Ab Initio Molecular Dynamics Study of the Structure and Properties of Nb-Doped Zr-Cu-Al Amorphous Alloys
Abstract
:1. Introduction
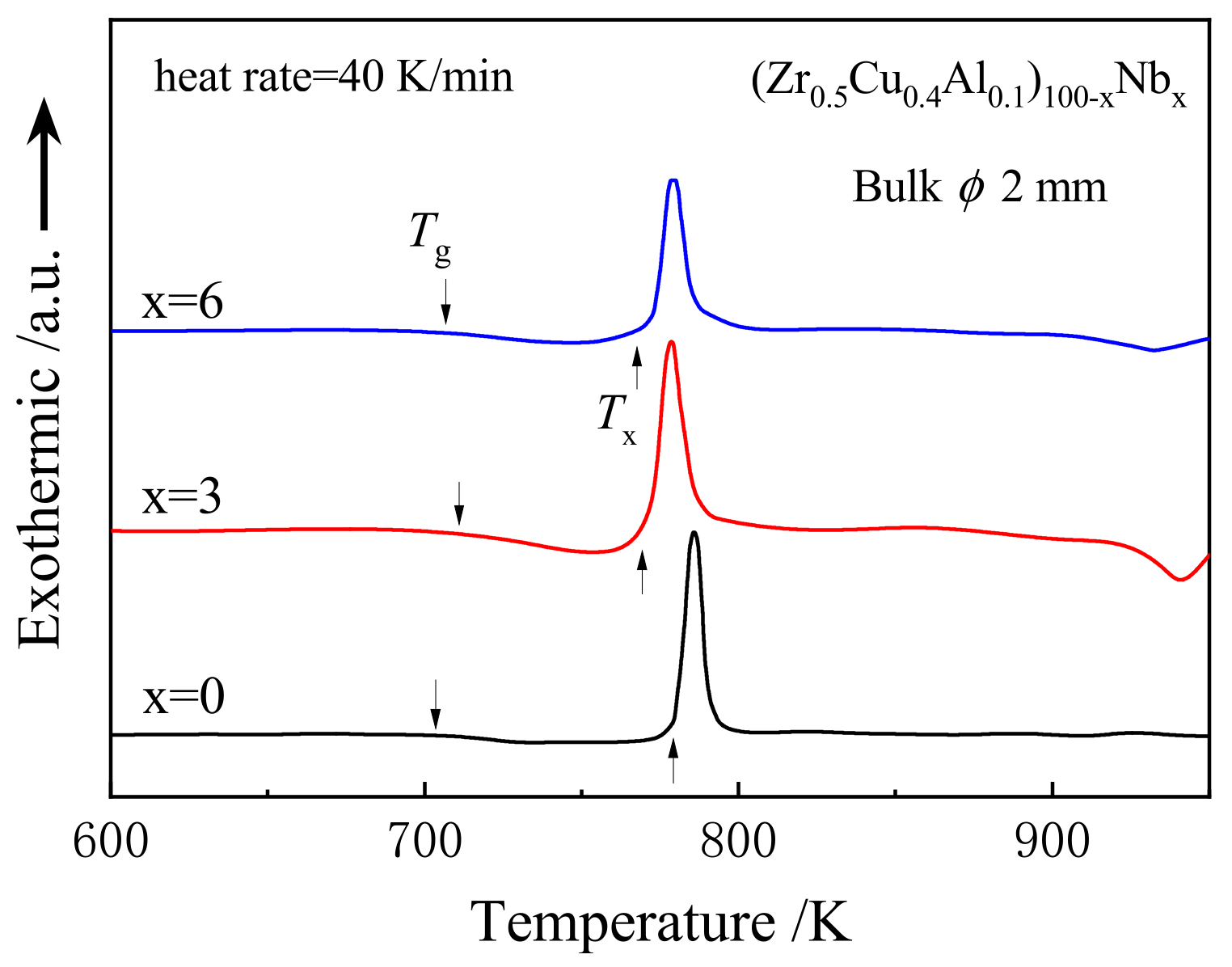
2. Experimental and Simulation Methods
3. Results and Analysis
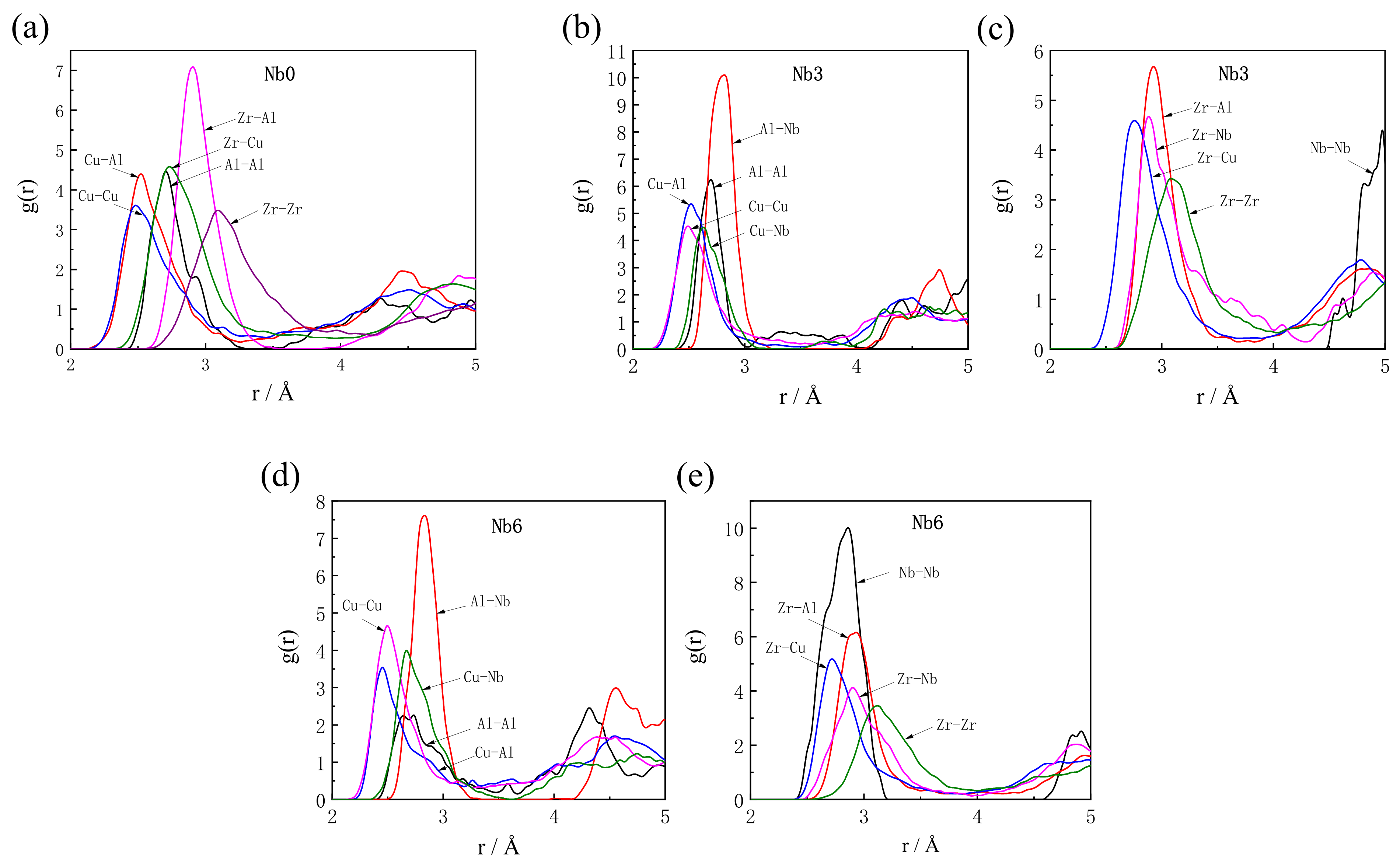
3.1. Analysis of Pair Correlation Functions
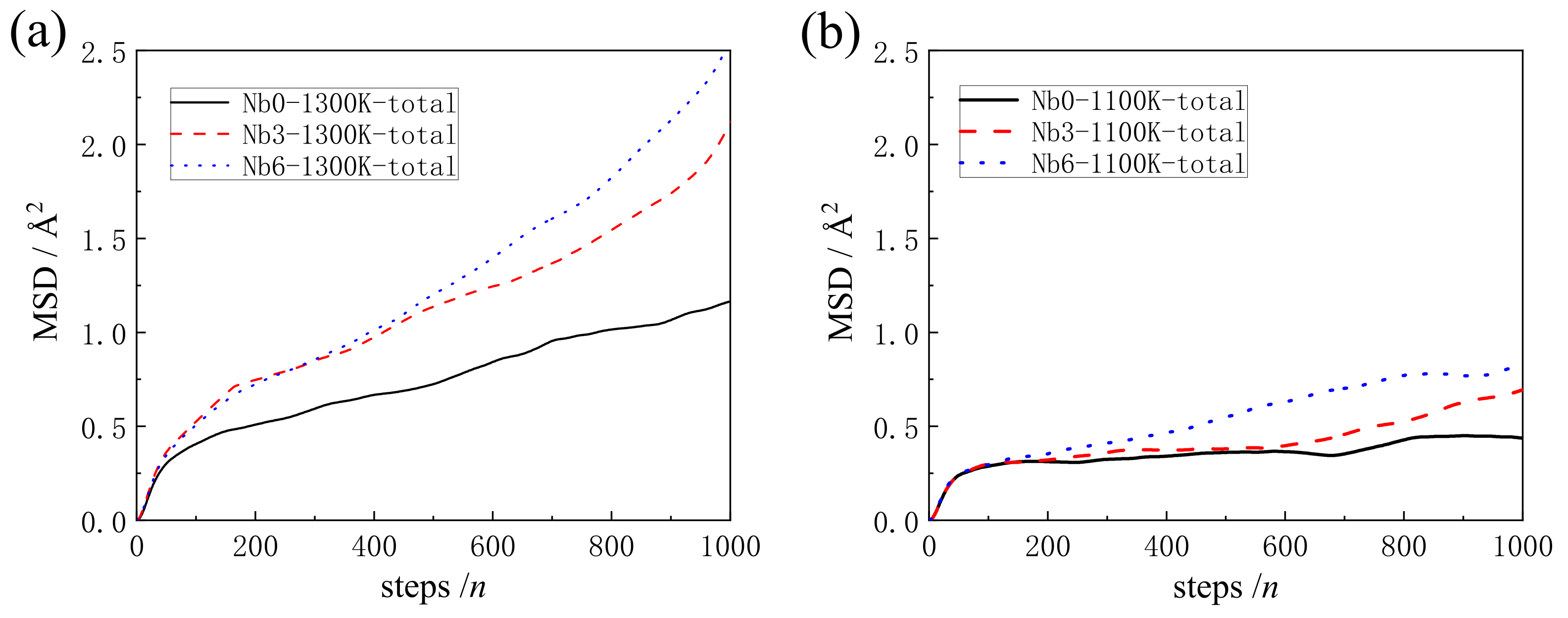
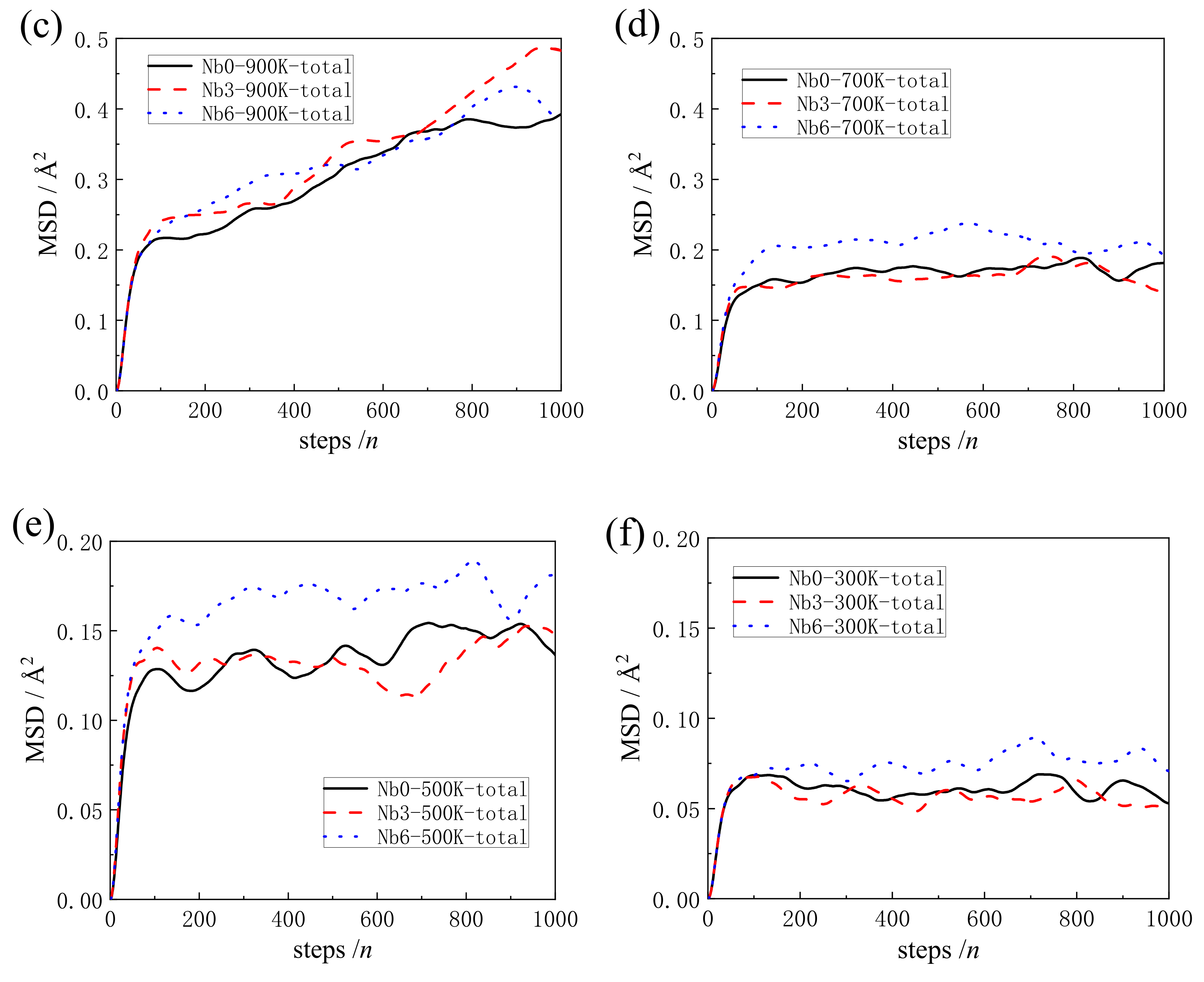
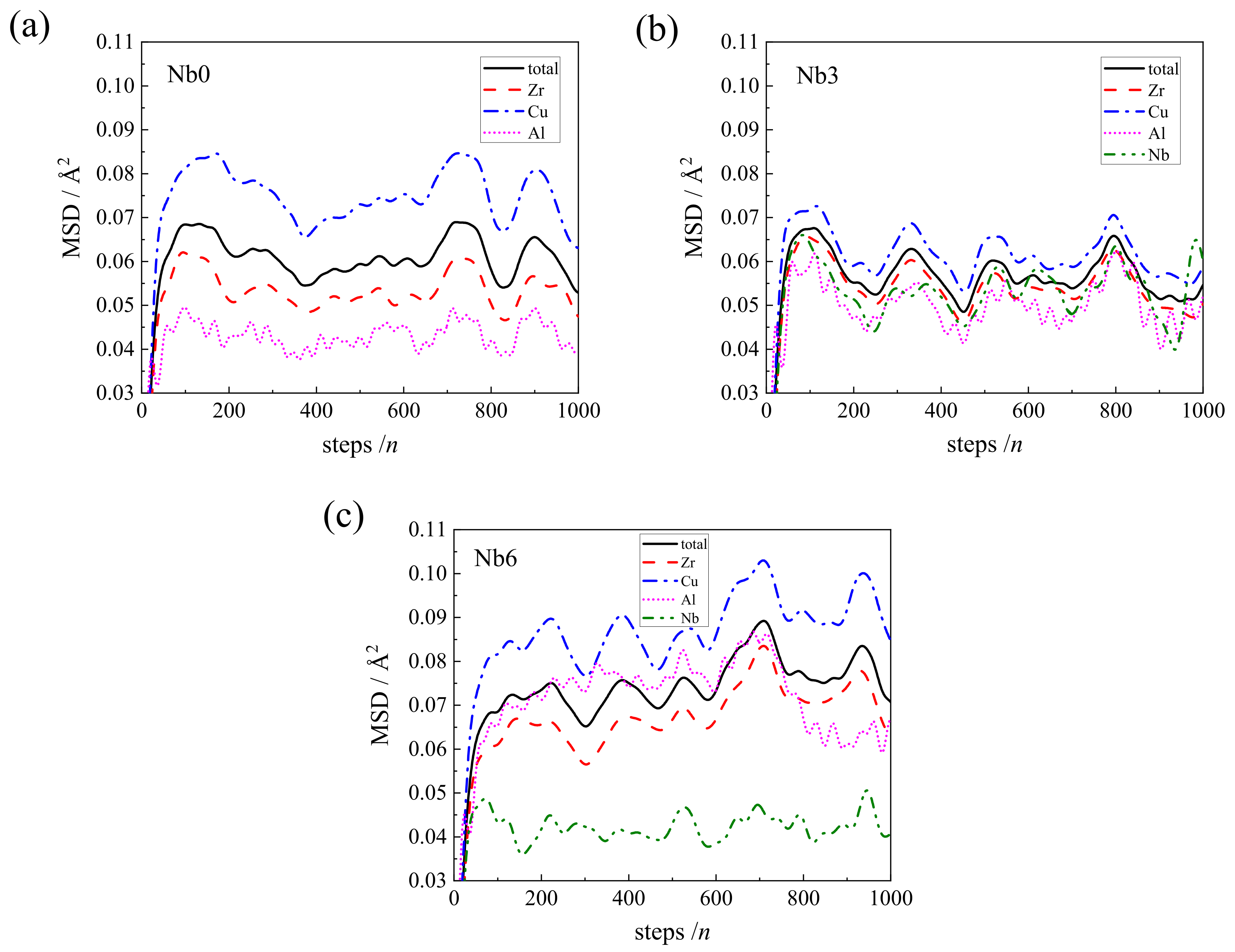
3.2. The Analysis of Mean Square Displacement
3.3. Analysis of Bonding Pairs
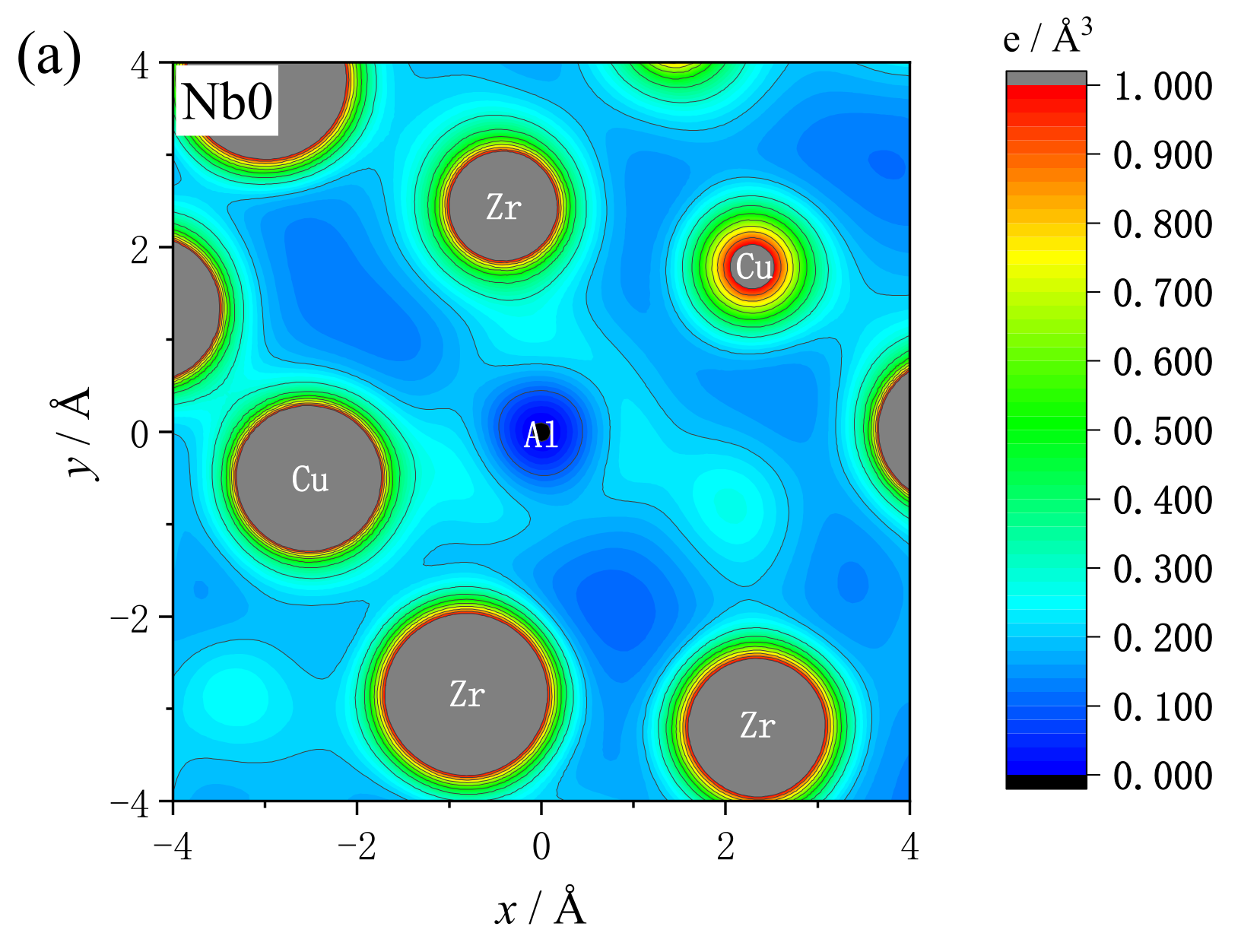
3.4. Analysis of Free Electron Density
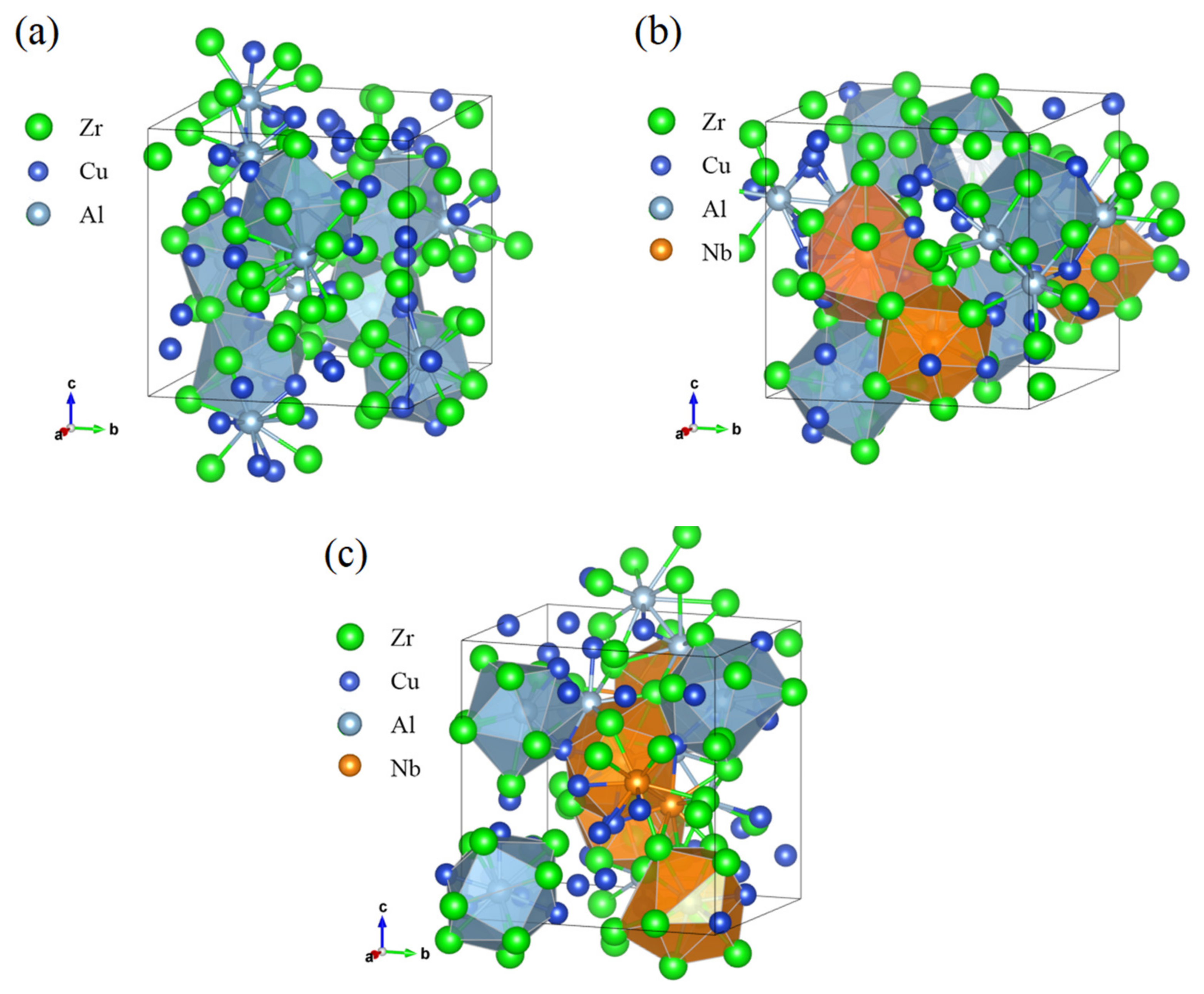
3.5. Analysis of Voronoi Polyhedra
4. Conclusions
- Al-centered icosahedral (and/or icosahedral-like) atomic structure are relatively more stable structure of atomic clusters in Zr50Cu40Al10;
- The structural forms of atomic clusters of the alloys are changed after adding 3 at.% Nb. Nb-centered icosahedral (-like) structures can form relatively stable clusters and coexist with Al-centered icosahedral (-like) structures, and an integrally more stable and close-packed structure can be formed via interconnection and matching of the two stable polyhedra, thereby enhancing the strength of the alloys. The overall heterogeneity of the structures is enhanced due to the two stable clusters, so the macroscopic plasticity of the alloys is improved;
- After 6 at.% Nb is added, the Al-centered icosahedral (-like) clusters in the alloys are replaced by more Nb-centered icosahedral (-like) clusters, and the stability and heterogeneity of the structures were partly reduced resulting in declined mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louzguine-Luzgin, D.V.; Louzguina-Luzgina, L.V.; Churyumov, A.Y. Mechanical Properties and Deformation Behavior of Bulk Metallic Glasses. Metals 2012, 3, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Trexler, M.M.; Thadhani, N.N. Mechanical properties of bulk metallic glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super Plastic Bulk Metallic Glasses at Room Temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Liu, Z.; Yu, C.; Dong, X.; He, L.; Gao, K.; Zhu, X.; Li, W.; Wang, C.; et al. Near-Net Forming Complex Shaped Zr-Based Bulk Metallic Glasses by High Pressure Die Casting. Materials 2018, 11, 2338. [Google Scholar] [CrossRef] [Green Version]
- Halim, Q.; Mohamed, N.A.N.; Rejab, M.R.M.; Naim, W.N.W.A.; Ma, Q. Metallic glass properties, processing method and development perspective: A review. Int. J. Adv. Manuf. Technol. 2021, 112, 1231–1258. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, W.; Zhang, T.; Yang, C.; Huang, X.; Liu, Z.; Wang, Z.; Li, W.; Li, L.; Liu, L. Large tensile plasticity in Zr-based metallic glass/stainless steel interpenetrating-phase composites prepared by high pressure die casting. Compos. Part B Eng. 2021, 224, 109226. [Google Scholar] [CrossRef]
- Abbasi, M.; Gholamipour, R.; Shahri, F. Glass forming ability and mechanical properties of Nb-containing Cu–Zr–Al based bulk metallic glasses. Trans. Nonferrous Met. Soc. China 2013, 23, 2037–2041. [Google Scholar] [CrossRef]
- Tam, M.; Pang, S.; Shek, C.H. Effects of niobium on thermal stability and corrosion behavior of glassy Cu–Zr–Al–Nb alloys. J. Phys. Chem. Solids 2006, 67, 762–766. [Google Scholar] [CrossRef]
- Tam, M.; Pang, S.; Shek, C.H. Corrosion behavior and glass-forming ability of Cu–Zr–Al–Nb alloys. J. Non-Cryst. Solids 2007, 353, 3596–3599. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Kou, S.Z.; Yuan, X.P.; Li, C.Y.; Yu, P.; Pu, Y.L.; Xu, J. Glass Forming Ability and Mechanical Properties of Cu-Zr-Al-Nb Amorphous Alloy. Rare Met. Mater. Eng. 2015, 44, 791–795. [Google Scholar] [CrossRef]
- Chen, S.; Todd, I. Enhanced plasticity in the Zr–Cu–Ni–Al–Nb alloy system by in-situ formation of two glassy phases. J. Alloys Compd. 2015, 646, 973–977. [Google Scholar] [CrossRef]
- Cao, G.; Liu, K.; Liu, G.; Zong, H.; Bala, H.; Zhang, B. Improving the glass-forming ability and the plasticity of Zr-Cu-Al bulk metallic glass by addition of Nb. J. Non-Cryst. Solids 2019, 513, 105–110. [Google Scholar] [CrossRef]
- Akbarpour, A.; Milkova, D.A.; Zanaeva, E.N.; Parkhomenko, M.S.; Cheverikin, V.V.; Lubenchenko, A.; Bazlov, A.I. Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses. Metals 2021, 11, 1514. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, B.; Zhang, Y.; He, A.; Zhang, T.; Li, F.; Dong, Y.; Wang, X. FeSiBPNbCu Bulk Nanocrystalline Alloys with High GFA and Excellent Soft-Magnetic Properties. Metals 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, S.; Gholamipour, R. Effect of Nb minor addition on the crystallization kinetics of Zr-Cu-Al-Ni metallic glass. J. Non-Cryst. Solids 2021, 560, 120731. [Google Scholar] [CrossRef]
- Cao, Q.; Peng, S.; Zhao, X.; Wang, X.; Zhang, D.; Jiang, J. Effect of Nb substitution for Cu on glass formation and corrosion behavior of Zr Cu Ag Al Be bulk metallic glass. J. Alloys Compd. 2016, 683, 22–31. [Google Scholar] [CrossRef]
- Jiang, S.-S.; Huang, Y.-J.; Wu, F.-F.; Xue, P.; Sun, J.-F. A CuZr-based bulk metallic glass composite with excellent mechanical properties by optimizing microstructure. J. Non-Cryst. Solids 2018, 483, 94–98. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Z.; Peng, Z. Serrated Behaviors and Plasticity of Nb-Alloyed Cu-Based Bulk Metallic Glasses. Met. Mater. Int. 2020, 26, 1483–1490. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-wave Basis. Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honeycutt, J.D.; Andersen, H.C. Molecular dynamics study of melting and freezing of small Lennard-Jones clusters. J. Phys. Chem. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Tournier, R.; Ojovan, M. Building and Breaking Bonds by Homogenous Nucleation in Glass-Forming Melts Leading to Transitions in Three Liquid States. Materials 2021, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Tlahuice-Flores, A.; Muñoz-Castro, A. Bonding and properties of superatoms. Analogs to atoms and molecules and related concepts from superatomic clusters. Int. J. Quantum Chem. 2019, 119, e25756. [Google Scholar] [CrossRef] [Green Version]
- Finney, J.L. Random packings and the structure of simple liquids. I. The geometry of random close packing. Proc. R. Soc. A 1970, 319, 479–493. [Google Scholar]
- Miracle, D.; Greer, A.; Kelton, K. Icosahedral and dense random cluster packing in metallic glass structures. J. Non-Cryst. Solids 2008, 354, 4049–4055. [Google Scholar] [CrossRef]
- Miracle, D.B. A structural model for metallic glasses. Nat. Mater. 2004, 3, 697–702. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liu, C.T.; Wang, W.H.; Inoue, A.; Sakurai, T.; Chen, M.W. Thermodynamic Origins of Shear Band Formation and the Universal Scaling Law of Metallic Glass Strength. Phys. Rev. Lett. 2009, 103, 065504. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Zhang, P.; Tang, Y. Ab Initio Molecular Dynamics Study of the Structure and Properties of Nb-Doped Zr-Cu-Al Amorphous Alloys. Metals 2021, 11, 1821. https://doi.org/10.3390/met11111821
Wei H, Zhang P, Tang Y. Ab Initio Molecular Dynamics Study of the Structure and Properties of Nb-Doped Zr-Cu-Al Amorphous Alloys. Metals. 2021; 11(11):1821. https://doi.org/10.3390/met11111821
Chicago/Turabian StyleWei, Hongqing, Ping Zhang, and Yi Tang. 2021. "Ab Initio Molecular Dynamics Study of the Structure and Properties of Nb-Doped Zr-Cu-Al Amorphous Alloys" Metals 11, no. 11: 1821. https://doi.org/10.3390/met11111821
APA StyleWei, H., Zhang, P., & Tang, Y. (2021). Ab Initio Molecular Dynamics Study of the Structure and Properties of Nb-Doped Zr-Cu-Al Amorphous Alloys. Metals, 11(11), 1821. https://doi.org/10.3390/met11111821






