High-Temperature Surface Oxide Growth Kinetics of Al–Si–Zr Bulk Alloys and Ribbons
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition of the Alloy
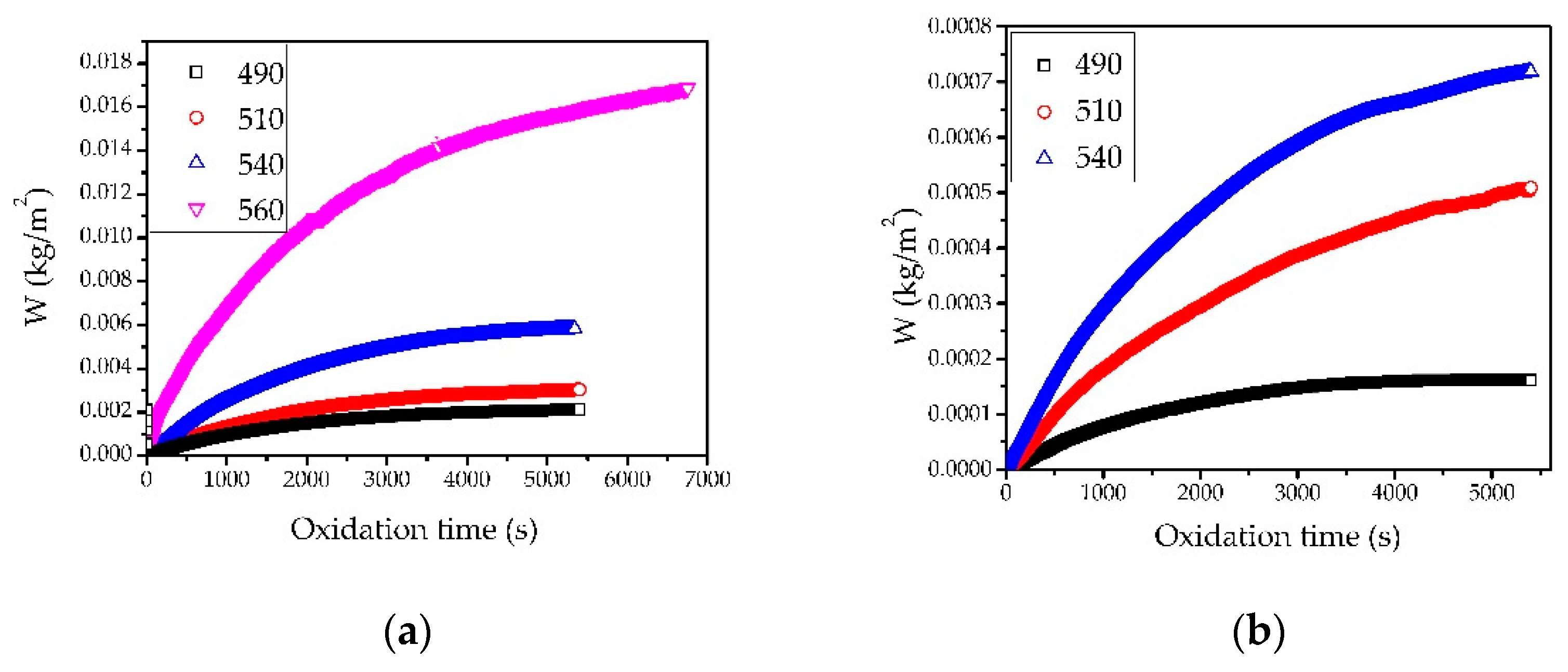
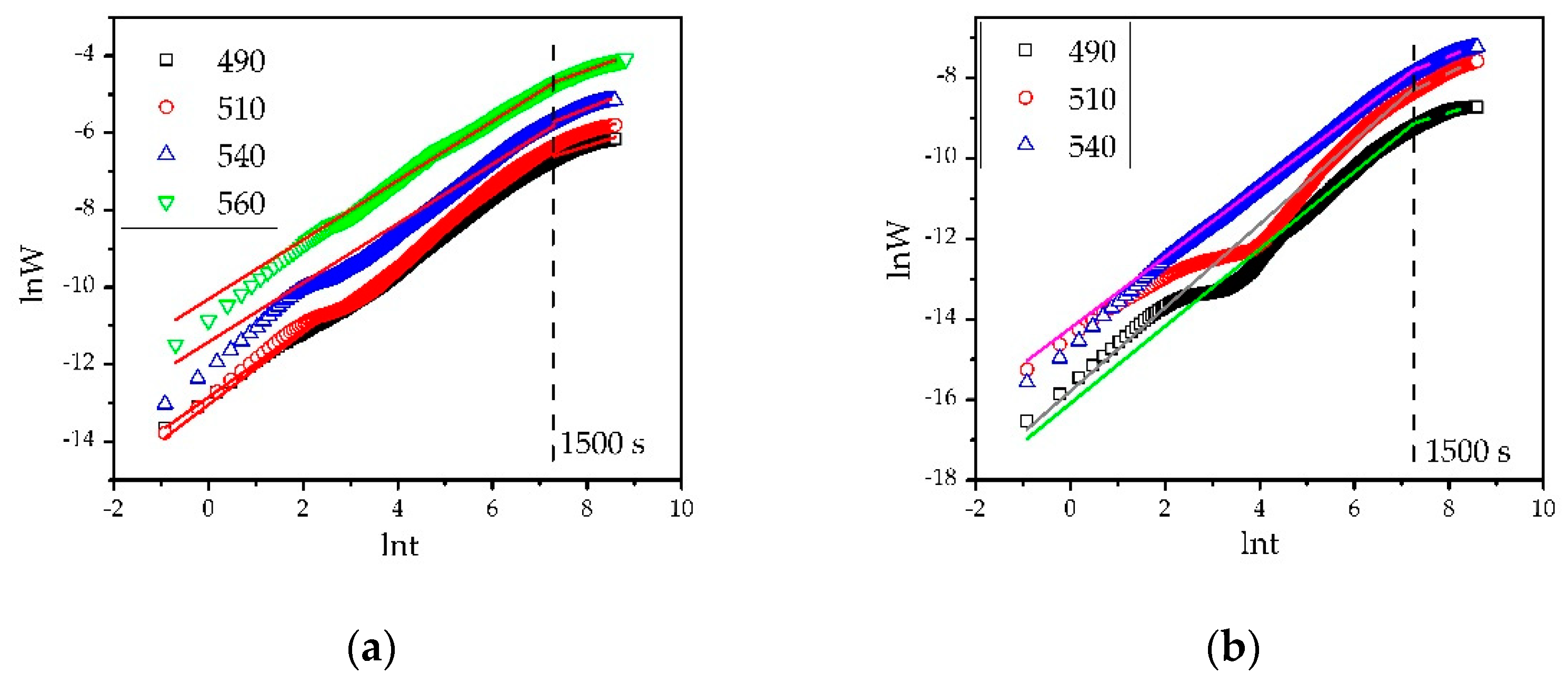
3.2. Oxidation Kinetics
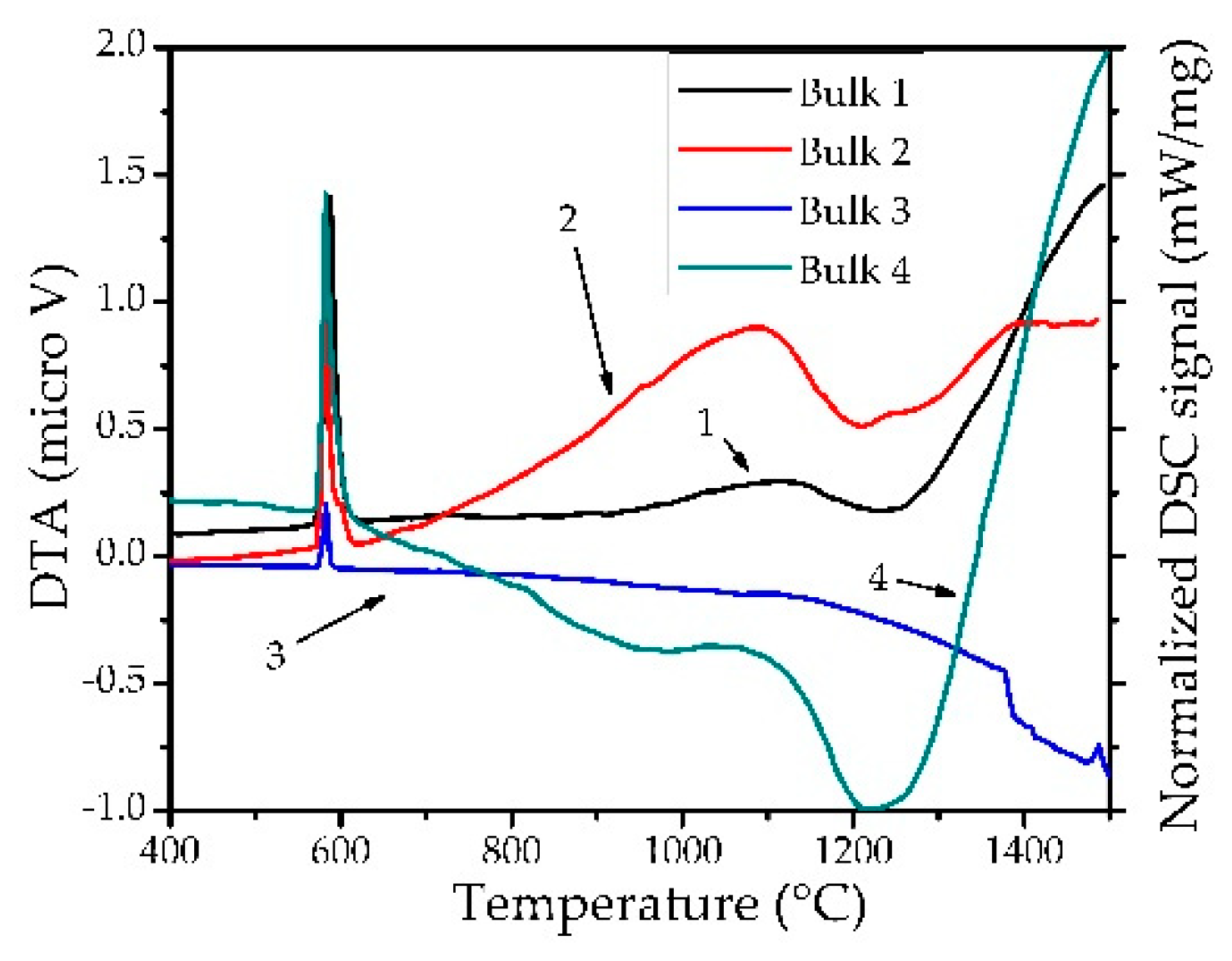
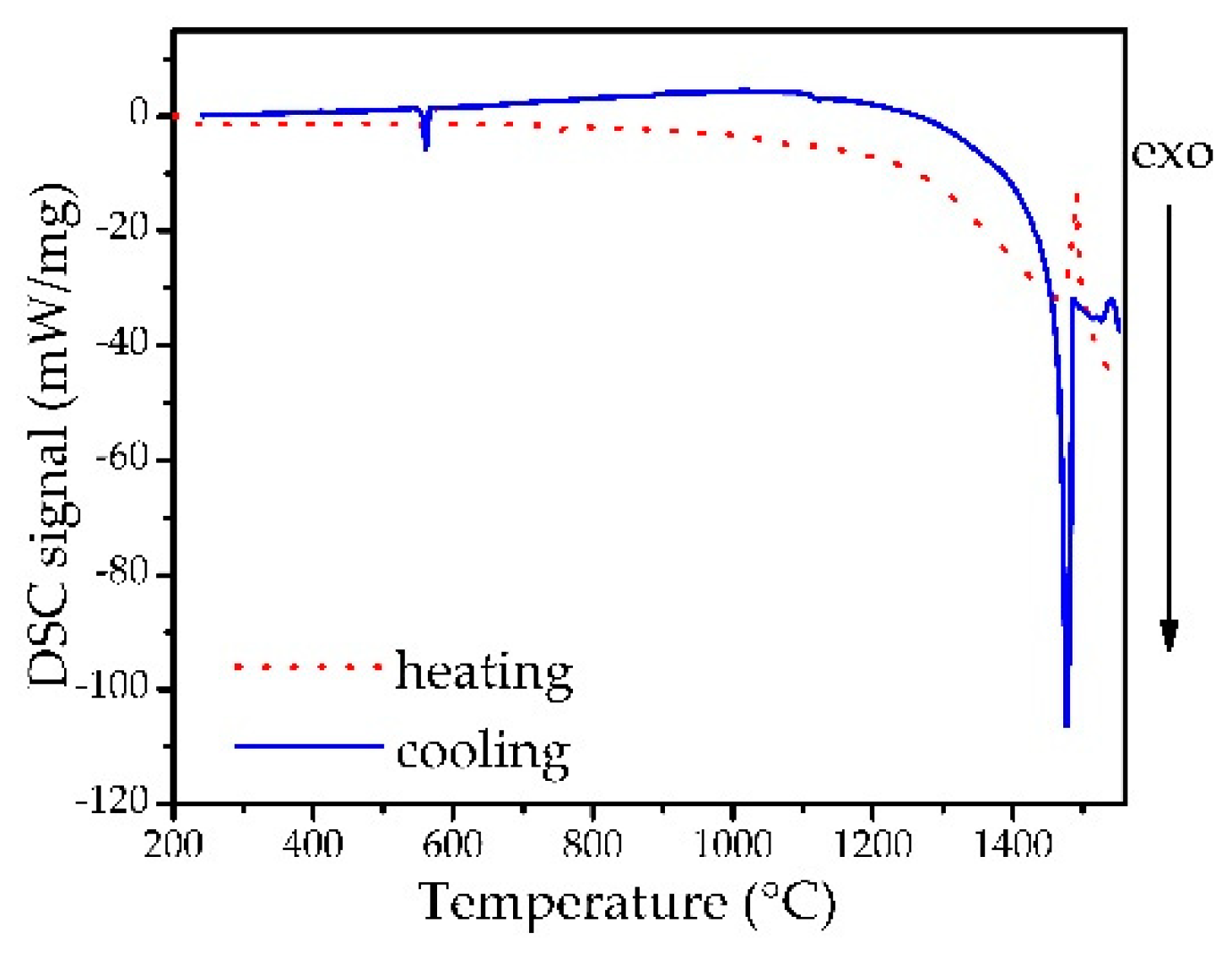
3.3. Thermal Studies
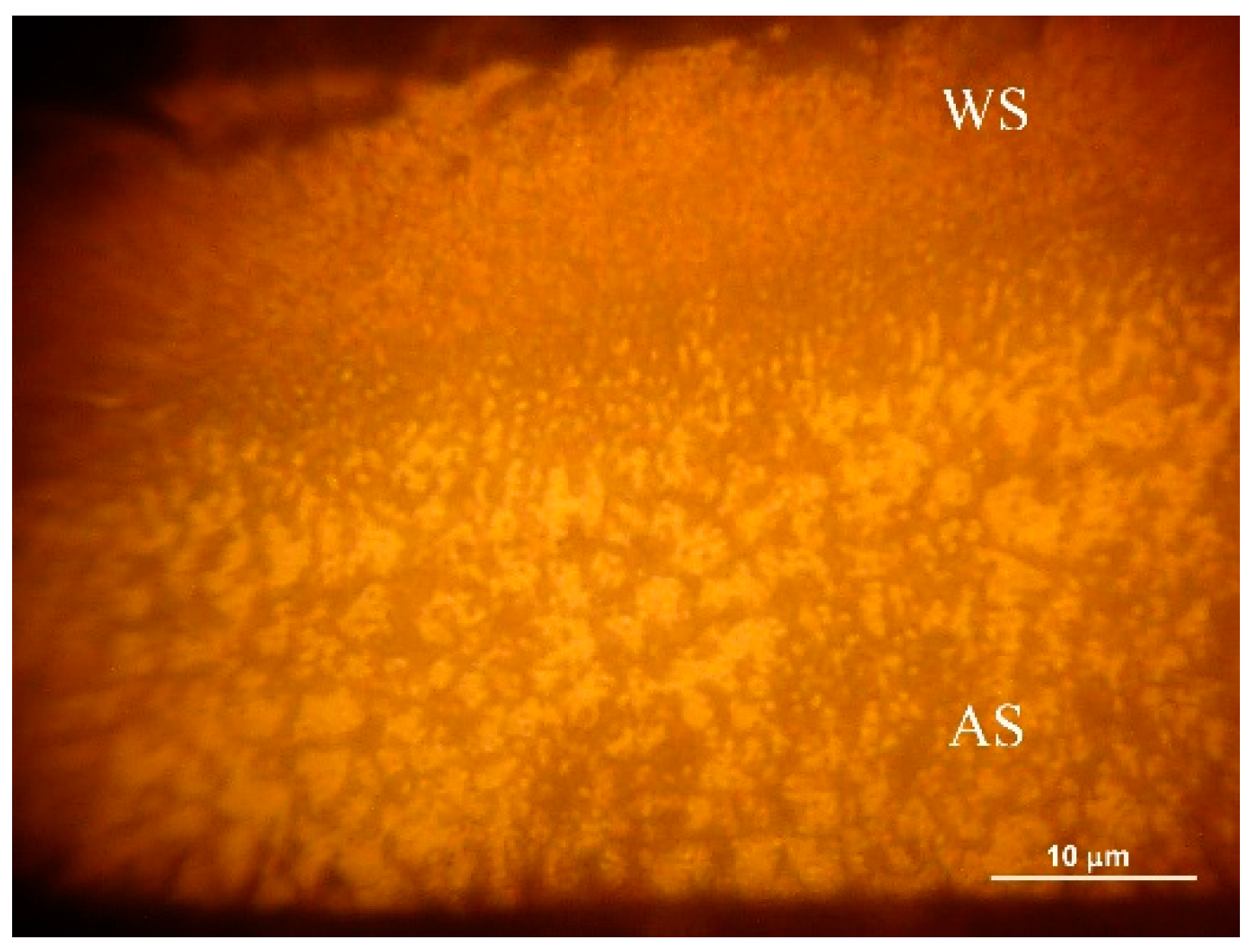
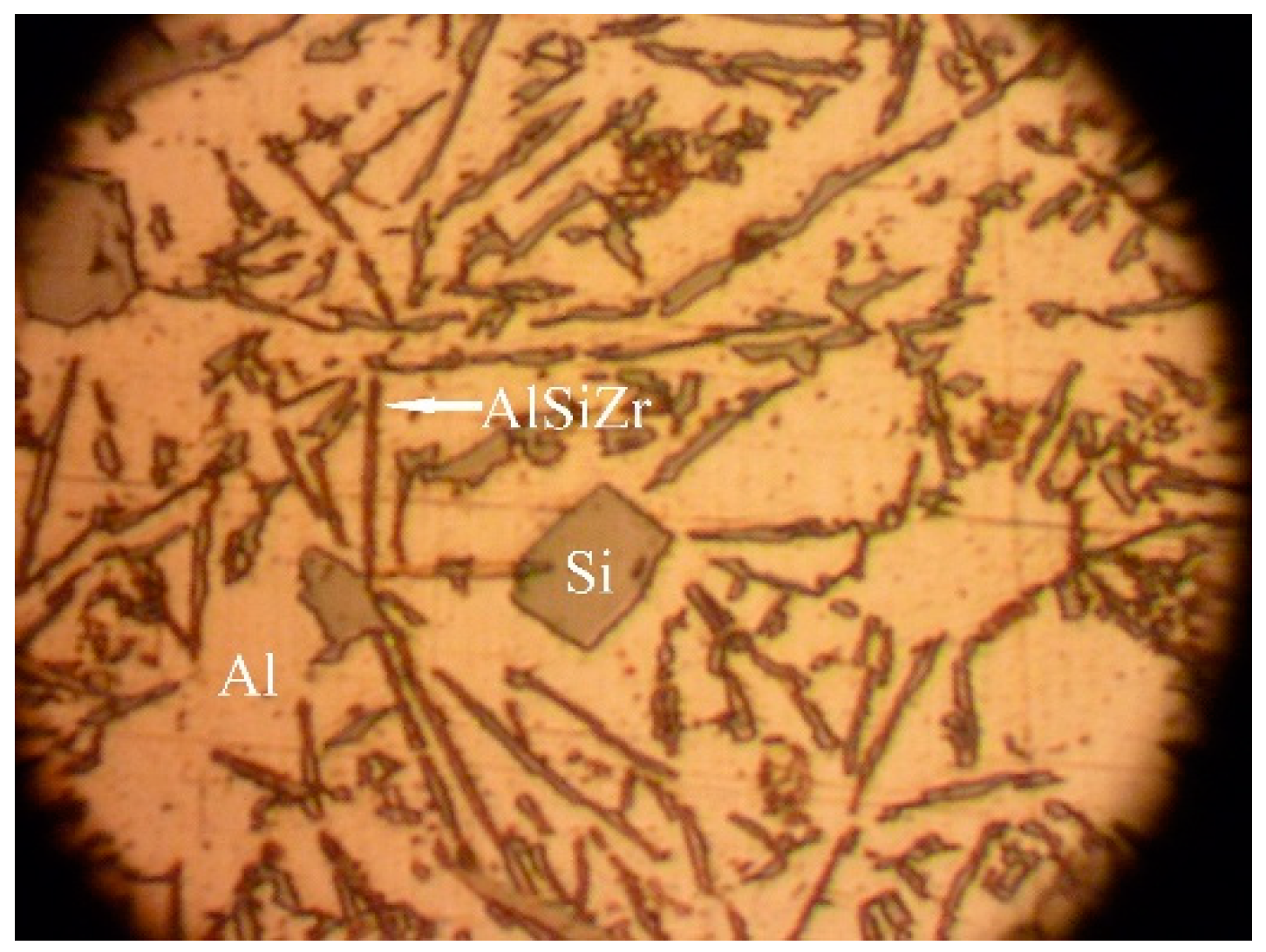
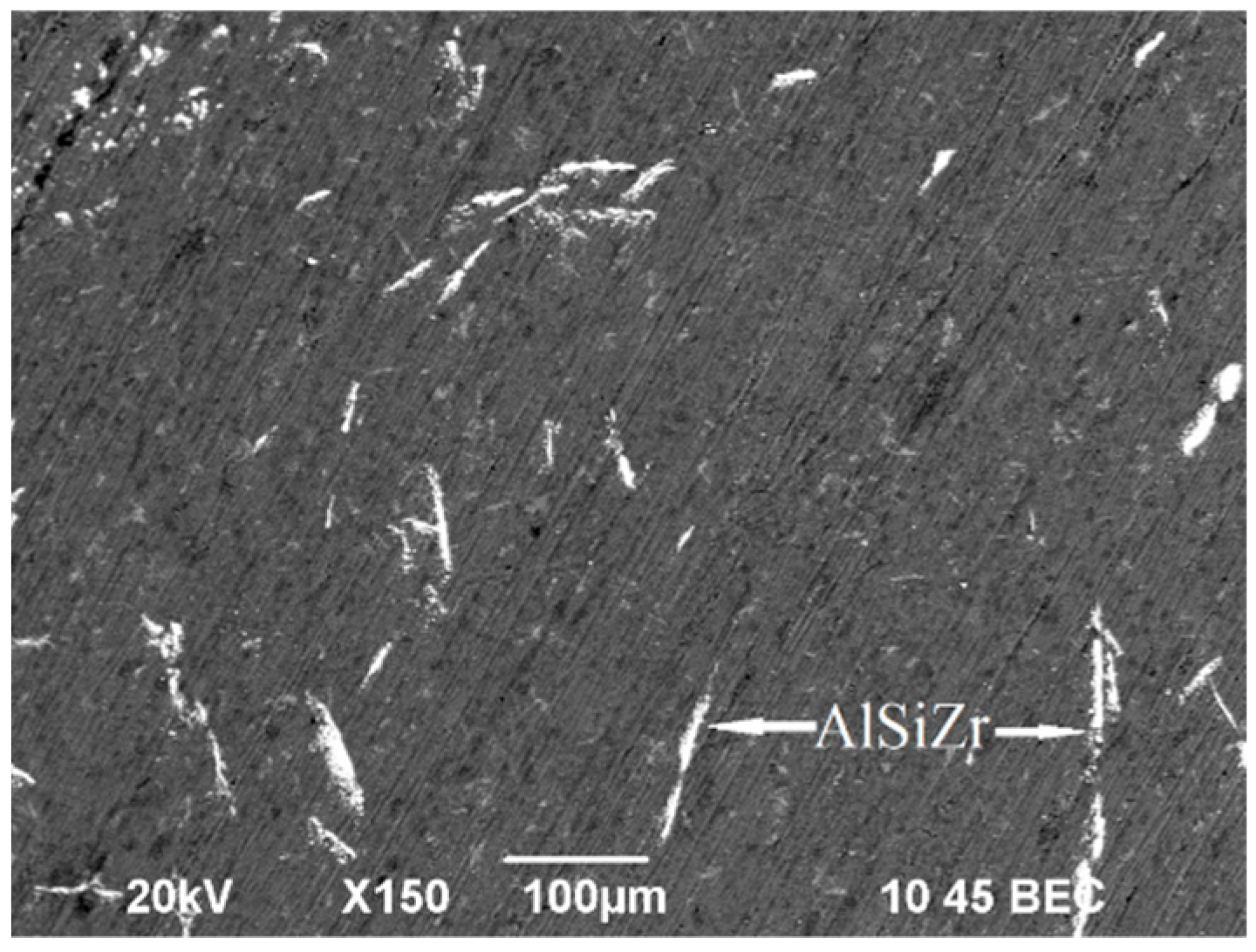
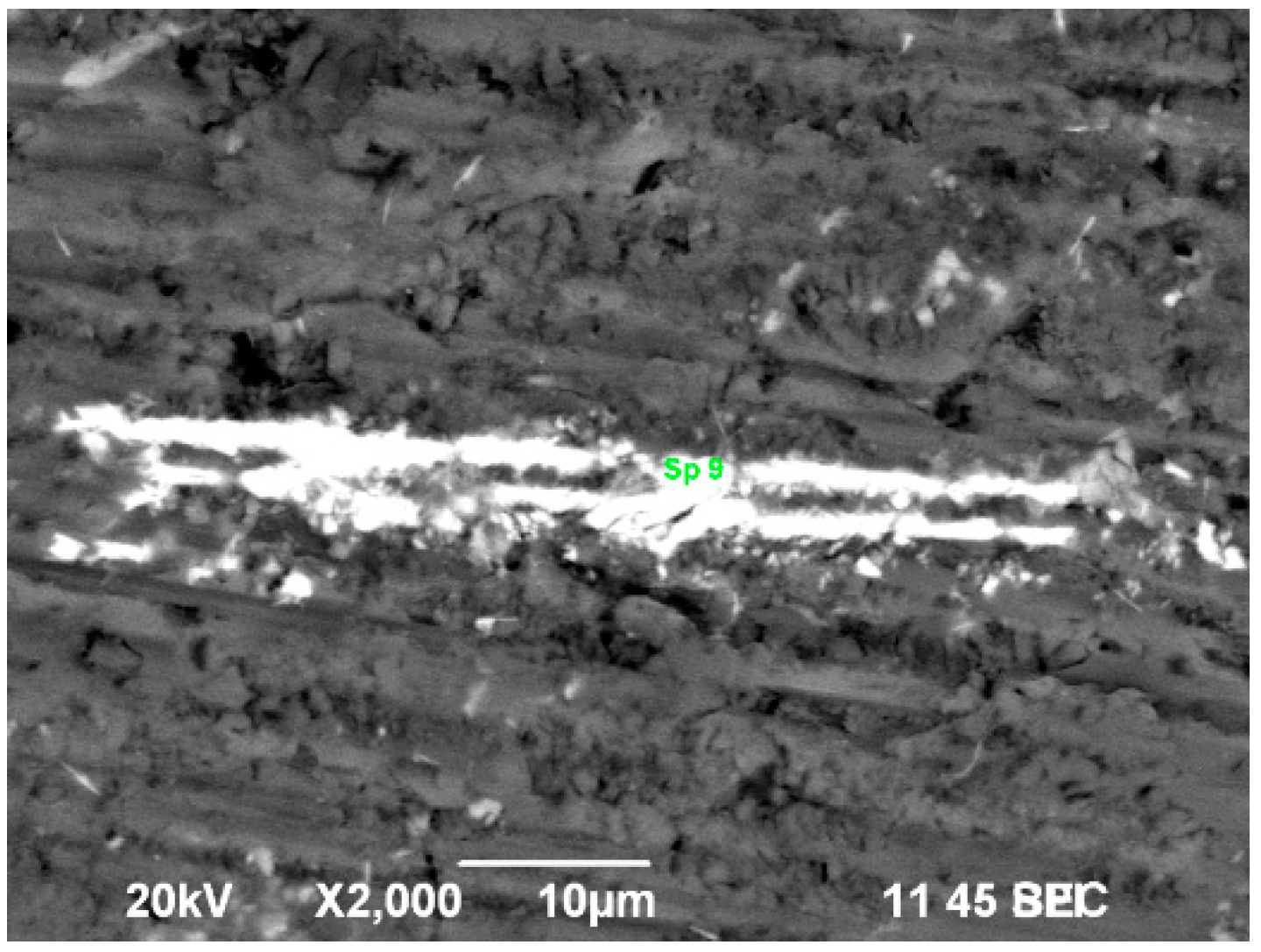
3.4. Optical and Scanning Electron Microscope Studies. Microhardness Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çadırlı, E.; Tecer, H.; Şahin, M.; Yılmaz, E.; Kırındı, T.; Gündüz, M. Effect of heat treatments on the microhardness and tensile strength of Al–0.25 wt.% Zr alloy. J. Alloys Compd. 2015, 632, 229–237. [Google Scholar] [CrossRef]
- Li, Q.; Ma, X.; Li, B.; Ma, J.; Feng, X.; Pei, R.; Liu, J. Influences of different Zr/Sc ratios on microstructure and mechanical properties of Al–2Si alloy. J. Rare Earths 2021, 39, 105–112. [Google Scholar] [CrossRef]
- Andrzejczuk, M.; Lewandowska, M.; Latuch, J.; Kurzydłowski, K.J. Multiscale characterization of nanostructured Al–Si–Zr alloys obtained by rapid solidification method. J. Mater. Sci. 2011, 46, 5454–5459. [Google Scholar] [CrossRef] [Green Version]
- Bao, G.; Zuo, M.; Li, D.; Li, Y.; Liu, X. The improvement of microstructures and mechanical properties of near eutectic Al–Si multicomponent alloy by an Al–8Zr–2P master alloy. Mater. Sci. Eng. A 2012, 531, 55–60. [Google Scholar] [CrossRef]
- Dang, J.; Huang, Y.; Cheng, J. Effect of Sc and Zr on microstructures and mechanical properties of as-cast Al–Mg–Si–Mn alloys. T. Nonferr. Metal Soc. 2009, 19, 540–544. [Google Scholar] [CrossRef]
- Bevilaqua, W.L.; Stadtlander, A.R.; Froehlich, A.R.; Lemos, G.V.B.; Reguly, A. High-temperature mechanical properties of cast Al–Si–Cu–Mg alloy by combined additions of cerium and zirconium. Mater. Res. Express. 2020, 7, 026532. [Google Scholar] [CrossRef]
- Reihanian, M.; Ranjbar, K.; Rashno, S. Microstructure and Impression Creep behaviour of Al–7Si–0.3Mg alloy with Zr addition. Met. Mater. Int. 2020, 27, 2530–2540. [Google Scholar] [CrossRef]
- Rahimian, M.; Amirkhanlou, S.; Blake, P.; Ji, S. Nanoscale Zr-containing precipitates; a solution for significant improvement of high-temperature strength in Al–Si–Cu–Mg alloys. Mater. Sci. Eng. A 2018, 721, 328–338. [Google Scholar] [CrossRef] [Green Version]
- McPherson, D.J.; Hansen, M. The System Zr–Al. Trans. ASM 1954, 46, 354–374. [Google Scholar]
- Murray, J.L.; McAlister, A.J. The Al–Si (Aluminum–Silicon) system. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Massalski, T. ASM CD-ROM. In Binary Phase Diagrams, 2nd ed.; ASM International: Almere, The Netherlands, 1996. [Google Scholar]
- Tong, G.; Dakuia, L.; Zuoshanb, W.; Xiangfaa, L. Evolution, microhardness of ZrAlSi intermetallic and its impact on the elevated-temperature properties in Al–Si alloys. Mater. Sci. Eng. A 2012, 552, 523–529. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Z.; Zhao, J.C. Thermodynamic assessment of the Al–Zr binary system. JPE 2001, 22, 544–551. [Google Scholar] [CrossRef]
- Litynska, L.; Abou-Ras, D.; Kostorz, G.; Duetkiewicz, J. TEM and HREM study of Al3Zr precipitates in an Al–Mg–Si–Zr alloy. J. Microsc. 2006, 223, 182–184. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Butterworths and Co., Ltd.: London, UK, 1976. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, M.; Wu, C.; Wang, J.; Su, X. Progress on phase equilibria of the AlSiZr system at 700 and 900 °C. J. Alloys Compd. 2017, 693, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Schob, O.; Nowotny, H.; Benesovsky, F. The Ternary Systems (Titanium, Zirconium, Hafnium)-Aluminum-Silicon. Planseeber. Pulvermetall. 1962, 10, 65–71. [Google Scholar]
- Beck, A.F.; Heine, M.A.; Caule, E.J.; Pryor, M.J. The kinetics of the oxidation of Al in oxygen at high temperature. Corros. Sci. 1967, 7, 1–22. [Google Scholar] [CrossRef]
- Petrova, A.; Stefanov, G.; Yaneva, S.; Avdeev, G.; Miteva, A.; Petrov, R. Influence of the inert particle additives on the properties of the nano-microcrystalline alloys on based on Al–Fe–V–Si. Metallurgist 2020, 64, 3–4. [Google Scholar] [CrossRef]
- Stoichev, N.; Petrov, K.; Yaneva, S.; Kovachev, P.; Tzvetanova, N. Micro-structural development in Al–Si microcrystalline alloys. Mater. Sci. Eng. A 2002, 337, 12–16. [Google Scholar] [CrossRef]
- Yaneva, S.; Stojanova, L.; Markow, T. Oxidation of Solid Aluminium Silicon Alloys in Air. Cryst. Res. Technol. 1987, 22, 251–258. [Google Scholar] [CrossRef]
- Lins, V.F.C.; Paula e Silva, E.M. Autocorrelation treatment of thermogravimetric data with the cochrane-orcutt method. Braz. J. Chem. Eng. 1997, 14, 179–184. [Google Scholar] [CrossRef]
- Knoll, A.; Smigiel, E.; Broll, N.; Cornet, A. Study of high temperature oxidation kinetics of steel using grazing X-ray reflectometry. In Advances in X-ray Analysis (AXA)-Denver X-ray Conferences; JCPDS-International Centre for Diffraction Data: Newtown Square, PA, USA, 1999; pp. 170–178. [Google Scholar]
- Kiradzhiyska, D.; Drajewicz, M.; Romanowska, J.; Volšak, D.; Gandova, V.; Vassilev, G. Thermochemical and electron microscope studies of fast-cooled cast Al–Si–Zr ribbons and Al–Cu–Nd alloys. Basic Inf. 2014, 1, 15–26. [Google Scholar]
- Stefanov, G.; Kiradzhiyska, D.; Avdeev, G. High-temperature oxidation of Al–Si–Zr alloys. In Proceedings of the NDT Days 2013, Sozopol, Bulgaria, 17–21 June 2013; Volume 2, pp. 305–308. (In Bulgarian). [Google Scholar]
- Kiradzhiyska, D.; Vassilev, G.; Mantcheva, R. Thermochemical studies of Al–Si–Zr alloys. In Proceedings of the Science and Youth Conference, Plovdiv, Bulgaria, 10–12 April 2014; pp. 319–322. [Google Scholar]


| Wet Chemistry and Spectral Methods | ICP-OES Methods | ||||||
|---|---|---|---|---|---|---|---|
| NB | cSi (Mass %) | cZr (Mass %) | cAl (Mass %) | NB | cZr ± RSD (Mass %) | NR | cZr ± RSD (Mass %) |
| 1B | 9.70 | 0.30 | 90.00 | 1B | 0.26 ± 0.01 | 1R | 0.27 ± 0.01 |
| 2B | 10.00 | 0.82 | 89.18 | 2B | 0.99 ± 0.06 | 2R | 1.09 ± 0.08 |
| 3B | 11.30 | 1.20 | 87.50 | 3B | 1.19± 0.05 | 3R | 1.16 ± 0.07 |
| 4B | 9.30 | 1.40 | 89.30 | 4B | 1.38 ± 0.06 | 4R | 1.34 ± 0.03 |
| NB | t ± SD (°C) | Duration (s) | NR | t ± SD (°C) | Duration (s) |
|---|---|---|---|---|---|
| 1B | 541.4 ± 0.4 | 5400 | 1R | 540.4 ± 0.4 | 5400 |
| 2B | 541.4 ± 0.4 | 5400 | 2R | 540.7 ± 0.4 | 5400 |
| 3B | 541.3 ± 0.4 | 5352 | 3R | 541.4 ± 0.4 | 5400 |
| 3B | 540.6 ± 0.3 | 7200 | - | - | - |
| 4B | 541.3 ± 0.4 | 5400 | 4R | 541.0 ± 0.4 | 5400 |
| 3B | 490.0 ± 0.4 | 5400 | 3R | 490.0 ± 0.4 | 5400 |
| 3B | 510.0 ± 0.4 | 5400 | 3R | 510.0 ± 0.4 | 5400 |
| 3B | 560.0 ± 0.4 | 5400 | - | - | - |
| No | t (°C) | n1 ± SDn1 | (k1,ef ± SDk1,ef) × 10–6 (kg/m2s) | n2 ± SDn2 | k2,ef ± SDk2,ef (kg/m2s) |
|---|---|---|---|---|---|
| 1B | 540 | 0.998 ± 0.002 | 1.887 ± 0.020 | 2.120 ± 0.004 | (2.230 ± 0.060) × 10−9 |
| 2B | 540 | 1.106 ± 0.002 | 0.758 ± 0.030 | 2.108 ± 0.004 | (2.900 ± 0.060) × 10−9 |
| 3B | 540 | 1.135 ± 0.001 | 1.158 ± 0.010 | 2.397 ± 0.004 | (9.530 ± 0.140) × 10−10 |
| 4B | 540 | 1.140 ± 0.001 | 0.870 ± 0.030 | 2.391 ± 0.004 | (5.680 ± 0.070) × 10−10 |
| 3B | 490 | 1.146 ± 0.001 | 0.355 ± 0.003 | 2.630 ± 0.005 | (1.930 ± 0.030) × 10−11 |
| 3B | 510 | 1.072 ± 0.002 | 0.841 ± 0.010 | 2.530 ± 0.005 | (8.840 ± 0.130) × 10−11 |
| 3B | 560 | 1.300 ± 0.002 | 1.499 ± 0.010 | 2.225 ± 0.003 | (2.000 ± 0.020) × 10−8 |
| 1R | 540 | 1.031 ± 0.001 | 0.199 ± 0.003 | 1.858 ± 0.003 | (3.056 ± 0.060) × 10−10 |
| 2R | 540 | 1.091 ± 0.001 | 0.176 ± 0.004 | 2.362 ± 0.004 | (1.100 ± 0.020) × 10−11 |
| 3R | 540 | 1.132 ± 0.001 | 0.102 ± 0.006 | 2.143 ± 0.004 | (3.852 ± 0.002) × 10−11 |
| 4R | 540 | 1.097 ± 0.002 | 0.930 ± 0.004 | 2.269 ± 0.004 | (2.940 ± 0.020) × 10−11 |
| 3R | 490 | 1.043 ± 0.002 | 0.051 ± 0.001 | 2.816 ± 0.004 | (4.788 ± 0.003) × 10−15 |
| 3R | 510 | 0.964 ± 0.002 | 0.245 ± 0.002 | 1.743 ± 0.004 | (3.578 ± 0.001) × 10−10 |
| (a) | |||||
|---|---|---|---|---|---|
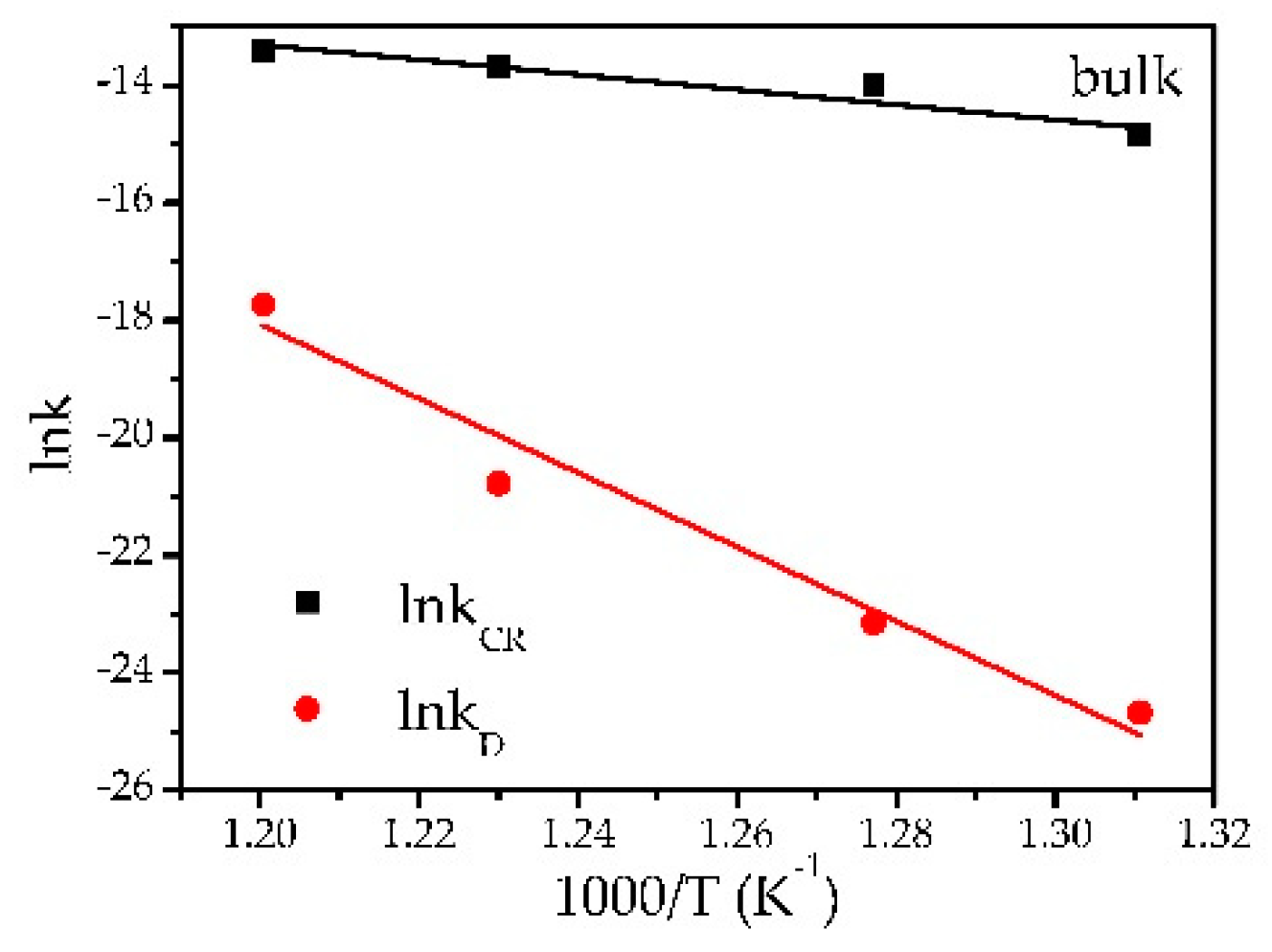
| No | t (°C) | lnkCR ± SD | lnkD ± SD | EA,CR ± SD (kJ/mol) | EA,D ± SD (kJ/mol) |
| 3B | 490 | −14.8501 ± 0.0067 | −24.6708 ± 0.0146 | 106.30 ± 20.45 R2 = 0.896 | 525.20 ± 64.85 R2 = 0.955 |
| 3B | 510 | −13.9881 ± 0.0089 | −23.1497 ± 0.0144 | ||
| 3B | 540 | −13.6688 ± 0.0062 | −20.7716 ± 0.0142 | ||
| 3B | 560 | −13.4102 ± 0.0077 | −17.7294 ± 0.0106 | ||
| 1B | 540 | −13.1797 ± 0.0078 | −19.9213 ± 0.0137 | - | - |
| 2B | 540 | −14.0926 ± 0.0071 | −19.6584 ± 0.0121 | - | - |
| 4B | 540 | −13.9528 ± 0.0081 | −21.2880 ± 0.0120 | - | - |
| (b) | |||||
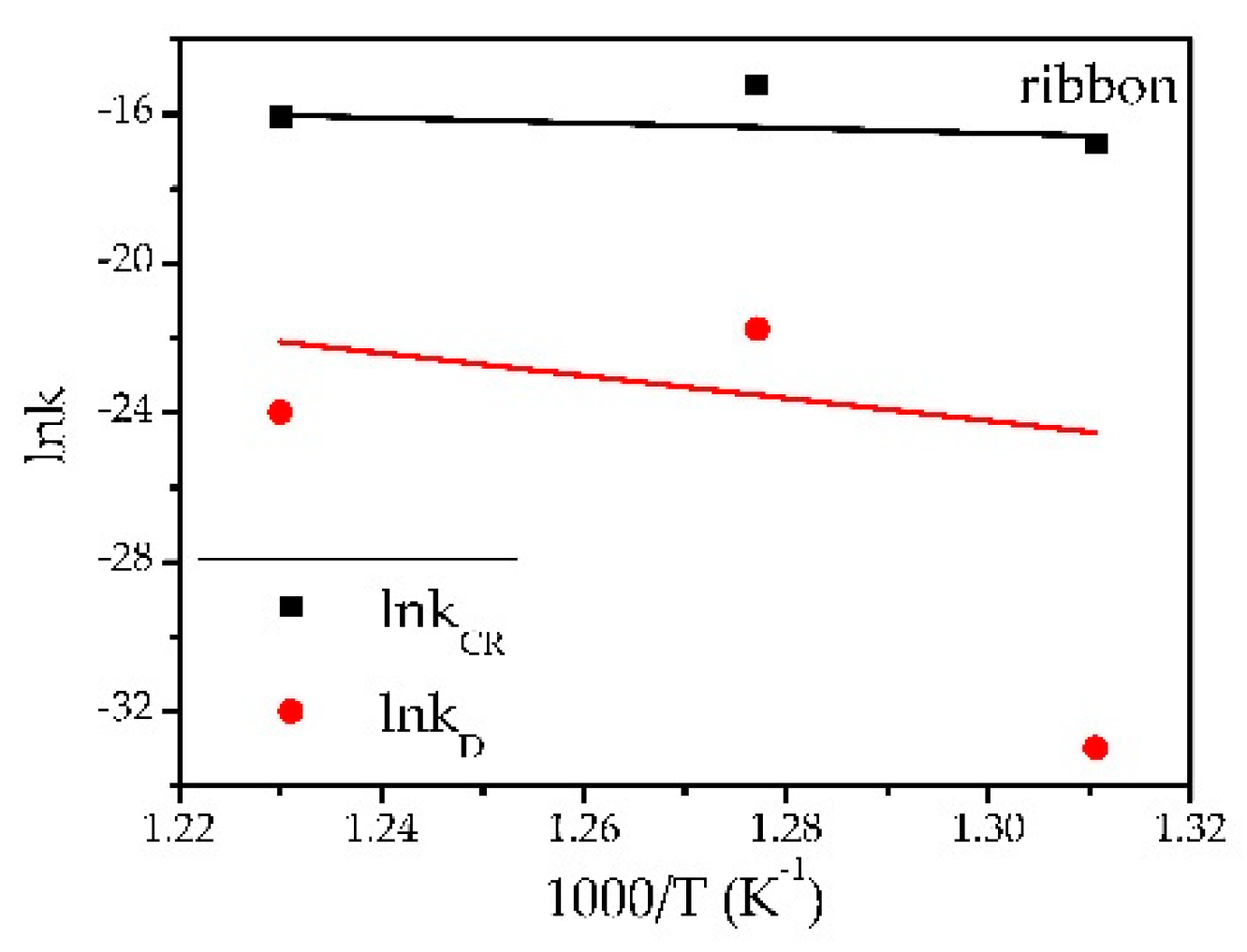
| No | t (°C) | lnkCR ± SD | lnkD ± SD | EA,CR (kJ/mol) | EA,D (kJ/mol) |
| 3R | 490 | −16.7894 ± 0.0087 | −32.9727 ± 0.0234 | 56.26 ± 9.88 R2 = 0.360 | 250.92 ± 123.37 R2 = 0.887 |
| 3R | 510 | −15.2211 ± 0.0155 | −21.7510 ± 0.0082 | ||
| 3R | 540 | −16.0989 ± 0.0057 | −23.9799 ± 0.0131 | ||
| 1R | 540 | −15.4276 ± 0.0157 | −21.9093 ± 0.0117 | - | - |
| 2R | 540 | −15.5500 ± 0.0167 | −25.2330 ± 0.0257 | - | - |
| 4R | 540 | −15.4594 ± 0.0127 | −24.2498 ± 0.0241 | - | - |
| No | P1 ± SD (°C) | ΔH1 ± SD (J g −1) | P2 ± SD (°C) | ΔH2 ± SD (J g −1) | P3 ± SD (°C) | ΔH3 ± SD (J g −1) |
|---|---|---|---|---|---|---|
| 1Bh | 574.7 ± 0.9 | 7.6 ± 0.5 | – | – | – | – |
| 1Bc | 580.7 ± 0.1 | −5.2 ± 0.3 | 586.8 ± 0.3 | 0.6 ± 0.7 | ≈1200 | 3.23 ± 0.6 |
| 2Bh | 574.6 ± 0.2 | 6.7 ± 0.7 | – | – | – | – |
| 2Bc | 574.2 ± 3.6 | −4.1 ± 0.3 | 594.2 ± 0.1 | −0.5 ± 0.2 | – | – |
| 4Bh | 574.5 ± 0.4 | 6.4 ± 0.6 | – | – | – | – |
| 4Bc | 580.5 ± 0.4 | −3.9 ± 0.1 | 588.2 ± 0.1 | −0.2 ± 0.1 | ≈1200 | 1.3 ± 0.4 |
| No | P1 (°C) | ΔH1 ± SD (J g −1) | P2 (°C) | ΔH2 ± SD (J g −1) | P3 (°C) | ΔH3 ± SD (J g −1) |
|---|---|---|---|---|---|---|
| 3Bh | 575.8 ± 0.4 | 17.8 ± 13.3 | 1475.5 ± 3.5 | 73.4 ± 74 | – | – |
| 3Bc | 572.1 ± 6.9 | −27.7 ± 21.7 | – | – | 1486.7 ± 1.6 | −333 ± 34 |
| No | Sp. | Before Oxidation | Sp. | After Oxidation | ||||
|---|---|---|---|---|---|---|---|---|
| Al (At.%) | Si (At.%) | Zr (At.%) | Al (At.%) | Si (At.%) | Zr (At.%) | |||
| 1B | 26 | 76.66 | 11.90 | 11.44 | 1 | 42.56 | 34.62 | 22.82 |
| 27 | 70.39 | 18.37 | 11.24 | 3 | 49.03 | 32.79 | 18.18 | |
| - | - | - | - | 4 | 41.29 | 47.11 | 11.61 | |
| - | - | - | - | 6 | 42.56 | 36.20 | 21.24 | |
| - | - | - | - | 7 | 49.89 | 32.63 | 17.48 | |
| 2B | 32 | 75.98 | 8.49 | 15.54 | 9 | 44.12 | 34.86 | 21.02 |
| 33 | 73.68 | 8.24 | 18.08 | 11 | 40.97 | 36.44 | 22.58 | |
| - | - | - | - | 12 | 39.52 | 37.36 | 23.12 | |
| 3B | 35 | 64.47 | 11.08 | 24.44 | 13 | 38.23 | 38.01 | 23.76 |
| 36 | 69.02 | 10.70 | 20.28 | 15 | 43.90 | 35.08 | 21.02 | |
| 37 | 71.01 | 10.21 | 18.78 | 16 | 39.83 | 37.56 | 22.61 | |
| - | - | - | - | 17 | 36.83 | 39.31 | 23.85 | |
| 4B | 39 | 64.84 | 10.49 | 24.67 | 19 | 39.96 | 34.84 | 25.20 |
| 40 | 74.43 | 8.46 | 17.11 | 20 | 35.14 | 40.28 | 24.58 | |
| 41 | 75.75 | 8.15 | 16.09 | 21 | 48.79 | 31.31 | 19.90 | |
| - | 80.00 | 6.94 | 13.06 | - | - | - | - | |
| Average | - | 72.38 | 10.28 | 17.34 | - | 42.18 | 36.56 | 21.26 |
| SD | - | 4.93 | 3.09 | 4.59 | - | 4.25 | 3.70 | 3.35 |
| NB | HV ± SD (kgf/mm2) | H ± SD (MPa) | NR | HV± SD (kgf/mm2) | H ± SD (MPa) |
|---|---|---|---|---|---|
| 1B | 54 ± 3 | 533 ± 32 | 1R | 99 ± 11 | 974 ± 105 |
| 2B | 55 ± 5 | 538 ± 53 | 2R | 100 ± 5 | 1077 ± 46 |
| 3B | 54 ± 4 | 534 ± 39 | 3R | 102 ± 12 | 996 ± 116 |
| 4B | 48 ± 2 | 473 ± 22 | 4R | 121 ± 2 | 1191 ± 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiradzhiyska, D.; Vassilev, G.; Mancheva, R.; Yaneva, S.; Milcheva, N. High-Temperature Surface Oxide Growth Kinetics of Al–Si–Zr Bulk Alloys and Ribbons. Metals 2021, 11, 1893. https://doi.org/10.3390/met11121893
Kiradzhiyska D, Vassilev G, Mancheva R, Yaneva S, Milcheva N. High-Temperature Surface Oxide Growth Kinetics of Al–Si–Zr Bulk Alloys and Ribbons. Metals. 2021; 11(12):1893. https://doi.org/10.3390/met11121893
Chicago/Turabian StyleKiradzhiyska, Denitsa, Gueorgui Vassilev, Rositsa Mancheva, Svetlana Yaneva, and Nikolina Milcheva. 2021. "High-Temperature Surface Oxide Growth Kinetics of Al–Si–Zr Bulk Alloys and Ribbons" Metals 11, no. 12: 1893. https://doi.org/10.3390/met11121893
APA StyleKiradzhiyska, D., Vassilev, G., Mancheva, R., Yaneva, S., & Milcheva, N. (2021). High-Temperature Surface Oxide Growth Kinetics of Al–Si–Zr Bulk Alloys and Ribbons. Metals, 11(12), 1893. https://doi.org/10.3390/met11121893








