New Analyzing Approaches for In Situ Interdiffusion Experiments to Determine Concentration-Dependent Diffusion Coefficients in Liquid Al–Au
Abstract
:1. Introduction
2. Materials and Methods
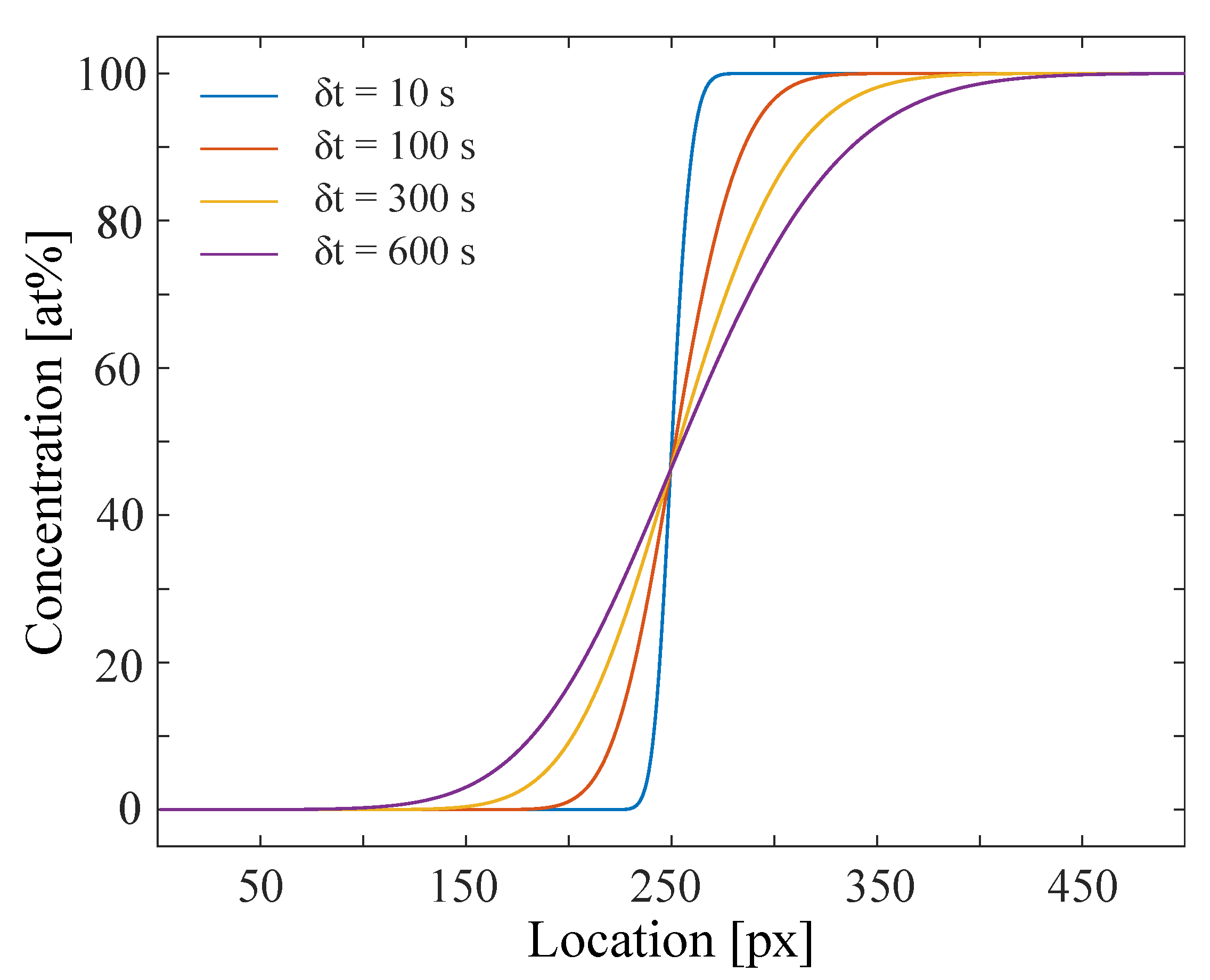
2.1. Simulation of Experiment-like Data Sets
2.2. Analyzing Models
2.2.1. Common Erf-Function Model
2.2.2. Left/Right Erf-Function Model
2.2.3. Local Erf-Function Approach
2.2.4. Numerical Fick’s Law Approach
2.3. In Situ Shear-Cell Experiment
3. Results
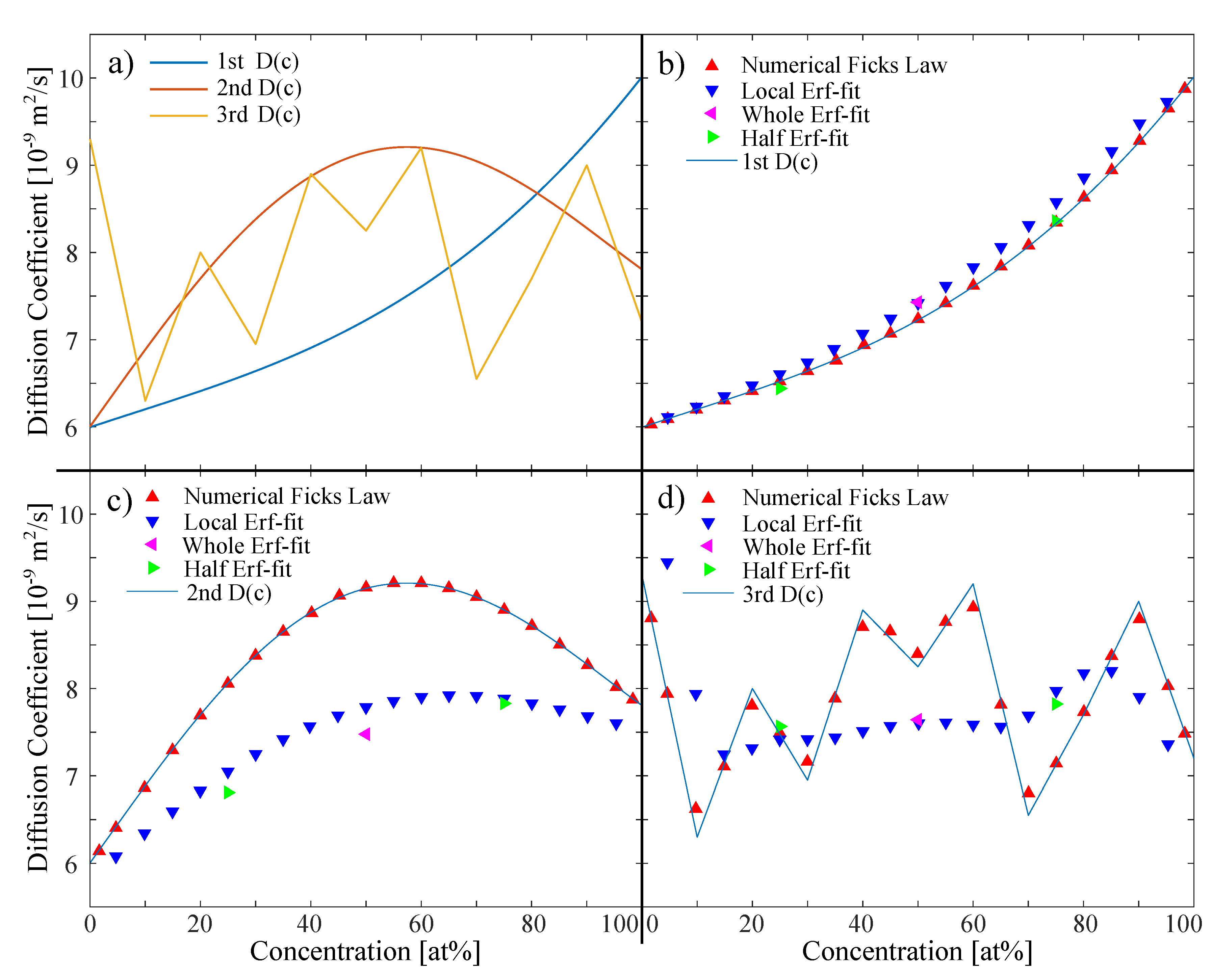
3.1. Noiseless Simulated In Situ Data
3.2. Robustness against Noise
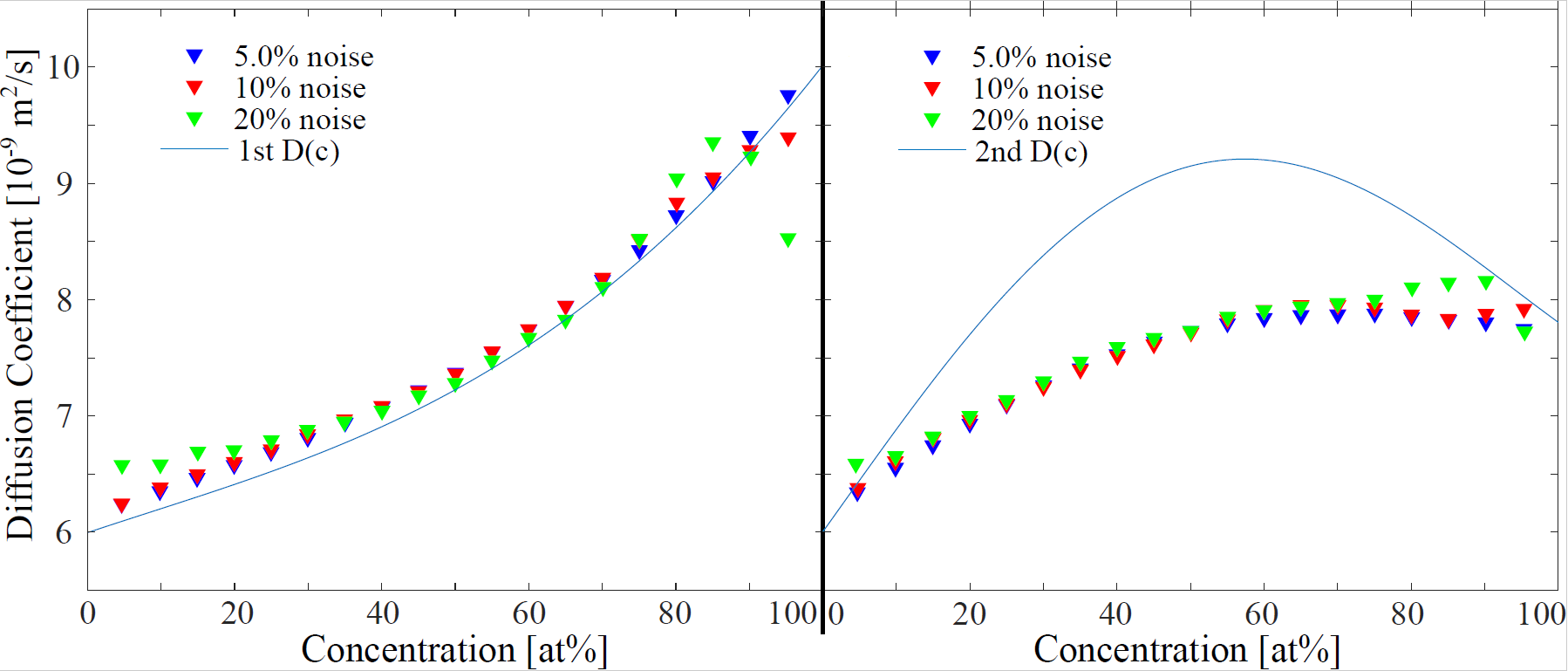
3.2.1. Noise on Local Erf-Fit
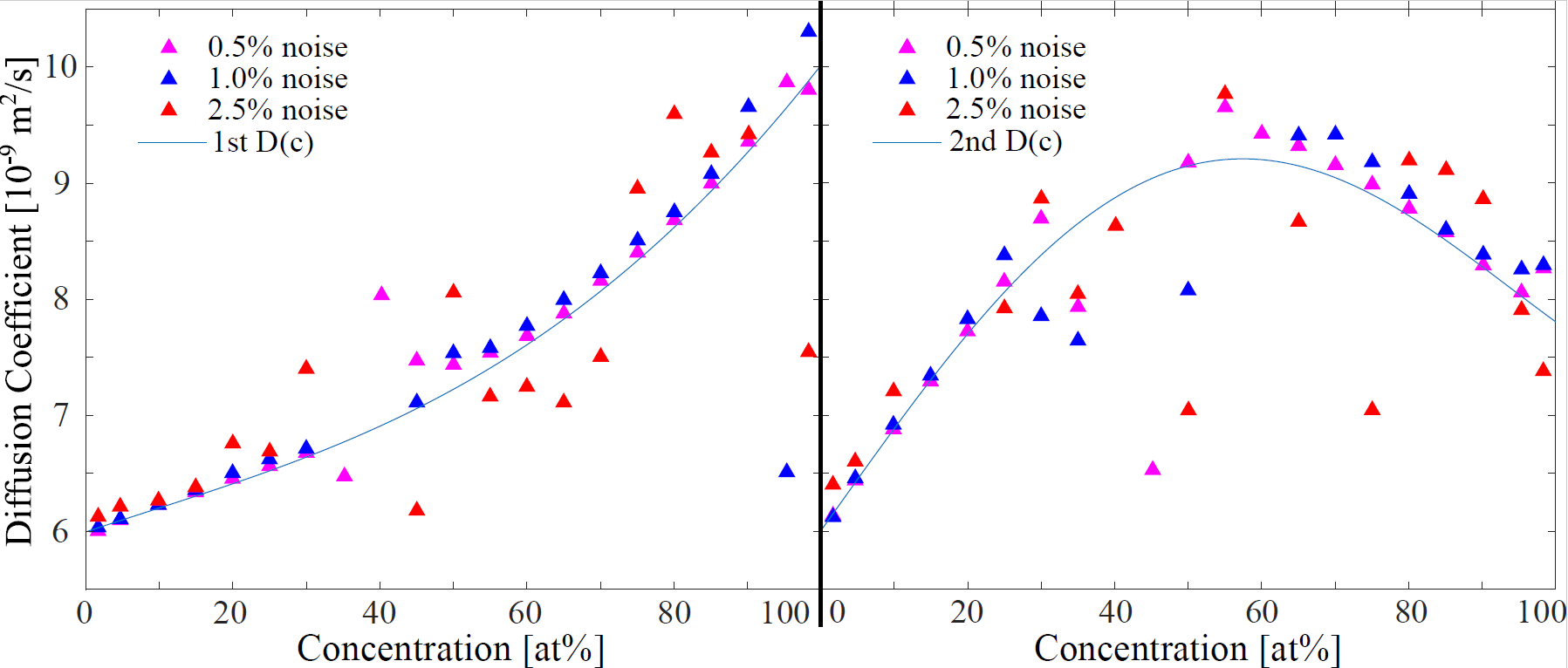
3.2.2. Noise on Numerical Fick’s Law Approach
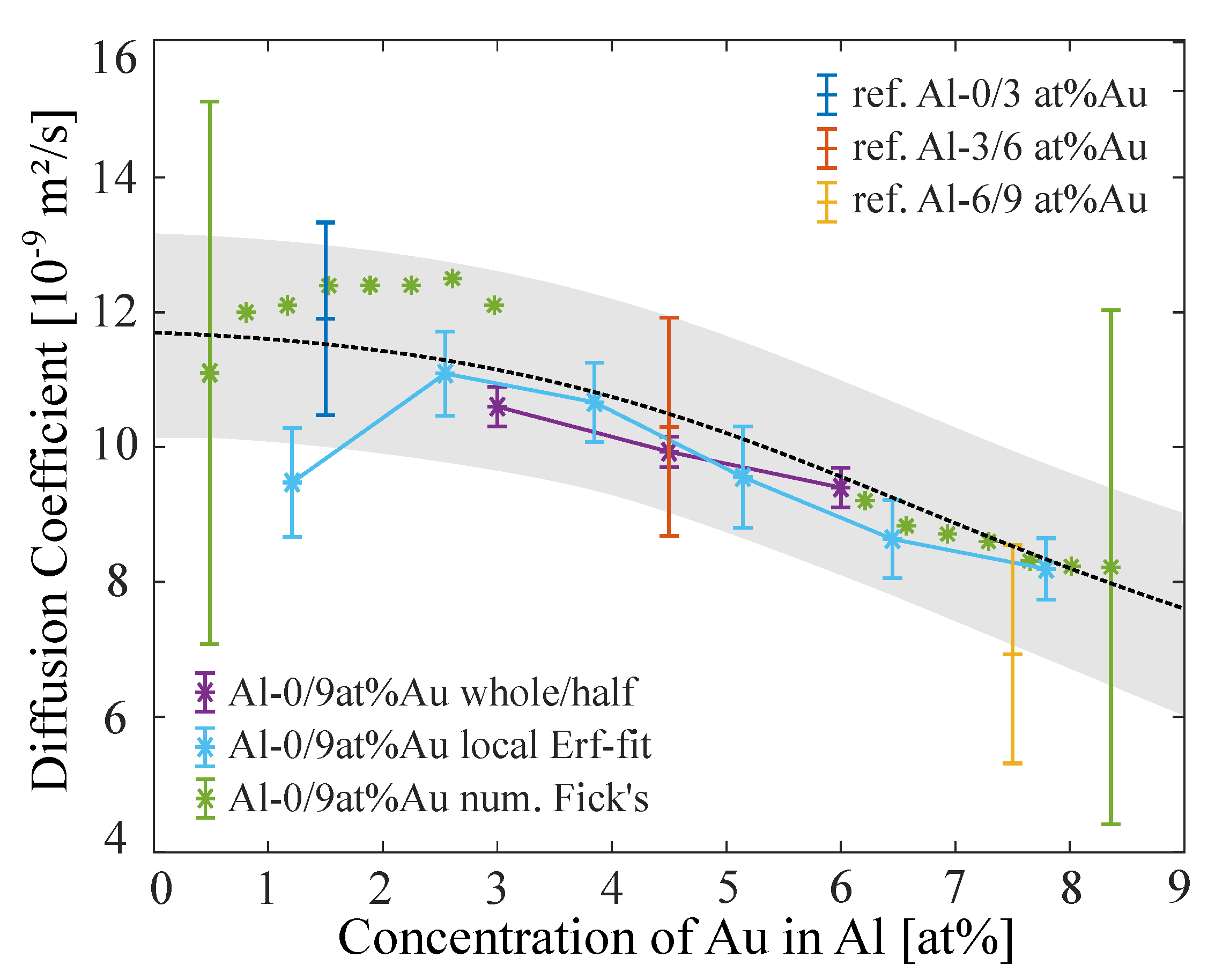
3.3. Performance on Experimental Data Sets of Al–Au System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Symeonidis, K.; Apelian, D.; Makhlouf, M. Controlled diffusion solidification: Application to metal casting. Metall. Sci. Technol. 2008, 26, 39–44. [Google Scholar]
- Miller, J.; Yuan, L.; Lee, P.; Pollock, T. Simulation of diffusion-limited lateral growth of dendrites during solidification via liquid metal cooling. Acta Mater. 2014, 69, 47–59. [Google Scholar] [CrossRef]
- Becker, M.; Klein, S.; Kargl, F. Free dendritic tip growth velocities measured in Al-Ge. Phys. Rev. Mater. 2018, 2, 073405. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Horbach, J.; Das, S.K.; Griesche, A.; Macht, M.P.; Frohberg, G.; Meyer, A. Self-diffusion and interdiffusion in Al 80 Ni 20 melts: Simulation and experiment. Phys. Rev. B 2007, 75, 174304. [Google Scholar] [CrossRef] [Green Version]
- Horbach, J.; Rozas, R.; Unruh, T.; Meyer, A. Improvement of computer simulation models for metallic melts via quasielastic neutron scattering: A case study of liquid titanium. Phys. Rev. B 2009, 80, 212203. [Google Scholar] [CrossRef]
- Han, X.; Schober, H. Transport properties and Stokes-Einstein relation in a computer-simulated glass-forming Cu33.3Zr66.7 melt. Phys. Rev. B 2011, 83, 224201. [Google Scholar] [CrossRef] [Green Version]
- Zöllmer, V.; Rätzke, K.; Faupel, F.; Meyer, A. Diffusion in a metallic melt at the critical temperature of mode coupling theory. Phys. Rev. Lett. 2003, 90, 195502. [Google Scholar] [CrossRef] [Green Version]
- Voigtmann, T.; Meyer, A.; Holland-Moritz, D.; Stüber, S.; Hansen, T.; Unruh, T. Atomic diffusion mechanisms in a binary metallic melt. EPL Europhys. Lett. 2008, 82, 66001. [Google Scholar] [CrossRef]
- Gotze, W. Complex dynamics of glass-forming liquids. Phys. J. 2009, 8, 52. [Google Scholar]
- Kuhn, P.; Horbach, J.; Kargl, F.; Meyer, A.; Voigtmann, T. Diffusion and interdiffusion in binary metallic melts. Phys. Rev. B 2014, 90, 024309. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Holland-Moritz, D.; Yang, F.; Voigtmann, T.; Kordel, T.; Hansen, T.; Meyer, A. Partial structure factors reveal atomic dynamics in metallic alloy melts. Phys. Rev. Mater. 2017, 1, 025603. [Google Scholar] [CrossRef]
- Meyer, A. Self-diffusion in liquid copper as seen by quasielastic neutron scattering. Phys. Rev. B 2010, 81, 012102. [Google Scholar] [CrossRef]
- Kargl, F.; Weis, H.; Unruh, T.; Meyer, A. Self diffusion in liquid aluminium. J. Phys. Conf. Ser. 2012, 340, 012077. [Google Scholar] [CrossRef]
- Sondermann, E.; Jakse, N.; Binder, K.; Mielke, A.; Heuskin, D.; Kargl, F.; Meyer, A. Concentration dependence of interdiffusion in aluminum-rich Al-Cu melts. Phys. Rev. B 2019, 99, 024204. [Google Scholar] [CrossRef]
- Belova, I.V.; Heuskin, D.; Sondermann, E.; Ignatzi, B.; Kargl, F.; Murch, G.E.; Meyer, A. Combined interdiffusion and self-diffusion analysis in Al-Cu liquid diffusion couple. Scr. Mater. 2018, 143, 40–43. [Google Scholar] [CrossRef]
- Griesche, A.; Macht, M.P.; Frohberg, G. First results from diffusion measurements in liquid multicomponent Al-based alloys. J. Non-Cryst. Solids 2007, 353, 3305–3309. [Google Scholar] [CrossRef]
- Suzuki, S.; Kraatz, K.H.; Frohberg, G. Reduction of convection in diffusion measurement using the shear cell by stabilization of density layering on the ground. J. Jpn. Soc. Microgravity Appl. 2011, 28, 100. [Google Scholar]
- Kargl, F.; Sondermann, E.; Weis, H.; Meyer, A. Impact of convective flow on long-capillary chemical diffusion studies of liquid binary alloys. High Temp. Press. 2013, 42, 3–21. [Google Scholar]
- Griesche, A.; Zhang, B.; Solórzano, E.; Garcia-Moreno, F. Note: X-ray radiography for measuring chemical diffusion in metallic melts. Rev. Sci. Instrum. 2010, 81, 056104. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Kargl, F. Diffusion of Mass in Liquid Metals and Alloys-Recent Experimental Developments and New Perspectives. Int. J. Microgravity Sci. Appl. 2013, 30, 30. [Google Scholar]
- Weis, H.; Kargl, F.; Kolbe, M.; Koza, M.; Unruh, T.; Meyer, A. Self-and interdiffusion in dilute liquid germanium-based alloys. J. Phys. Condens. Matter 2019, 31, 455101. [Google Scholar] [CrossRef]
- Neumann, C.; Sondermann, E.; Kargl, F.; Meyer, A. Compact high-temperature shear-cell furnace for in-situ diffusion measurements. J. Phys. Conf. Ser. 2011, 327, 012052. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Zhu, C.; Zhang, B. A sliding cell technique for diffusion measurements in liquid metals. AIP Adv. 2014, 4, 037102. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, J.; Geng, Y.; Zhu, C.; Zhang, B. A multi-slice sliding cell technique for diffusion measurements in liquid metals. Rev. Sci. Instrum. 2017, 88, 093905. [Google Scholar] [CrossRef] [PubMed]
- Griesche, A.; Macht, M.; Frohberg, G. Diffusion in Metallic Melts. Defect Diffus. Forum 2007, 266, 101–108. [Google Scholar] [CrossRef]
- Griesche, A.; Zhang, B.; Horbach, J.; Meyer, A. Interdiffusion and Thermodynamic Forces in Binary Liquid Alloys. Mater. Sci. Forum 2010, 649, 481–486. [Google Scholar] [CrossRef]
- Masaki, T.; Fukazawa, T.; Matsumoto, S.; Itami, T.; Yoda, S. Measurements of diffusion coefficients of metallic melt under microgravity—Current status of the development of shear cell technique towards JEM on ISS. Meas. Sci. Technol. 2005, 16, 327. [Google Scholar] [CrossRef]
- Griesche, A.; Kraatz, K.H.; Frohberg, G.; Mathiak, G.; Willnecker, R. Liquid diffusion measurements with the shear cell technique-study of shear convection. Microgravity Res. Apl. Phys. Sci. Biotechnol. 2001, 454, 497. [Google Scholar]
- Sondermann, E.; Neumann, C.; Kargl, F.; Meyer, A. Compact high-temperature shear-cell furnace for in-situ interdiffusion measurements. High Temp. Press. 2013, 42, 012052. [Google Scholar]
- Ahmed, T.; Belova, I.; Evteev, A.; Levchenko, E.; Murch, G. Comparison of the Sauer-Freise and Hall methods for obtaining interdiffusion coefficients in binary alloys. J. Phase Equilibria Diffus. 2015, 36, 366–374. [Google Scholar] [CrossRef]
- Olaye, O.; Ojo, O. A New Analytical Method for Computing Concentration-Dependent Interdiffusion Coefficient in Binary Systems with Pre-existing Solute Concentration Gradient. J. Phase Equilibria Diffus. 2021, 42, 303–314. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, J.C. Extracting interdiffusion coefficients from binary diffusion couples using traditional methods and a forward-simulation method. Intermetallics 2013, 34, 132–141. [Google Scholar] [CrossRef]
- Wierzba, B.; Skibiński, W. The generalization of the Boltzmann–Matano method. Phys. A Stat. Mech. Appl. 2013, 392, 4316–4324. [Google Scholar] [CrossRef]
- Olaye, O.; Ojo, O. Time variation of concentration-dependent interdiffusion coefficient obtained by numerical simulation analysis. Materialia 2021, 16, 101056. [Google Scholar] [CrossRef]
- Sondermann, E.; Kargl, F.; Meyer, A. Influence of cross correlations on interdiffusion in Al-rich Al-Ni melts. Phys. Rev. B 2016, 93, 184201. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Cahoon, J. Interdiffusion of copper and iron in liquid aluminum. J. Phase Equilibria Diffus. 2011, 32, 226–234. [Google Scholar] [CrossRef]
- Engelhardt, M.; Meyer, A.; Yang, F.; Simeoni, G.; Kargl, F. Self and chemical diffusion in liquid Al-Ag. Defect Diffus. Forum 2016, 367, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Griesche, A.; Meyer, A. Diffusion in Al-Cu melts studied by time-resolved X-ray radiography. Phys. Rev. Lett. 2010, 104, 035902. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Zhang, L. A novel approach to eliminate the effect of external stress on interdiffusivity measurement. Materials 2017, 10, 961. [Google Scholar] [CrossRef] [Green Version]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiller, T.; Sondermann, E.; Meyer, A. New Analyzing Approaches for In Situ Interdiffusion Experiments to Determine Concentration-Dependent Diffusion Coefficients in Liquid Al–Au. Metals 2021, 11, 1772. https://doi.org/10.3390/met11111772
Schiller T, Sondermann E, Meyer A. New Analyzing Approaches for In Situ Interdiffusion Experiments to Determine Concentration-Dependent Diffusion Coefficients in Liquid Al–Au. Metals. 2021; 11(11):1772. https://doi.org/10.3390/met11111772
Chicago/Turabian StyleSchiller, Toni, Elke Sondermann, and Andreas Meyer. 2021. "New Analyzing Approaches for In Situ Interdiffusion Experiments to Determine Concentration-Dependent Diffusion Coefficients in Liquid Al–Au" Metals 11, no. 11: 1772. https://doi.org/10.3390/met11111772
APA StyleSchiller, T., Sondermann, E., & Meyer, A. (2021). New Analyzing Approaches for In Situ Interdiffusion Experiments to Determine Concentration-Dependent Diffusion Coefficients in Liquid Al–Au. Metals, 11(11), 1772. https://doi.org/10.3390/met11111772





