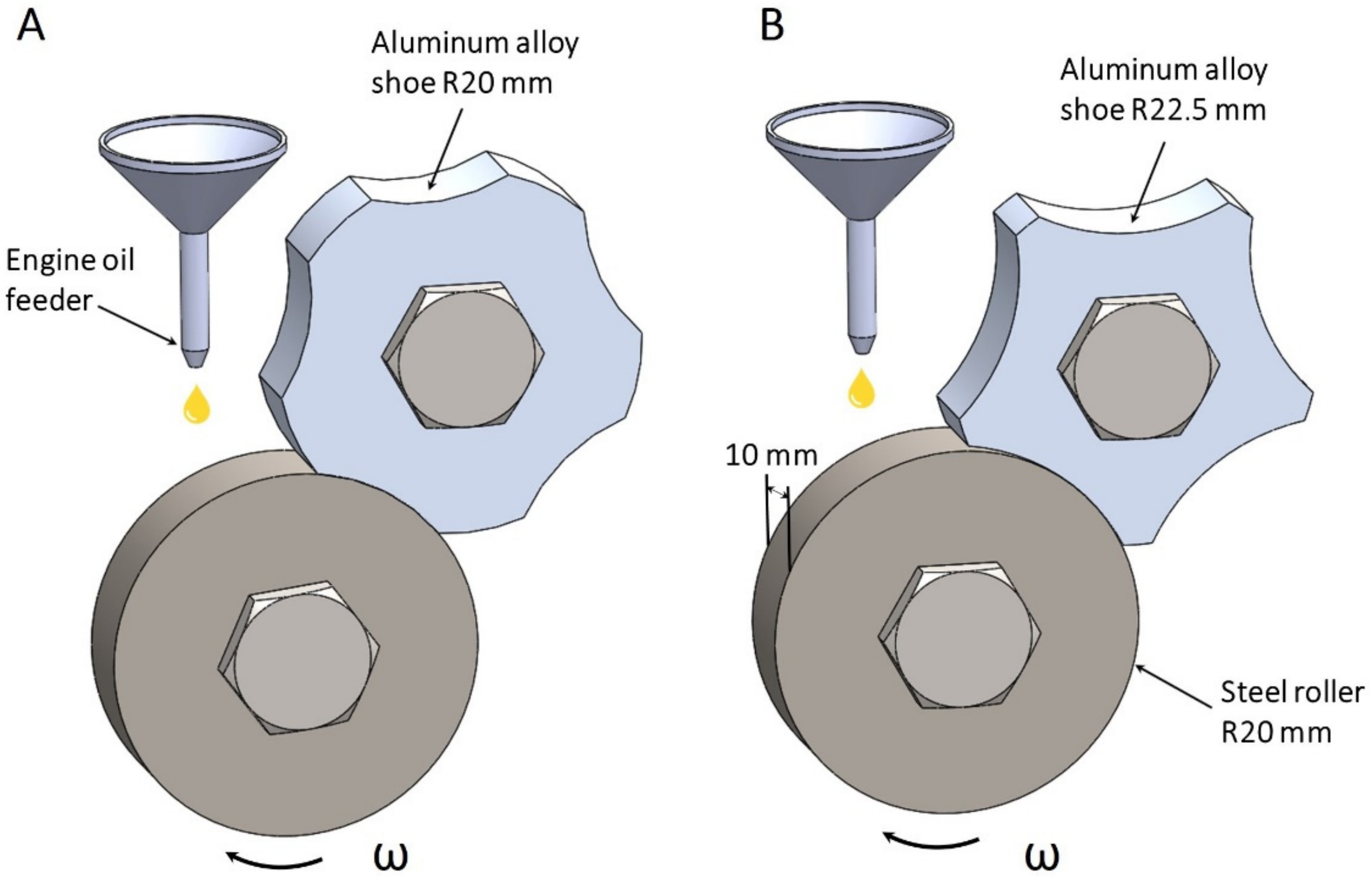
Today, most monometallic plain bearings are produced by casting bronze alloys to meet demanded mechanical and tribological properties. However, for bronze bearings, a significant disadvantage is the Rebinder effect in an emergency, where molten copper droplets become a surface-active substance for steel, causing the appearance of cracks at the boundaries of steel grains up to the complete fracture of the steel counter body. Therefore, research on the development of new antifriction aluminum alloys that can replace bronze in the manufacture of monometallic plain bearings is relevant. Moreover, replacing bronze with aluminum alloy gives a noticeable economic effect since aluminum is 2.5 times cheaper than copper in terms of unit weight cost and 3.3 times lighter.
Various bearings require different combinations of the service characteristics of antifriction materials, which results in the necessity for fabrication of a whole range of aluminum alloys with different running-in ability, scuff, and wear resistance as well as to minimize wear of the steel counter body. To extend the service life of bearings and provide their resistance to wear different technological methods are used. The coating is one of the most common processes. So far, different materials and products have been developed and investigated for these purposes. The possibility of production of multilayer coatings in a titanium-nitride/chrome-nitride system with high hardness (up to 57 GPa) was reported by Sobol et al. [
5]. Another work showed that the developed coating composition based on Ti–TiN–TiAlCrN drastically improved the operational life of the cutting inserts (more than double) [
6]. Ren et al. demonstrated that diamond-like carbon (DLC) coatings could be considered as promising surface coatings of engine parts in terms of friction and wear performance [
7]. Summer et al. reported that various polymer-coated bearings tested provide enhanced friction and wear performance in comparison to other bearing types (e.g., lead-based electroplated) [
8]. The production of complex-shaped dielectric products of superhard and tough nanocomposite coatings with wide interfaces, which were developed with a combined source of metal atoms and pulsed beams of fast neutral molecules with the energy of 50–200 eV, was described in [
9]. The formation and microstructure of the Ti–TiN–(Ti, Al, Si)N coating were investigated. It was found that the (Ti, Al, Si)N nanolayer structure of the coating made it possible to restrain the growth of grains within the nanometric range [
10,
11,
12]. However, despite their diversity and variety of deposition techniques, coatings alone cannot be always effective in both sliding performance and wear resistance characteristics of materials (bearings, cutting tools, engines, automotive brakes) especially when working in boundary and mixed friction lubrication conditions (start-stop cycles) under high load applications. In addition, plain bearings are usually designed to have loading capacity to withstand heavy weight from the shaft. Thus, in order to perform both the antifriction and load-bearing functions, a certain set of mechanical and tribological properties of the alloys from which the bearing materials are made is required. To balance colliding properties, new alloys are being developed by adding different elements responsible for mechanical and tribological performance. Magnesium, zinc, and nickel are used as major alloying elements in the vast majority of Al–Sn–Cu antifriction alloys. Copper [
13,
14] is added to aluminum alloys to strengthen them. The strengthening maximum is between 4% and 6% Cu, depending upon the influence of other constituents present. The mechanical alloying of Mg to Al–Sn alloys was reported to increase strength and wear performance by improving the distribution of Sn. Lu et al. found that the appropriate addition of Mg could increase wettability and improve the distribution of Sn phase, strength, and ductility of the alloy [
15]. The addition of zinc to aluminum (in conjunction with some other elements, primarily magnesium and/or copper) produces heat-treatable aluminum alloys with enhanced strength. Zinc substantially increases strength and allows precipitation hardening. Low melting zinc phases show chemical activity that contributes to the formation of protective secondary structures on the rubbing surface, and increases the running-in ability and wear resistance [
16,
17]. Nickel is added to aluminum–copper and aluminum–silicon alloys to improve hardness and strength at elevated temperatures and to reduce the coefficient of thermal expansion [
18,
19]. Tin [
20,
21,
22,
23] and lead [
24,
25,
26,
27] are present as soft structural components in these alloys that act as solid lubricants. Therefore, to achieve high values of both mechanical and tribological properties, the composition of Al-based alloys can include many alloying elements and form different intermetallic phases such as CuAl
2, Al
3Ni, Al
3Ni
2, Al
7Cu
4Ni, Al
9FeNi, AlFeMnSi, and Al
5Cu
2Mg
8Si
6, etc. The addition of chromium (Сr) and molybdenum (Mo) improves the strength and wear resistance of the alloys. Nasef et al. reported that Al-based alloys with 10 wt.% of Cr showed higher wear resistance and improved mechanical properties than compositions without chrome [
28]. The tensile and aging properties of the Al–Si–Cu–Mg alloy modified with additions of Cr, Ti, V, and Zr were examined by Shaha et al. [
29]. It was shown that the modified alloy formed the different dispersoid phases with a variety of morphologies that enhance the strength of the alloys at elevated temperatures. The presence of Mo enhances the strength properties of the Al-based alloys [
30,
31]. In addition, molybdenum interacts with sulfur (S), which is usually part of the chemical compositions of lubricant and forms molybdenum sulfide (MoS
2), which in turn could significantly improve the tribological performance of friction surfaces [
32,
33]. Due to the synergistic interaction of all the constituent alloying elements with each other and with the aluminum matrix, special friction layers (tribofilms) are formed on friction surfaces referred to as “secondary structures” by the authors of the present work.
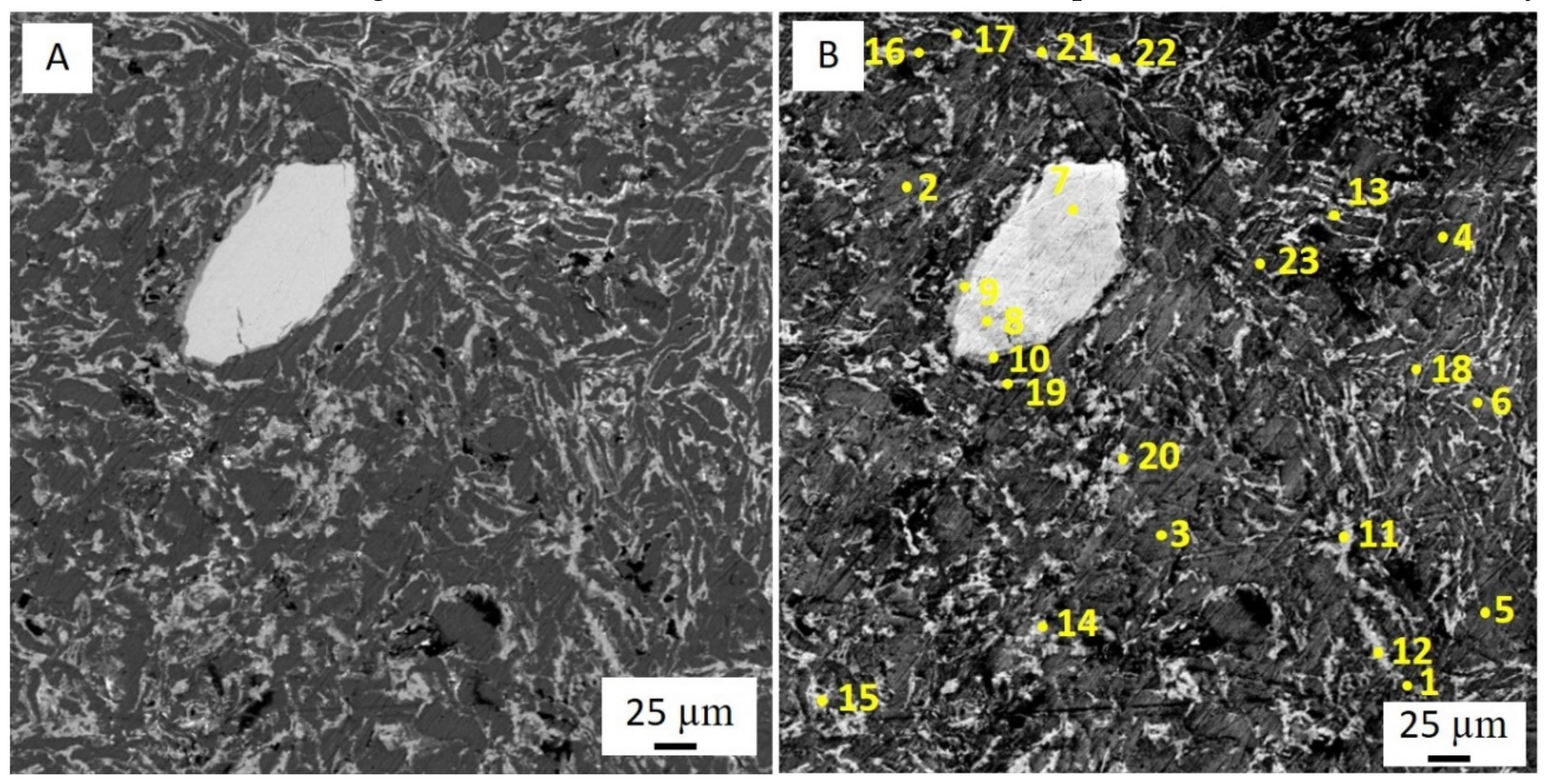
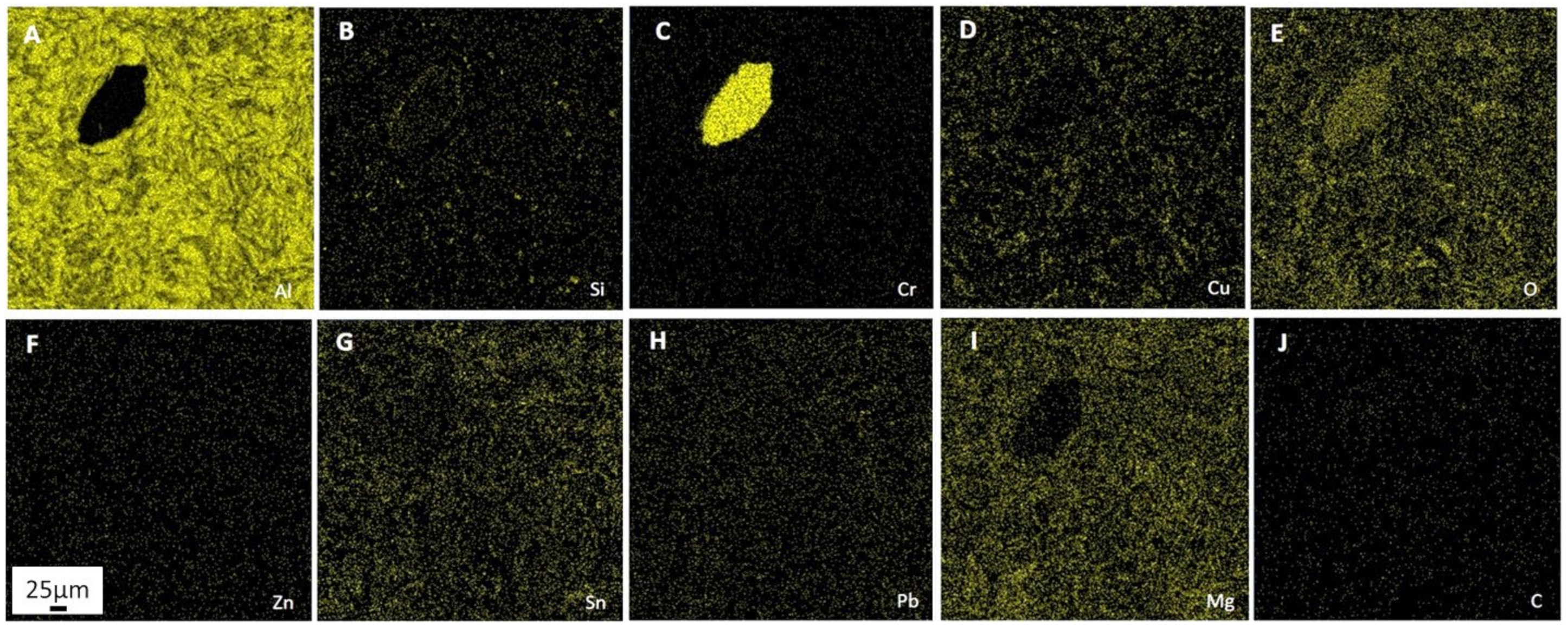
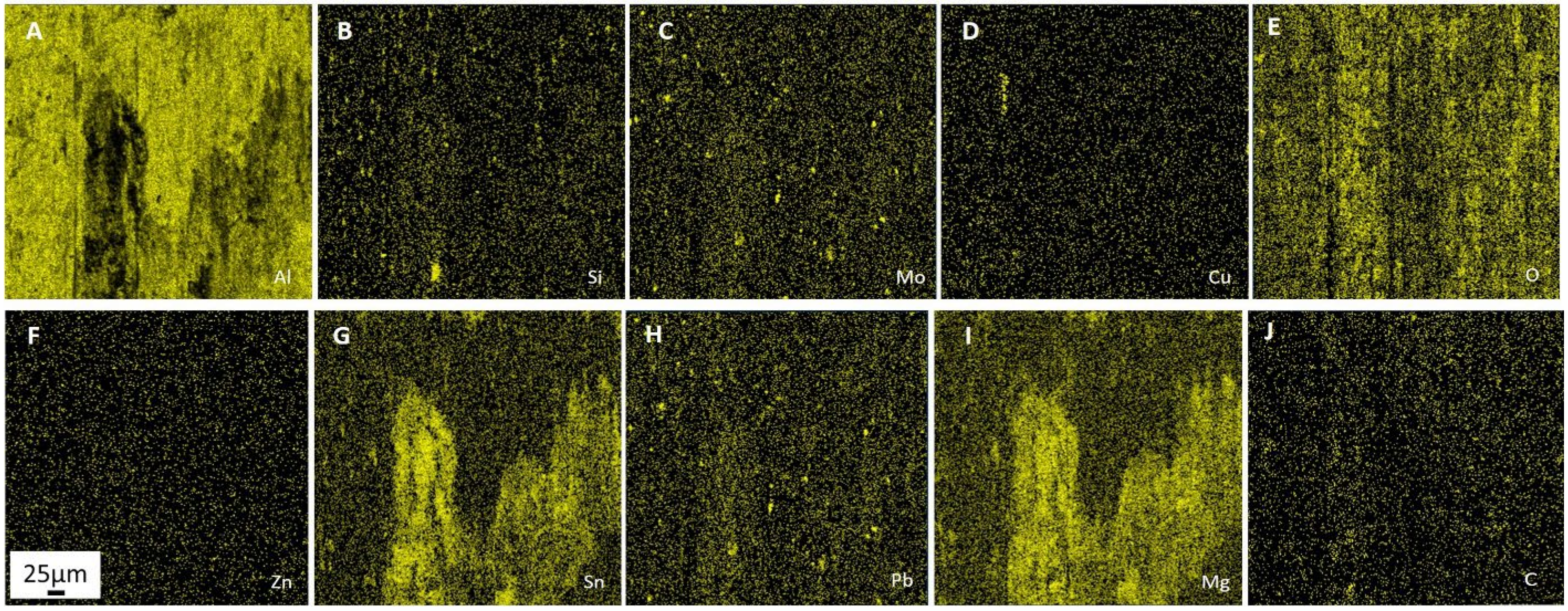
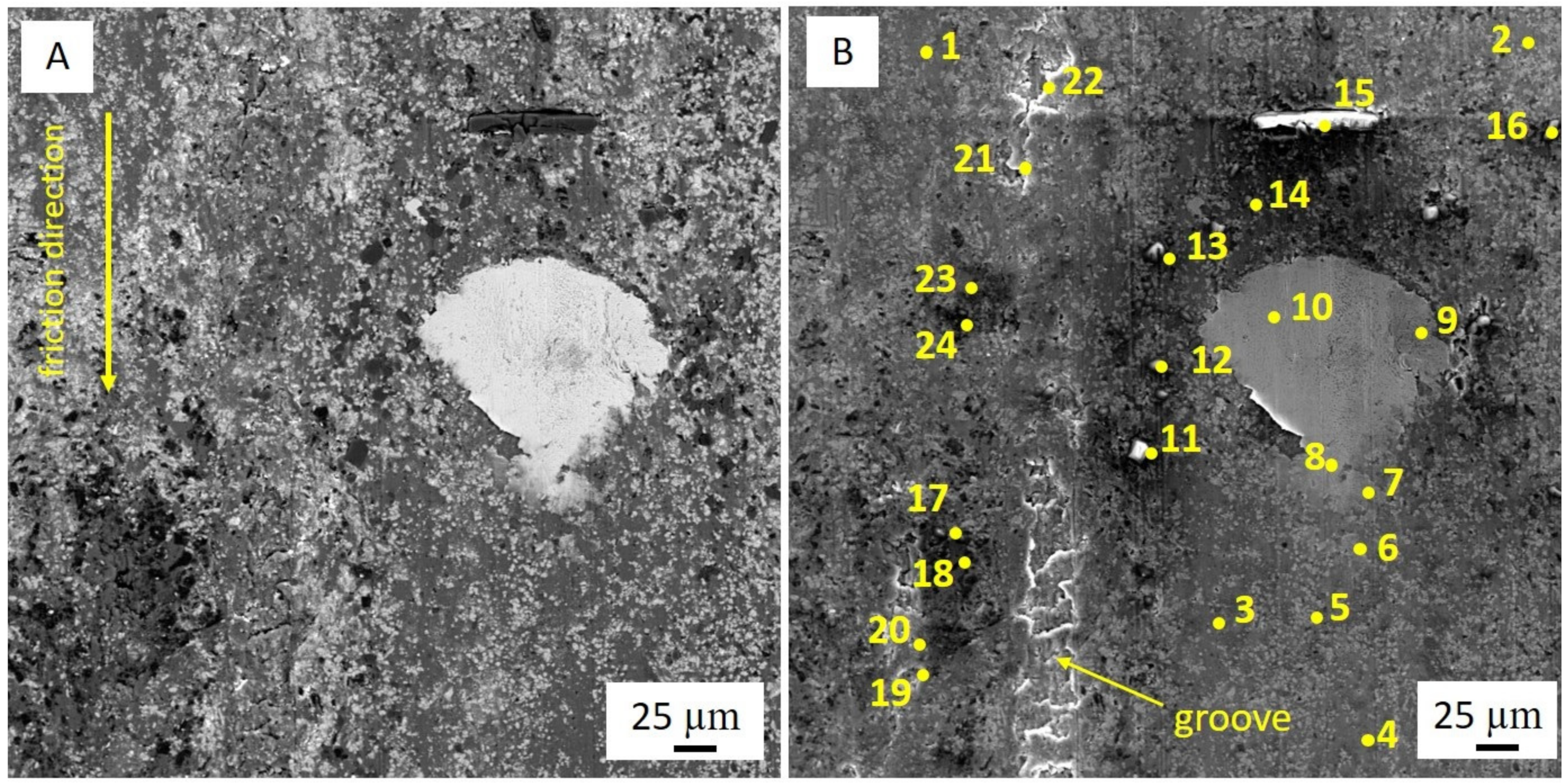
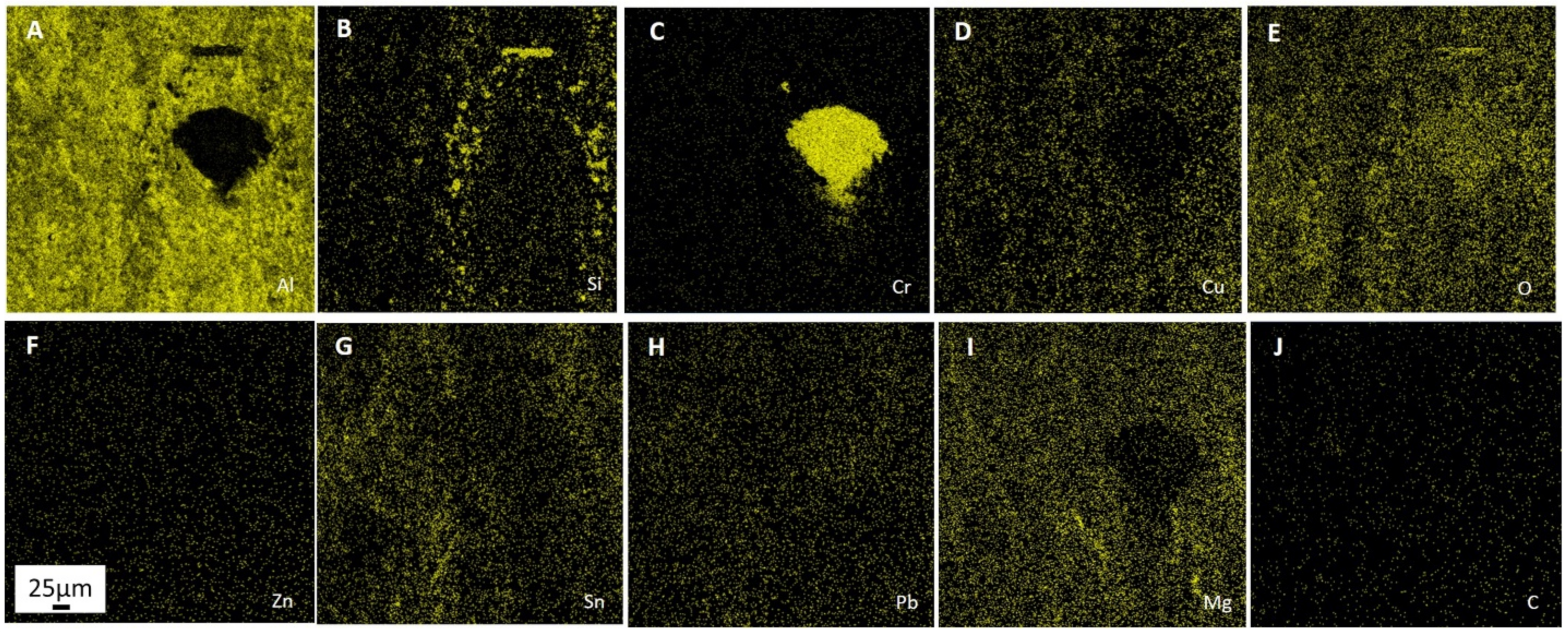
Processes of friction and wear are realized on a background of the raised gradient relations of temperature, stresses, the concentration of alloying elements, and defects of a crystal structure and represent a complex set of physical and chemical phenomena as a consequence of what the self-organization processes appear on the friction surface in tribopairs [
34,
35]. The essence of this phenomenon is that under the influence of an external perturbation any thermodynamically open nonlinear system is rearranged in such a way that its response maximally compensates for the internal cause that caused such an internal change [
36,
37]. The following processes are manifestations of self-organization in friction: formation of secondary structures with a different, in the limit higher strength and wear resistance in comparison to the initial one; development of equilibrium roughness irrespective of the initial microgeometry of friction surfaces; increase in the actual contact area due to linear wear and, as a consequence, decrease in contact loads; realization of selective transfer, etc. The external form of self-organization is the reduction and stabilization of practically all energy, force, and tribotechnical parameters of friction and wear process, in particular friction coefficient, temperature, wear rate, etc. The enforcement addition of appropriate chemical elements to the tribosystem assists in the formation of necessary secondary structures. Studies on the role of chemical elements in secondary structures have been carried out [
38,
39]. The studies showed an example of optimization of magnesium content in antifriction material in terms of ensuring its presence in secondary structures to reduce wear rate [
40,
41]. In addition, it was reported that chromium and molybdenum), which are part of the steel, were detected in secondary structures on the friction surfaces of aluminum-based alloy [
39]. The presence of these elements accelerates the processes of self-organization and adaptation on the rubbing surface. Therefore, the improved mechanical and wear properties of multicomponent aluminum alloys can be achieved by adding low-melting and refractory elements. However, it should be pointed out that traditional metallurgical methods make it practically impossible to simultaneously alloy aluminum with both refractories (chromium and molybdenum) and low-melting metals (tin, lead, zinc). The former is distributed unevenly, while the latter burn out at high temperatures. Therefore, alternative manufacturing methods are needed.
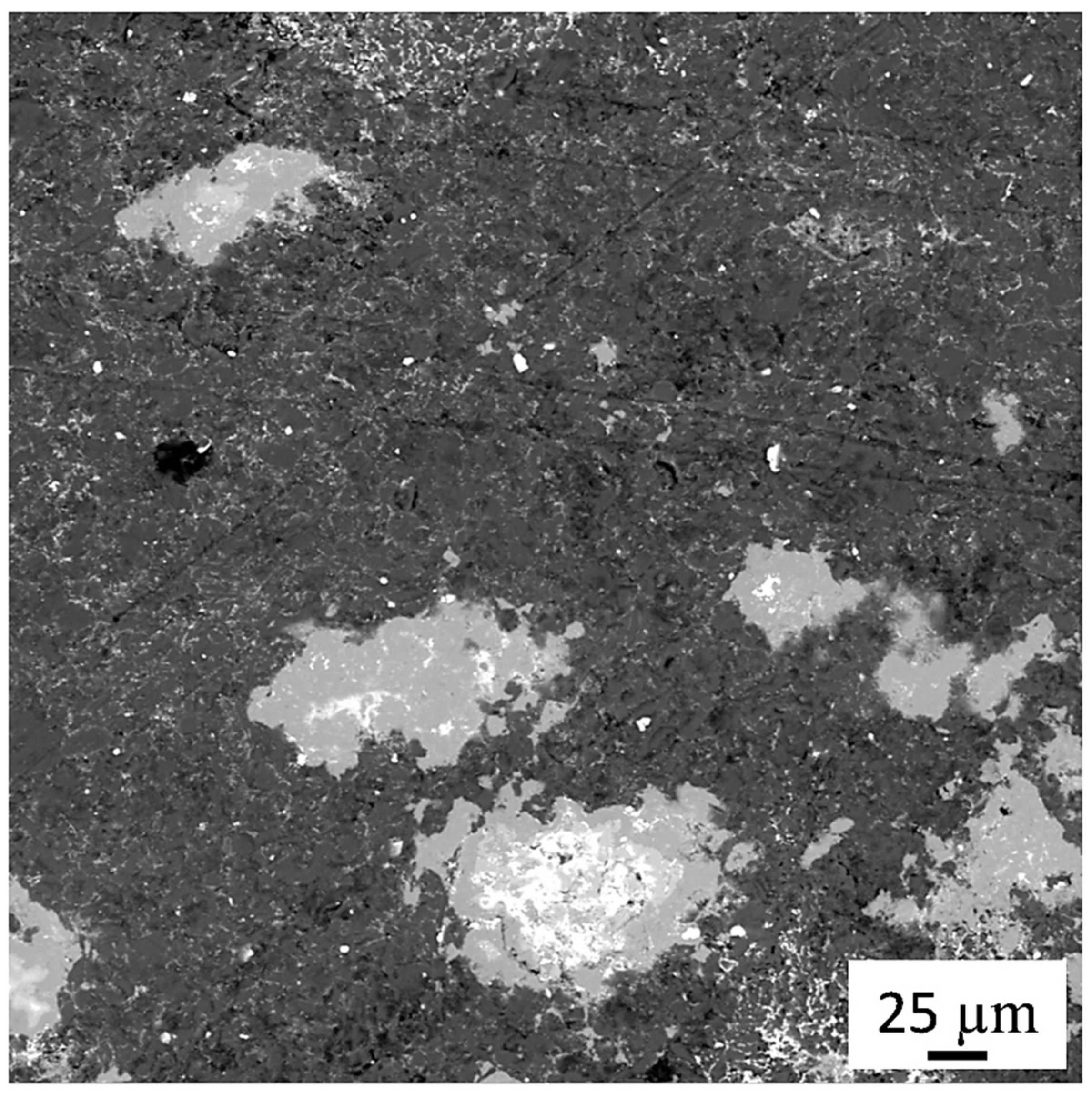
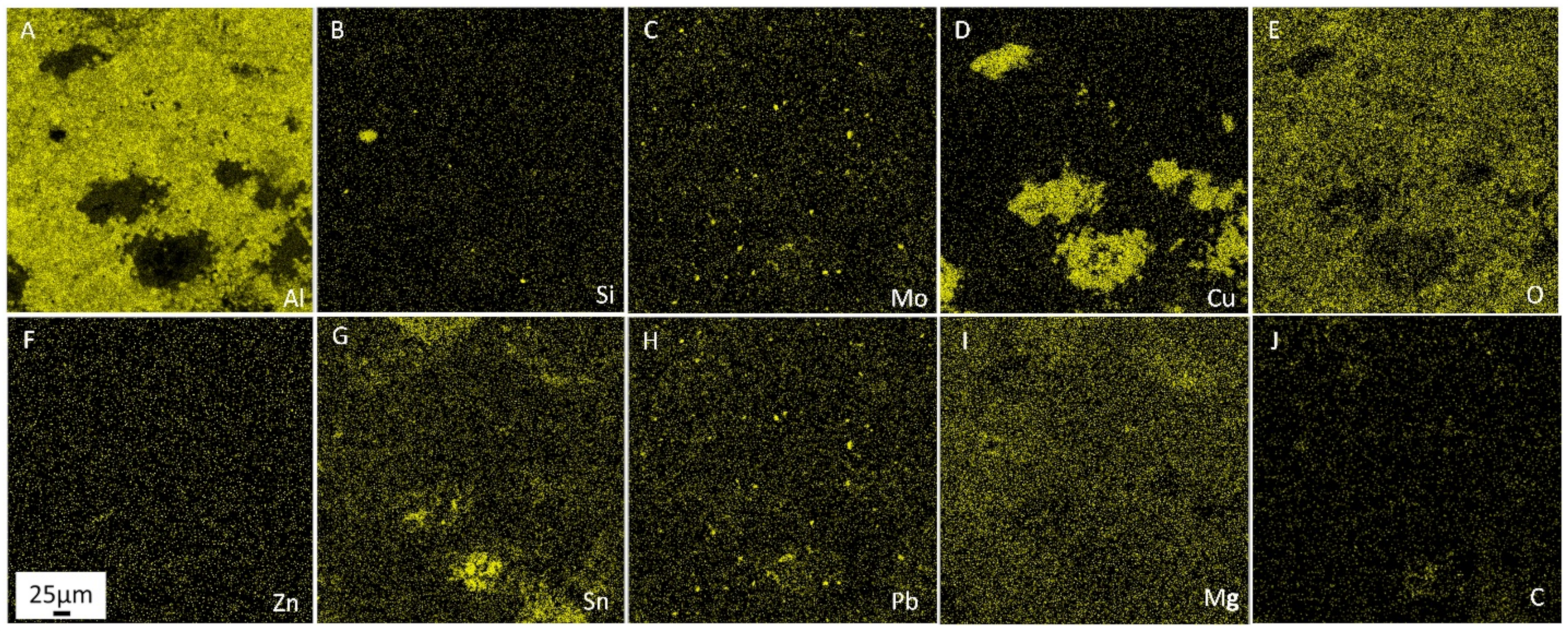
In addition, the oxide film continuity on the aluminum particles is broken during this process. The oxide-free surfaces easily interact with each other, which further increases the strength of the sintered material. The separated oxide film particles, which have higher hardness and wear resistance compared to the aluminum matrix, form fairly evenly distributed conglomerates within the alloy. As a result, there is an additional resource for improving tribotechnical properties-alloy becomes more consistent with the first rule of Charpy, according to which the antifriction material should consist of hard, evenly distributed strong inclusions and softer and more ductile matrix.
Then for the fabrication of bulk samples derived from mixtures prepared by mechanical alloying spark plasma sintering (SPS) technique was used. This consolidation method offers considerable advantages such as faster and shorter processing time compared to conventional sintering methods, due to simultaneous application of mechanical pressure and electric pulse. The advantages of this technique are associated with a high densification rate and fine microstructure and consequently better properties [
46,
47,
48,
49]. Moreover, SPS allows the breaking of the continuity of oxide film on the surface of aluminum particles. In our previous study, we had confirmed the effectiveness of the MA and spark plasma sintering method for the preparation of tailored mixtures of metallic powders, enabling microstructural characteristics that could be favorable for the formation of secondary structures on the tribosurfaces [
48]. However, the evaluation of the mechanical and tribological behavior of the obtained compositions was not presented.
The present investigation aims to analyze the impact of Cr and Mo on mechanical properties of SPS-ed multicomponent aluminum alloys derived from powders synthesized by MA technology, evaluate the effect of the presence of these elements on the formation of favorable secondary structures on the friction surfaces, and tribological behavior of shaft-plain bearing couple. To facilitate their reach into secondary structures these elements were added to the raw powder mixture. Of particular interest are the studies on microstructure, mechanical properties, and tribological behavior of SPS-ed multicomponent aluminum alloys.