Kinetic and Interaction Studies of Adenosine-5′-Triphosphate (ATP) Hydrolysis with Polyoxovanadates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
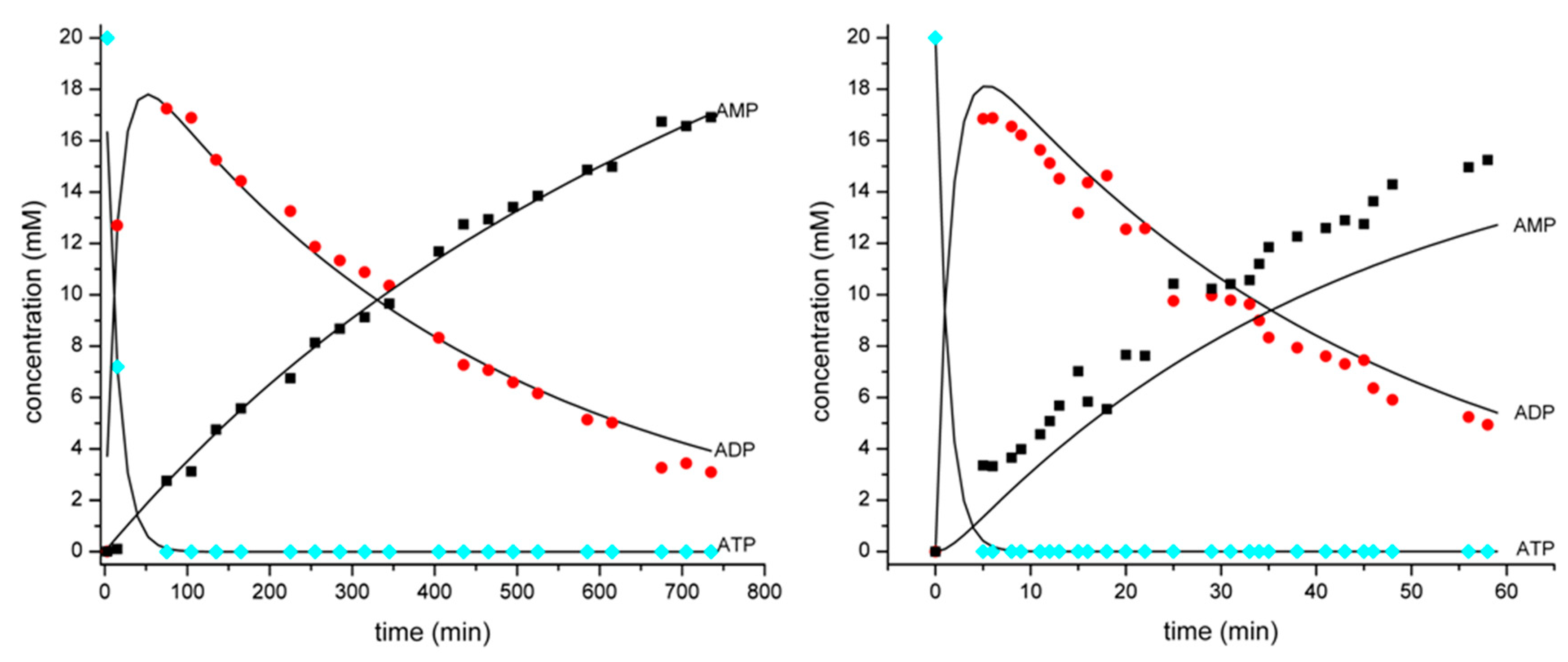
3.1. Development of a Method for the Determination of Rate Constants of ATP Hydrolysis
3.2. Effect of pH and Temperature on the Dephosphorylation of ATP
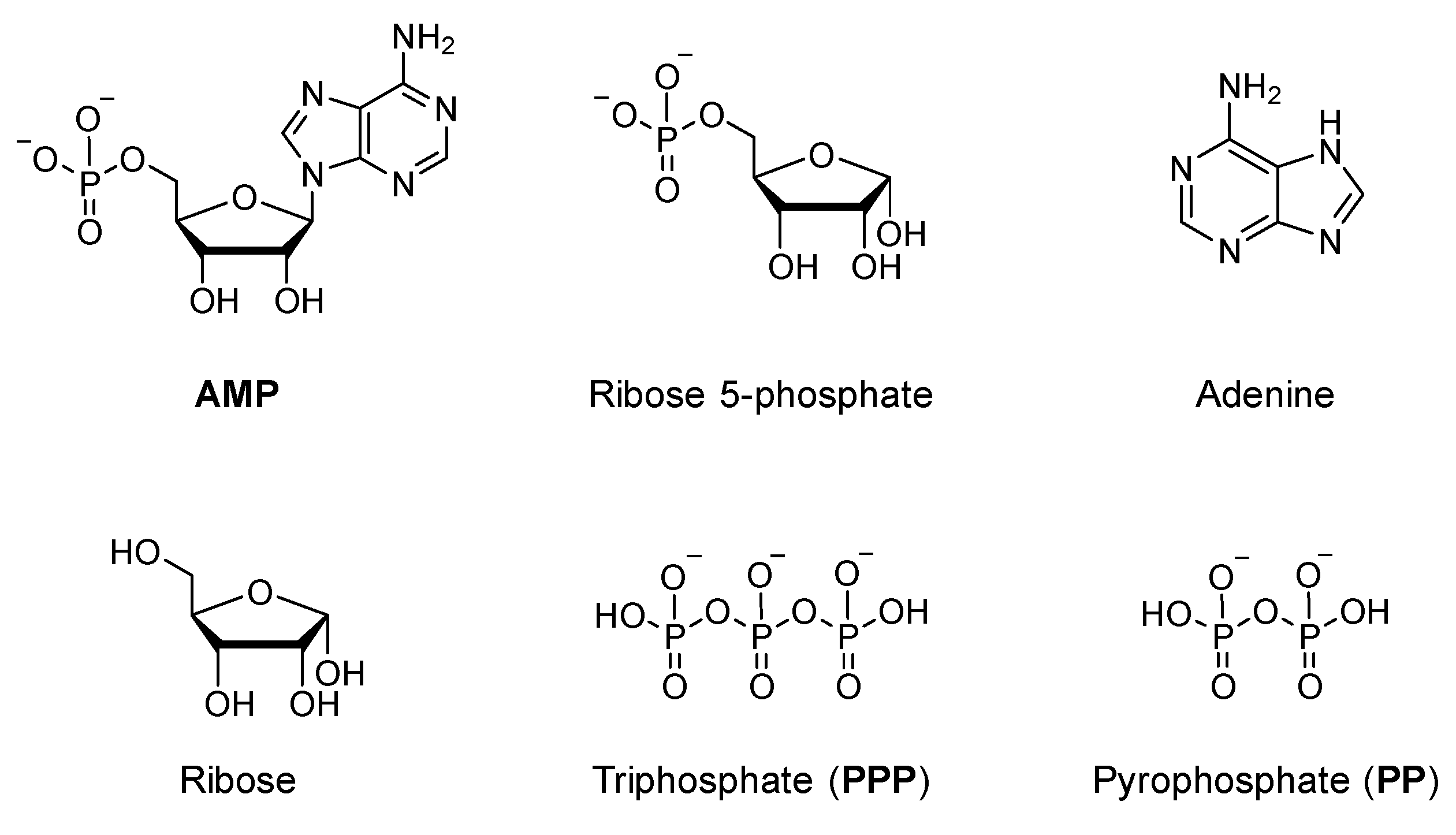
3.3. NMR Study of ATP–Vanadate Interactions
3.3.1. 31P and 51V NMR snapshots of reaction mixtures
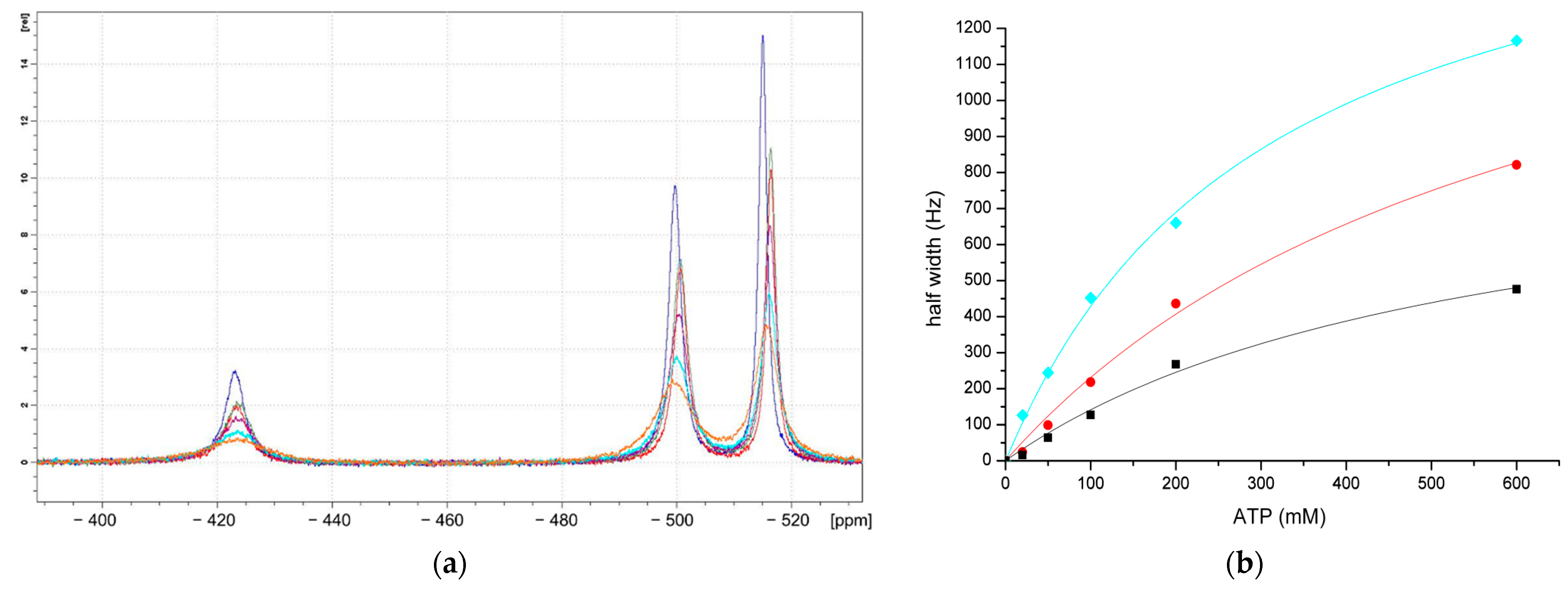
3.3.2. Binding between ATP and Decavanadate Studied by 51V-NMR Spectroscopy
3.3.3. Interaction between Vanadates and Other Substrates Structurally Related to ATP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Arefian, M.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. A survey of the different roles of polyoxometalates in their interaction with amino acids, peptides and proteins. Dalton Trans. 2017, 46, 6812–6829. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [Green Version]
- Guedes, G.; Wang, S.; Santos, H.A.; Sousa, F.L. Polyoxometalate Composites in Cancer Therapy and Diagnostics. Eur. J. Inorg. Chem. 2020, 2020, 2121–2132. [Google Scholar] [CrossRef]
- Rehder, D. Biological and medicinal aspects of vanadium. Inorg. Chem. Commun. 2003, 6, 604–617. [Google Scholar] [CrossRef]
- Rehder, D. Influence of Vanadium Compounds on Cellular Functions. In Bioinorganic Vanadium Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 157–201. [Google Scholar]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The (biological) speciation of vanadate(V) as revealed by 51V NMR: A tribute on Lage Pettersson and his work. J. Inorg. Biochem. 2015, 147, 25–31. [Google Scholar] [CrossRef]
- Steens, N.; Ramadan, A.M.; Parac-Vogt, T.N. When structural and electronic analogy leads to reactivity: The unprecedented phosphodiesterase activity of vanadates. Chem. Commun. 2009, 965–967. [Google Scholar] [CrossRef]
- Steens, N.; Ramadan, A.M.; Absillis, G.; Parac-Vogt, T.N. Hydrolytic cleavage of DNA-model substrates promoted by polyoxovanadates. Dalton Trans. 2010, 39, 585–592. [Google Scholar] [CrossRef]
- Walsh, C.T.; Tu, B.P.; Tang, Y. Eight Kinetically Stable but Thermodynamically Activated Molecules that Power Cell Metabolism. Chem. Rev. 2018, 118, 1460–1494. [Google Scholar] [CrossRef]
- Ahmann, F.R.; Garewal, H.S.; Schifman, R.; Celniker, A.; Rodney, S. Intracellular adenosine triphosphate as a measure of human tumor cell viability and drug modulated growth. In Vitro Cell. Dev. Biol. 1987, 23, 474–480. [Google Scholar] [CrossRef]
- Lu, X.; Errington, J.; Chen, V.J.; Curtin, N.J.; Boddy, A.V.; Newell, D.R. Cellular ATP Depletion by LY309887 as a Predictor of Growth Inhibition in Human Tumor Cell Lines. Clin. Cancer Res. 2000, 6, 271–277. [Google Scholar] [PubMed]
- Martin, D.S.; Bertino, J.R.; Koutcher, J.A. ATP Depletion + Pyrimidine Depletion Can Markedly Enhance Cancer Therapy: Fresh Insight for a New Approach. Cancer Res. 2000, 60, 6776–6783. Available online: https://cancerres.aacrjournals.org/content/canres/60/24/6776.full.pdf (accessed on 15 September 2021). [PubMed]
- Granot, J. Nuclear magnetic resonance studies of catecholamines. Ternary complexes with adenosine 5’-triphosphate and divalent metal ions in aqueous solution. J. Am. Chem. Soc. 1978, 100, 2886–2891. [Google Scholar] [CrossRef]
- Tetas, M.; Lowenstein, J.M. The Effect of Bivalent Metal Ions on the Hydrolysis of Adenosine Di- and Triphosphate*. Biochemistry 1963, 2, 350–357. [Google Scholar] [CrossRef]
- Wilson, J.E.; Chin, A. Chelation of divalent cations by ATP, studied by titration calorimetry. Anal. Biochem. 1991, 193, 16–19. [Google Scholar] [CrossRef]
- Granot, J.; Fiat, D. Proton magnetic resonance study of divalent metal ions binding to adenosine 5′-triphosphate. J. Am. Chem. Soc. 1977, 99, 70–79. [Google Scholar] [CrossRef]
- Absillis, G.; Cartuyvels, E.; Van Deun, R.; Parac-Vogt, T.N. Hydrolytic Cleavage of an RNA-Model Phosphodiester Catalyzed by a Highly Negatively Charged Polyoxomolybdate [Mo7O24]6− Cluster. J. Am. Chem. Soc. 2008, 130, 17400–17408. [Google Scholar] [CrossRef]
- Cartuyvels, E.; Absillis, G.; Parac-Vogt, T.N. Questioning the paradigm of metal complex promoted phosphodiester hydrolysis: [Mo7O24]6− polyoxometalate cluster as an unlikely catalyst for the hydrolysis of a DNA model substrate. Chem. Commun. 2008, 85–87. [Google Scholar] [CrossRef]
- Lokeren, L.V.; Cartuyvels, E.; Absillis, G.; Willem, R.; Parac-Vogt, T.N. Phosphoesterase activity of polyoxomolybdates: Diffusion ordered NMR spectroscopy as a tool for obtaining insights into the reactivity of polyoxometalate clusters. Chem. Commun. 2008, 2774–2776. [Google Scholar] [CrossRef]
- Martins, F.F.; Sánchez-González, Á.; Lanuza, J.; Miras, H.N.; Lopez, X.; Bandeira, N.A.G.; Gil, A. Probing the Catalytically Active Species in POM-catalysed DNA-model Hydrolysis. Chem. Eur. J. 2021, 27, 8977–8984. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef] [PubMed]
- Selling, A.; Andersson, I.; Pettersson, L.; Schramm, C.M.; Downey, S.L.; Grate, J.H. Multicomponent Polyanions. 47. The Aqueous Vanadophosphate System. Inorg. Chem. 1994, 33, 3141–3150. [Google Scholar] [CrossRef]
- Ishikawa, E.; Yamase, T. 31P NMR and isothermal titration calorimetry studies on polyoxomolybdates-catalyzed hydrolysis of ATP. J. Inorg. Biochem. 2006, 100, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Gresser, M.J.; Tracey, A.S.; Parkinson, K.M. Vanadium(V) oxyanions: The interaction of vanadate with pyrophosphate, phosphate, and arsenate. J. Am. Chem. Soc. 1986, 108, 6229–6234. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Castro, M.M.C.A. Multinuclear NMR study of the interaction of vanadate with mononucleotides, ADP, and ATP. J. Inorg. Biochem. 1989, 37, 213–232. [Google Scholar] [CrossRef]
- Kato, R.; Kobayashi, A.; Sasaki, Y. The heteropolyvanadate of phosphorus. Crystallographic and NMR studies. Inorg. Chem. 1982, 21, 240–246. [Google Scholar] [CrossRef]
- Andersson, I.; Gorzsás, A.; Kerezsi, C.; Tóth, I.; Pettersson, L. Speciation in the aqueous H+/H2VO4−/H2O2/phosphate system. Dalton Trans. 2005, 3658–3666. [Google Scholar] [CrossRef]
- Richter, J.; Rehder, D. Modeling the Transition State of Biological Phosphoester Cleavage: The Complexation of Vanadate(V) by Nucleosides. Z. Naturforsch. B 1991, 46, 1613–1620. [Google Scholar] [CrossRef]
- Angus-Dunne, S.J.; Batchelor, R.J.; Tracey, A.S.; Einstein, F.W.B. The Crystal and Solution Structures of the Major Products of the Reaction of Vanadate with Adenosine. J. Am. Chem. Soc. 1995, 117, 5292–5296. [Google Scholar] [CrossRef]
- Elvingson, K.; Crans, D.C.; Pettersson, L. Speciation in Vanadium Bioinorganic Systems. 4. Interactions between Vanadate, Adenosine, and ImidazoleAn Aqueous Potentiometric and 51V NMR Study. J. Am. Chem. Soc. 1997, 119, 7005–7012. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Chen, Y. Critical evaluation of stability constants for nucleotide complexes with protons and metal ions and the accompanying enthalpy changes. Pure Appl. Chem. 1991, 63, 1015–1080. [Google Scholar] [CrossRef]
- Sigel, H.; Griesser, R. Nucleoside 5′-triphosphates: Self-association, acid–base, and metal ion-binding properties in solution. Chem. Soc. Rev. 2005, 34, 875–900. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, C.; Milea, D.; Pettignano, A.; Sammartano, S. Modeling ATP protonation and activity coefficients in NaClaq and KClaq by SIT and Pitzer equations. Biophys. Chem. 2006, 121, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The Bioinorganic Chemistry of Vanadium. Angew. Chem. Int. Ed. 1991, 30, 148–167. [Google Scholar] [CrossRef]
- Comba, P.; Helm, L. The solution structure and reactivity of decavanadate. Helv. Chim. Acta 1988, 71, 1406–1420. [Google Scholar] [CrossRef]
- Crans, D.C.; Rithner, C.D.; Theisen, L.A. Application of time-resolved vanadium-51 2D NMR for quantitation of kinetic exchange pathways between vanadate monomer, dimer, tetramer, and pentamer. J. Am. Chem. Soc. 1990, 112, 2901–2908. [Google Scholar] [CrossRef]
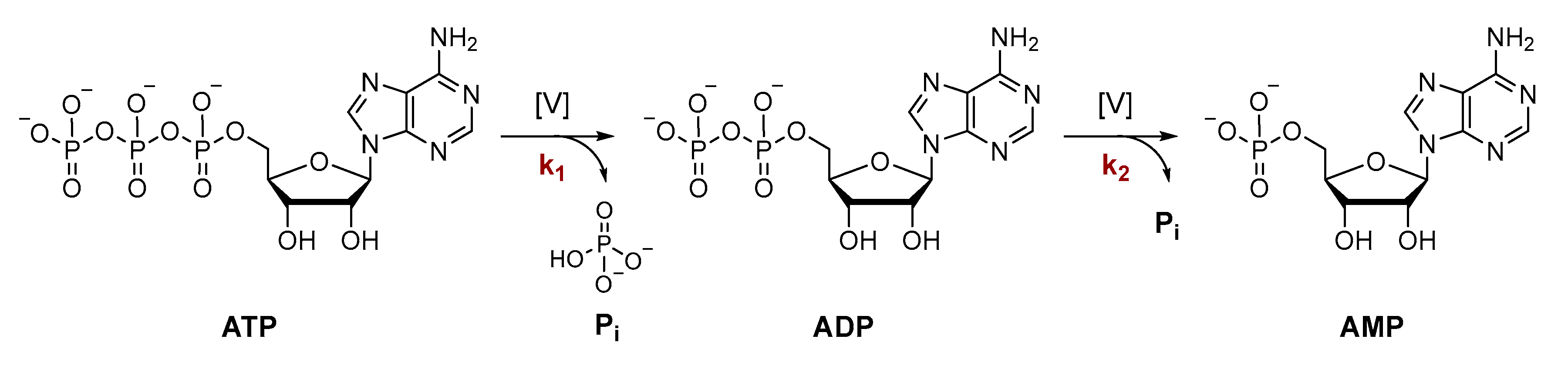
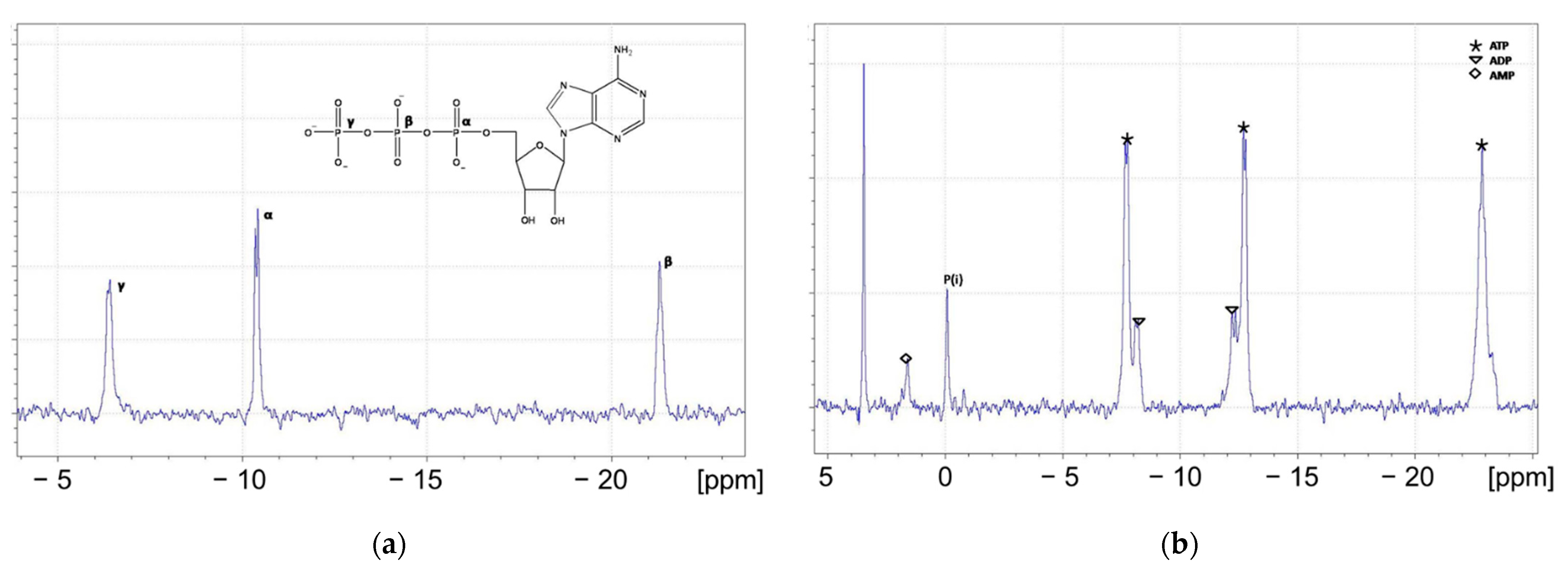


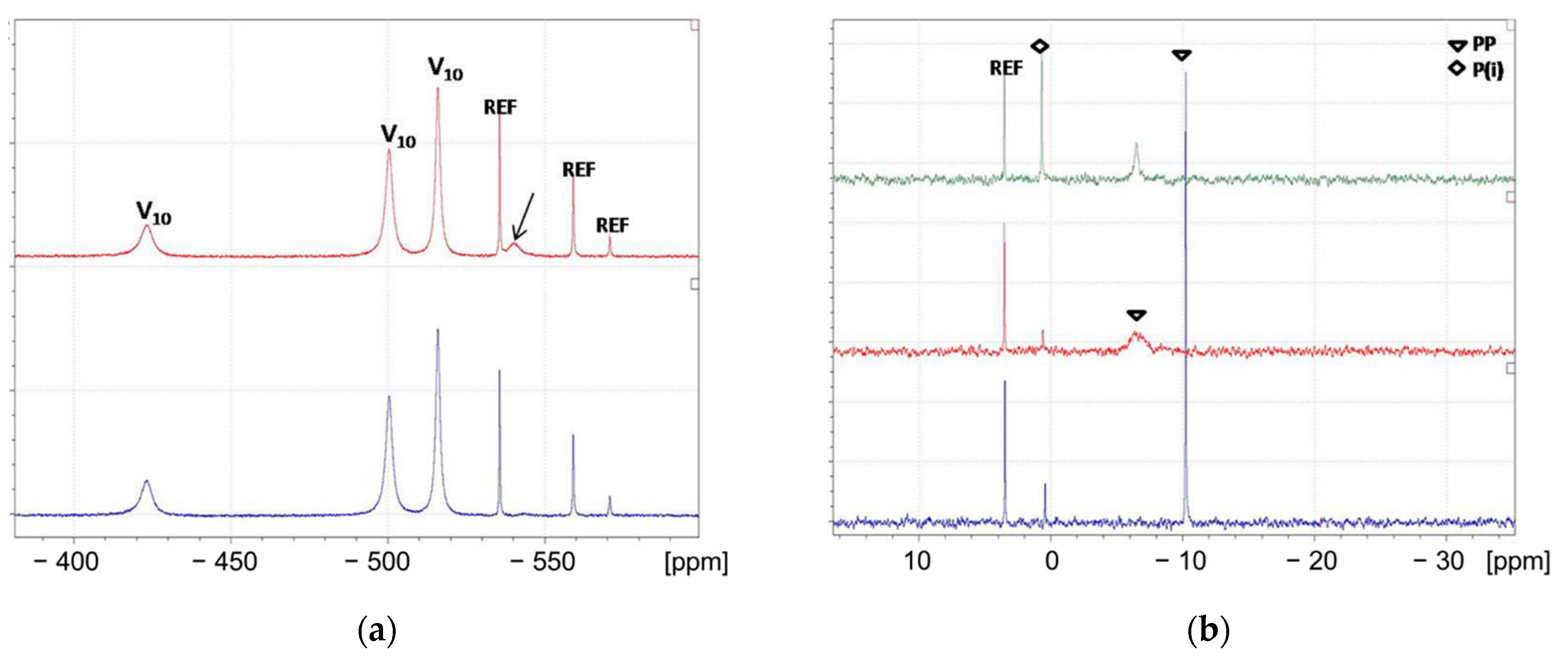

| Region | Chemical Shift Range | Species Monitored |
|---|---|---|
| R1 | 3 ppm → −0.5 ppm | AMP, free phosphate (Pi) |
| R2 | −4 ppm → −12 ppm | ADP (2 P), ATP (γ-P, α-P) |
| R3 | −15 ppm → −25 ppm | ATP (β-P) |
| Quantity | Reaction Time | ||||
|---|---|---|---|---|---|
| t = 0 | t = 15 min | t = 45 min | t = 225 min | t = 735 min | |
| Integration values R1 | 0 | 0.217 | 0.375 | 0.558 | 0.897 |
| Integration values R2 | 0.666 | 0.663 | 0.625 | 0.442 | 0.103 |
| Integration values R3 | 0.333 | 0.120 | 0 | 0 | 0 |
| ATP (relative value) | 0.333 | 0.120 | 0 | 0 | 0 |
| ADP (relative value) | 0 | 0.212 | 0.313 | 0.221 | 0.052 |
| AMP (relative value) | 0 | 0.002 | 0.021 | 0.112 | 0.282 |
| ATP (absolute value, mM) | 20 | 7.20 | 0 | 0 | 0 |
| ADP (absolute value, mM) | 0 | 12.70 | 18.75 | 13.25 | 3.09 |
| AMP (absolute value, mM) | 0 | 0.10 | 1.25 | 6.75 | 16.91 |
| pH | 25 °C | 37 °C | 50 °C | |||
|---|---|---|---|---|---|---|
| k1 (×10−5) | k2 (×10−5) | k1 (×10−5) | k2 (×10−5) | k1 (×10−5) | k2 (×10−5) | |
| 2 | 131 | 4.67 | 177 | 34 | 1073 | 38.33 |
| 4 | 1.23 | 0.53 | 5.01 | 5.92 | 11.67 | 12.10 |
| 6 | n.d. | n.d. | 1.15 | 1.25 | 3.53 | 2.35 |
| 7 | n.d. | n.d. | 0.13 | 0.17 | 0.43 | 0.43 |
| Compound | pKa1/pKa2/pKa3 | pH 2 | pH 4 | pH 6 | pH 7 |
|---|---|---|---|---|---|
| ATP | 6.5/4.0 | H2ATP2− | H2ATP2−/HATP3− | HATP3− | ATP4− |
| ADP | 6.2/3.8 | H2ADP− | H2ADP−/HADP2− | HADP2− | ADP3− |
| AMP | 6.1/3.7 | H2AMP | H2AMP/HAMP− | HAMP−/AMP2- | AMP2− |
| V10, V4, V5 | 5.5/3.5/2.0 (V10) | H3V10O283−/H2V10O284− | HV10O285- | V10O286−/V4O124−/V5O105− | V4O124−/V5O105− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Azambuja, F.; Steens, N.; Parac-Vogt, T.N. Kinetic and Interaction Studies of Adenosine-5′-Triphosphate (ATP) Hydrolysis with Polyoxovanadates. Metals 2021, 11, 1678. https://doi.org/10.3390/met11111678
de Azambuja F, Steens N, Parac-Vogt TN. Kinetic and Interaction Studies of Adenosine-5′-Triphosphate (ATP) Hydrolysis with Polyoxovanadates. Metals. 2021; 11(11):1678. https://doi.org/10.3390/met11111678
Chicago/Turabian Stylede Azambuja, Francisco, Nele Steens, and Tatjana N. Parac-Vogt. 2021. "Kinetic and Interaction Studies of Adenosine-5′-Triphosphate (ATP) Hydrolysis with Polyoxovanadates" Metals 11, no. 11: 1678. https://doi.org/10.3390/met11111678
APA Stylede Azambuja, F., Steens, N., & Parac-Vogt, T. N. (2021). Kinetic and Interaction Studies of Adenosine-5′-Triphosphate (ATP) Hydrolysis with Polyoxovanadates. Metals, 11(11), 1678. https://doi.org/10.3390/met11111678








