The Recovery of Metallic Tin from an Industrial Tin-Bearing By-Product Containing Na2SO4 by Reduction Smelting Process
Abstract
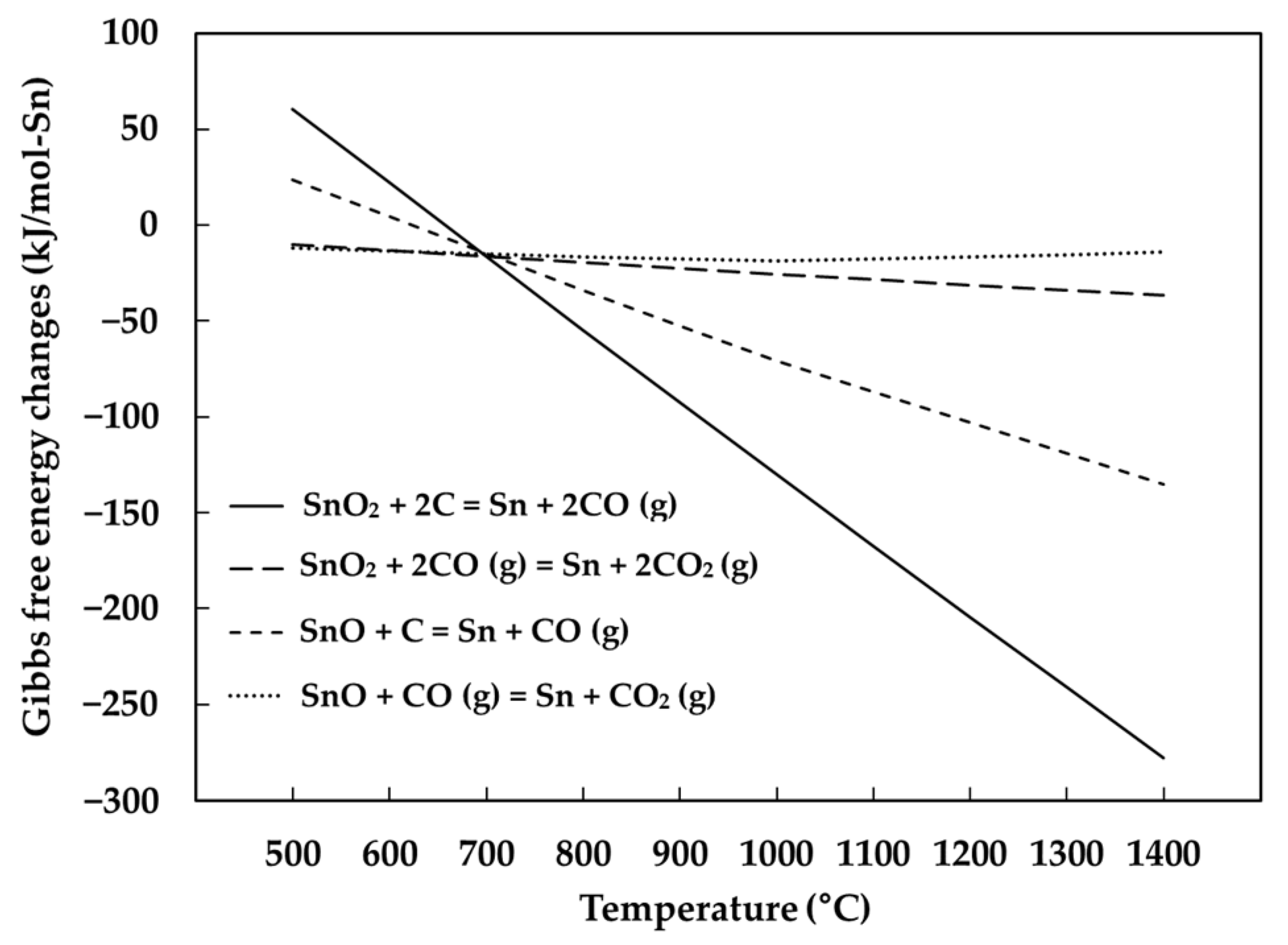
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Apparatus and Procedures
3. Results
3.1. The Effect of Basicity (Na2O/SiO2)
3.2. The Effect of Temperature
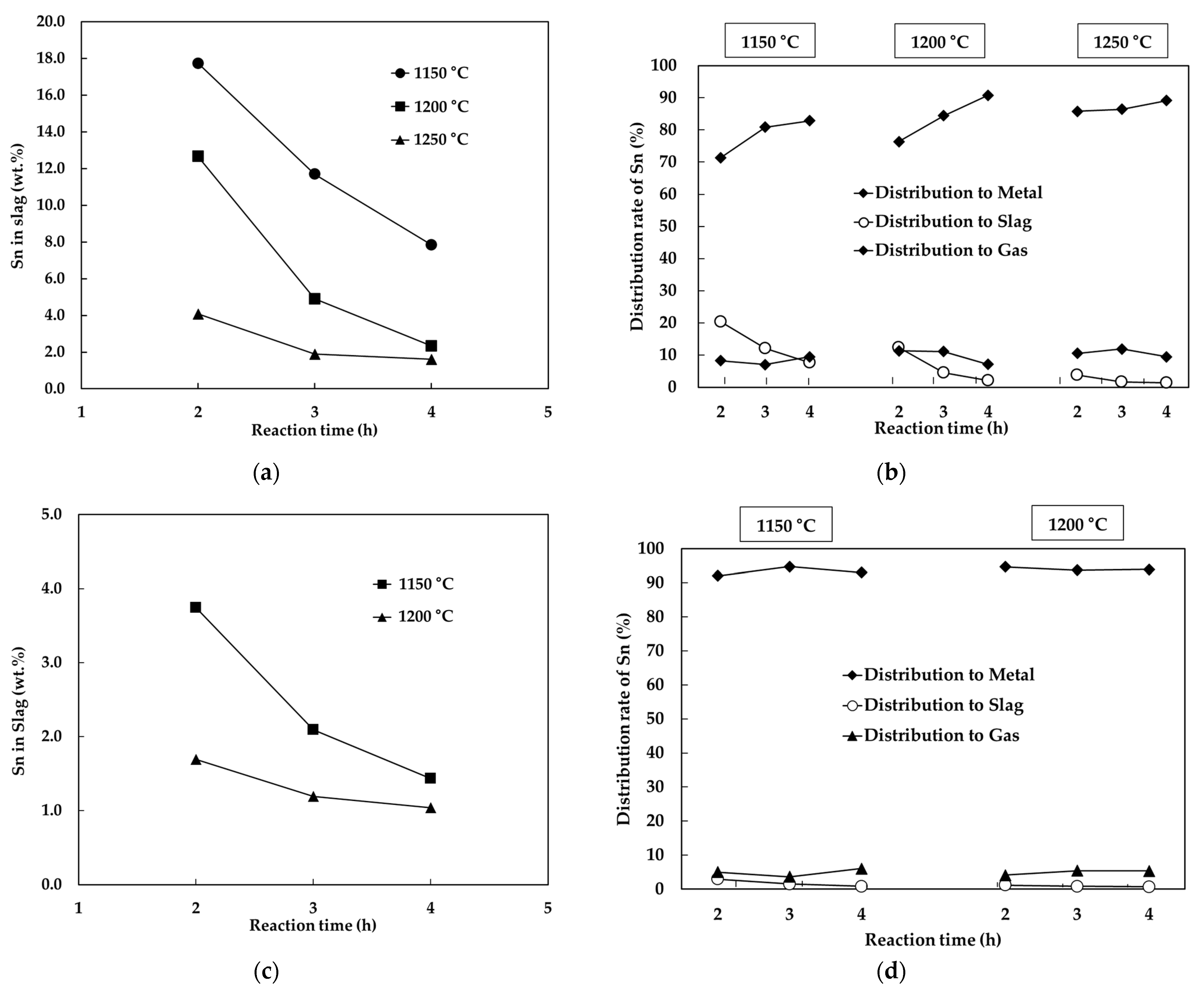
3.3. The Effect of Reaction Time
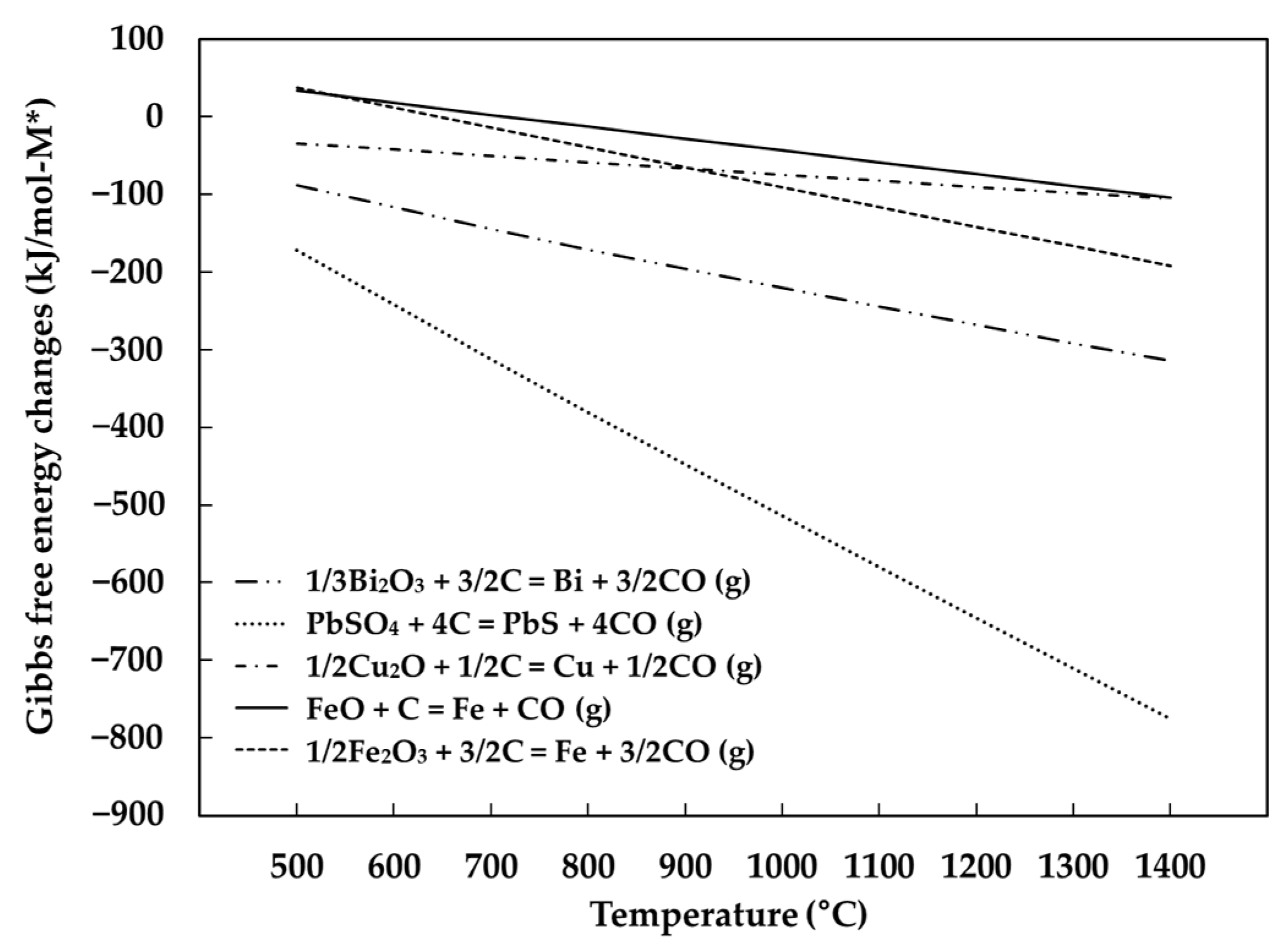
3.4. The Behavior of Impurities in Metals
4. Conclusions
- Na2SO4 could be completely removed in slag in the form of sodium silicate in the presence of proper SiO2.
- The reduction behavior of SnO2 strongly depended on Na2O/SiO2 ratio. As the amount of SiO2 increased, tin content in slag increased due to the formation of SnO·SiO2, and tin was volatilized in the form of SnS (g) and SnO (g) in the high temperature. Therefore, the recovery of tin decreased with increase of SiO2.
- The effect of temperature on the recovery of tin showed that as temperature increased, tin content in slag decreased, but the distribution rate of tin into the gas phase increased. Therefore, the recovery of tin increased with temperature but rather decreased from a certain temperature which was 1300 °C at 0.40 of Na2O/SiO2 and 1250 °C at 0.44 of Na2O/SiO2.
- The effect of reaction time on the recovery of tin showed that as time increased, the residual tin content in slag decreased, more tin was distributed to the metal phase, and the distribution of tin into the gas phase was little changed with reaction time at the same temperature and basicity.
- The maximum recovery rate of tin was 94.8% at the experimental conditions of 1150 °C, 3 h and 1200 °C, 2 h with 0.55 of Na2O/SiO2 ratio.
- The major impurities in produced metals were Bi, Pb, Cu, Fe. The distribution of the impurities at the maximum tin recovery condition showed that most of Bi, Pb, Cu were distributed to the metal phase, but 51% of Fe was distributed to the metal phase due to the reaction of FeO and SiO2.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, Z.; Zhang, Y.; Liu, B.; Lu, M.; Li, G.; Jiang, T. Extraction and separation of tin from tin-bearing secondary resources: A review. JOM 2017, 69, 2364–2372. [Google Scholar] [CrossRef]
- Tin Recycling-International Tin Association. Available online: https://www.internationaltin.org/recycling (accessed on 14 June 2021).
- Wan, X.; Fellman, J.; Jokilaakso, A.; Klemettinen, L.; Marjakoski, M. Behavior of Waste Circuit Board (WPCB) Materials in the Copper Matte Smelting Process. Metals 2018, 8, 887. [Google Scholar] [CrossRef]
- Bieler, T.R.; Lee, T.K. Lead-Free Solder. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–12. [Google Scholar]
- Sukhomlinov, D.; Klemettinen, L.; O’brien, H.; Taskinen, P.; Jokilaakso, A. Behavior of Ga, In, Sn, and Te in copper Matte Smelting. Metall. Mater. Tranctions B 2019, 50B, 2073–2732. [Google Scholar] [CrossRef]
- Klemettinen, L.; Avarmaa, K.; O’brien, H.; Taskinen, P.; Jokilaakso, A. Behavior of Tin and Antimony in Secondary Smelting Process. Minerals 2019, 9, 39. [Google Scholar] [CrossRef]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Recoveries of rare elements Ga, Ge, In and Sn from waste electric and electronic equipment through secondary copper smelting. Waste Manag. 2018, 71, 400–410. [Google Scholar] [CrossRef]
- Avarmaa, K.; Taskinel, P. The Influence of Aluminum on Indium and Tin Behavior During Secondary Copper Smelting, Extraction 2018. In Proceedings of the First Global Conference on Extractive Metallurgy, Ottawa, ON, Canada, 26–29 August 2018; pp. 1061–1071. [Google Scholar]
- Avarmaa, K.; Klemettinen, L.; O’brien, H.; Taskinen, P. The behavior of tin in black copper smelting conditions with different iron-silicate based slags. In Proceedings of the EMC 2019, Dusseldorf, Germany, 23–26 June 2019; pp. 497–510. [Google Scholar]
- Anindya, A.; Swinbourne, D.; Reuter, M.A.; Matusewicz, R. Tin distribution during smelting of WEEE with copper scrap. In Proceedings of the EMC 2009, Innsbruck, Austria, 28 June–1 July 2009; pp. 555–568. [Google Scholar]
- Forsén, O.; Aromaa, J.; Lundström, M. Primary Copper Smelter and Refinery as a Recycling Plant—A System Integrated Approach to Estimate Secondary Raw Material Tolerance. Recycling 2017, 2, 19. [Google Scholar] [CrossRef]
- Jing, J.; Guo, Y.; Chen, F.; Zheng, F.; Yang, L. Volatilization Behavior of Tin During Carbothermic Reduction Of Tin-Bearing Middling To Recover Tin. J. Min. Metall. Sect. B-Metall. 2018, 54 B, 419–426. [Google Scholar] [CrossRef]
- Pommier, L.W. Effect of Coke in the Volatilization of Tin. JOM 1979, 31, 161–162. [Google Scholar] [CrossRef]
- López, F.A.; García-Díaz, I.; Largo, O.R.; Polonio, F.G.; Llorens, T. Recovery and Purification of Tin from Tailings from the Penouta Sn–Ta–Nb Deposit. Minerals 2018, 8, 20. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Shelkova, N.E.; Mogutnov, B.M. Thermodynamics of Na2O-SiO2 Melts. Inorg. Mater. 2000, 36, 529–543. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; You, Z.; Lv, X. Kinetics study on non-isothermal carbothermic reduction of nickel laterite ore in presence of Na2SO4. Powder Technol. 2020, 362, 486–492. [Google Scholar] [CrossRef]
- Lv, W.; Bai, C.; Lv, X.; Hu, K.; Lv, X.; Xiang, J.; Song, B. Carbothermic reduction of ilmenite concentrate in semi-molten state by adding sodium sulfate. Powder Technol. 2018, 340, 354–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Su, Z.; Chen, J.; Li, G.; Jiang, T. Effect of Na2CO3 on the preparation of metallic tin from cassiterite roasted under strong reductive atmosphere. J. Min. Metall. Sect. B-Metall. 2016, 52 B, 9–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Liu, B.; Zhou, Y.; Jiang, T.; Li, G. Reduction behavior of SnO2 in the tin-bearing iron concentrates under CO–CO2 atmosphere. Part II: Effect of quartz. Powder Technol. 2016, 291, 337–343. [Google Scholar] [CrossRef]
- Koike, K.; Fujii, Y.; Otomo, T.; Taguchi, N. Activity Measurements of SnO in the Molten SnO-SiO2 System. J. Soc. Mater. Eng. Resour. Jpn. 1997, 10, 9–18. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Grau, A.E.; Flengas, S.N. Activities of SnO in the SnO-SiO System. J. Electrochem. Soc. 1976, 123, 852–856. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, L.; Yu, Y. Tin recovery from a low-grade tin middling with high Si content and low Fe content by reduction–sulfurization roasting with anthracite coal. Int. J. Miner. Metall. Mater. 2021, 28, 210–220. [Google Scholar] [CrossRef]
- Zhang, R.J.; Li, L.; Yu, Y. Reduction Roasting of Tin-Bearing Iron Concentrates Using Pyrite. ISIJ Int. 2016, 6, 953–959. [Google Scholar] [CrossRef]
- Padilla, R.; Sohn, H.Y. The reduction of Stannic Oxide with Carbon. Metall. Tract. B 1979, 10B, 109–115. [Google Scholar] [CrossRef]
- Michell, A.R.; Parker, R.H. The reduction of SnO2 and Fe2O3 by solid carbon. Miner. Eng. 1988, 1, 53–66. [Google Scholar] [CrossRef]
- Takeda, Y.; Yazawa, A. Activity of FeO and Solubility of Copper in NaO0.5-SiO2-FeO Slag Saturated with Solid Iron. Trans. Jpn. Inst. Met. 1988, 29, 224–235. [Google Scholar] [CrossRef][Green Version]
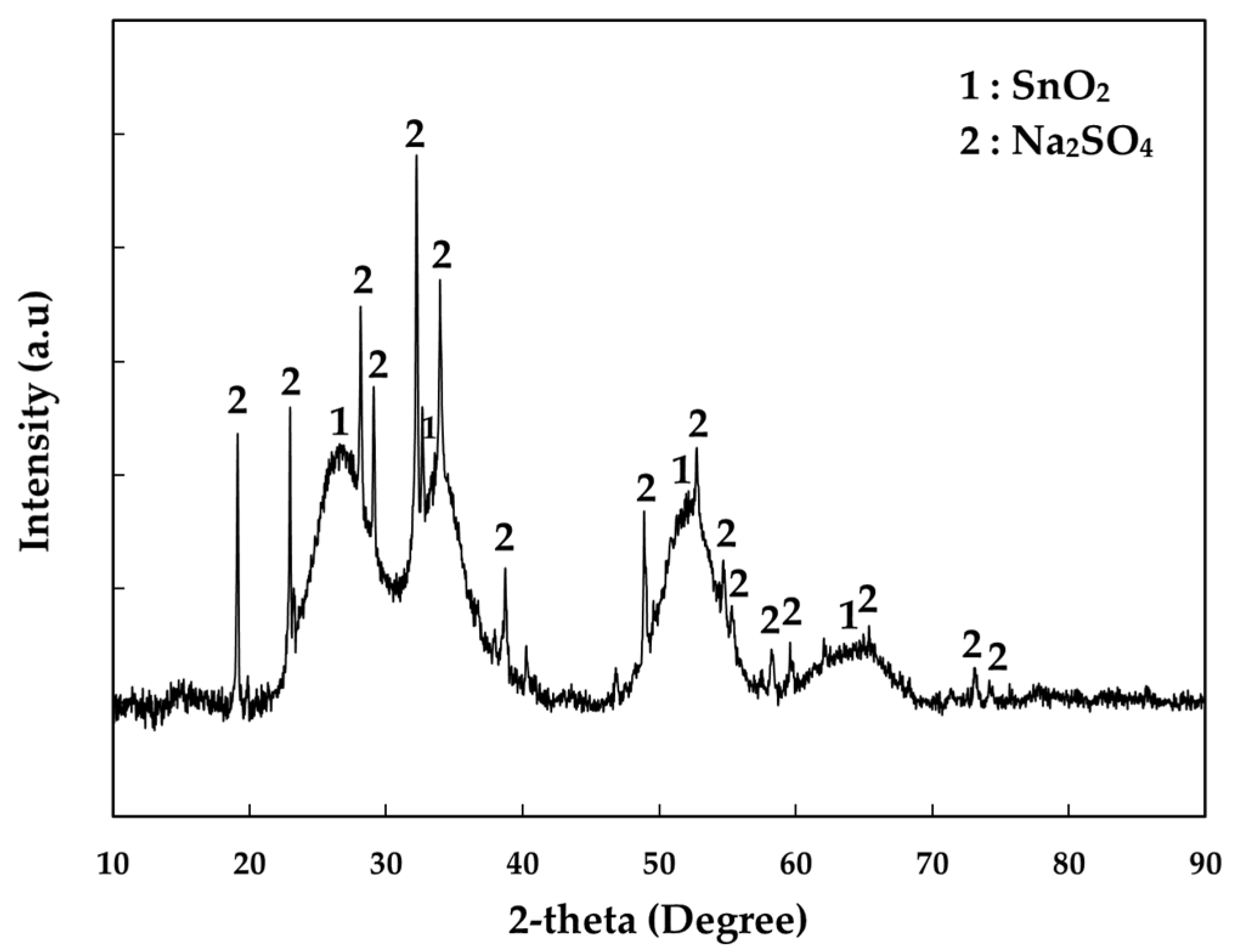
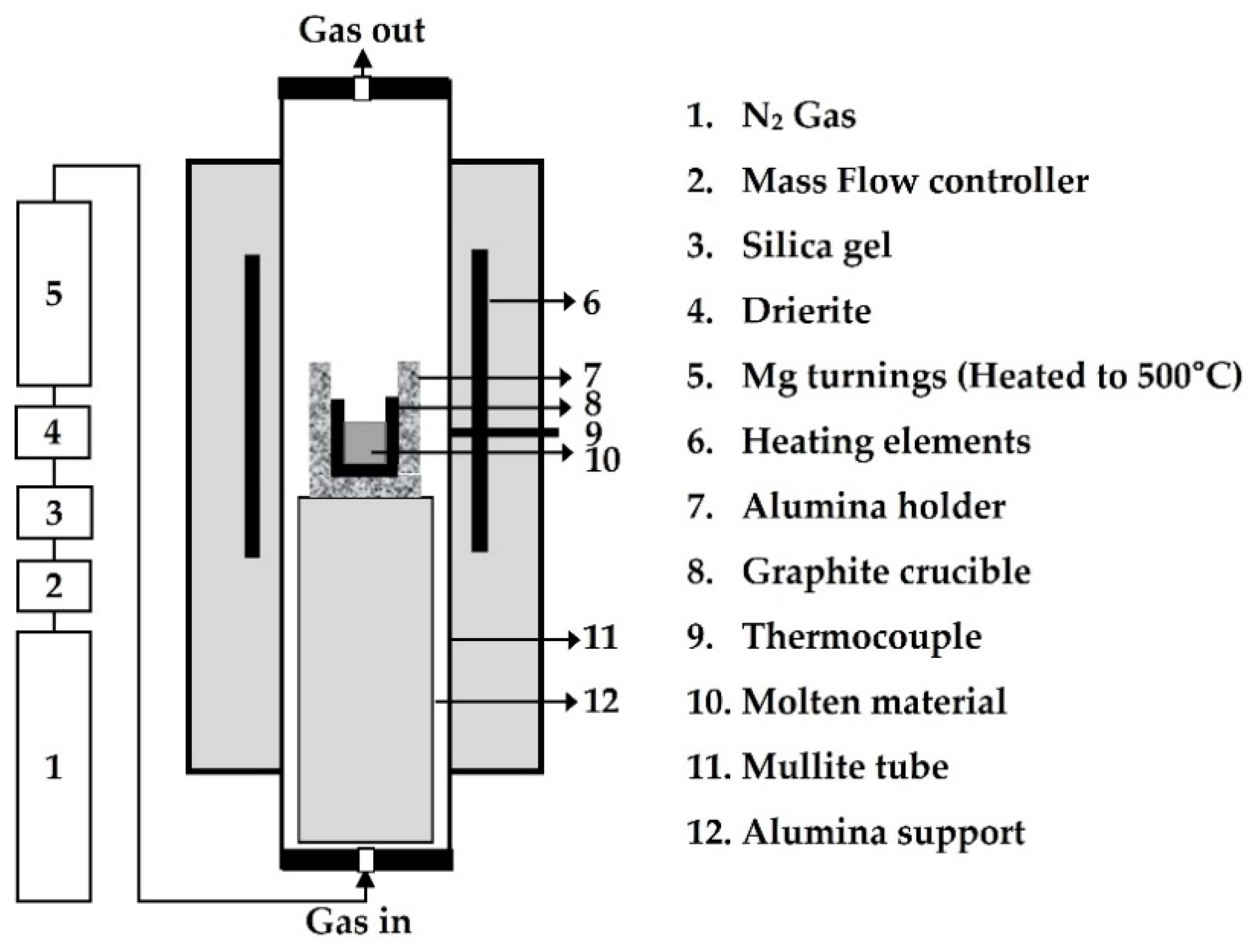
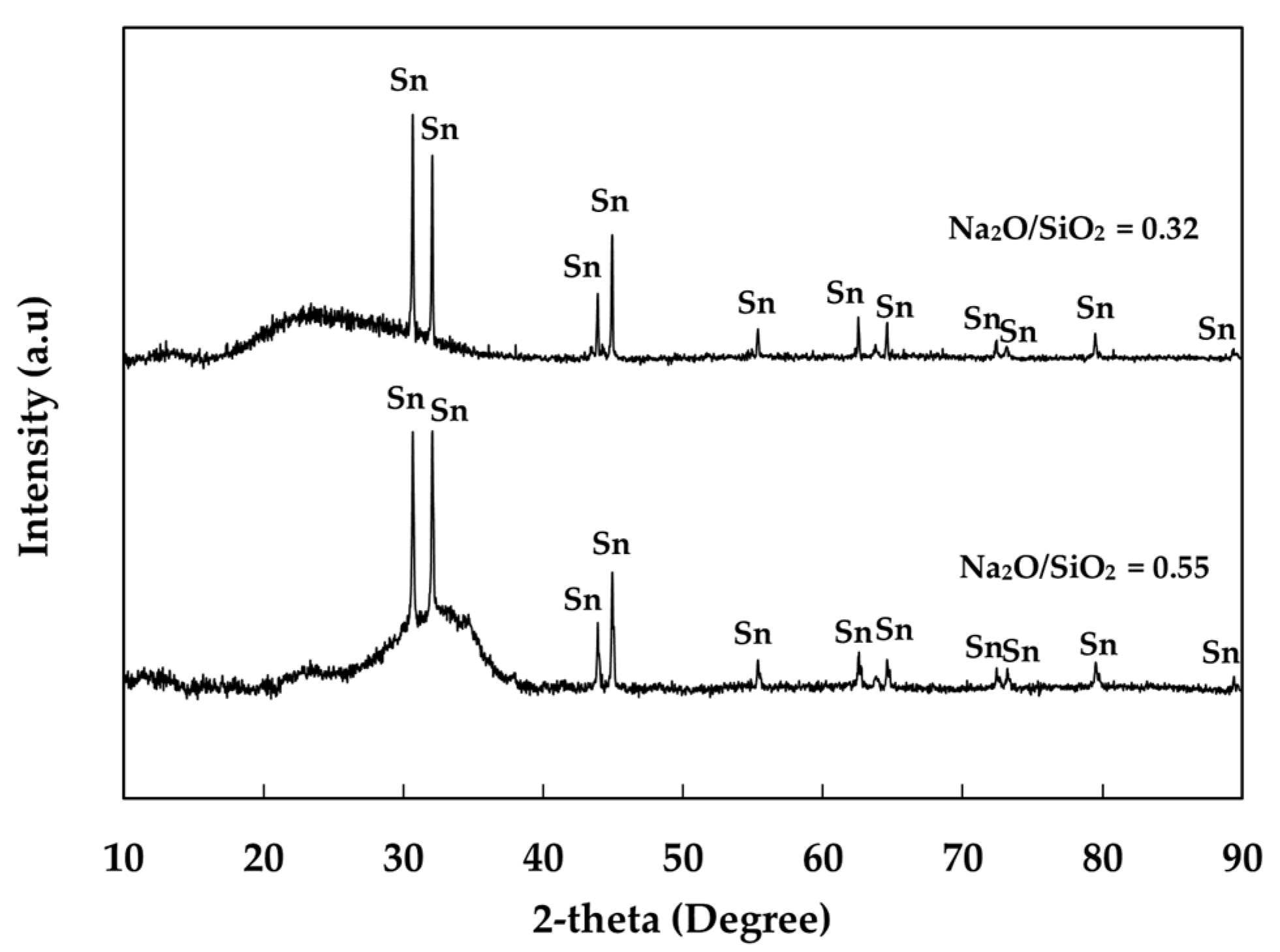

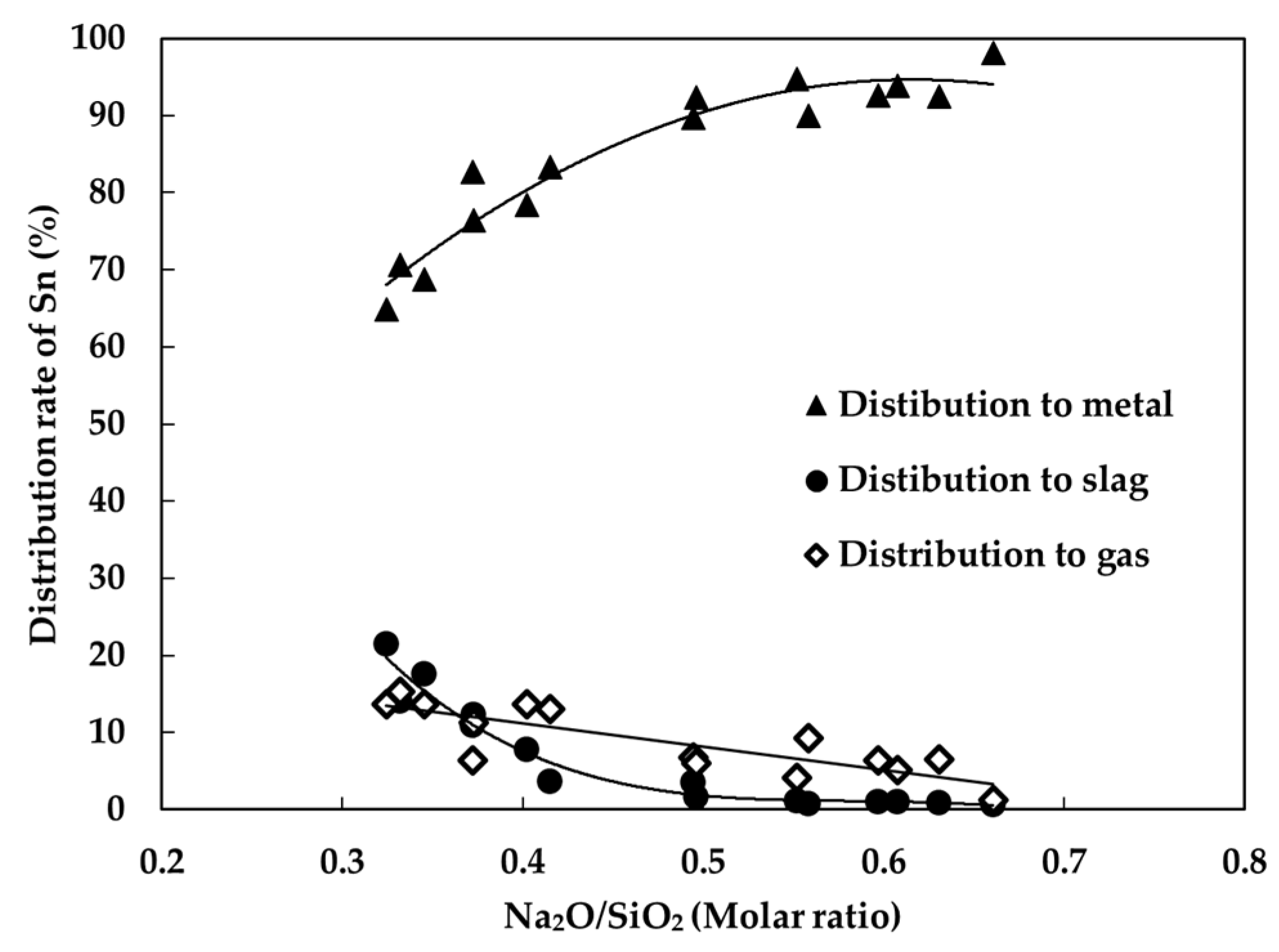



| Element | Al | Bi | Cu | Fe | Na | Pb | Sn | Te | Zn | S | O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt. (%) | 0.590 | 2.03 | 0.190 | 0.230 | 7.19 | 1.46 | 42.4 | 0.310 | 0.350 | 3.49 | Balance |
| Na2O/SiO2 | 0.320 | 0.330 | 0.350 | 0.370 | 0.400 | 0.420 | 0.490 | 0.500 | 0.550 | 0.560 | 0.600 | 0.610 | 0.630 | 0.660 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sn in Metal (g) | 4.12 | 4.49 | 4.37 | 4.86 | 4.99 | 5.29 | 5.70 | 5.87 | 6.02 | 5.72 | 5.89 | 5.96 | 5.88 | 6.24 |
| Sn in Slag (g) | 1.37 | 0.890 | 1.11 | 0.780 | 0.490 | 0.240 | 0.220 | 0.100 | 0.750 | 0.0470 | 0.0660 | 0.0660 | 0.0580 | 0.0360 |
| Elements (wt.%, ppm) | Al | Bi | Cu | Fe | Na | Pb | Sn | Si | O | |
|---|---|---|---|---|---|---|---|---|---|---|
| Na2O/SiO2 = 0.55, (1200 °C, 2 h) | Metal | - | 4.45 | 0.520 | 0.270 | 170 * | 3.26 | Balance | - | - |
| Slag | 1.90 | 80.0 * | 250 * | 0.380 | 23.9 | 120 * | 1.70 | 26.4 | Balance | |
| Na2O/SiO2 = 0.55, (1150 °C, 3 h) | Metal | - | 4.40 | 0.450 | 0.180 | 270 * | 3.24 | Balance | - | - |
| Slag | 2.00 | 230 * | 170 * | 0.500 | 23.3 | 180 * | 2.10 | 25.5 | Balance | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Sohn, H. The Recovery of Metallic Tin from an Industrial Tin-Bearing By-Product Containing Na2SO4 by Reduction Smelting Process. Metals 2021, 11, 1697. https://doi.org/10.3390/met11111697
Chang J, Sohn H. The Recovery of Metallic Tin from an Industrial Tin-Bearing By-Product Containing Na2SO4 by Reduction Smelting Process. Metals. 2021; 11(11):1697. https://doi.org/10.3390/met11111697
Chicago/Turabian StyleChang, Jongshin, and Hosang Sohn. 2021. "The Recovery of Metallic Tin from an Industrial Tin-Bearing By-Product Containing Na2SO4 by Reduction Smelting Process" Metals 11, no. 11: 1697. https://doi.org/10.3390/met11111697
APA StyleChang, J., & Sohn, H. (2021). The Recovery of Metallic Tin from an Industrial Tin-Bearing By-Product Containing Na2SO4 by Reduction Smelting Process. Metals, 11(11), 1697. https://doi.org/10.3390/met11111697






