Ternary Copper Complex of L-Glutamine and Phenanthroline as Counterions of Cyclo-Tetravanadate Anion: Experimental–Theoretical Characterization and Potential Antineoplastic Activity
Abstract
1. Introduction
2. Results
2.1. Infrared Spectroscopy
2.2. Visible Spectroscopy
2.3. Computational Calculations
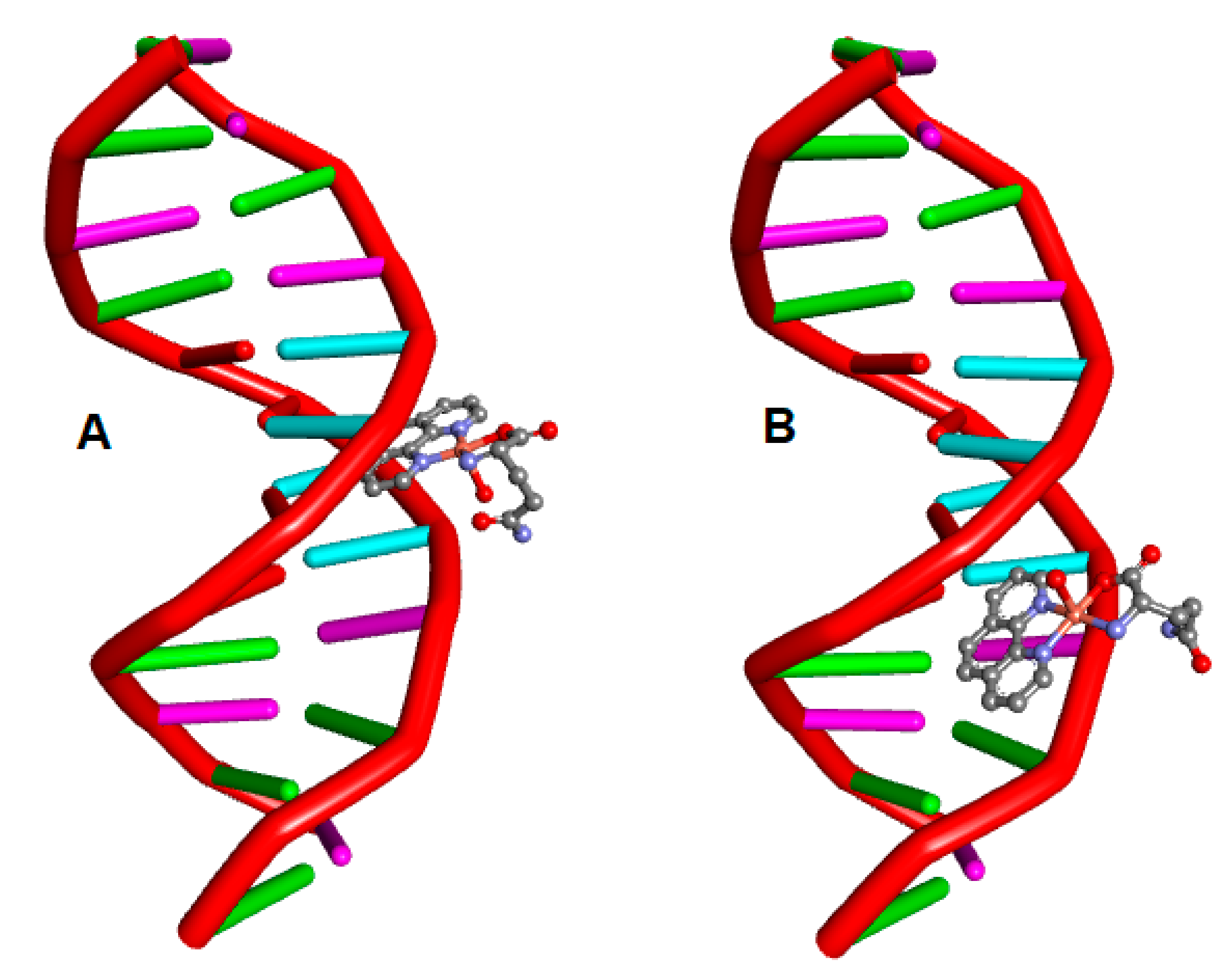
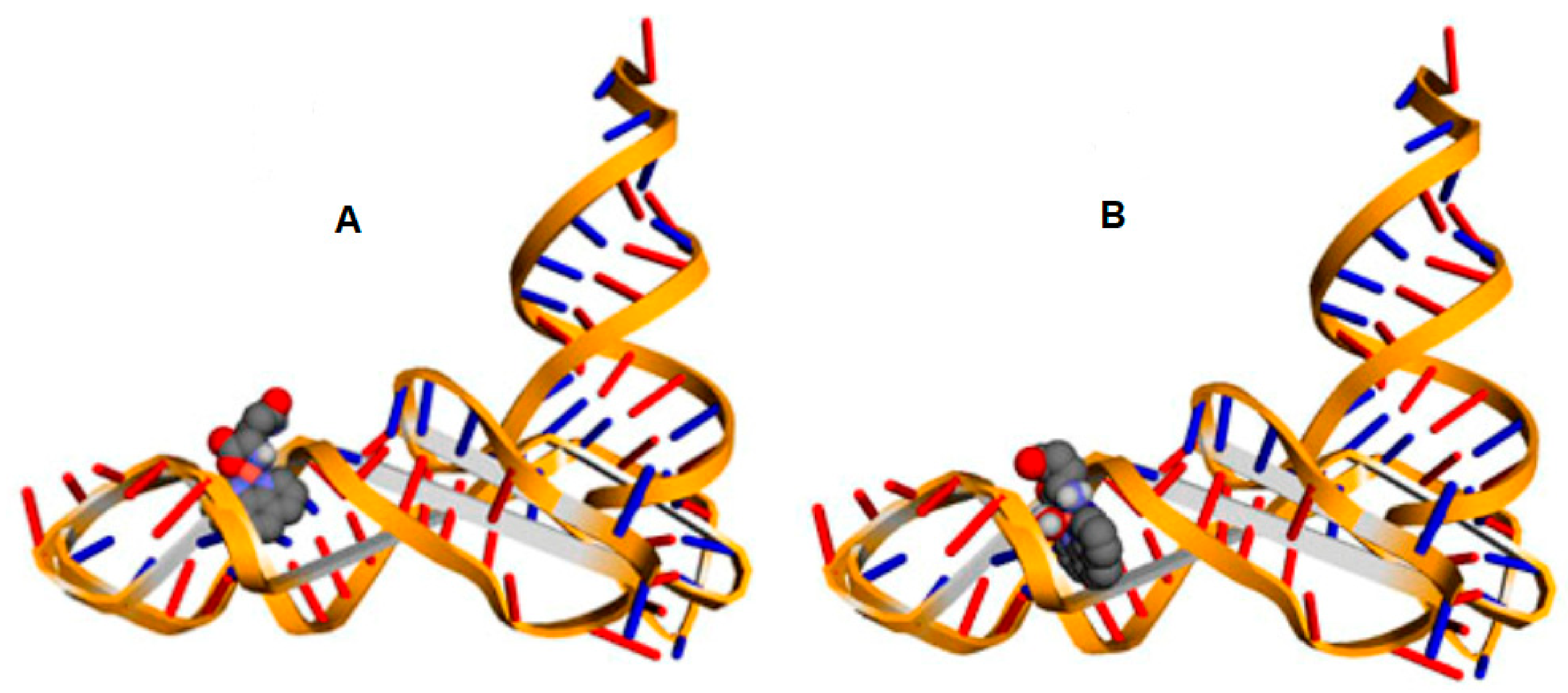
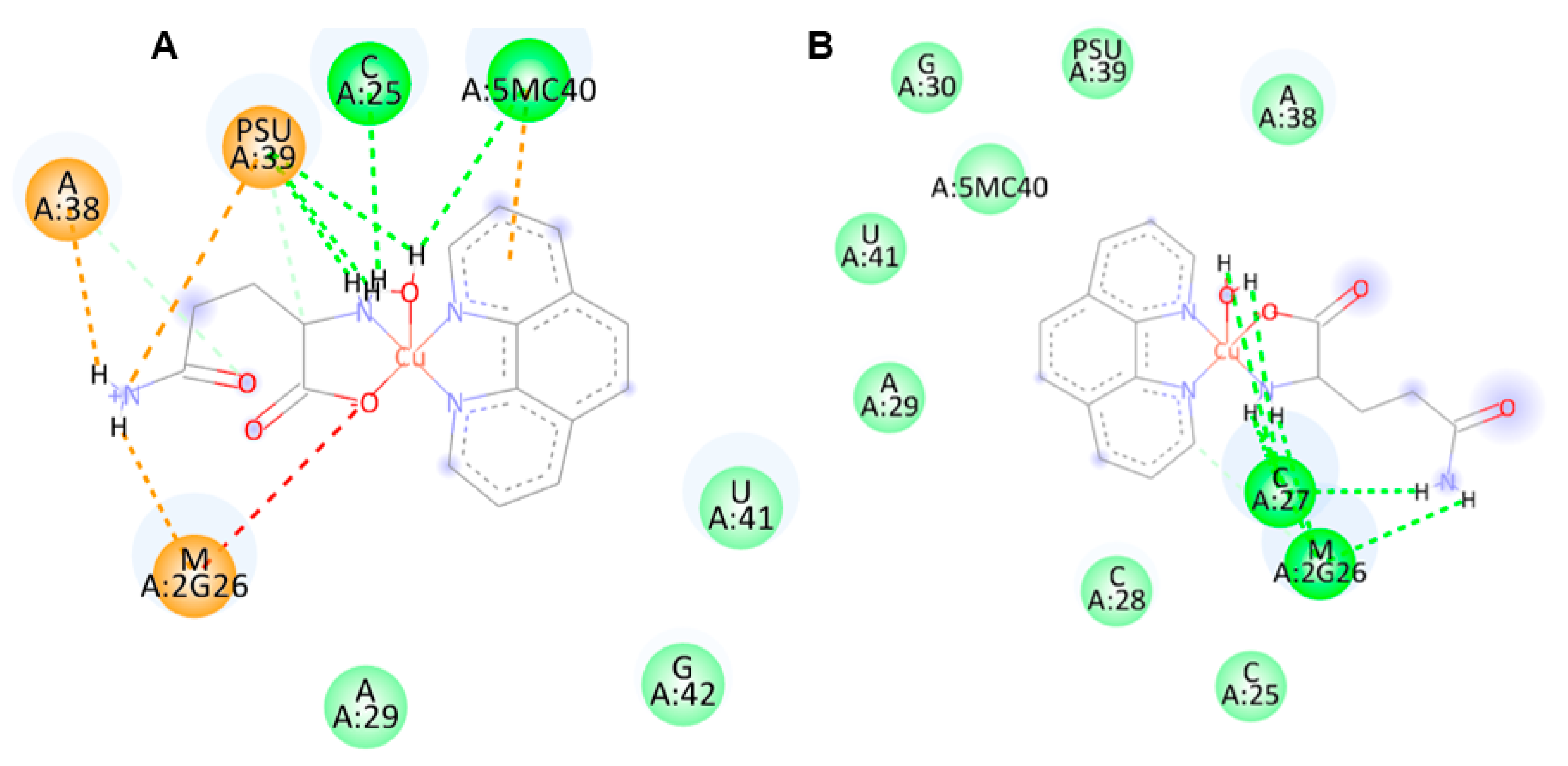
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. General Considerations
4.2. Crystallization and Synthesis
4.3. Single Crystal X-ray Diffraction
4.4. Computational Methods
4.5. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Zhu, W.; Yang, W.; Chen, Z.; Song, J.; Song, X.; Chen, X.; Yang, H. Engineered Nanoscale Vanadium Metallodrugs for Robust Tumor-Specific Imaging and Therapy. Adv. Funct. Mater. 2021, 31, 2010337. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- OPS/OMS, Programa de Cáncer. Available online: https://www.paho.org/es/campanas/dia-mundial-contra-cancer-2021-yo-soy-voy (accessed on 24 September 2021).
- Spreckelmeyer, S.; Orvig, C.; Casini, A. Cellular transport mechanisms of cytotoxic metallodrugs: An overview beyond cisplatin. Molecules 2014, 19, 15584–15610. [Google Scholar] [CrossRef]
- Ndagi, U.; Ndumiso, M.; Mahmoud, E.S. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The Status of Platinum Anticancer Drugs in the Clinic and in Clinical Trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Valdéz-Camacho, J.R.; Ramírez-Solís, A.; Escalante, J.; Ruiz-Azuara, L.; Ho, M. Theoretical determination of half-wave potentials for phenanthroline-, bipyridine-, acetylacetonate-, and glycinate-containing copper (II) complexes. J. Mol. Model. 2020, 26, 191. [Google Scholar] [CrossRef]
- Krajčiová, D.; Melník, M.; Havránek, E.; Forgácsová, A.; Mikuš, P. Copper compounds in nuclear medicine and oncology. J. Coord. Chem. 2014, 67, 1493–1519. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bravo-Gómez, M.E. Copper Compounds in Cancer Chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper Complexes in Cancer Therapy. Met. Ions Life Sci. 2018, 18, 469–506. [Google Scholar] [CrossRef]
- Dhakshanamoorthy, S.; Krishnan, M.M.; Arumugham, M.N. Synthesis, characterization, DNA binding/cleavage, anticancer and antimicrobial activity of ternary copper(II) complexes. Asian J. Res. Chem. 2017, 10, 312–318. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Arumugham, M.N. Synthesis, characterization DNA binding and biological activity of Copper(II) complexes with mixed ligands. J. Chem. Biol. Phys. Sci. 2017, 7, 896–905. [Google Scholar]
- Tabti, R.; Tounsi, N.; Gaiddon, C.; Bentouhami, E.; Désaubry, L. Progress in Copper Complexes as Anticancer Agents. Med. Chem. 2017, 7, 875–879. [Google Scholar] [CrossRef]
- İnci, D.; Aydin, R. A review of DNA binding activities of metal (II) complexes containing aromatic amino acids and intercalating ligands. In Natural Sciences: Methods and Applications; Iksad Publishing House: Ankara, Turkey, 2021; Volume 89, Chapter 4. [Google Scholar]
- Sigman, D.S.; Graham, D.R.; Aurora, V.D.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979, 254, 12269–12272. [Google Scholar] [CrossRef]
- Mancin, F.; Scrimin, P.; Tecilla, P.; Tonellato, U. Artificial metallonucleases. Chem. Commun. 2005, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.R.; Iranzo, O. Synthetic metallonucleases for RNA cleavage. Curr. Opin. Chem. Biol. 2004, 8, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mancin, F.; Scrimin, P.; Tecilla, P. Progress in artificial metallonucleases. Chem. Commun. 2012, 48, 5545–5559. [Google Scholar] [CrossRef] [PubMed]
- McGivenrn, T.J.P.; Marmion, A.C.J. Copper complexes as artificial DNA metallonucleases: From Sigman’s reagent to a next-generation anticancer agent? Inorg. Chim. Acta 2018, 472, 12–39. [Google Scholar] [CrossRef]
- Dupureur, C.M. An Integrated Look at Metallonuclease Mechanism. Curr. Chem. Biol. 2008, 2, 159–173. [Google Scholar]
- Yu, Z.; Cowan, J.A. Metal complexes promoting catalytic cleavage of nucleic acids—Biochemical tools and therapeutics. Curr. Opin. Chem. Biol. 2018, 43, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Das, R.K. DNA Cleavage by Transition Metal Complexes and its Applications—A Review. Int. J. Pharm. Biol. Sci. 2019, 9, 1089–1106. [Google Scholar]
- Williams, D.E.; Grant, K.B. Metal-Assisted Hydrolysis Reactions Involving Lipids: A Review. Front. Chem. 2019, 7, 14. [Google Scholar] [CrossRef]
- Lüdtke, C.; Sobottka, S.; Heinrich, J.; Liebing, P.; Wedepohl, S.; Sarkar, B.; Kulak, N. Forty Years after the Discovery of Its Nucleolytic Activity: [Cu(phen)2]2+ Shows Unattended DNA Cleavage Activity upon Fluorination. Chem.-Eur. J. 2020, 27, 3273–3277. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Yan, M.; Wang, H.; Zhang, C. Novel copper complexes as potential proteasome inhibitors for cancer treatment (Review). Mol. Med. Rep. 2017, 15, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, C.; Martoniati, A.; Pelinski, L.; Cailliau, K. Phenanthroline-Cu(II) complexes modulated by amino acids can induce cancer cell apoptosis via mitochondrial signaling. Cancers 2020, 12, 2863. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Uivarosi, V.; Olar, R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules 2020, 25, 5830. [Google Scholar] [CrossRef]
- Zehra, S.; Tabassum, S.; Arjmand, F. Biochemical pathways of copper complexes: Progress over the past 5 years. Drug Discov. Today 2021, 26, 1086–1096. [Google Scholar] [CrossRef]
- Erxleben, A. Interactions of copper complexes with nucleic acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and Its Complexes in Medicine: A Biochemical Approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Ng, C.H.; Kong, S.M.; Tiong, Y.L.; Maah, M.J.; Sukram, N.; Ahmad, M.; Khoo, A.S.B. Selective anticancer copper(II)-mixed ligand complexes: Targeting of ROS and proteasomes. Metallomics 2014, 6, 892–906. [Google Scholar] [CrossRef]
- Alvarez, N.; Viña, D.; Leite, C.M.; Mendes, L.F.S.; Batista, A.A.; Ellena, J.; Costa-Filho, A.; Facchin, G. Synthesis and structural characterization of a series of ternary copper(II)-L-dipeptide-neocuproine complexes. Study of their cytotoxicity against cancer cells including MDA-MB-231, triple-negative breast cancer cells. J. Inorg. Biochem. 2019, 203, 110930. [Google Scholar] [CrossRef]
- Mroueh, M.; Daher, C.; Hariri, E.; Demirdjian, S.; Isber, S.; Choi, E.S.; Mirtamizdoust, B.; Hammud, H.H. Magnetic property, DFT calculation, and biological activity of bis[(μ(2)-chloro)chloro(1,10-phenanthroline)copper(II)] complex. Chem.-Biol. Interact. 2015, 231, 53–60. [Google Scholar] [CrossRef]
- Miranda-Calderón, J.E.; Macías-Rosales, L.; Gracia-Mora, I.; Ruíz-Azuara, L.; Faustino-Vega, A.; Gracia-Mora, J.; Bernad-Bernad, M.J. Effect of casiopein III-ia loaded into chitosan nanoparticles on tumor growth inhibition. J. Drug Deliv. Sci. Technol. 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Reina, M.; Hernández-Ayala, L.F.; Bravo-Gómez, M.E.; Gómez, V.; Ruiz-Azuara, L. Second generation of Casiopeinas®: A joint experimental and theoretical study. Inorg. Chim. Acta 2021, 517, 120201. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Gomes, J.; Oliveira da Silva, A.; Ayala, A.P.; Santos-Oliveira, R.; Silva, A.; Ferreira, F. Copper(II):phenanthroline complexes with L-asparagine and L-methionine: Synthesis, crystal structure and in-vitro cytotoxic effects on prostate, breast, and melanoma cancer cells. Polyhedron 2020, 191, 114807. [Google Scholar]
- Zhang, S.; Chun, X.; Chen, Y.; Zhou, J. Synthesis, Crystal Structure and DNA Cleavage Activity of a Ternary Copper(II) Complex of Dipyrido[3,2-d:2′,3′-f]-quinoxaline and Glycine. Chin. J. Chem. 2011, 29, 65–71. [Google Scholar] [CrossRef]
- Nakahata, D.H.; De Paiva, R.E.F.; Lustri, W.R.; Ribeiro, C.M.; Pavan, F.R.; Da Silva, G.G.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Corbi, P.P. Sulfonamide-containing copper(II) metallonucleases: Correlations with in vitro antimycobacterial and antiproliferative activities. J. Inorg. Biochem. 2018, 187, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Azuara, L.; Fuentes Noriega, I.; Espinoza Guillén, A.; García Conde, D.; Cortés Guzmán, F. Composición Parental de Casiopeína y Sus Usos de la Misma; Gaceta Digital del Instituto de Química UNAM: México City, Mexico, 2017. [Google Scholar]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; González-Vergara, E. Metformin-decavanadate treatment ameliorates hyperglycemia and redox balance of the liver and muscle in a rat model of alloxan-induced Diabetes. New J. Chem. 2019, 43, 17850–17862. [Google Scholar] [CrossRef]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic or anticancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar]
- Aureliano, M. The role of decavanadate in antitumor activity. Glob. J Cancer Ther. 2017, 3, 012–014. [Google Scholar] [CrossRef]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stepniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef]
- Lu, L.-P.; Suo, F.-Z.; Feng, Y.-L.; Song, L.-L.; Li, Y.; Li, Y.-J.; Wang, K.-T. Synthesis and biological evaluation of vanadium complex as novel antitumor agents. Eur. J. Med. Chem. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Griffin, E.; Levina, A.; Lay, P.A. Vanadium(V) tris-3,5-di-tert-butylcatecholato complex: Links between speciation and antiproliferative activity in human pancreatic cancer cells. J. Inorg. Biochem. 2019, 201, 110815. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.; Arru, C.; Serra, M.; Galleri, G.; Sanna, D.; Garribba, E.; Palmieri, G.; Rozzo, C. Antiproliferative activity of vanadium compounds: Effects on the major malignant melanoma molecular pathways. Metallomics 2019, 11, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Vieira, A.P.; Wijetunga, A.; Kaur, R.; Koehn, J.T.; Crans, D.C.; Lay, P.A. A Short-Lived but Highly Cytotoxic Vanadium(V)Complex as a Potential Drug Lead for Brain Cancer Treatment by Intratumoral Injections. Angew. Chem. 2020, 132, 15968–15972. [Google Scholar] [CrossRef]
- León, I.L.; Ruiz, M.C.; Franca, C.A.; Parajón-Costa, B.S.; Baran, E.J. Metvan, bis(4,7-Dimethyl-1,10-phenanthroline) sulfatooxidovanadium(IV): DFT and Spectroscopic Study—Antitumor Action on Human Bone and Colorectal Cancer Cell Lines. Biol. Trace Elem. Res. 2019, 191, 81–87. [Google Scholar] [CrossRef]
- Althumairy, D.; Postal, K.; Barisas, B.G.; Nunes, G.G.; Roess, D.A.; Crans, D.C. Polyoxometalates function as indirect activators of a G protein-coupled receptor. Metallomics 2020, 12, 1044–1061. [Google Scholar] [CrossRef]
- Althumairy, D.; Murakami, H.A.; Zhang, D.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Effects of vanadium(IV) compounds on plasma membrane lipids lead to G protein-coupled receptor signal transduction. J. Inorg. Biochem. 2020, 203, 110873. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yu, H.; Gao, Y.; Guo, W.; Li, Z.; Chen, Y.; Pan, Q.; Ren, M.; Han, X.; Guo, C. Surface-engineered vanadium nitride nanosheets for an imaging-guided photothermal/photodynamic platform of cancer treatment. Nanoscale 2019, 11, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Huang, R.T.; Liang, J.; Zhou, B.; Guo, X.; Shen, X.C.; Jiang, B.P. Natural Polyphenol-vanadium Oxide Nanozymes for Synergistic Chemodynamic/Photothermal Therapy. Chem. Eur. J. 2020, 26, 15159. [Google Scholar] [CrossRef] [PubMed]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coordination. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Santos, M.F.A.; Correia, I.; Sanna, D.; Sciortino, G.; Garriba, E. Binding of vanadium ions and complexes to proteins and enzymes in aqueous solution. Coord. Chem. Rev. 2021, 449, 214192. [Google Scholar] [CrossRef]
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health Benefits of Vanadium and Its Potential as an Anticancer Agent. Met. Ions Life Sci. 2018, 18, 251–279. [Google Scholar]
- Redher, D. The potentiality of vanadium in medicinal applications. Inorg. Chim. Acta 2020, 504, 119445. [Google Scholar]
- Alves de Lima, L.M.; Da Silva, A.K.J.; De Mendonca, T.F.; Da Silva, J.P.; Moura, S.V.N.; Batista, E.K.; Lira, E.C.; Belian, M.F. “Redescobrindo e redesenhando” estratégias para obtenção de complexos de vanádio com atividade anti-diabética. Rev. Virtual Quim. 2021, 13, 933–952. [Google Scholar] [CrossRef]
- Samart, N.; Althumairy, D.; Zhang, D.; Roess, D.; Crans, D.C. Initiation of a novel mode of membrane signaling: Vanadium facilitated signal transduction. Coord. Chem. Rev. 2020, 416, 213286. [Google Scholar] [CrossRef]
- Lima, L.M.A.; Murakami, H.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Treviño, S.; Sánchez-Gaytán, B.L.; Sáchez-Mora, E.; Castro, M.E.; Meléndez-Bustamante, F.J.; Méndez-Rojas, M.A.; González-Vergara, E. Decavanadate Salts of Cytosine and Metformin: A Combined Experimental-Theoretical Study of Potential Metallodrugs against Diabetes and Cancer. Front. Chem. 2018, 6, 402. [Google Scholar] [CrossRef]
- Cheng, M.; Li, N.; Hu, N.W.K.; Xiao, Z.; Wu, P.; Wei, Y. Synthesis, structure and antitumor studies of a novel decavanadate complex with a wavelike two-dimensional network. Polyhedron 2018, 155, 313–319. [Google Scholar] [CrossRef]
- Silva-Nolasco, A.M.; Camacho, L.; Saavedra-Díaz, R.O.; Hernández-Abreu, O.; León, I.E.; Sánchez-Lombardo, I. Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and In Vitro Cytotoxicity and Insulin-Like Activity. Inorganics 2020, 8, 67. [Google Scholar] [CrossRef]
- Ksiksi, R.; Abdelkafi-Koubaa, Z.; Mlayah-Bellalouna, S.; Aissaoui, D.; Marrakchi, N.; Srairi-Abid, N.; Zid, M.F.; Graia, M. Synthesis, structural characterization and antitumoral activity of (NH4)4Li2V10O28.10H2O compound. J. Mol. Struct. 2021, 1229, 129492. [Google Scholar] [CrossRef]
- Louati, M.; Ksiksi, R.; Elbini-Dhouib, I.; Mlayah-Bellalouna, S.; Doghri, R.; Srairi-Abid, N.; Zid, M.-F. Synthesis, structure, and characterization of a novel decavanadate, Mg(H2O)6(C4N2H7)4V10O28.4H2O. J. Mol. Struct. 2021, 1242, 130711. [Google Scholar] [CrossRef]
- Irving, E.; Tagalakis, A.D.; Maeshima, R.; Hart, S.L.; Eaton, S.; Lehtonen, A.; Stoker, A.W. The liposomal delivery of hydrophobic oxidovanadium complexes imparts highly effective cytotoxicity and differentiating capacity in neuroblastoma tumor cells. Sci. Rep. 2020, 10, 16660. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Swanson, C.H.; Smith, G.C.; Moschona, A.; Hadjispyrou, S.; Salifoglou, A.; Pantazaki, A.A.; Pelecanou, M.; Litsardakis, G. Magnetic cationic liposomal nanocarriers for the efficient drug delivery of a curcumin-based vanadium complex with anticancer potential. J. Inorg. Biochem. 2019, 199, 110778. [Google Scholar] [CrossRef]
- Amante, C.; Sousa-Coelho, D.; Luísa, A.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Correia, I.; Borovic, S.; Cavaco, I.; Matos, C.P.; Roy, S.; Santos, H.M.; Fernandes, L.; Capelo, J.L.; Ruiz-Azuara, L.; Costa Pessoa, J. Evaluation of the binding of four anti-tumor Casiopeínas® to human serum albumin. J. Inorg. Biochem. 2017, 175, 284–297. [Google Scholar] [CrossRef]
- Nunes, P.; Correia, I.; Cavaco, I.; Marques, F.; Pinheiro, T.; Avecilla, F.; Costa Pessoa, J. Therapeutic potential of vanadium complexes with 1, 10-phenanthroline ligands, quo vadis? Fate of complexes in cell media and cancer cells. J. Inorg. Biochem. 2021, 217, 111350. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Lay, P.A. Stabilities and Biological Activities of Vanadium Drugs: What is the Nature of the Active Species? Chem. Asian J. 2017, 12, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements, and toxins in media and bodily fluids control in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Vanadium (V/IV)–Transferrin Binding Disrupts the Transferrin Cycle and Reduces Vanadium Uptake and Antiproliferative Activity in Human Lung Cancer Cells. Inorganic Chemistry 2020, 59, 16143–16153. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, G.; Maréchal, J.D.; Garribba, E. Integrated approaches to characterize the systems formed by vanadium with proteins and enzymes. Inorg. Chem. Front. 2021, 8, 1951–1974. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Noriega, L.; Sánchez-Gaytán, B.L.; Castro, M.E.; Meléndez-Bustamante, F.J.; González-Vergara, E. Cyclo-tetravanadate bridged copper complexes as potential double bullet pro-metallodrugs for cancer treatment. J. Inorg. Biochem. 2020, 208, 111081. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Sánchez-Gaytán, B.L.; Cerro-López, M.; Mendoza, A.; Castro, M.E.; Meléndez-Bustamante, F.J.; González-Vergara, E. Synthesis and Experimental-Computational Characterization of a Copper/Vanadium Compound with Potential Anticancer Activity. Crystals 2020, 10, 492. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; García-García, A.; González-Vergara, E.; Cepeda, J.; Rodríguez-Diéguez, A. Magneto-structural correlations of cyclo-tetravanadates functionalized with mixed-ligand copper (ii) complexes. New J. Chem. 2021, 45, 5081–5092. [Google Scholar] [CrossRef]
- Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Méndez-Rojas, M.A.; Melendez, F.J.; Castro, M.E.; González-Vergara, E. Synthesis, Crystal Structure, and Computational Methods of Vanadium and Copper Compounds as Potential Drugs for Cancer Treatment. Molecules 2020, 25, 4679. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van de Street, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Sharma, R.P.; Ajnesh, S.; Venugopalan, P.; Dansby-Sparks, R.; Xue, Z.-L.; Rossetti, S.; Ferreti, V. Stabilization of tetrameric metavanadate ion by tris(1,10-phenanthroline)cobalt(III): Synthesis, spectroscopic, and X-ray structural study of [Co(phen)3]3(V4O12)2Cl·27H2O. J. Coord. Chem. 2019, 63, 3016–3027. [Google Scholar] [CrossRef][Green Version]
- Kucsera, R.; Joniaková, D.; Zúrková, L. Thermal properties of [MII(phen)3]2V4O12 phen 22H2O (MII = Co, Ni, Cu, phen = 1,10-phenanthroline). J. Therm. Anal. Calorim. 2004, 78, 263–272. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bìcer, E.; Dege, N.; Coskun, E. Synthesis, characterization, and crystal structure of a novel decavanadate salt, [V0.50(H2O)5]2[H2(V10O28)]·4(H2O). J. Chil. Chem. Soc. 2017, 62, 3610–3614. [Google Scholar] [CrossRef][Green Version]
- Guilherme, L.R.; Massabni, A.C.; Dametto, A.C.; De Souza Correa, R.; Suman de Araujo, A. Synthesis, Infrared Spectroscopy and Crystal Structure Determination of a New Decavanadate. J. Chem. Crystallogr. 2010, 40, 897–901. [Google Scholar] [CrossRef]
- Rakovsky, E.; Zurková, L.; Marek, J. Synthesis, Crystal Structure, and IR Spectroscopic Characterization of 1,6-Hexanediammonium Dihydrogendecavanadate. Mon. Chem. 2002, 133, 277–283. [Google Scholar] [CrossRef]
- Wery, A.S.J.; Gutierrez-Zorrila, J.M.; Roman, P. Influence of protonation on crystal packing and thermal behavior of tert-butylammonium decavanadates. Polyhedron 1996, 15, 4555–4564. [Google Scholar] [CrossRef]
- Román, P.; San José, A.; Luque, A.; Gutiérrez-Zorrila, J.M. Observation of a Novel Cyclic Tetrametavanadate Anion Isolated from Aqueous Solution. Inorg. Chem. 1993, 32, 775–776. [Google Scholar] [CrossRef]
- Paredes-García, P.; Gaune, S.; Saldías, M.; Garland, M.T.; Baggio, R.; Vega, A.; Salah El Fallah, M.; Escuer, A.; Le Fur, E.; Venegas-Yazigi, D.; et al. Solvatomorphs of dimeric transition metal complexes based on the V4O12 cyclic anion as a building block: Crystalline packing and magnetic properties. Inorg. Chim. Acta 2008, 361, 3681–3689. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, D.; Wang, M.H. Bis[tris(2,2-bipyridyl-κ2N, N)cobalt(II)] cyclo-tetravanadate undecahydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Wilson, R.M.; Randall, C.S.; Sokoloski, T.D. Second derivative infrared spectroscopy as a non-destructive tool to assess the purity and structural integrity of proteins. Pharm. Res. 1995, 12, 446–450. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef]
- Smith, J.C.; Gonzalez-Vergara, E.; Vincent, J.B. Detection of structural changes upon oxidation in multinuclear Mn-oxo-carboxylate assemblies by Fourier transform infrared spectroscopy: Relationship to photosystem II. Inorg. Chim. Acta 1997, 225, 99–103. [Google Scholar] [CrossRef]
- Schilt, A.A.; Taylor, R.C. Infra-red spectra of 1:10-phenanthroline metal complexes in the rock salt region below 2000 cm−1. J. Inorg. Nucl. Chem. 1959, 9, 211–221. [Google Scholar] [CrossRef]
- Baskaran, S.; Krishnan, M.M.; Arumugham, M.N.; Kumar, R. DFT analysis and DNA binding, cleavage of copper(II) complexes. J. Mol. Liq. 2016, 221, 1045–1053. [Google Scholar] [CrossRef]
- Barán, E.; Viera, I.; Torre, M.H. Vibrational spectra of the Cu(II) complexes of l-asparagine and l-glutamine. Spectrochim. Acta Part A 2007, 66, 114–117. [Google Scholar] [CrossRef]
- Valora, G.; Munzi, G.; Bonomo, R.P. Ternary copper(II) complexes with 1, 10-phenanthroline and various aminoacidates: A spectroscopic and voltammetric study in aqueous solution. J. Inorg. Biochem. 2019, 191, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984; p. 356. [Google Scholar]
- Agudelo, D.; Bourassa, P.; Beauregard, M.; Bérubé, G.; Tajmir-Riahi, H.A. tRNA binding to antitumor drug doxorubicin and its analogue. PLoS ONE 2013, 8, e69248. [Google Scholar] [CrossRef]
- García-Ramos, J.C.; Tovar-Tovar, A.; Hernández-Lima, J.; Cortés-Guzmán, F.; Moreno-Esparza, R.; Ruiz-Azuara, L. A new kind of intermolecular stacking interaction between copper(II) mixed chelate complex (Casiopeína III-ia) and adenine. Polyhedron 2011, 30, 2697–2703. [Google Scholar] [CrossRef]
- Parveen, S.; Fatima, K.; Zehra, S.; Arjmand, F. RNA-targeted Cu(II)-based potential antitumor drug entity: Comprehensive structural, biological {DNA/RNA binding, cleavage, cytotoxicity} and computational studies. J. Biomol. Struct. Dyn. 2020, 39, 6070–6083. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The early years of 2, 2′-bipyridine—A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [PubMed]
- Bencini, A.; Lippolis, V. 1,10-Phenanthroline: A versatile building block for the construction of ligands for various purposes. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar] [CrossRef]
- Alvarez, N.; Kramer, M.G.; Ellena, J.; Costa-Filho, A.; Torre, M.H.; Facchin, G. Copper-diimine coordination compounds as potential new tools in the treatment of cancer. Cancer Rep. Rev. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Tabatabaee, M.; Ahadiat, G.; Molčanov, K. Tetrakis (2-amino-4-methylpyridinium) cyclo-tetra-μ2-oxido-tetrakis [dioxidovanadate (V)] tetrahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m1090. [Google Scholar] [CrossRef]
- Wéry, A.S.J.; Gutiérrez-Zorrilla, J.M.; Luque, A.; Ugalde, M.; Román, P. Phase transitions in metavanadates. Polymerization of tetrakis (tert-butylammonium)-cyclo-tetrametavanadate. Chem. Mater. 1996, 8, 408–413. [Google Scholar] [CrossRef]
- Chen, B.; Huang, X.; Wang, B.; Lin, Z.; Hu, J.; Chi, Y.; Hu, C. Three New Imidazole-Functionalized Hexanuclear Oxidovanadium Clusters with Exceptional Catalytic Oxidation Properties for Alcohols. Chem.-A Eur. J. 2013, 19, 4408–4413. [Google Scholar] [CrossRef]
- Li, C.; Zhong, D.; Huang, X.; Shen, G.; Li, Q.; Du, J.; Dou, J. Two organic–inorganic hybrid polyoxovanadates as reusable catalysts for Knoevenagel condensation. New J. Chem. 2019, 43, 5813–5819. [Google Scholar] [CrossRef]
- Huang, M.H.; Bi, L.H.; Dong, S.J. Bis[tris(2, 2′-bipyridyl-κ2N, N′) iron (II)] cyclo-tetravanadate decahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online. 2004, 60, m153–m155. [Google Scholar] [CrossRef]
- Zhang, X.M. catena-Poly[[bis [triaqua [2, 5-bis (4-pyridyl)-1, 3, 4-thiadiazole] cobalt (II)]]-μ4-tetravanadato]. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m1411–m1413. [Google Scholar] [CrossRef]
- Xiao, D.; Hou, Y.; Wang, E.; Lü, J.; Li, Y.; Xu, L.; Hu, C. Dehydrogenative coupling of 2, 2′-bipyridine: Hydrothermal synthesis and crystal structure of a novel polyoxovanadate decorated with the 2, 2′; 6′, 2″; 6″, 2′′′-quaterpyridine ligand. Inorg. Chem. Commun. 2004, 7, 437–439. [Google Scholar] [CrossRef]
- Xie, A.; Xu, D.; Xu, Y.; Zhou, K. Synthesis, Crystal Structure and Electrochemical Behaviour of Tris (1, 10-phenanthroline) zinc (II) Tetrametavanadate Octahydrate. Acta Chim. Sin. 1997, 55, 853–859. [Google Scholar]
- Xing, Y.; Zhang, Y.; Sun, Z.; Ge, M.; Yuan, H.; Zhang, B. Synthesis, Structure Characterization, and Quantum Chemistry of a Discrete Cluster [{Zn (2, 2′-bipy) 3} 2V4O12]•11H2O (2, 2′-bipy = 2, 2′-bipyridine). Synth. React. Inorg. Met.-Org., Nano-Met. Chem. 2005, 35, 747–753. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Shi, L.-F.; Gao, G.-G.; Li, C.-B.; Han, L. Synthesis and Crystal Structure of a Novel Tetravanadate Modified.by Chiral Racemoid Zirconium Complex. Jiegou Huaxue 2006, 25, 979–984. [Google Scholar]
- Sun, Y.; Xu, Y.; Liu, S.; Wang, J.; Gao, D.; Zhang, G. Four inorganic–organic hybrid complexes built from tetravanadate and macrocyclic oxamide. J. Coord. Chem. 2013, 66, 2516–2528. [Google Scholar] [CrossRef]
- Yucesan, G.; Armatas, N.G.; Zubieta, J. Hydrothermal synthesis of molecular oxovanadium compounds. The crystal and molecular structures of [VO2(terpy)] NO3,[VO(terpy)(OH3PC6H5)2],[{Cu(H2O)(terpy)}V2O6],[{Cu(ttbterpy)}V2O6] and [{Cu (ttbterpy)} VO2 (HO3PCH2PO3)]•H2O (terpy = 2, 2′: 6′, 2 ″-terpyridine; ttbterpy = 4, 4′, 4 ″-tri-tert-butyl-2, 2′: 6′, 2″-terpyridine). Inorg. Chim. Acta 2006, 359, 4557–4564. [Google Scholar]
- Smith, T.M.; Freund, S.R.; Lau, A.; Varges, J.; Spinu, L.; Zubieta, J. Investigations of organic–inorganic hybrid materials: Ligand influences on the structures of copper-vanadium oxide materials. Inorg. Chim. Acta 2014, 414, 91–96. [Google Scholar] [CrossRef]
- Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Méndez-Rojas, M.A.; Melendez, F.J.; Castro, M.E.; González-Vergara, E. Unpublished work. 2021.
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate Complexes from Phenylate Phenathrolines to Be Used as Anticancerigenic Agents. European Patent EP 0434444A1, 26 June 1991. [Google Scholar]
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate from Methylate Phenanthroline Complexes to Be Used as Anticancerigenic Agents. USA Patent No. 5,576,326, 19 November 1996. [Google Scholar]
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate Complexes from Phenylate Phenanthroline to Be Used as Anticancerigenic Agents. USA Patent No. 35458, 18 February 1997. [Google Scholar]
- Ruiz-Azuara, L.; Bastian, G.; Bravo-Gómez, M.E.; Cañas, R.C.; Flores-Alamo, M.; Fuentes, I.; Mejia, C.; García-Ramos, J.C.; Serrano, A. Abstract CT408: Phase I study of one mixed chelates copper(II) compound, Casiopeína CasIIIia with antitumor activity and its mechanism of action. Cancer Res. 2014, 74 (suppl. 19), CT408. [Google Scholar] [CrossRef]
- Patra, A.K.; Roy, S.; Chakravarty, A.R. Synthesis, crystal structures, DNA binding and cleavage activity of L-glutamine copper(II) complexes of heterocyclic bases. Inorg. Chim. Acta 2009, 362, 1591–1599. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Lin, F.; Zhu, L.; Huang, F.; Zhao, L.; Ou, R. Casiopeina II-gly acts on lncRNA MALAT1 by miR-17-5p to inhibit FZD2 expression via the Wnt signaling pathway during the treatment of cervical carcinoma. Oncol. Rep. 2019, 42, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Ugone, V.; Pisanu, F.; Sanna, D.; Garriba, E. Interaction of the potent antitumoral compounds Casiopeinas® with blood serum and cellular bioligands. J. Inorg. Biochem. 2021, 224, 111566. [Google Scholar] [CrossRef]
- Becco, L.; García-Ramos, J.C.; Azuara, L.R.; Gambino, D.; Garat, B. Analysis of the DNA interaction of copper compounds belonging to the Casiopeínas® antitumoral series. Biol. Trace Elem. Res. 2014, 161, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard Puschmann, J.A.K. OLEX2: A complete structure solution, refinement, and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence, and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Methods of Electronic Structure Theory. In Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations—Potentials for the transition-metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, B; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. Gauss View, Version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Keith, T.A. TK Gristmill Software; Version 19.02.13; AIMAll: Overland Park, KS, USA, 2019. [Google Scholar]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.A.; Peek, M.E.; Zhou, F.X.; Bertrand, J.A.; VanDerveer, D.; Williams, L.D. Water ring structure at DNA interfaces: Hydration and dynamics of DNA-anthracycline complexes. Biochemistry 1994, 33, 3649–3659. [Google Scholar] [CrossRef]
- Sussman, J.L.; Holbrook, S.R.; Warrant, R.W.; Church, G.M.; Kim, S.H. Crystal structure of yeast phenylalanine transfer RNA. I. Crystallographic refinement. J. Mol. Biol. 1978, 123, 607–630. [Google Scholar] [CrossRef]
- Gilad, Y.; Senderowitz, H. Docking studies on DNA intercalators. J. Chem. Inf. Model. 2014, 54, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Biovia Discovery Studio. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 24 September 2021).
- García-García, A.; Noriega, L.; Meléndez-Bustamante, F.J.; Castro, M.E.; Sánchez-Gaytán, B.L.; Choquesillo-Lazarte, D.; González-Vergara, E.; Rodríguez-Diéguez, A. 2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity. Inorganics 2021, 9, 67. [Google Scholar] [CrossRef]
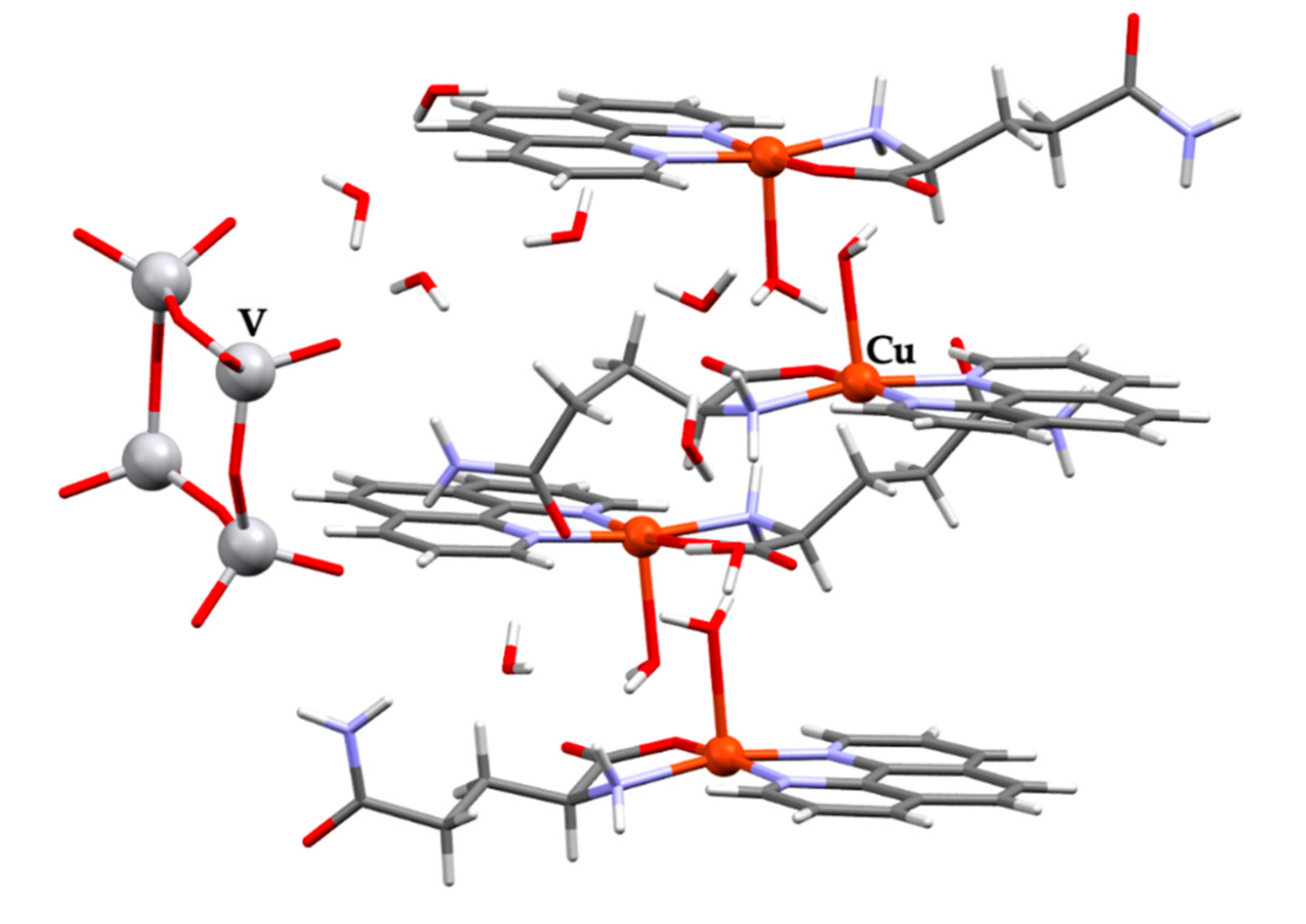
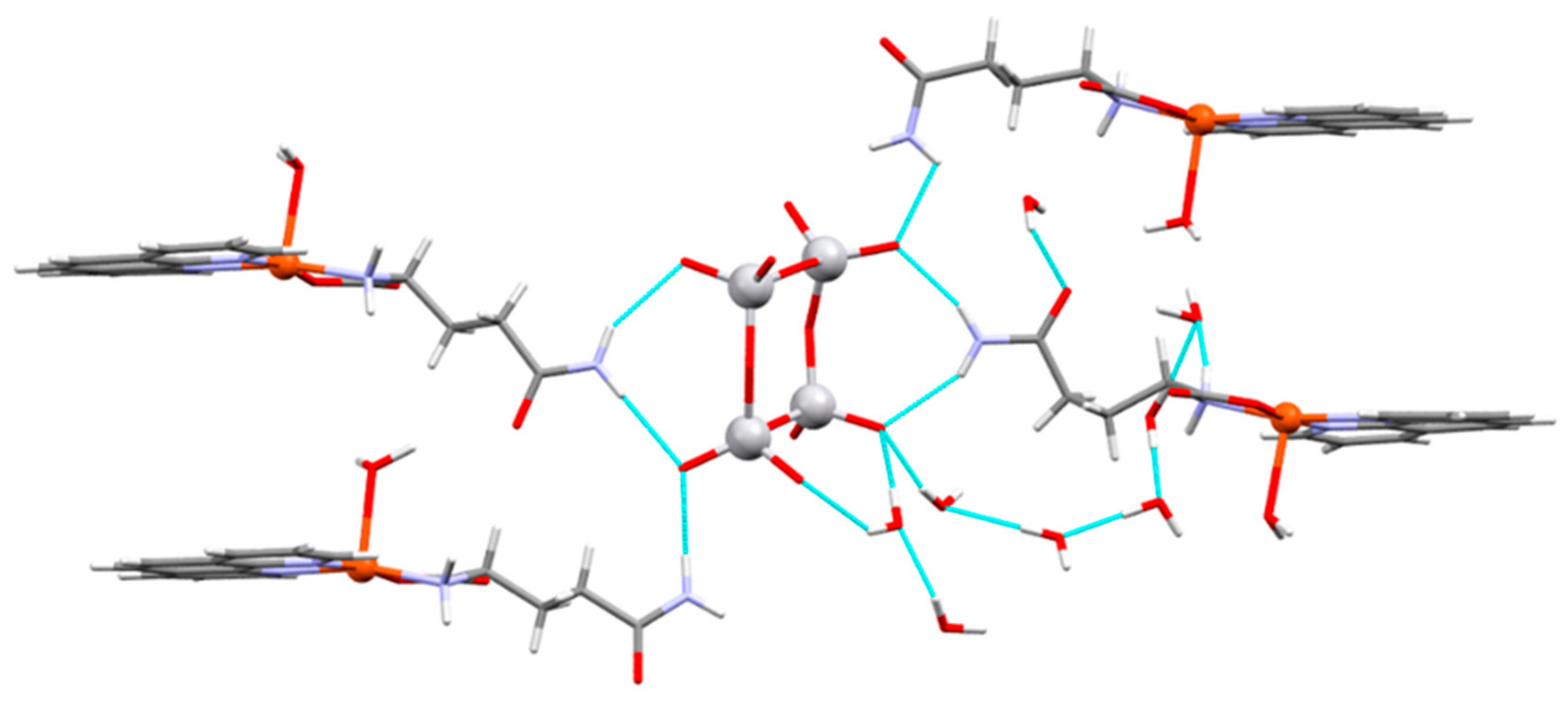

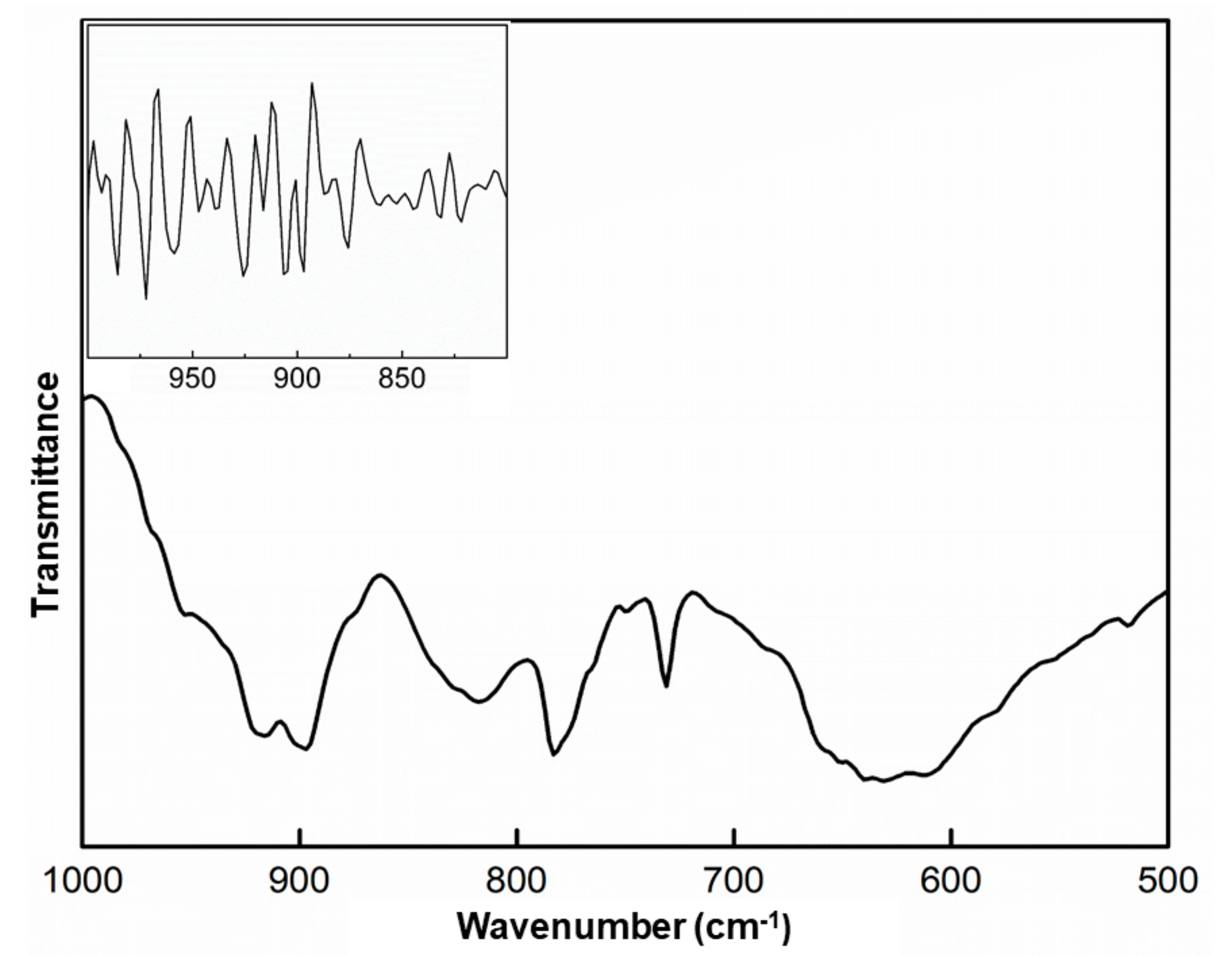
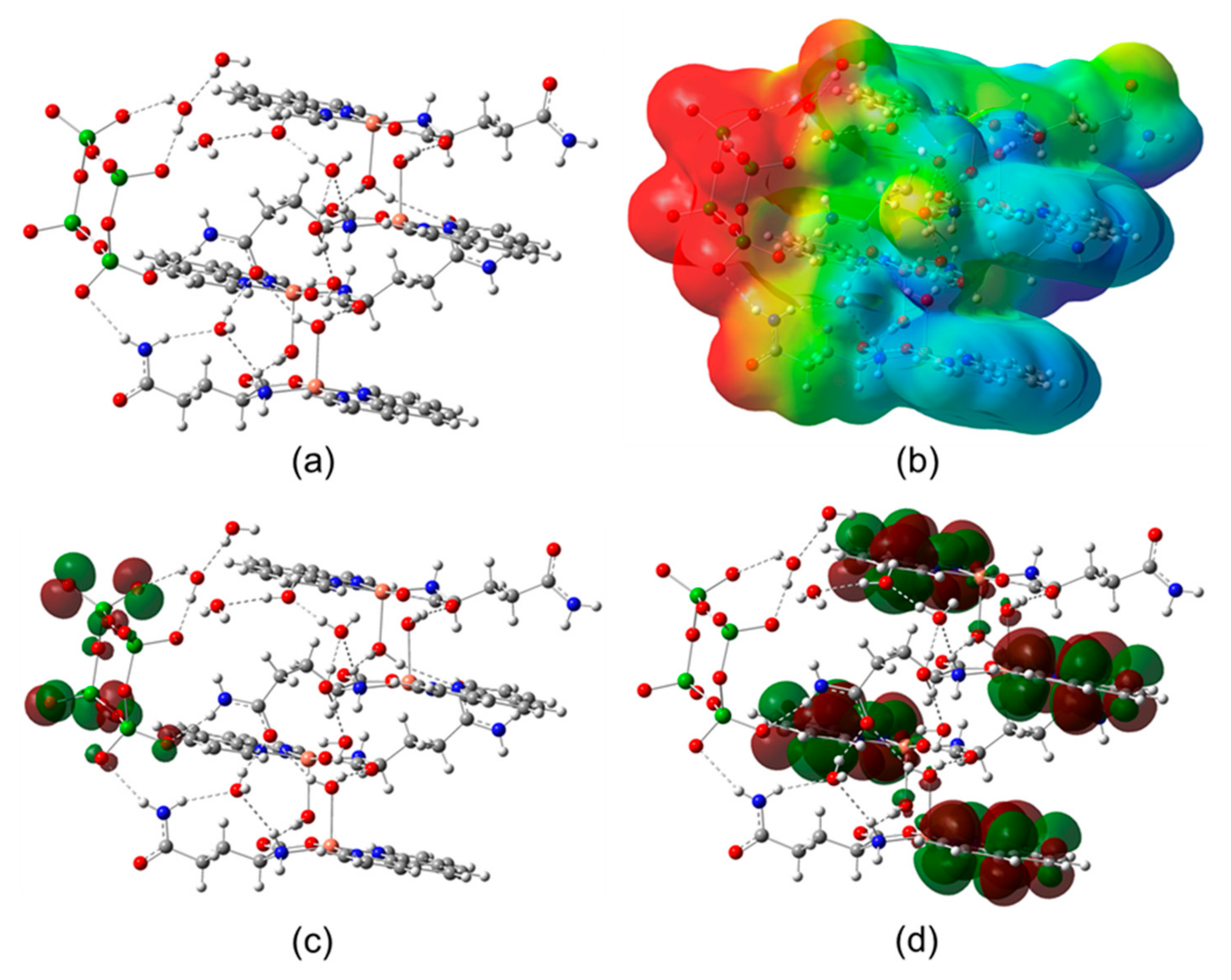
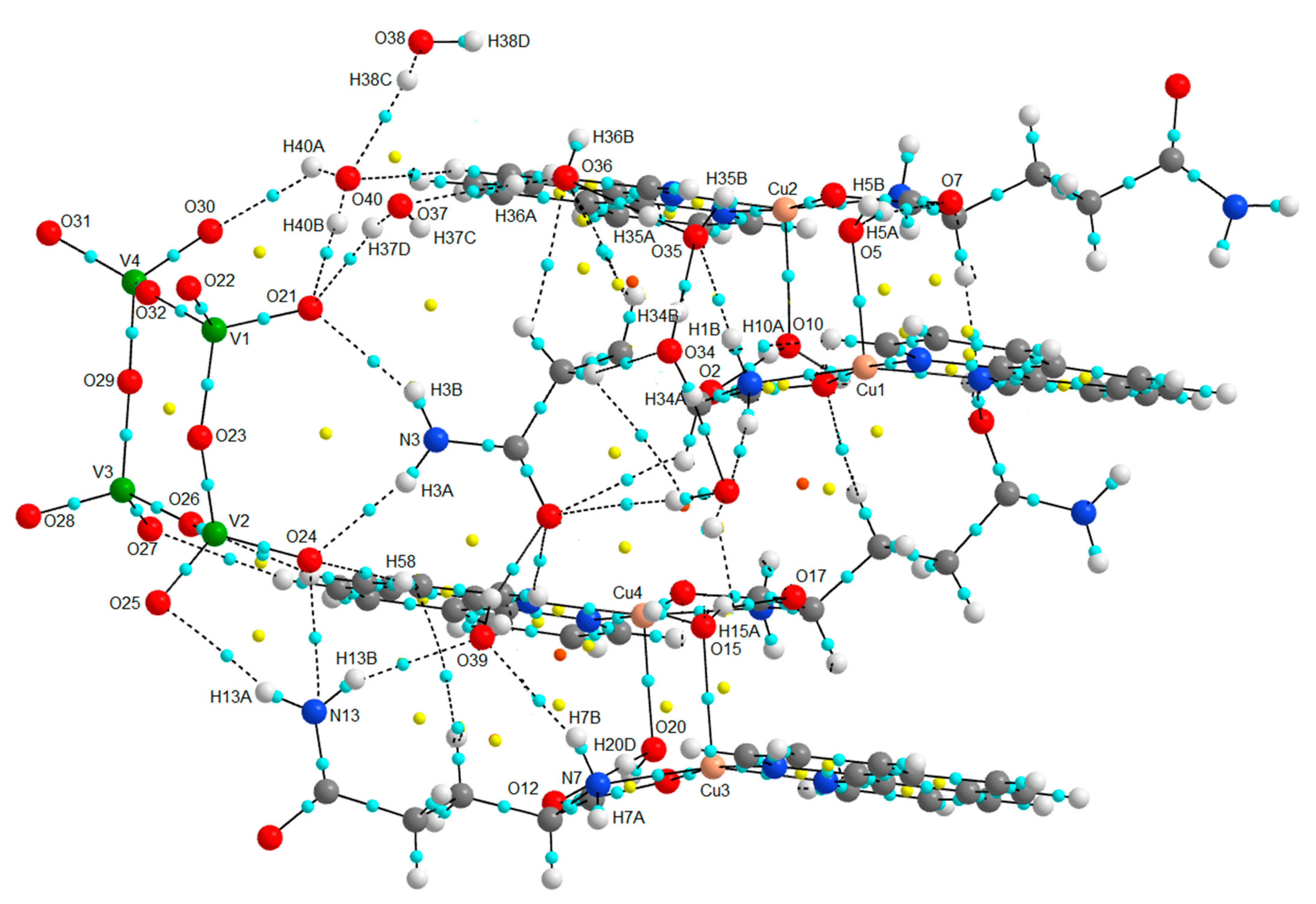

| Chemical formula | (C17H19CuN4O4)4V4O12∙8(H2O) |
| Mr | 2167.49 |
| Crystal system, space group | Triclinic, P1 |
| Temperature (K) | 293.15 |
| a, b, c (Å) | 12.3849 (3), 14.1023 (3), 14.1516 (3) |
| α,β,γ (°) | 68.967 (2), 71.327 (2), 77.252 (2) |
| V (Å3) | 2169.86 (9) |
| Z | 1 |
| Radiation type | Mo Kα |
| µ (mm−1) | 1.47 |
| Crystal size (mm) | 0.35 × 0.17 × 0.10 |
| Absorption correction | Gaussian |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 59,756; 21,452; 17,760 |
| Rint | 0.058 |
| (sin θ/λ)max (Å−1) | 0.667 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.045, 0.111, 1.02 |
| No. of reflections | 21,452 |
| No. of parameters | 1171 |
| H-atom treatment | H-atom parameters constrained research papers |
| Δmax Δmin (e Å−3) | 1.03, −0.29 |
| EHOMO | ELUMO | μ | χ | η | s | ω | |
|---|---|---|---|---|---|---|---|
| Compound 1 | –4.8697 | –2.6351 | –3.7524 | 3.7524 | 2.2346 | 0.4475 | 3.1506 |
| Compounds for Comparison | Interaction Energy (kcal/mol) ADN 1BNA | Interaction Energy (kcal/mol) ADN 151D | Interaction Energy (kcal/mol) tRNA 6TNA | Interaction Type |
|---|---|---|---|---|
| * Doxorubicin | −11.09 | −11.54 | −9.82 | H-bond, π-interaction, van der Waals |
| * [Cu(acac)(dmbipy)]1+ CAS III ia | −7.47 | −8.76 | −6.16 | H-bond, π-interaction, van der Waals |
| * [Cu(phen)(Gly)(H2O)]+ CAS II-Gly | −10.57 | −8.86 | −9.47 | H-bond,π-interaction, van der Waals |
| * [Cu(hydroxynaphthaldehyde)(H2O)] | −11.56 | −9.12 | –7.98 | H-bond, π-interaction, van der Waals |
| * [Cu(Metf)(bipy)(H2O)]2+ | −9.69 | −7.05 | −12.76 | H-bond, salt bridges, van der Waals |
| * [Cu(Gly)(Impy)(H2O)]+ | −8.82 | −6.73 | −8.83 | H-bond, π-interaction, van der Waals |
| [Cu(Lys)(phen)(H2O)]2+ | −11.03 | −9.98 | −8.86 | H-bond, salt bridge, π-interaction, van der Waals |
| [Cu(Orn)(bipy)(H2O)]2+ | −11.12 | −9.68 | –13.15 | H-bond, salt bridge, π-interaction, van der Waals, Attractive charges |
| [Cu(Gly)(phen)(H2O)]+ | −9.5 | −8.52 | –9.12 | H-bond, π-interaction, van der Waals |
| [Cu(Orn)(phen)(H2O)]2+ | −11.05 | −9.43 | –13.05 | H-bond, salt bridge, π-Interaction, van der Waals, Attractive charges |
| [Cu(Lys)(bipy)(H2O)]2+ | −11.04 | −8.72 | –12.23 | H-bond, salt bridge, π-Interaction, van der Waals Attractive charges |
| Compound | Binding Energies (kcal/mol) | Interactions |
|---|---|---|
| 1BNA | ||
| anti-[Cu(L-Gln)(phen)(H2O] | −9.39 | H-bond (3), van der Waals |
| syn-[Cu(L-Gln)(phen)(H2O] | −9.37 | H-bond (3), van der Waals, Salt bridge, attractive charge |
| 151D | ||
| anti-[Cu(L-Gln)(phen)(H2O] | −8.79 | H-bond (3), van der Waals, π-π |
| syn-[Cu(L-Gln)(phen)(H2O] | −8.35 | H-bond (3) van der Waals, π-π |
| 6TNA | ||
| anti-[Cu(L-Gln)(phen)(H2O] | −9.64 | H-bond (6), van der Waals |
| syn-[Cu(L-Gln)(phen)(H2O] | −10.26 | H-bond (6), salt bridge, attractive charge, π-anion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Mendoza, A.; Meléndez-Bustamante, F.J.; Castro, M.E.; González-Vergara, E. Ternary Copper Complex of L-Glutamine and Phenanthroline as Counterions of Cyclo-Tetravanadate Anion: Experimental–Theoretical Characterization and Potential Antineoplastic Activity. Metals 2021, 11, 1541. https://doi.org/10.3390/met11101541
Corona-Motolinia ND, Martínez-Valencia B, Noriega L, Sánchez-Gaytán BL, Mendoza A, Meléndez-Bustamante FJ, Castro ME, González-Vergara E. Ternary Copper Complex of L-Glutamine and Phenanthroline as Counterions of Cyclo-Tetravanadate Anion: Experimental–Theoretical Characterization and Potential Antineoplastic Activity. Metals. 2021; 11(10):1541. https://doi.org/10.3390/met11101541
Chicago/Turabian StyleCorona-Motolinia, Nidia D., Beatriz Martínez-Valencia, Lisset Noriega, Brenda L. Sánchez-Gaytán, Angel Mendoza, Francisco J. Meléndez-Bustamante, María Eugenia Castro, and Enrique González-Vergara. 2021. "Ternary Copper Complex of L-Glutamine and Phenanthroline as Counterions of Cyclo-Tetravanadate Anion: Experimental–Theoretical Characterization and Potential Antineoplastic Activity" Metals 11, no. 10: 1541. https://doi.org/10.3390/met11101541
APA StyleCorona-Motolinia, N. D., Martínez-Valencia, B., Noriega, L., Sánchez-Gaytán, B. L., Mendoza, A., Meléndez-Bustamante, F. J., Castro, M. E., & González-Vergara, E. (2021). Ternary Copper Complex of L-Glutamine and Phenanthroline as Counterions of Cyclo-Tetravanadate Anion: Experimental–Theoretical Characterization and Potential Antineoplastic Activity. Metals, 11(10), 1541. https://doi.org/10.3390/met11101541







