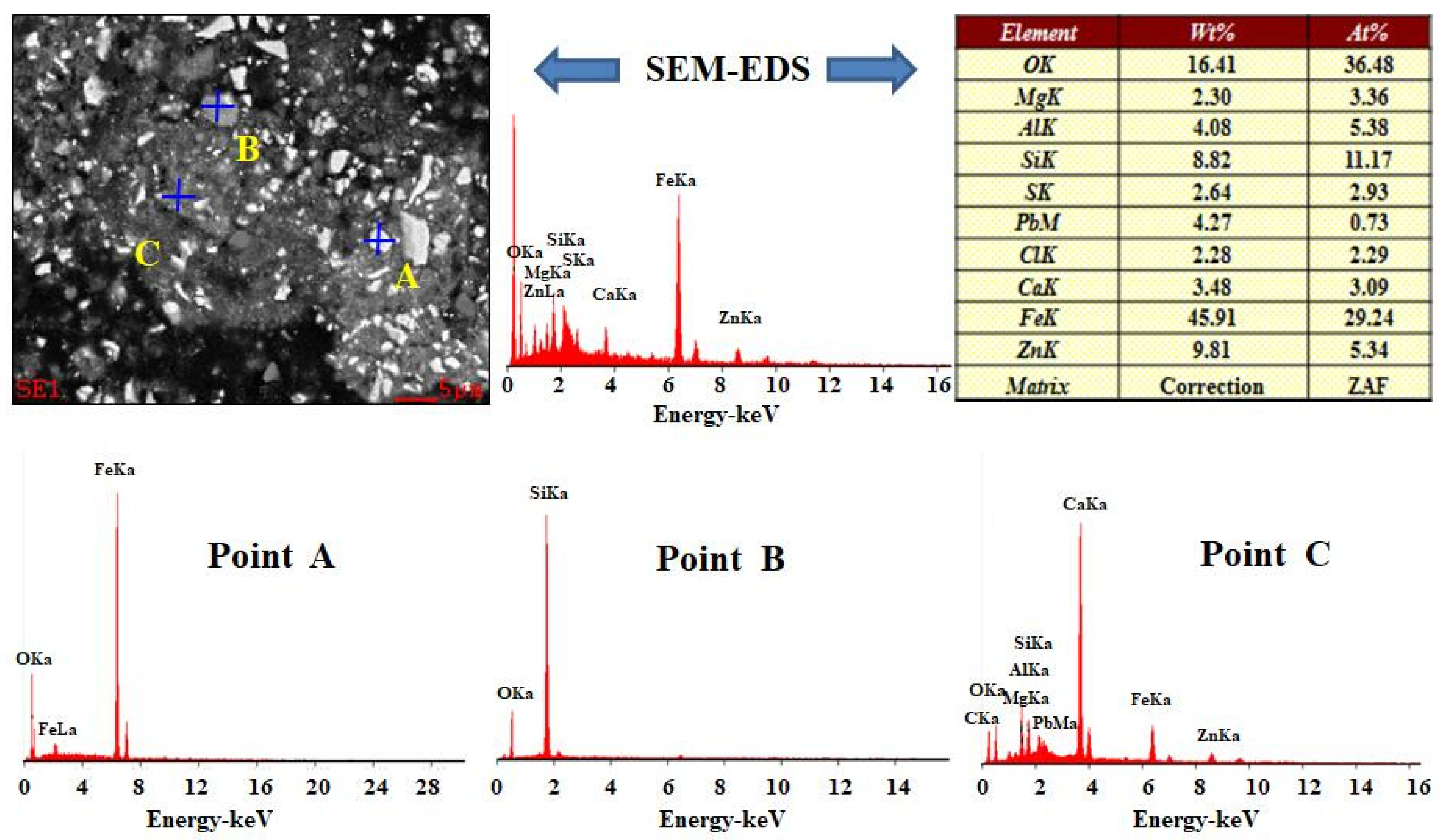
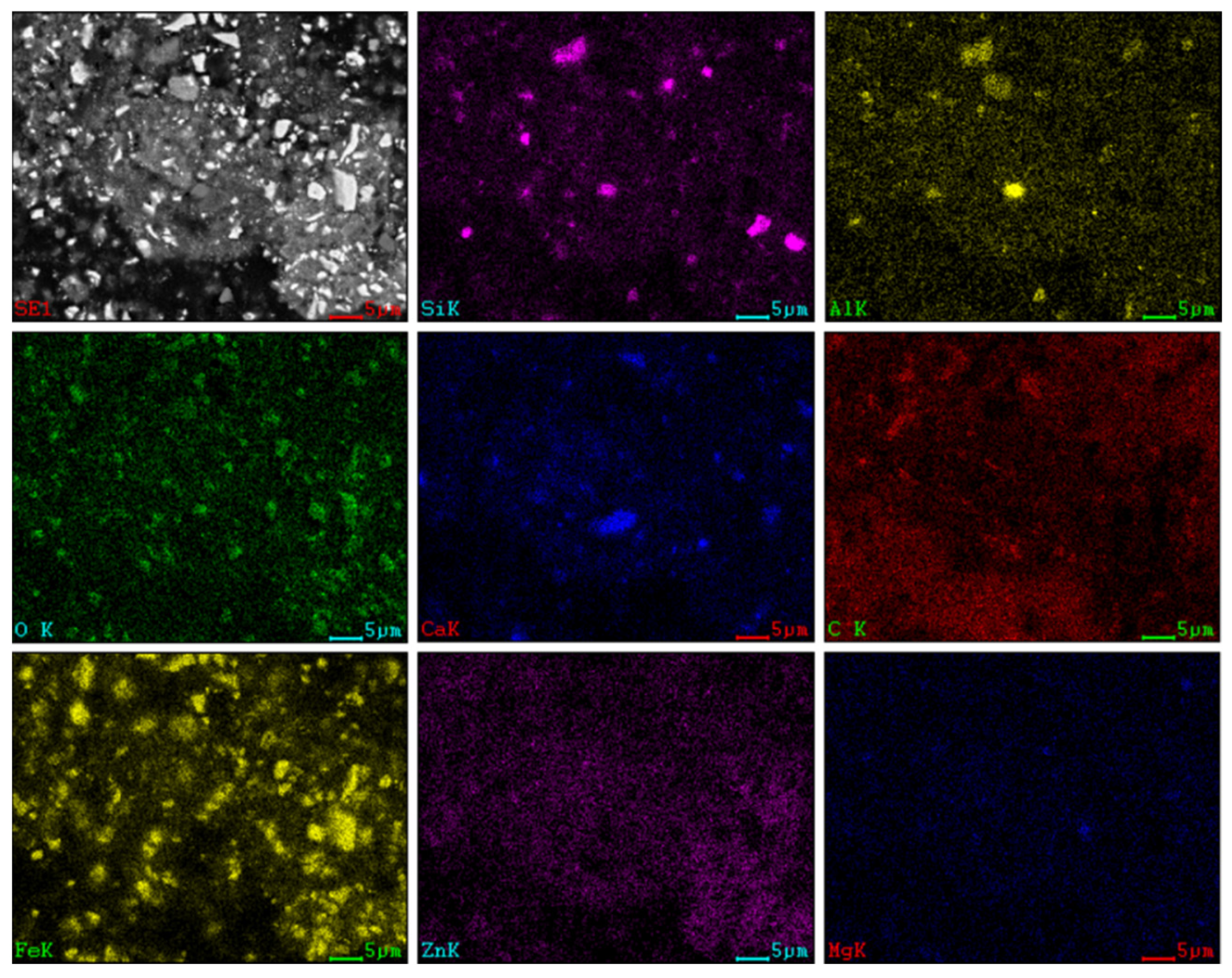

| Chemical Composition | ||||||
|---|---|---|---|---|---|---|
| Compositions | Zn | Fe | C | Pb | Mg | Al2O3 |
| Content (w%) | 24.74 | 21.66 | 9.14 | 1.13 | 1.14 | 2.22 |
| Compositions | CaO | Cl | Si | S | Bi | In |
| Content (w%) | 4.10 | 2.94 | 2.66 | 1.39 | 0.97 | 0.035 |
| Samples | D10 (μm) | D50 (μm) | D90 (μm) | D98 (μm) | Volume Average Particle Size (μm) | Area Average Particle Size (μm) | Surface Area to Volume Ratio (m2/cm3) |
|---|---|---|---|---|---|---|---|
| A | 10.26 | 21.42 | 44.67 | 68.15 | 23.40 | 19.40 | 2.48 |
| B | 10.44 | 21.80 | 45.33 | 68.93 | 23.78 | 19.74 | 2.60 |
| C | 11.68 | 24.27 | 50.17 | 75.21 | 26.35 | 22.03 | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.; Zheng, X.; Gao, L.; Li, K.; Omran, M.; Chen, G. Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals 2021, 11, 1922. https://doi.org/10.3390/met11121922
Ma A, Zheng X, Gao L, Li K, Omran M, Chen G. Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals. 2021; 11(12):1922. https://doi.org/10.3390/met11121922
Chicago/Turabian StyleMa, Aiyuan, Xuemei Zheng, Lei Gao, Kangqiang Li, Mamdouh Omran, and Guo Chen. 2021. "Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment" Metals 11, no. 12: 1922. https://doi.org/10.3390/met11121922
APA StyleMa, A., Zheng, X., Gao, L., Li, K., Omran, M., & Chen, G. (2021). Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals, 11(12), 1922. https://doi.org/10.3390/met11121922








