Abstract
Tin anode slime is a by-product of the tin electrolytic refining process. This study investigated a route to separate Sn, Sb, Bi, and Cu from tin anode slime after leaching with hydrochloric acid. In the solvent extraction process with tributyl phosphate, Sb and Sn were extracted into the organic phase. Bi and Cu were unextracted and remained in the liquid phase. In the stripping experiment, Sb and Sn were stripped and separated with HCl and HNO3. Bi and Cu in the aqueous phase were also separated with chemical precipitation procedure by controlling pH value. The purities of Sn, Sb, Cu solution and the Bi-containing solid were 96.25%, 83.65%, 97.51%, and 92.1%. The recovery rates of Sn, Sb, Cu, and Bi were 76.2%, 67.1%, and 96.2% and 92.4%.
1. Introduction
Tin anode slime is a by-product of the tin electro-refining process. It is precipitated at the bottom of the refining tank and mainly contains Sn, Sb, Bi, Cu, Ag, and Si. In recent years, there has been an increasing interest in recovering secondary resources to achieve the goal of material sustainability [1,2]. Owing to the valuable and complex metals in tin anode slime, developing an appropriate and environmentally friendly process to recover tin anode slime is required. However, research into treating copper anode slime have been studied in detail [3,4,5]. But there are relatively few studies focusing on recovering tin anode slime with a hydrometallurgical method [6].
One of the main components of tin anode slime, bismuth, is rapidly used in the field of semiconductors, the pharmaceutical industry, and in cosmetic products [7,8,9]. Additionally, another component, antimony, was widely used as flame retardant synergist, opacifying agent, and catalyst [10,11,12]. As the US Geological Survey described [13,14,15], bismuth production is often a byproduct of the lead ore process, and only accounts for 0.001% of the Earth’s crust constitution. Antimony is also a critical metal on the Earth. Secondary antimony resources supplied about 14% of US domestic consumption, but it is mostly recovered in lead smelters of the lead-acid battery industry. Thus, not only in terms of environmental perspective but also for avoiding the shortage of bismuth and antimony resources, investigating a proper method to treat and recover tin anode slime is a considerable issue.
In the whole recovery process, suitable separation procedures could make the recovery system much effective and complete. In the present, the studies of separating bismuth, antimony, and tin from other metals or mediums by the solvent extraction method have been investigated. Sarkar et al. used Cyanex302 (bis (2,4,4-trimethylpentyl) monothiophosphinic acid) with toluene to separate Sn, Sb, and Bi in HNO3 media. Bi and Sb were extracted into the organic phase and stripped with HNO3 and H2SO4, respectively. In the contrast, Sn was unextracted and remained in the aqueous phase. The recovery rates of Bi, Sb, and Sn were 98.4%, 98.3%, and 99.2%. [16] Bandekar et al. separated Sn, Sb, and Bi by using PC-88A (2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester) with toluene in HCl solution. Their result showed that Sn and Sb were extracted, and Bi was unextracted. After the extraction procedure, Sn and Sb were stripped with HCl and H2SO4, respectively. The recovery rates of Sn, Sb, and Bi were 99.2%, 99.14%, and 100% [17]. Ahn et al. separated and purified Sn from impurities including Sb, Bi, Cu, and other metals in hydrochloric acid solution. Through the extraction process with tributyl phosphate, two scrubbing processes with HCl, and a stripping process with NaOH, the purity of the final Sn solution was 98.9%, and the recovery rate of Sn was 67.9% [18]. Except for the solvent extraction method, a chemical precipitation procedure was also an effective process to separate metals in solution. Ha et al. used H2O as a precipitating reagent to separate Bi from leaching liquor containing Cu [19]. Mizoguchi et al. used the precipitation process by controlling the pH value with ammonia vapor to separate Bi from Cu and Fe in sulfuric acid media [20].
In this study, the solvent extraction method and chemical precipitation process were selected to separate Sn, Sb, Bi, and Cu from tin anode slime after hydrochloric leaching. Tributyl phosphate was chosen as the extractant of the solvent extraction process. Sn and Sb ions were extracted into the organic phase and separated through the stripping procedure with different stripping agents. Bi and Cu ions that remained in the aqueous phase were separated by the chemical precipitation process. The parameters of every process were investigated, and the optimal condition of each procedure was also determined.
2. Materials and Methods
2.1. Materials
In this study, tin anode slime was provided by a material company in Taiwan, and the main components of tin anode slime are shown in Table 1. After the leaching process with 3.0 M hydrochloric acid, 0.1 M NaClO3, a liquid solid ratio of 30 mL/g, 45 °C, and 30 min for reaction, the residues of tin anode slime containing silver and silicon were filtered out. The leaching efficiencies of Sn, Sb, Bi, and Cu were 85.2%, 96.93%, 97.63%, and 99.8% [21]. According to the result of HCl leaching, the simulated solution was prepared by dissolving the chlorides of metals with hydrochloric acid. The concentration of the simulated solution was presented in Table 2. All the chlorides were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO, USA).

Table 1.
The components of tin anode slime.

Table 2.
The concentrations of the simulated solution.
Tributyl phosphate (TBP), the extractant of solvent extraction procedure, was purchased from Alfa Aesar (Haverhill, MA, USA), and the diluent, kerosene, was supplied by CPC Corporation (Kaohsiung, Taiwan). In the stripping process, HCl and HNO3 were supplied by Fluka Honeywell (Charlotte, NC, USA), and H2SO4 was purchased from PanReac AppliChem (Chicago, IL, USA). Sodium hydroxide, which was used to control the pH value in the precipitation experiment, was supplied by Showa Chemical (Tokyo, Japan).
2.2. Equipment and Experiment Conditions
The concentrations of samples in this study were analyzed by ICP-OES (Industry Coupled Plasma Optical Emission Spectrometry, PerkinElmer Optima 2100DV; PerkinElmer Inc., Waltham, MA, USA), and pH value was detected by pH meter (SUNTEX pH meter sp-2300; SUNTEX Instruments Co., Ltd., Saitama, Japan).
All experiments of this study were operated in room temperature and normal atmospheric pressure.
2.3. Solvent Extraction with TBP
The components of the simulated solution, Sn, Sb, Bi, and Cu, were divided into two groups through the solvent extraction process. The first group was Sn and Sb, which would be extracted into the organic phase by TBP. The second group was Bi and Cu, which remained in the aqueous phase. In this process, 10 mL of simulated solution and an appropriate volume of TBP solution based on the O/A ratio were placed in a separating funnel and shaken by a funnel shaker at 3000 rpm. The parameters of solvent extraction experiment included the concentration of HCl, TBP concentration, O/A ratio, and reaction time. The extraction efficiency of TBP was calculated by Equation (1).
2.4. Stripping Process
After the solvent extraction process, Sb and Sn were extracted into the organic phase. In the stripping procedure, Sb and Sn were stripped with different stripping agents to separate. The organic phase solution and stripping agent were taken into separating funnel and shaken at 3000 rpm. Selection of the stripping agent, the concentration of stripping agent, O/A ratio, and stripping time were investigated. The stripping percentage was determined by Equation (2).
2.5. Precipitation Experiment
Through the solvent extraction and stripping process, the precipitation procedure with a controlled pH value was chosen to separate Bi and Cu in the aqueous phase. In this process, 10 mL of aqueous phase solution was taken into a beaker and stirred with a magnetic mixer at 300 rpm. The pH value was adjusted by sodium hydroxide solution and measured by a pH meter. After precipitation, the solution was filtered by a vacuum filter and the precipitation rate was calculated by Equation (3).
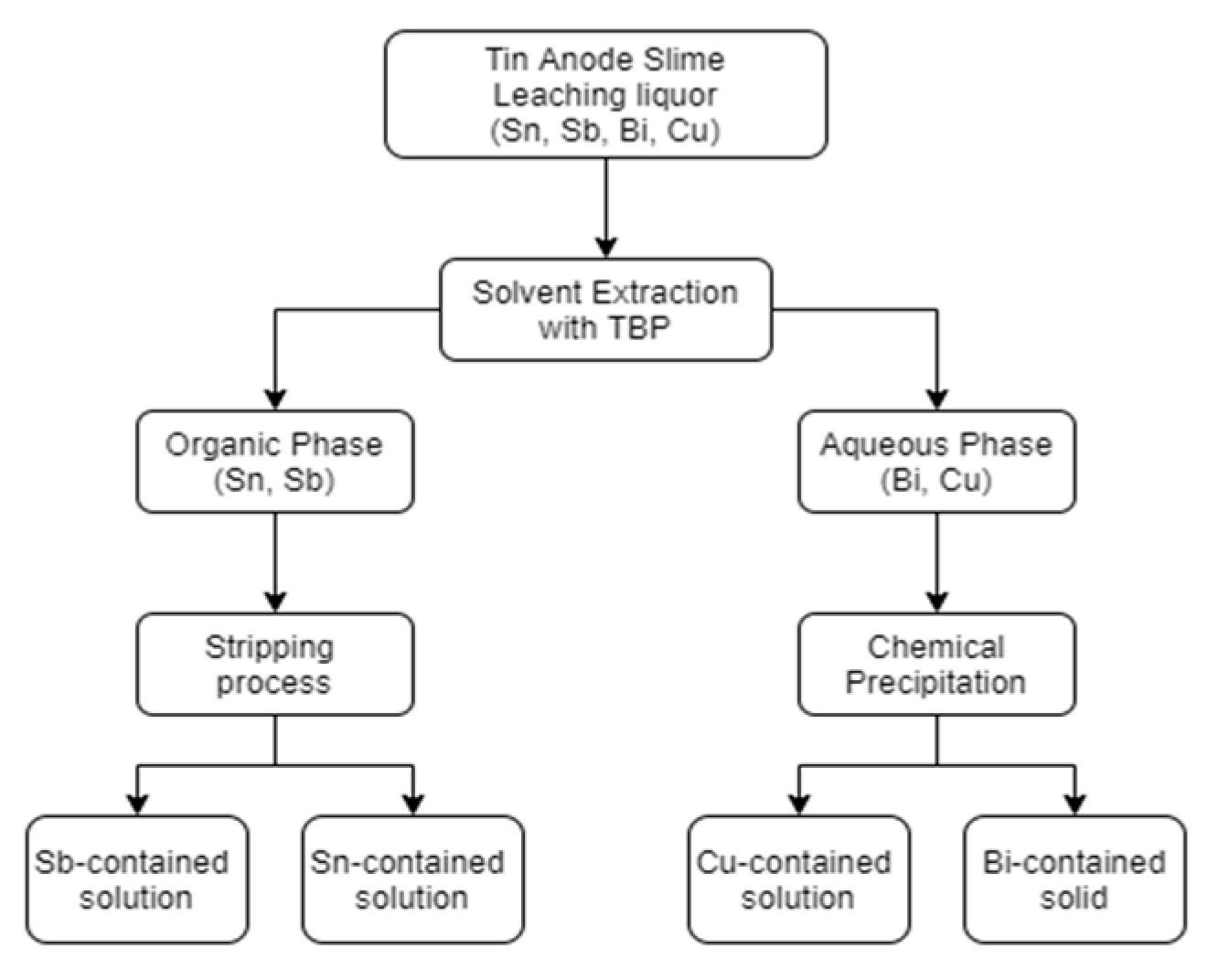
2.6. Flow Chart
The flow chart of separation process is shown in Figure 1. The most important steps are solvent extraction and chemical precipitation.
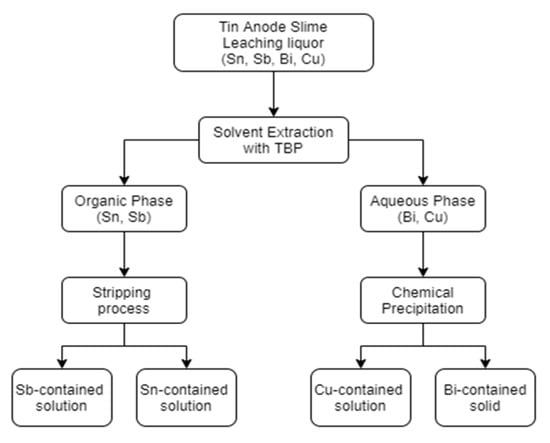
Figure 1.
Flow chart of separation processes in this study.
3. Results and Discussions
3.1. Solvent Extraction with TBP
3.1.1. Effect of HCl Concentration
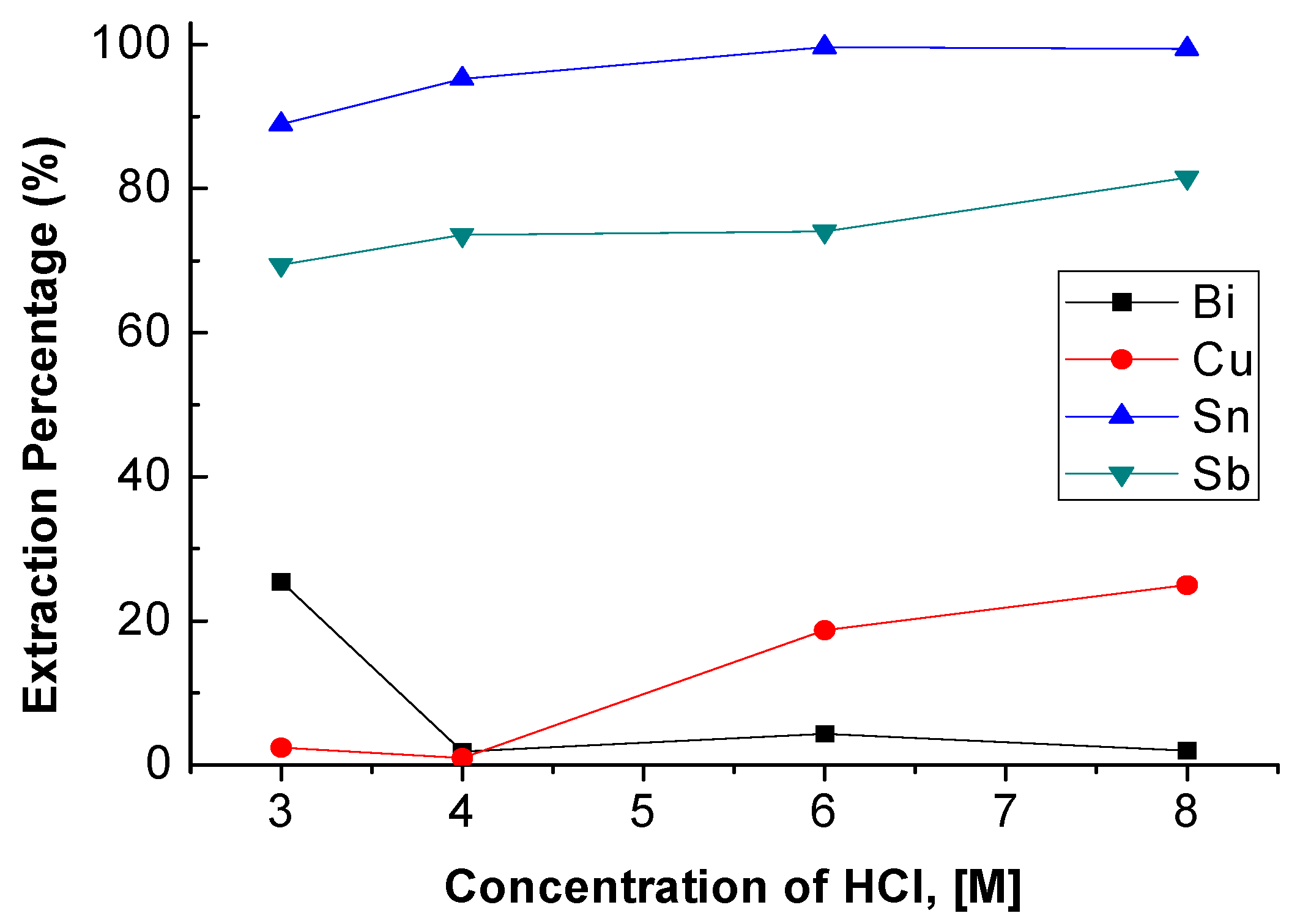
The concentration of HCl was set from 3 M to 8 M with 0.5 M TBP and an O/A ratio = 1 at 5 min reaction time. Figure 2 shows the result that the extraction percentages of Sn, Sb, and Cu rose slightly with the increase in HCl concentration. However, the extraction percentage of Bi was decreased. Compared with other research, the variation of extraction percentage showed similar correlations between HCl concentration and metal ions [18]. In order to extract Sb and Sn into the organic phase and keep Bi and Cu in the aqueous phase. The optimal condition of HCl concentration was chosen as 4 M.
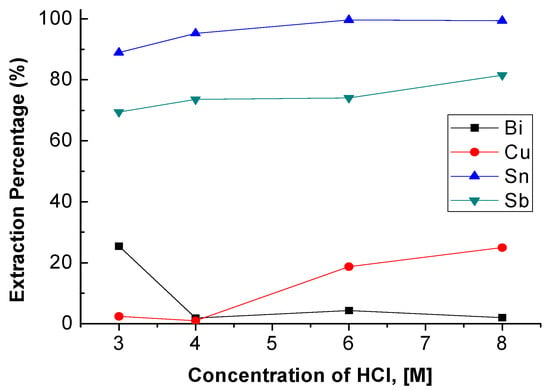
Figure 2.
Extraction percentages of metals at different concentrations of HCl. (Tributyl phosphate TBP) = 0.5 M, O/A ratio = 1, reaction time = 5 min.
3.1.2. Effect of TBP Concentration
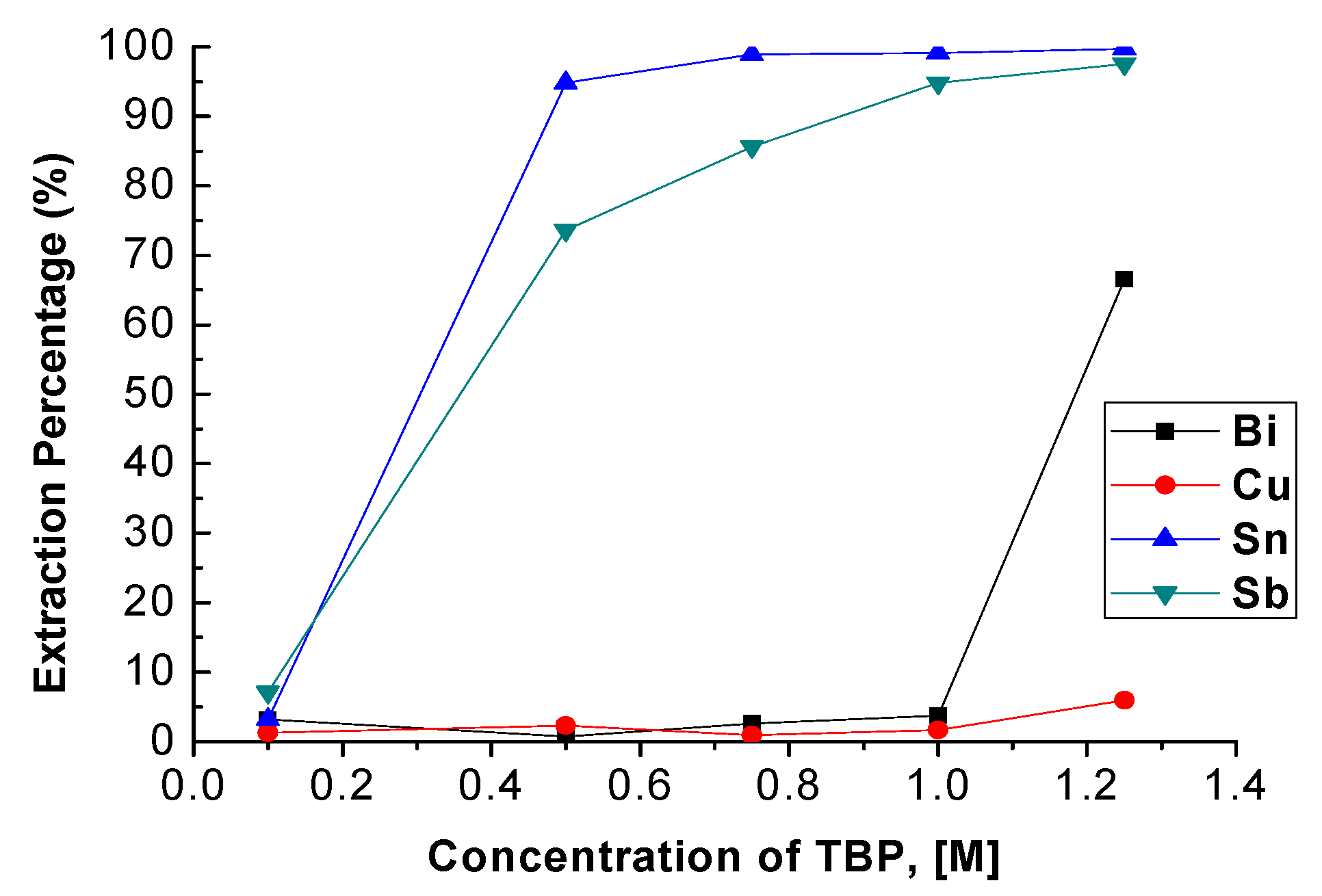
TBP concentration was varied from 0.1 M to 1.25 M with 4 M HCl, an O/A ratio = 1, and the reaction time was 5 min. Fuerstenau et al. revealed that the extraction percentage of Sn was increased from 90.0% to 97.3% with the increase in TBP concentration from 15 vol% to 35 vol% [22]. As Figure 3 depicts, the extraction percentages of Sn and Sb were also increased from 4.79% to 99.72% and 7.14% to 97.57% with the increase in TBP concentration. However, the extraction efficiency of Bi was dramatically increased when the concentration of TBP was higher than 1.0 M, and the extraction percentage of Cu was also increased slightly. To avoid the co-extraction of Bi and Cu, the optimal TBP concentration was chosen as 1.0 M with which to carry out the extraction process.
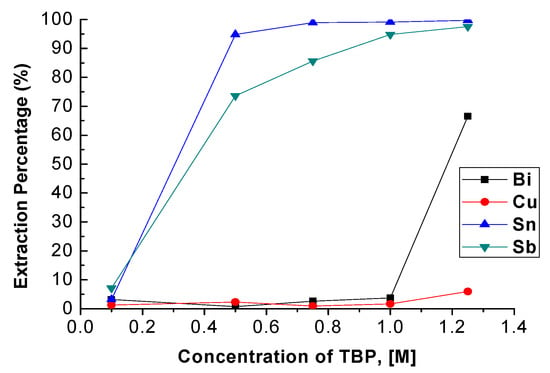
Figure 3.
Extraction percentage of metals at different concentrations of TBP. (HCl) = 4 M, O/A ratio = 1, reaction time = 5 min.
3.1.3. Effect of O/A Ratio
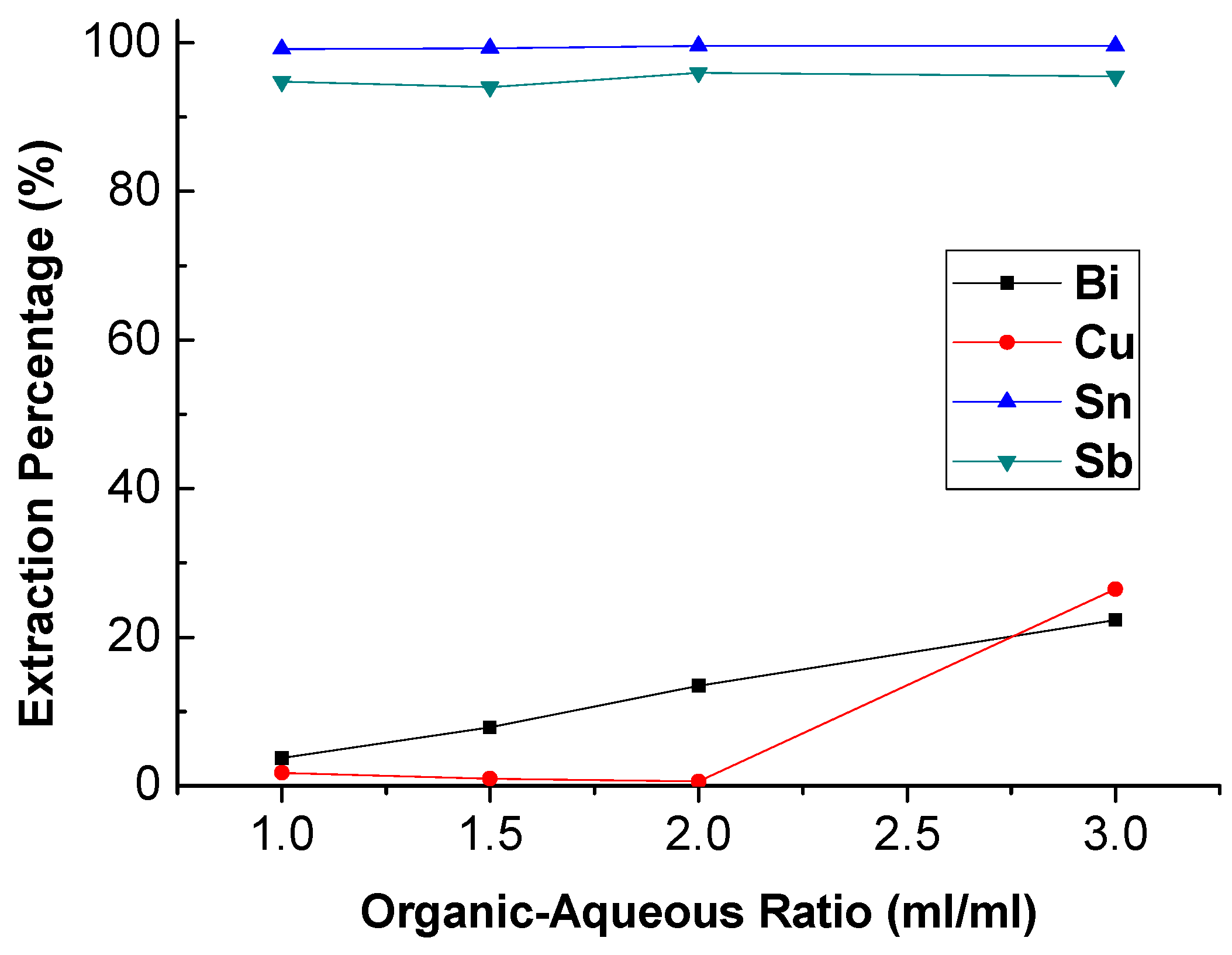
To investigate the effect of the O/A ratio on extraction, the O/A ratio was studied from 1.0 to 3.0 with 4 M HCl and 1 M TBP at 5 min reaction time. The result was presented in Figure 4. It can be seen that the extraction efficiency of Bi and Cu was increased obviously with the increase in O/A ratio. Under this condition, the reaction of trapping Sn and Sb ion by TBP was almost equilibrium. Thus, the excess TBP started to grab Bi and Cu ions with the increase in O/A ratio. For the purpose of separation, the optimal O/A ratio was 1.0.
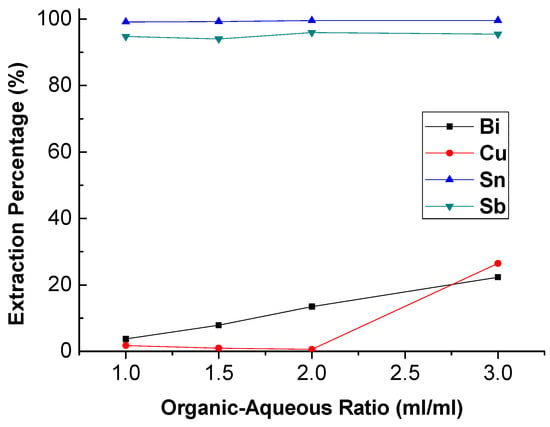
Figure 4.
Extraction percentage of metals at different O/A ratio. (HCl) = 4 M, (TBP) = 1 M, reaction time = 5 min.
3.1.4. Effect of Reaction Time
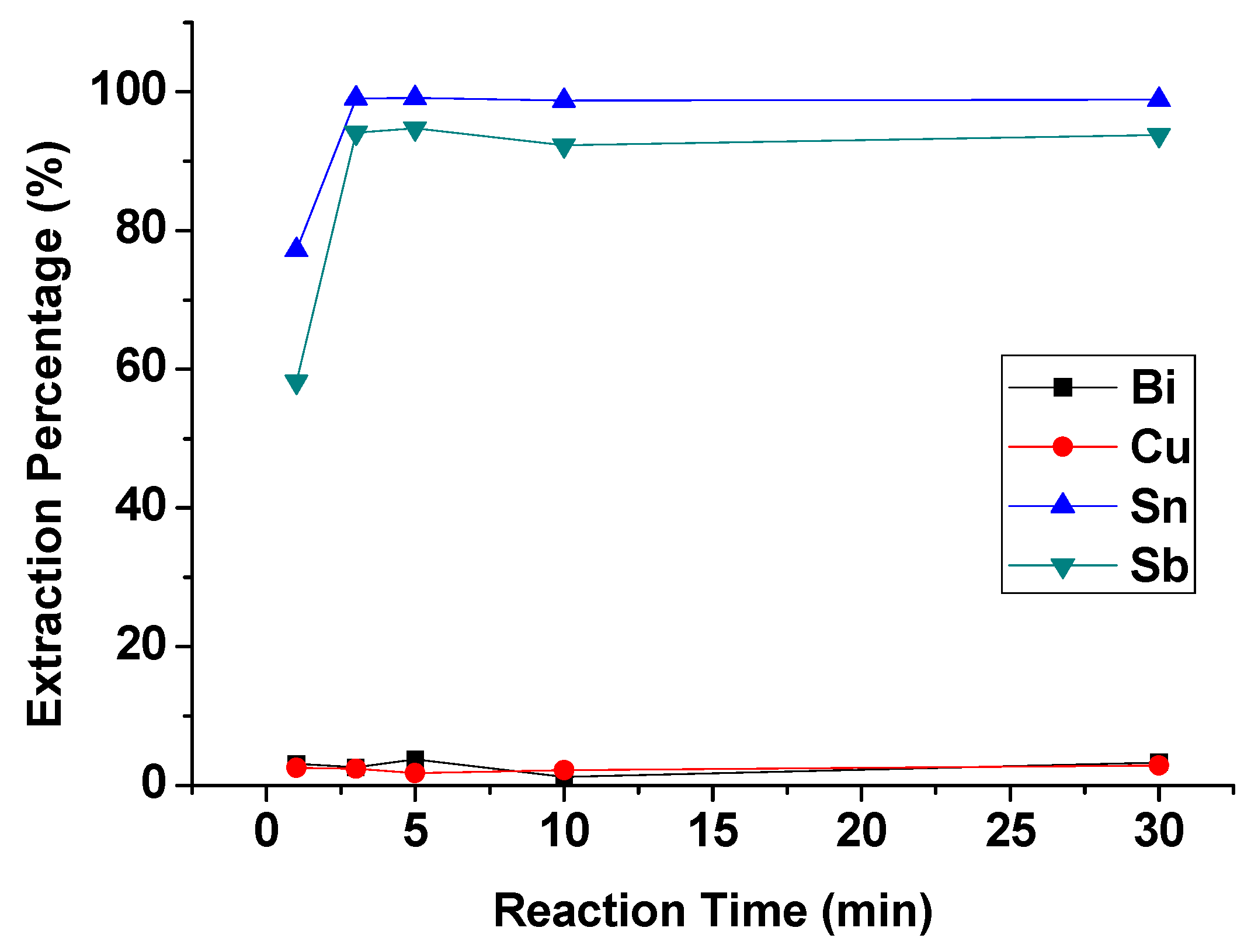
To evaluate the effect of reaction time on the extraction process, reaction time was set from 1 min to 30 min with 4 M HCl, 1 M TBP, and an O/A ratio = 1. Figure 5 showed that the extraction moved towards equilibrium in 3 min. Thus, the optimal reaction time was set at 3 min.
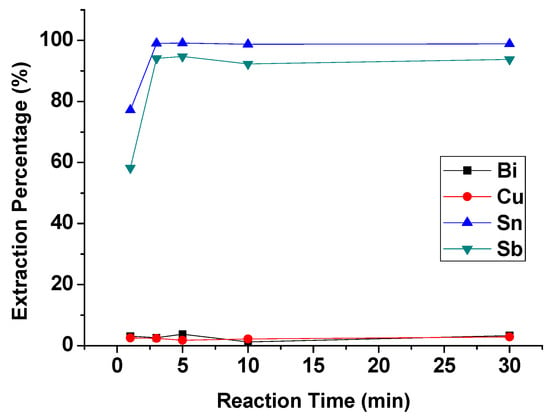
Figure 5.
Extraction percentage of metals at different reaction times. (HCl) = 4 M, (TBP) = 1 M, O/A ratio = 1.
3.1.5. Results of Solvent Extraction with TBP
The solvent extraction process was carried out with the optimal conditions: 4 M HCl, 1 M TBP, and an O:A ratio = 1 at 3 min reaction time. Extraction percentages of Sn, Sb, Bi, and Cu are shown in Table 3. Sn and Sb were extracted into the organic phase successfully while Bi and Cu were almost unextracted and remained in the aqueous phase.

Table 3.
Extraction efficiency of Sn, Sb, Bi, and Cu with TBP at optimal conditions.
3.2. Stripping Experiment—Sb Stripping
3.2.1. Selection of Stripping Agent
To separate Sb and Sn in the organic phase after solvent extraction process with TBP, investigating an appropriate stripping agent is essential. Table 4 showed the results of stripping percentages with different stripping agents. The concentration of each stripping agent was 9 M, the O/A ratio = 1, and the reaction time was 5 min. As the result, HNO3 was effective in stripping Sn and Sb, and H2SO4 was poor at stripping in this condition. However, HCl could strip Sb up to 40.02% while the extraction efficiency of Sn was only 1.27%. For the sake of separating Sb and Sn, HCl was chosen as the stripping agent in the following experiment.

Table 4.
Stripping efficiency of Sn and Sb with different 9 M stripping agents.
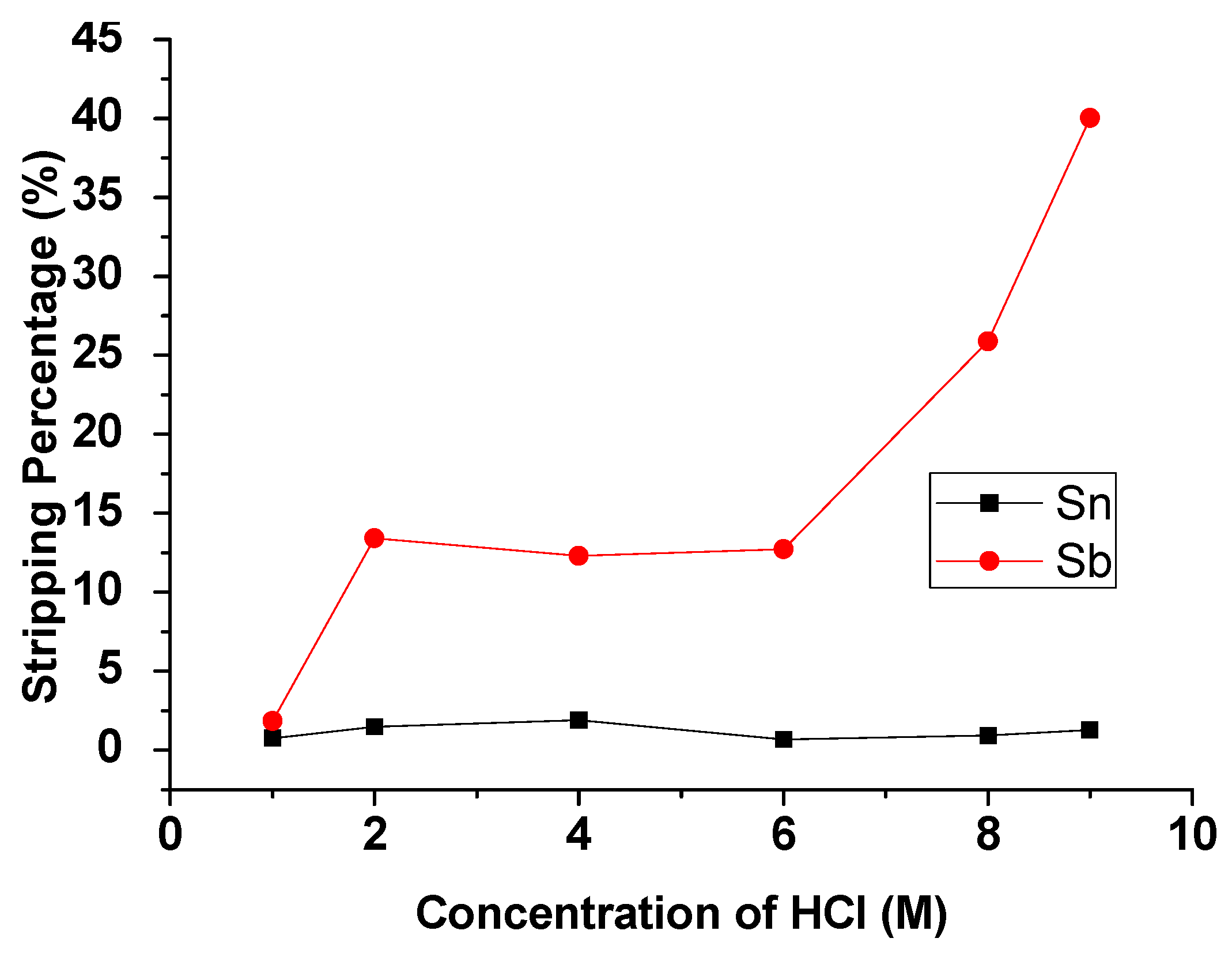
3.2.2. Effect of HCl Concentration
The concentration of HCl was studied from 1 M to 9 M with an O/A ratio = 1 at 5 min reaction time. Figure 6 presents the result that the stripping percentage of Sb was increased significantly with the increase in HCl concentration. In contrast, the stripping efficiency of Sn remained the same. For separating Sb and Sn, 9 M HCl was chosen as the optimal concentration for the stripping experiment.
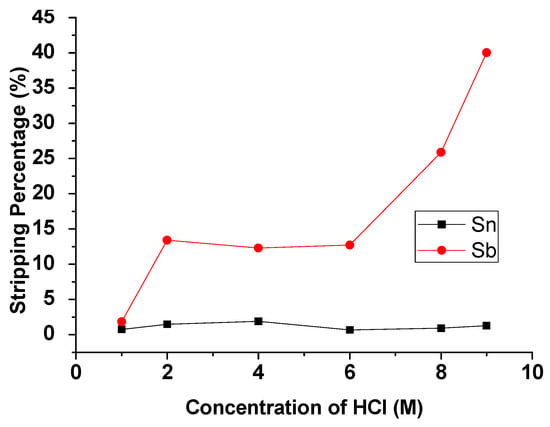
Figure 6.
Stripping percentage of Sn and Sb at different concentrations of HCl. O/A ratio = 1, reaction time = 5 min.
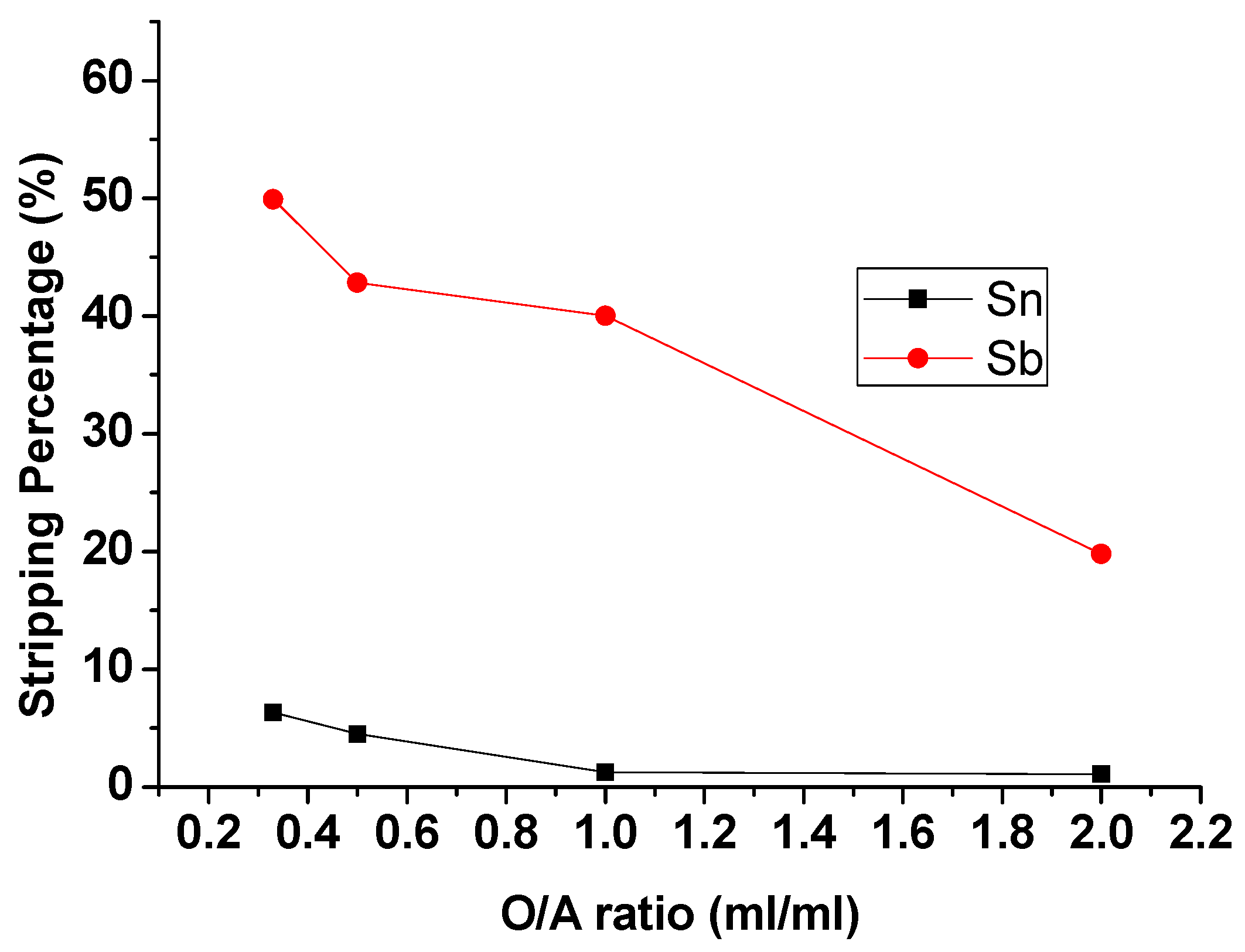
3.2.3. Effect of O/A Ratio
To evaluate the effect of the O/A ratio on the stripping process, the O/A ratio was set from 0.33 to 2 with 9 M HCl and 5 min reaction time. Figure 7 showed that the stripping percentages of Sb and Sn were decreased with the increase in O/A ratio. Although the Stripping efficiency of Sb at O/A ratio 0.33 was highest and up to 49.91%, the stripping efficiency of Sn was also increased to 6.35%. To strip and purify Sb completely in this process, an O/A ratio of 1 was chosen as the optimal condition.
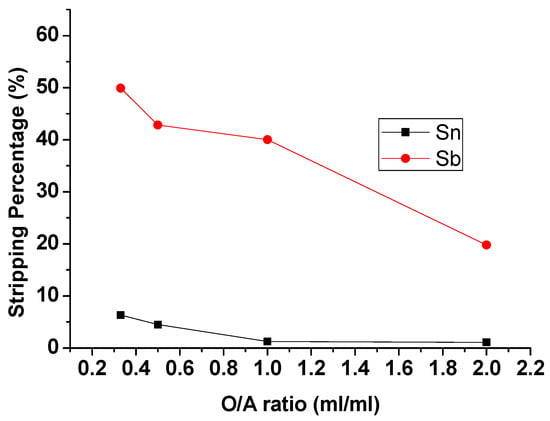
Figure 7.
Stripping percentage of Sn and Sb at different O/A ratios. (HCl) = 9 M, Reaction time = 5 min.
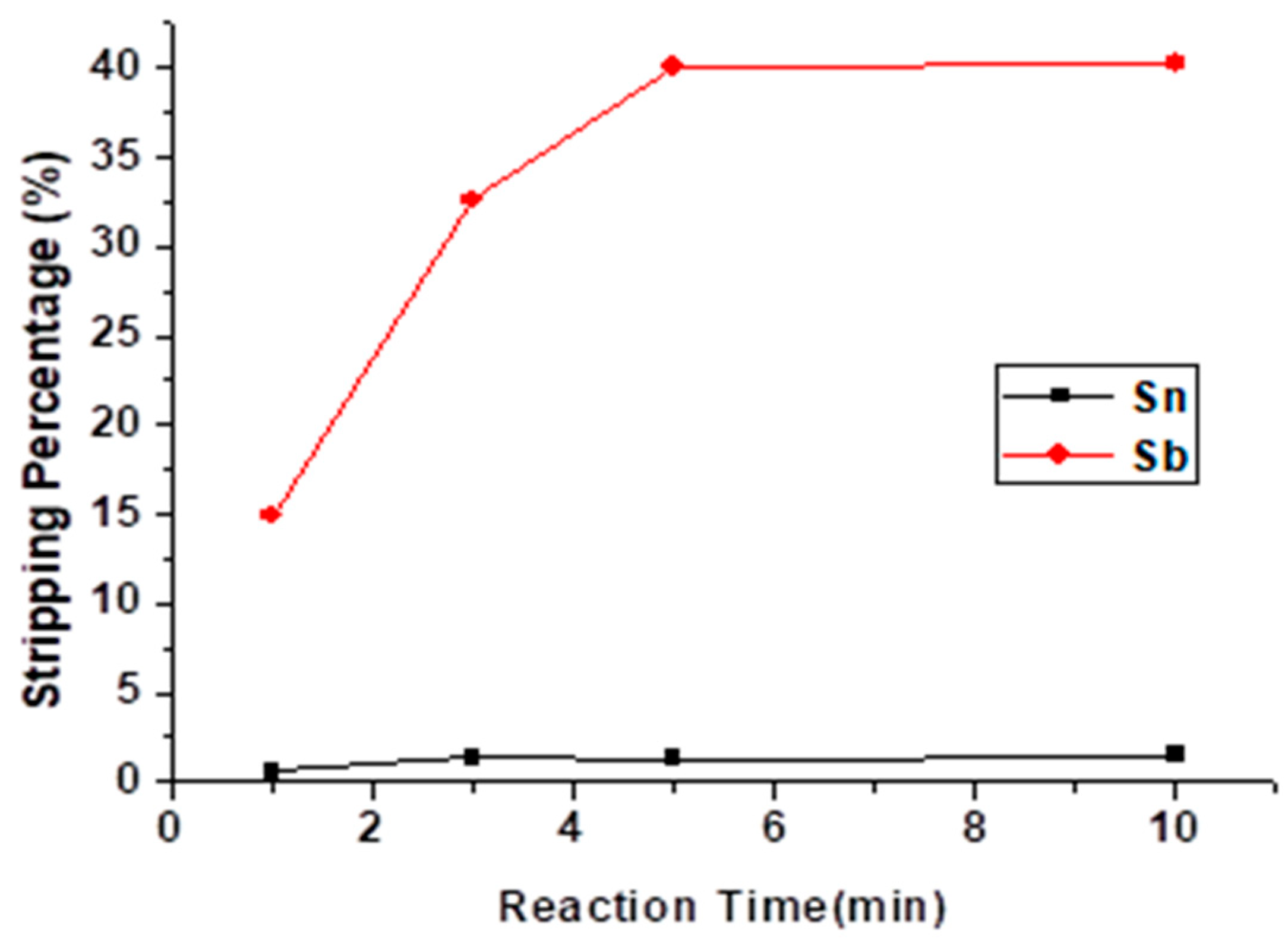
3.2.4. Effect of Reaction Time
The reaction time of the stripping experiment was studied from 1 min to 10 min, and the result is shown in Figure 8. The stripping reaction tended to move towards equilibrium at 5 min, and the stripping percentage of Sb did not rise anymore. Thus, the optimal reaction time of the stripping experiment was 5 min.
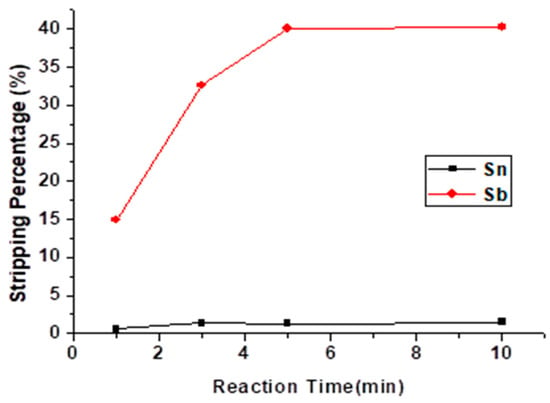
Figure 8.
Stripping percentage of Sn and Sb at different reaction times. (HCl) = 9 M, O/A ratio = 1.
3.2.5. Stripping Efficiency of Repetitive Operation
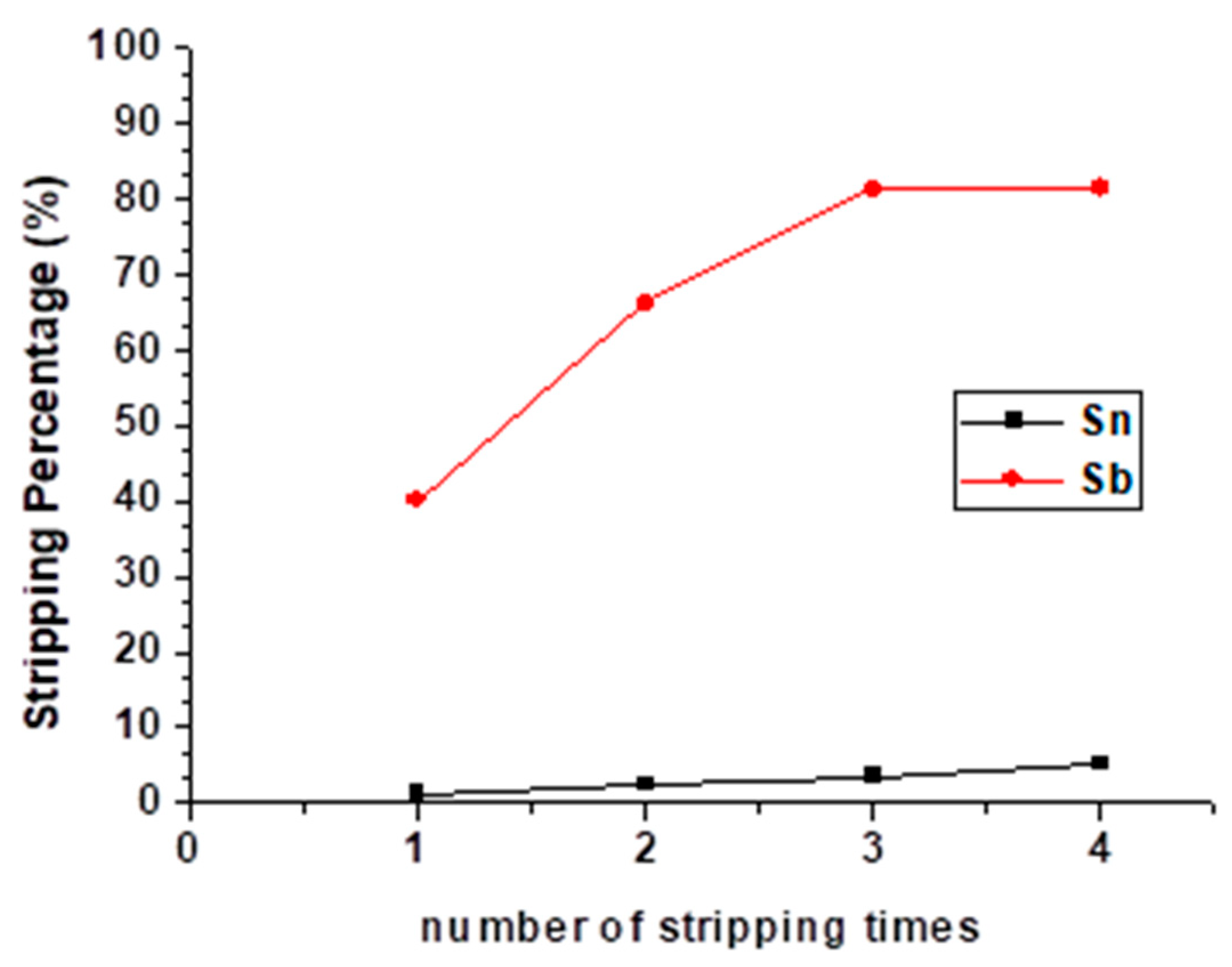
Since the stripping efficiency of Sb was only 40.02% even in the optimal condition, to raise the stripping percentages and separate Sb and Sn, repetitive stripping was studied. The number of stripping times was set from one to four with 9 M HCl, O/A ratio = 1 at 5 min reaction time, and the result is shown in Figure 9. Although the stripping percentage of Sb was increased the first three times, there was no significant change the fourth time. Moreover, the stripping efficiency of Sn was still increased the fourth time. To avoid the decrease in purity, we chose three times as the optimal condition of the repetitive stripping process.
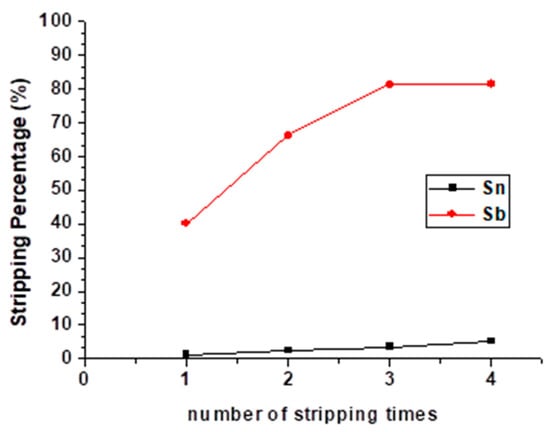
Figure 9.
Stripping percentage of Sn and Sb at different numbers of stripping times. (HCl) = 9 M, O/A ratio = 1, reaction time = 5 min.
3.2.6. Optimal Condition and Result of HCl Stripping
The optimal conditions of the HCl stripping process are shown in Table 5. In this condition, the stripping percentage of Sb was 81.36%, and Sn was 3.48%.

Table 5.
Optimal condition of the HCl stripping experiment.
3.3. Stripping Experiment—Sn Stripping
3.3.1. Effect of HNO3 Concentration
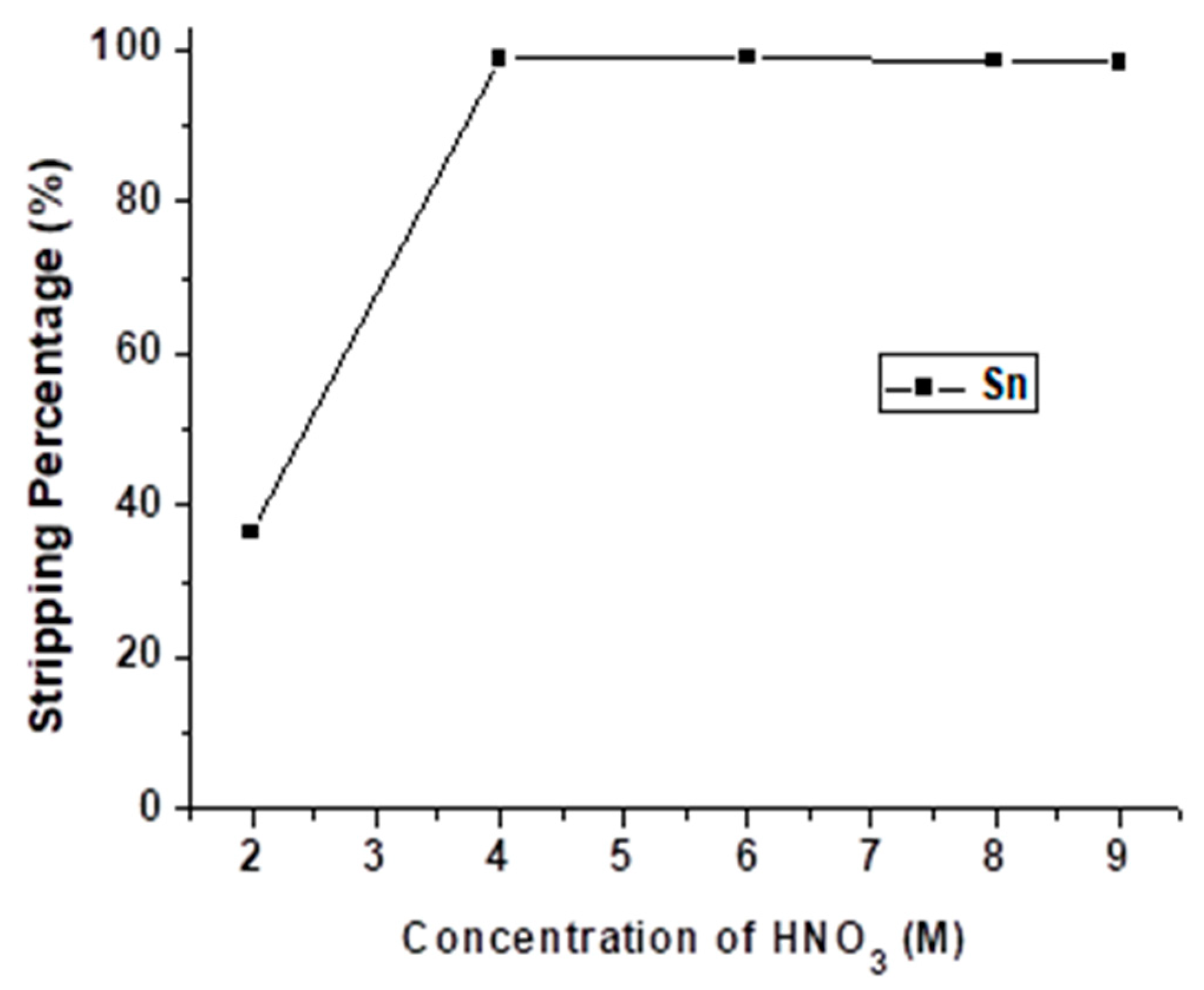
Through the stripping process with HCl, Sb was stripped out from the organic phase. As Table 4 describes, HNO3 was able to strip Sn effectively. Thus, HNO3 was chosen as the next stripping agent to operate the Sn stripping experiment. The concentration of HNO3 was varied from 2 M to 9 M with O/A ratio = 0.5 at 5 min reaction time, and the result is shown in Figure 10. The stripping percentage of Sn was decreased when the HNO3 concentration was lower than 4 M. Thus, 4 M HNO3 was chosen as the optimal condition in this process.
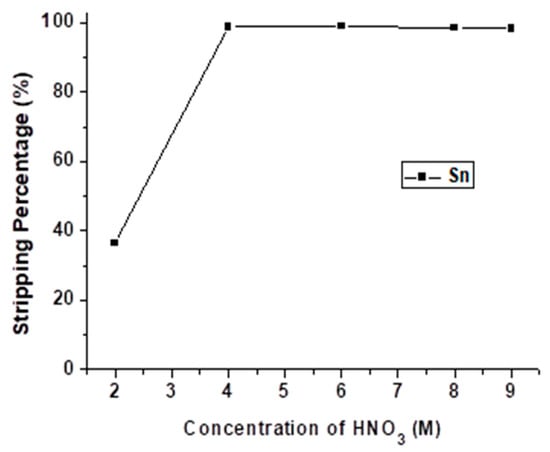
Figure 10.
Stripping percentage of Sn at different concentrations of HNO3. O/A ratio = 0.5, reaction time = 5 min.
3.3.2. Effect of the O/A Ratio
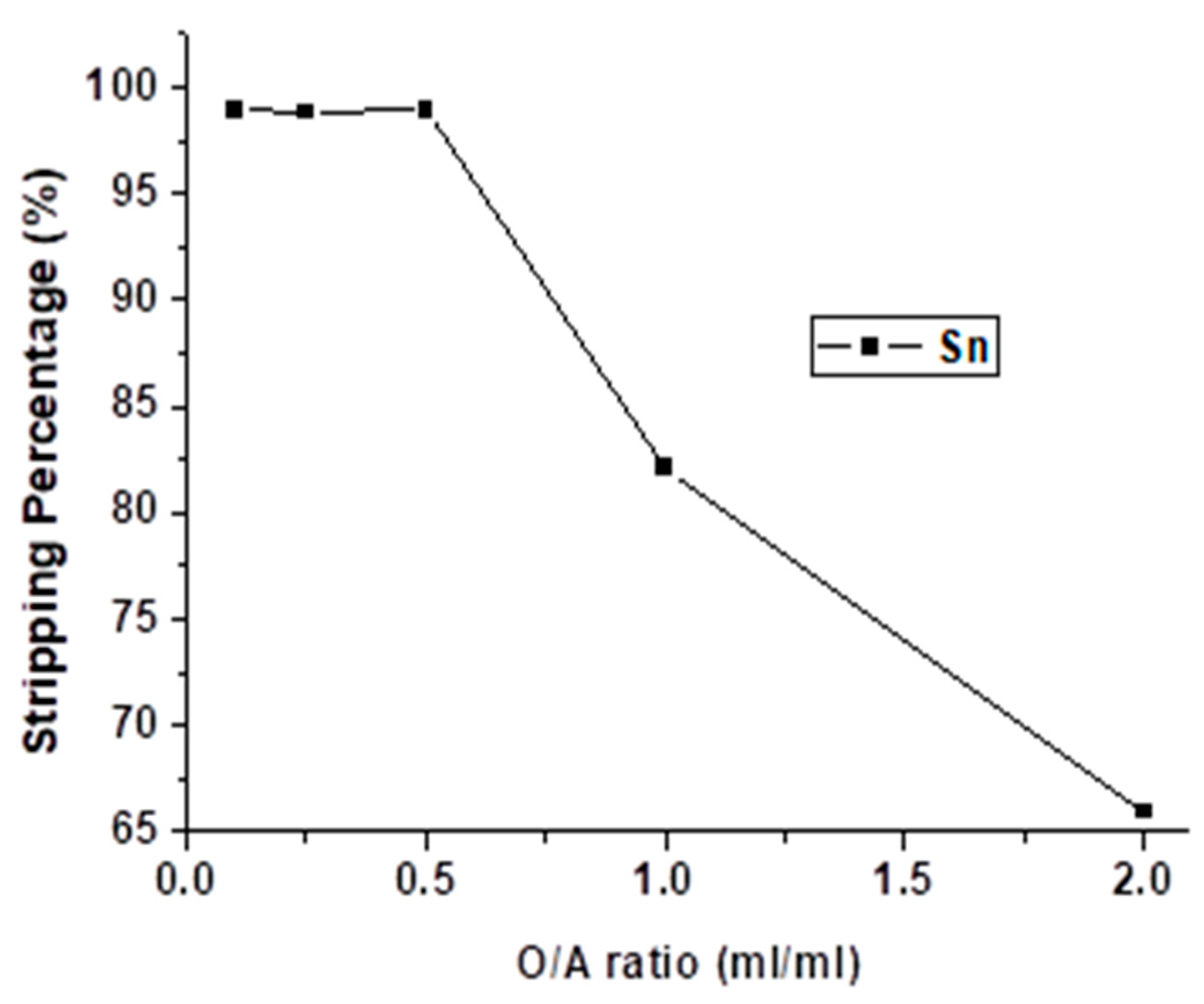
The effect of the O/A ratio was studied from 0.1 to 2 with 4 M HNO3 and 5 min for reacting. As the result shows in Figure 11, the stripping percentage of Sn was decreased dramatically when the O/A ratio was higher than 0.5. The optimal O/A ratio was chosen 0.5 so as to decrease the usage of HNO3 without the depression of stripping efficiency.
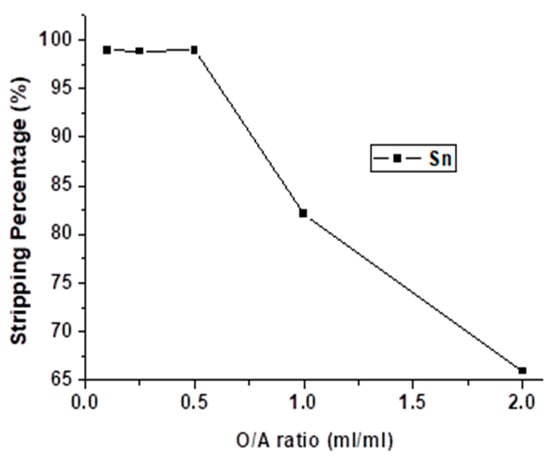
Figure 11.
Stripping percentage of Sn at different O/A ratios. (HNO3) = 4 M, reaction time = 5 min.
3.3.3. Effect of Reaction Time
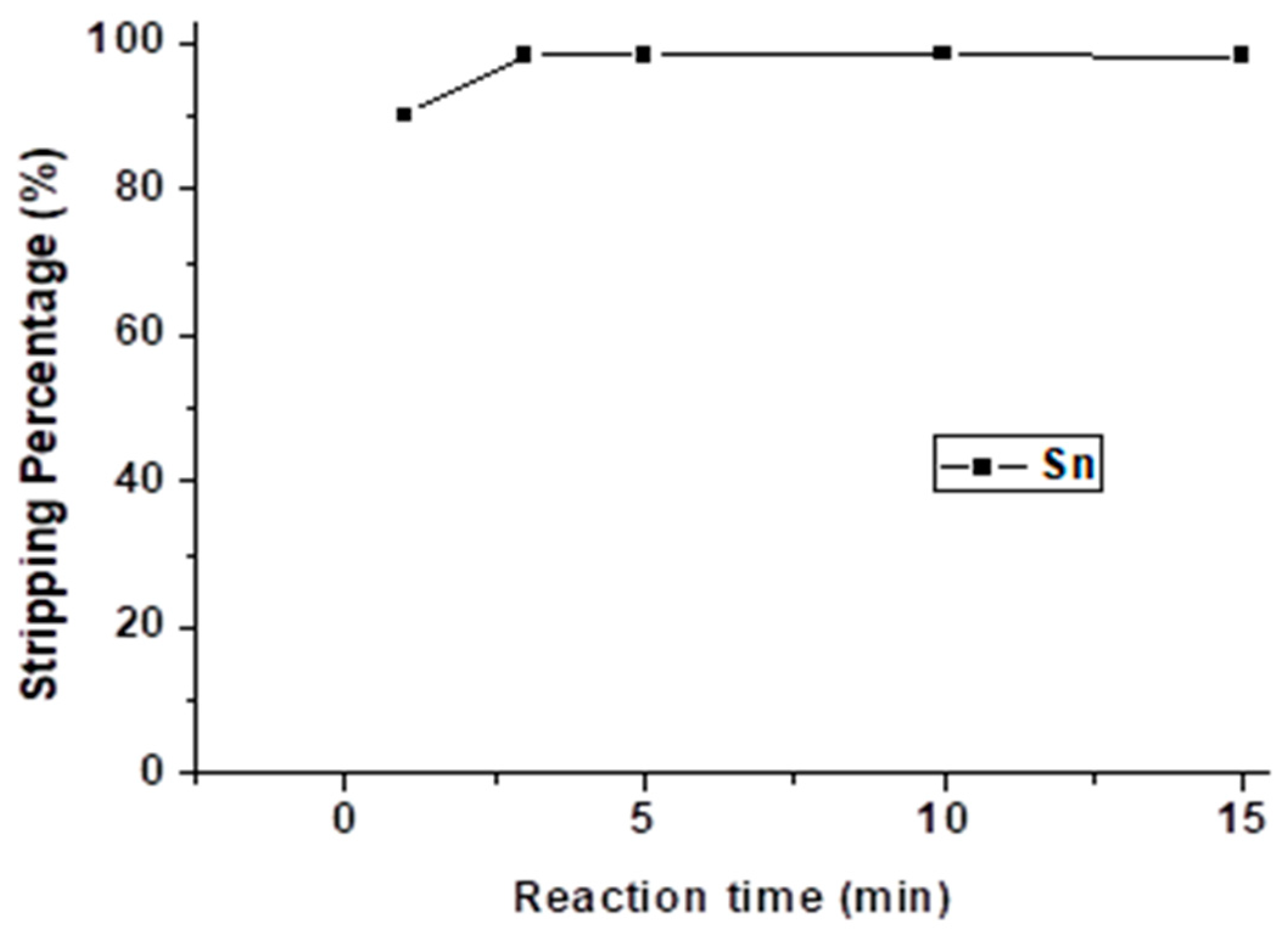
Figure 12 shows the effect of reaction time, and the reaction time was set from 1 min to 15 min with 4 M HNO3 and an O/A ratio = 0.5. The stripping process reached equilibrium at 3 min. Hence, we selected 3 min as the optimal reaction time.
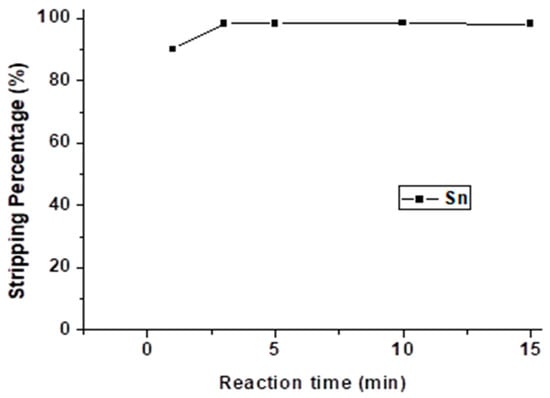
Figure 12.
Stripping percentage of Sn at different reaction times. (HNO3) =4 M, O/A ratio = 0.5.
3.3.4. Result of the Stripping Process with HNO3
Table 6 presents the optimal condition of the Sn stripping process. Under this condition, the stripping percentage of Sn was 98.91%. Compared with the study of Ahn et al. that used 2.0 M NaOH and 0.5 M HCl as the stripping agents to operate the stripping experiment, the stripping efficiencies of Sn were 99.3% and 82.4%, respectively [18]. Therefore, HNO3 was also a suitable stripping agent to strip Sn after TBP extraction.

Table 6.
Optimal conditions of the stripping experiment with HNO3.
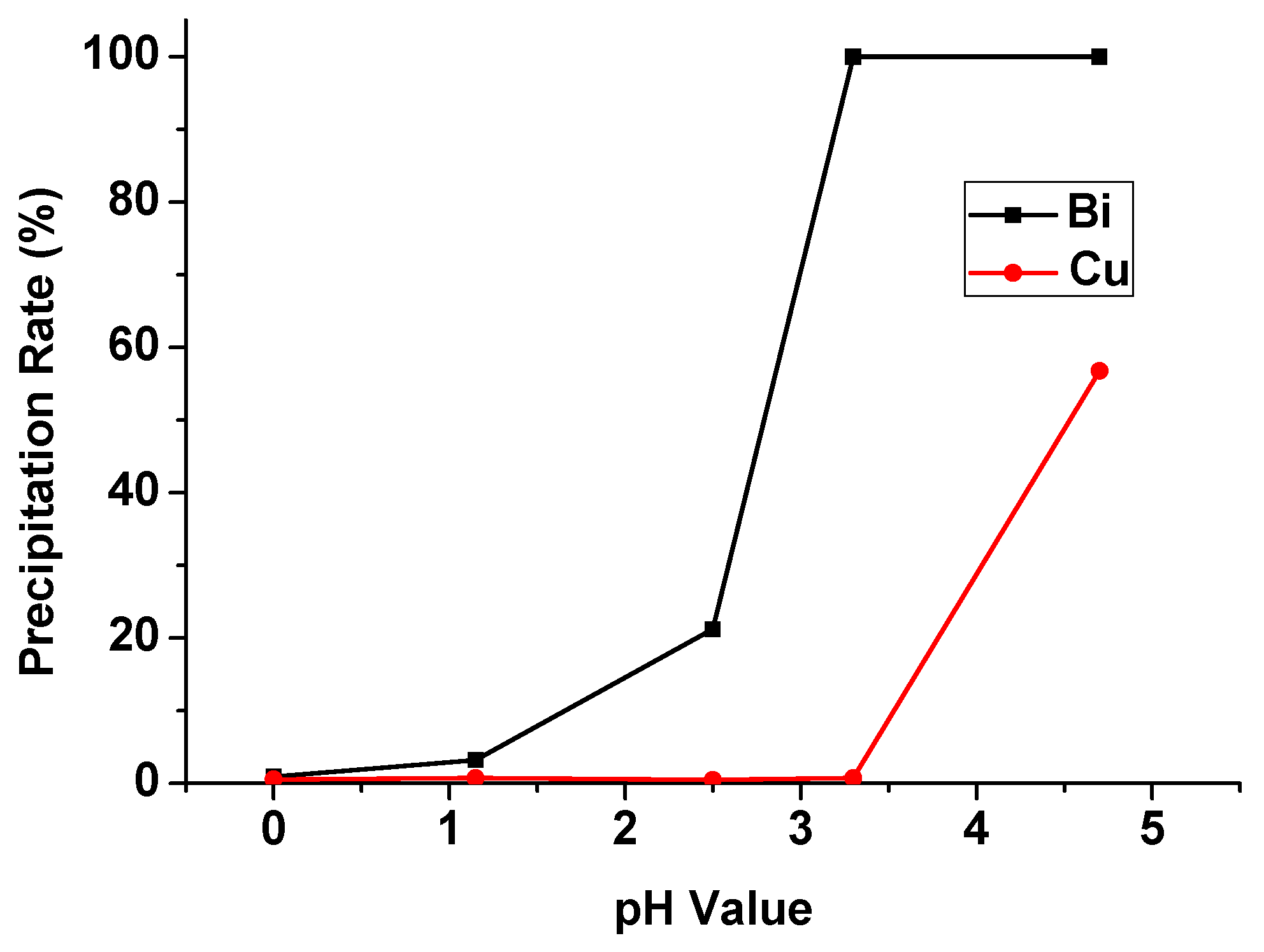
3.4. Chemical Precipitation Experiment
In the chemical precipitation process, the pH value of the aqueous phase after solvent extraction was set from 0 to 4.5, and the precipitation percentages of Bi and Cu were shown in Figure 13. The precipitation rate of Bi and Cu were increased significantly with the increase in pH value. In order to separate Bi and Cu in this process, we chose a pH value of 3.3 as the optimal condition. Under this condition, the precipitation rate of Bi was 99.8%, and Cu was 0.71%. This result corresponded with other research precipitating Bi from sulfuric acid media. The optimal pH value was also around 3 and Bi was precipitated completely [20]. The separation method of Bi and Cu in the aqueous phase was investigated.
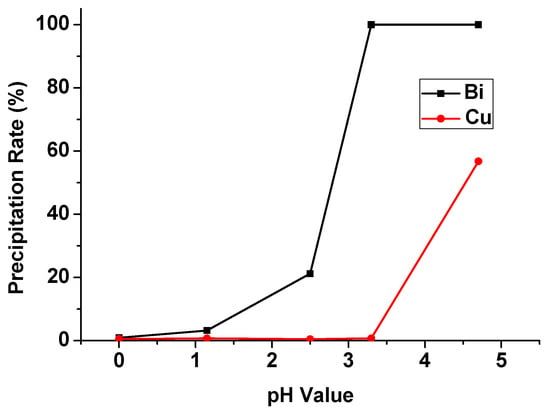
Figure 13.
Precipitation percentage of Bi and Cu at different pH values.
4. Conclusions
This study investigated a hydrometallurgy route to separate Sn, Sb, Bi, and Cu from tin anode slime in hydrochloric acid media. Moreover, this method could treat and recover tin anode slime suitably and made valuable metals reusable. It would be also useful to approach the goal of resource circulation. With the solvent extraction process using TBP, Sb stripping procedures with HCl, Sn stripping process with HNO3, and Bi precipitation processes with NaOH, Sn, Sb, Bi, and Cu were separated successfully under the optimal conditions investigated in this study. After separation, the components of Sn, Sb, Cu solutions, and Bi-containing solid are shown in Table 7.

Table 7.
Components of Sn, Sb, Cu solutions and Bi-containing solid.
The purities of Sn, Sb, Cu solution and Bi-containing solid were 96.25%, 83.65%, 97.51%, and 92.10%. The recovery rates of Sn, Sb, Bi, and Cu were 76.2%, 67.1%, 96.2%, and 92.4%.
Author Contributions
Conceptualization, W.-S.C., S.M. and C.-H.L.; methodology, S.M. and C.-H.L.; data curation, S.M.; writing—original draft preparation, S.M.; writing—review and editing, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, Z.; Zhang, Y.; Liu, B.; Lu, M.; Li, G.; Jiang, T. Extraction and separation of tin from tin-bearing secondary resources: A review. JOM 2017, 69, 2364–2372. [Google Scholar] [CrossRef]
- Shibata, J.; Okuda, A. Recycling technology of precious metals. Shigen-to-Sozai 2002, 118, 1–8. [Google Scholar] [CrossRef]
- Kilic, Y.; Kartal, G.; Timur, S. An investigation of copper and selenium recovery from copper anode slimes. Int. J. Miner. Process. 2013, 124, 75–82. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.L.; Yu, Y.; Fu, G.Y.; Han, P.W.; Sun, Z.H.I.; Ye, S.F. An environmentally friendly process to selectively recover silver from copper anode slime. J. Clean. Prod. 2018, 187, 708–716. [Google Scholar] [CrossRef]
- Ranjbar, R.; Naderi, M.; Omidvar, H.; Amoabediny, G. Gold recovery from copper anode slime by means of magnetite nanoparticles (MNPs). Hydrometallurgy 2014, 143, 54–59. [Google Scholar] [CrossRef]
- Hongbo, L.; Jiufa, Y.; Qiwang, Z.; Min, Z. Thermodynamics of Separation Stibium from Tin Anode Slime by Sulfidation Roasting. Chin. J. Rare Met. 2013, 37, 845–850. [Google Scholar]
- Murmu, P.; Kennedy, J.; Suman, S.; Chong, S.; Leveneur, J.; Storey, J.; Rubanov, S.; Ramanath, G. Multifold improvement of thermoelectric power factor by tuning bismuth and antimony in nanostructured n-type bismuth antimony telluride thin films. Mater. Des. 2019, 163, 107549. [Google Scholar] [CrossRef]
- Ishihara, S. On bismuth resources of Japan and world. Resour. Geol. 2008, 58, 131–138. [Google Scholar]
- Rohe, O. Bismuth—The new ecologically green metal for modern lubricating engineering. Ind. Lubr. Tribol. 2002, 54, 153–164. [Google Scholar]
- Ishihara, S.; Ohno, T. Present status of antimony mineral resource of this world. Resour. Geol. 2012, 62, 91–97. [Google Scholar]
- Anderson, C.G. The metallurgy of antimony. Geochemistry 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Tsunemi, K.; Wada, H. Substance Flow Analysis of Antimony for Risk Assessment of Antimony and Antimony Compounds in Japan. J. Jpn. Inst. Met. 2008, 72, 91–98. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018.
- U.S. Geological Survey. Mineral Commodity Summaries 2019; U.S. Geological Survey: Reston, VA, USA, 2019.
- U.S. Geological Survey. Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020.
- Sarkar, S.G.; Dhadke, P.M. Solvent extraction separation of antimony (III) and bismuth (III) with bis(2,4,4-trimethylpentyl) monothiophosphinic acid (cyanex302). Sep. Purif. Technol. 1999, 15, 131–138. [Google Scholar] [CrossRef]
- Bandekar, S.V.; Dhadke, P.M. Solvent extraction separation of tin (IV) with 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC-88A). Talanta 1998, 46, 1181–1186. [Google Scholar] [CrossRef]
- Ahn, J.W.; Lee, J.C. Separation of Sn, Bi, As, Cu, Pb and Zn from Hydrochloric Acid Solution by Solvent Extraction Process Using TBP (tri-n-Butylphosphate) as an Extractant. Mater. Trans. 2011, 52, 2228–2232. [Google Scholar] [CrossRef]
- Ha, T.K.; Kwon, B.H.; Park, K.S.; Mohapatre, D. Selective leaching and recovery of bismuth as Bi2O3 from copper smelter converter dust. Sep. Purif. Technol. 2015, 142, 116–122. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Sobukawa, T.; Okabe, T. Recovery of Bismuth by Precipitation from Mixed Solution of Bismuth Sulfate, Iron Sulfate and Sulduric Acid. J. Soc. Chem. Ind. 1971, 74, 17–21. [Google Scholar]
- Chen, W.-S.; Zhong, Y.-F.; Wang, L.-P. Oxidation leaching of tin anode slime by controlling potential. IOP Conf. Ser. Earth Environ. Sci. 2019, 345, 012014. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Wang, G. Selective separation of tin from a chloride leach solution. Hydrometallurgy 1997, 46, 229–234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).