Microstructure Design and Its Effect on Mechanical Properties in Gamma Titanium Aluminides
Abstract
:1. Introduction
2. Typical Phases and Microstructures in TiAl Alloys
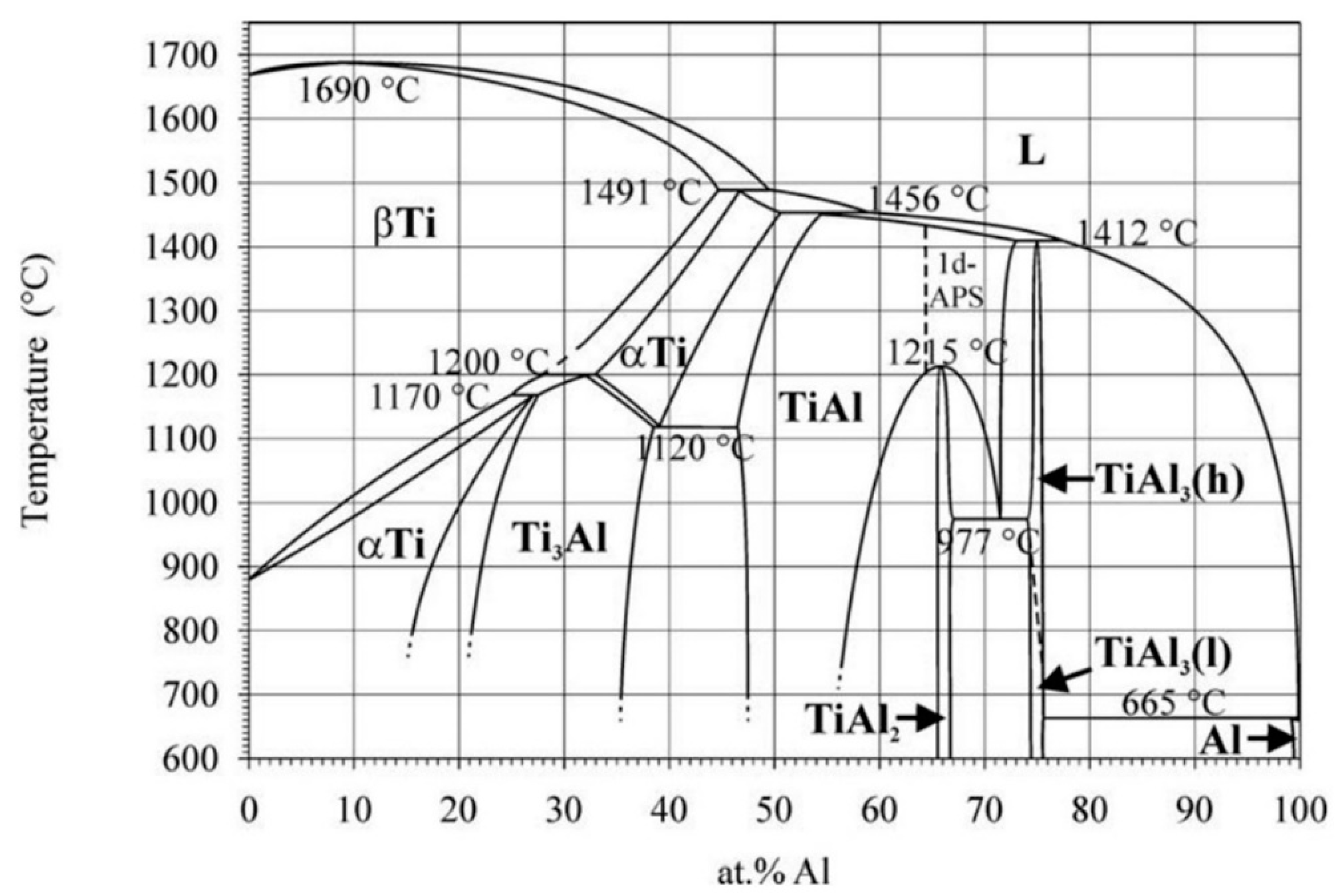
2.1. Phases in the Ti–Al System
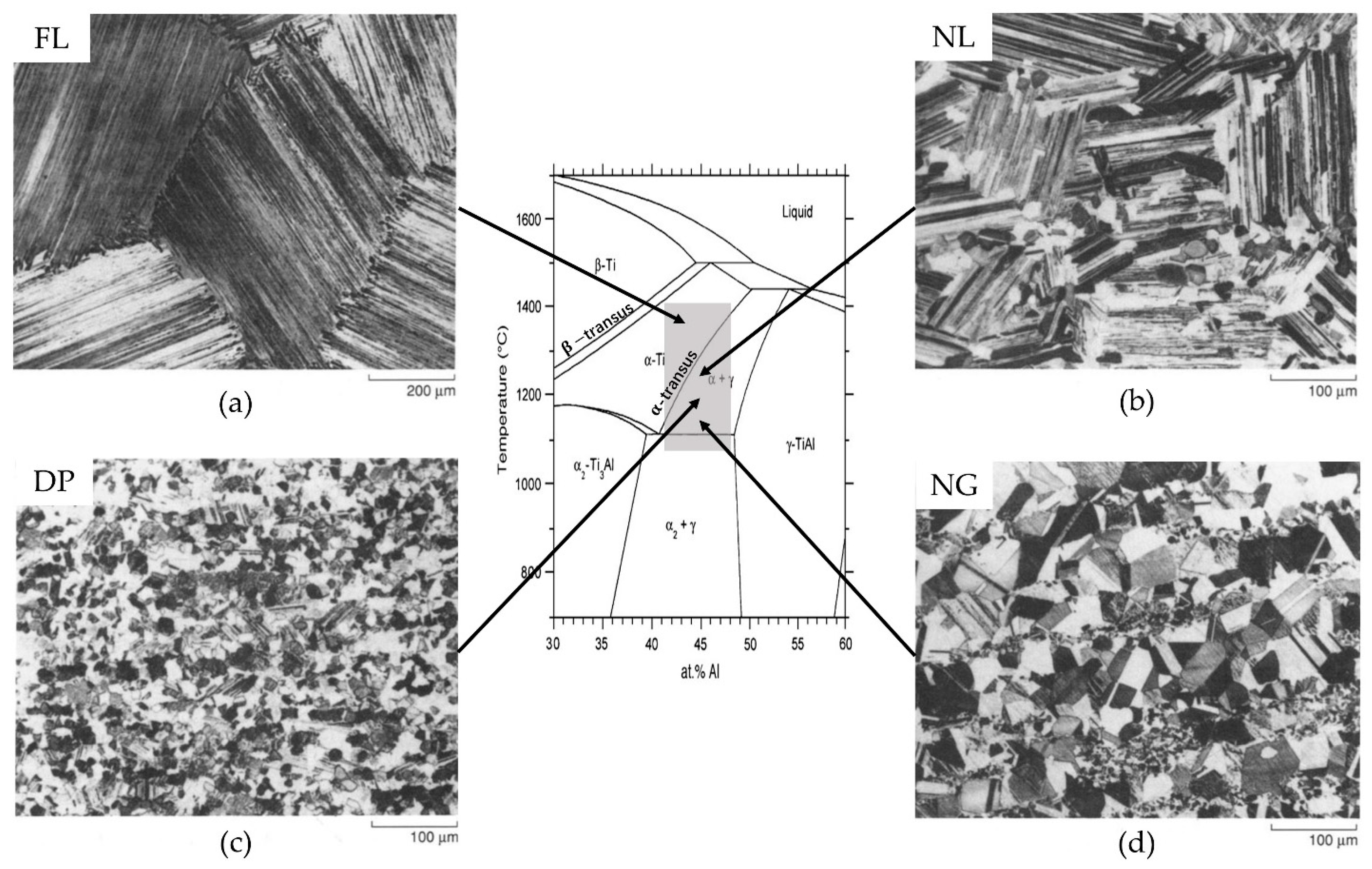
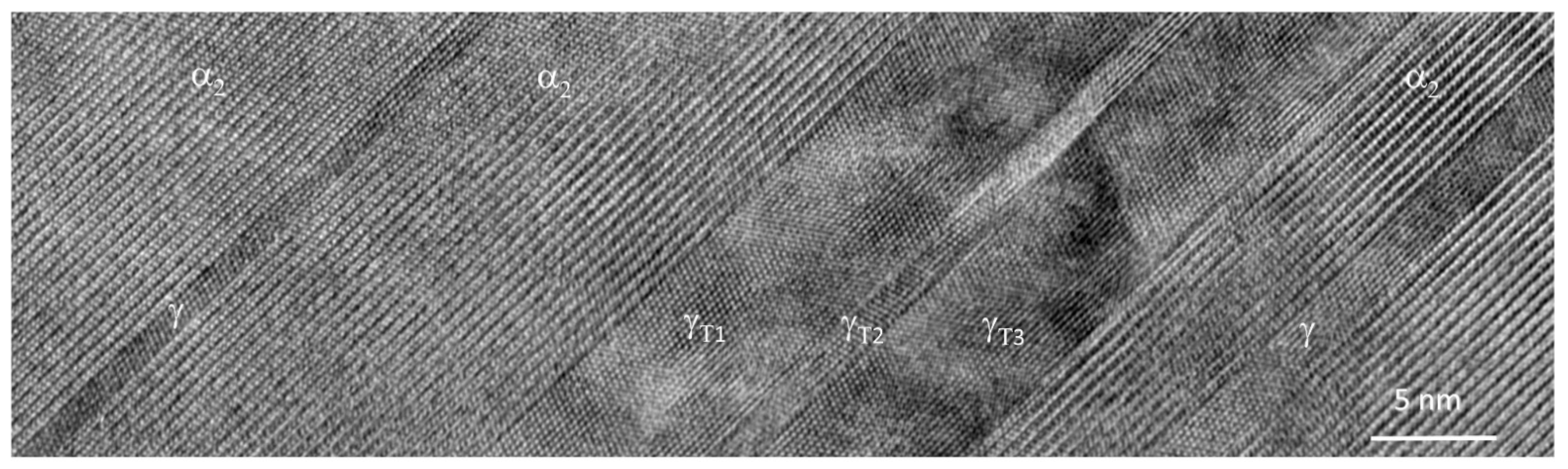
2.2. Typical Microstructures and Their Characteristics
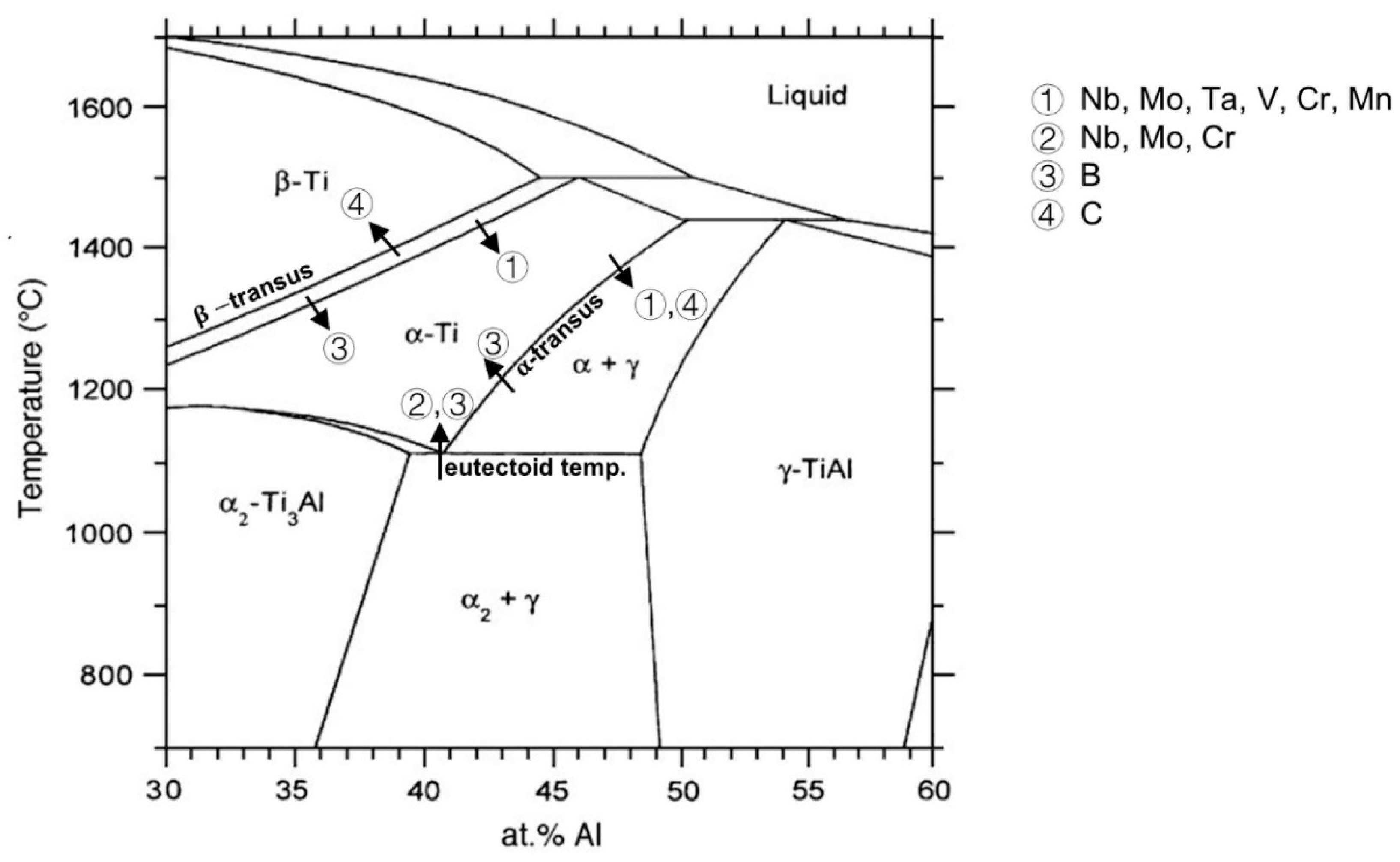
3. Effects of Alloying Elements on Microstructures
3.1. Nb, Mo, Ta, W, Mn, V and Cr
3.2. B, C and Si
4. Microstructure Evolution in Processing and Heat Treatments
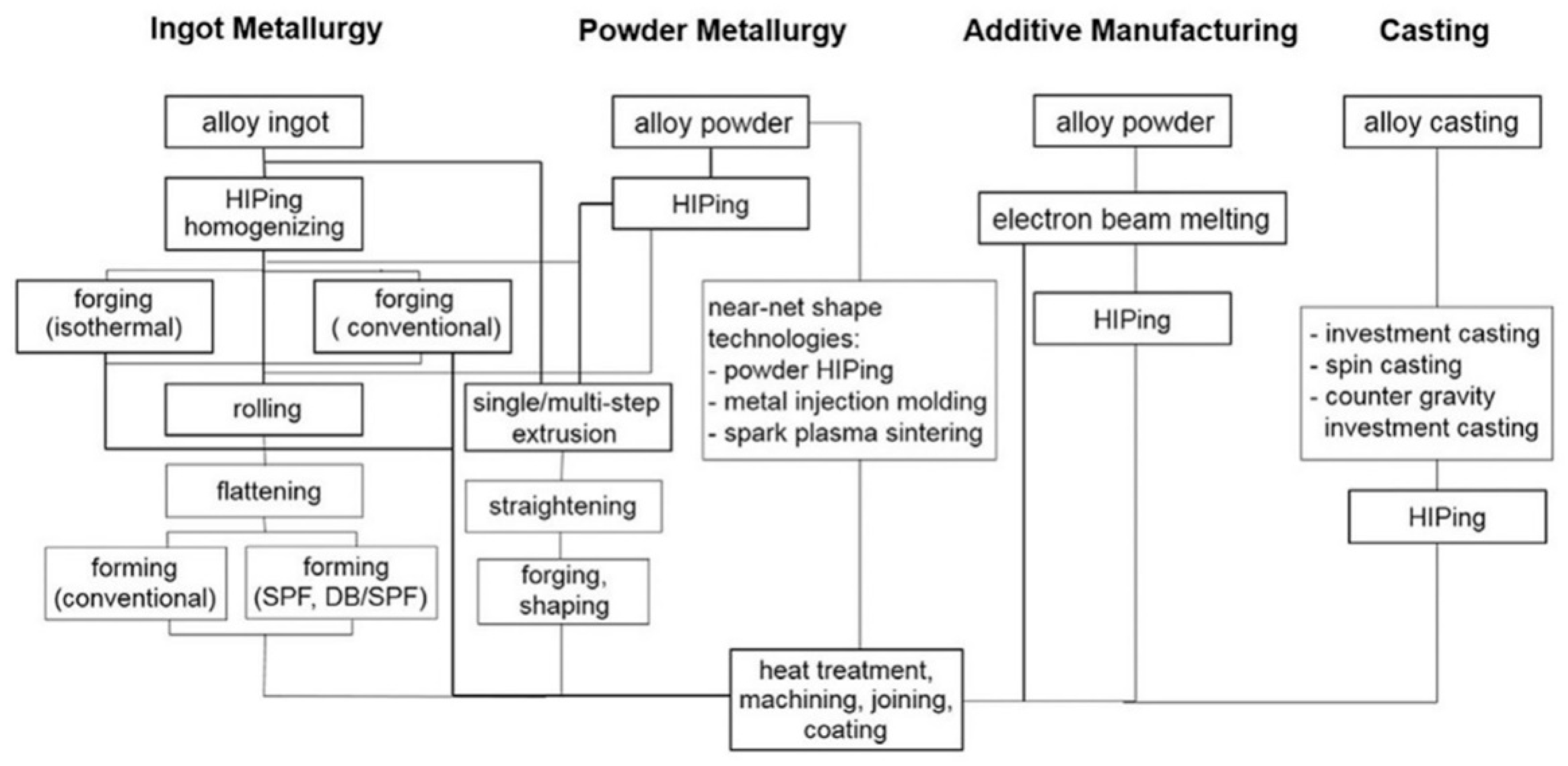
4.1. Ingot Metallurgy
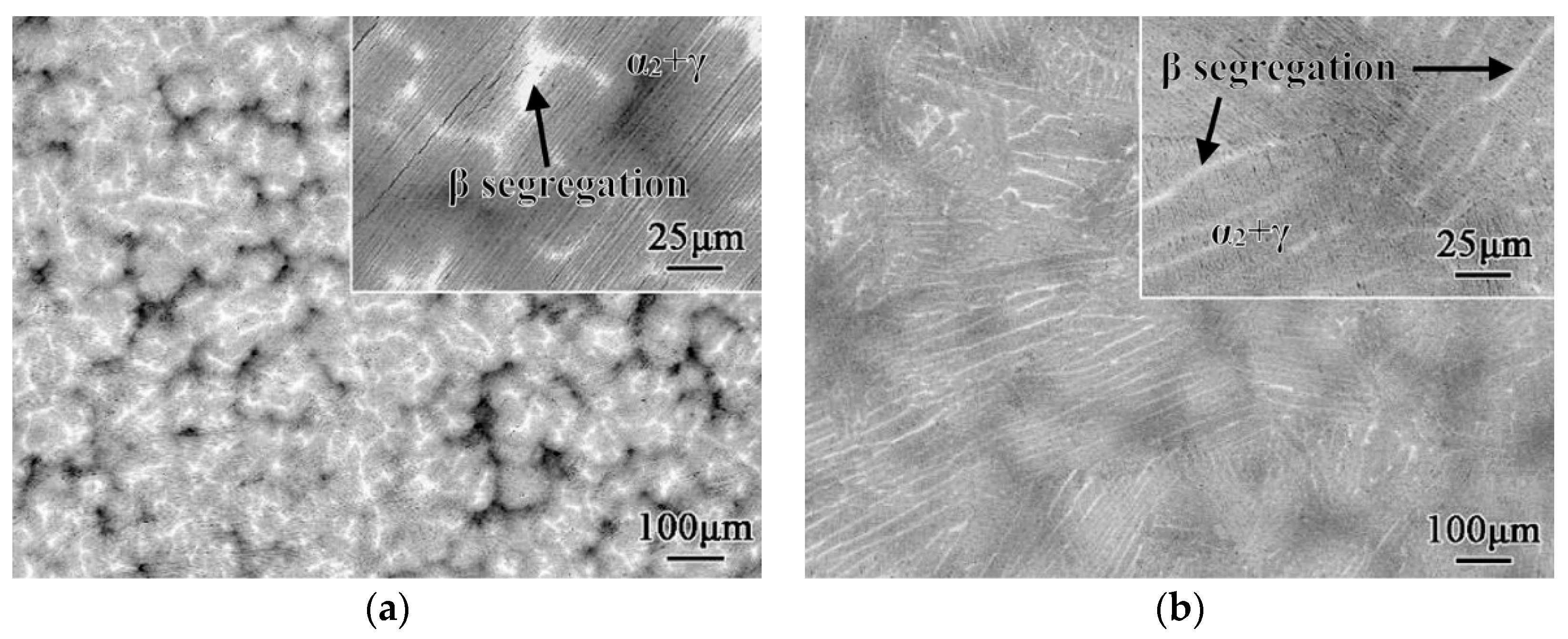
4.1.1. Peritectic Solidified Ingot
4.1.2. 𝛽 Solidified Ingot
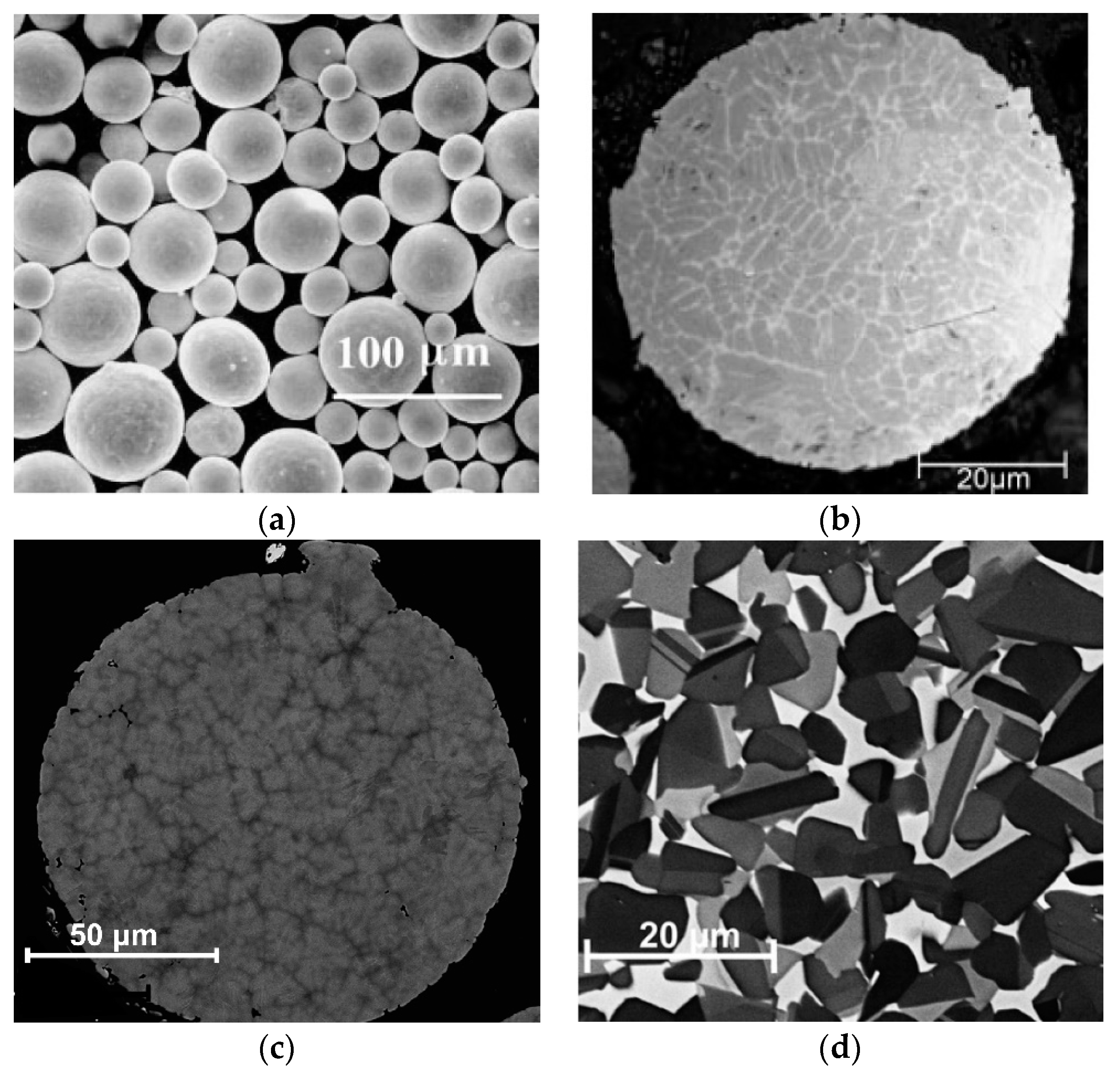
4.2. Powder Metallurgy and Compaction
4.3. Additive Manufacturing and Casting
4.4. Heat Treatments
5. Effect of Microstructures on Mechanical Properties
5.1. Creep Resistance
5.2. Dctility
5.3. Strength and Hardness
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.W. Ordered intermetallic alloys, part III: Gamma titanium aluminides. JOM 1994, 46, 30–39. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Reith, M.; Franke, M.; Schloffer, M.; Körner, C. Processing 4th generation titanium aluminides via electron beam based additive manufacturing–characterization of microstructure and mechanical properties. Materialia 2020, 14, 100902. [Google Scholar] [CrossRef]
- Wimler, D.; Lindemann, J.; Reith, M.; Kirchner, A.; Allen, M.; Vargas, W.G.; Franke, M.; Klöden, B.; Weißgärber, T.; Güther, V.; et al. Designing advanced intermetallic titanium aluminide alloys for additive manufacturing. Intermetallics 2021, 131. [Google Scholar] [CrossRef]
- Klein, T.; Usategui, L.; Rashkova, B.; Nó, M.L.; San Juan, J.; Clemens, H.; Mayer, S. Mechanical behavior and related microstructural aspects of a nano-lamellar TiAl alloy at elevated temperatures. Acta Mater. 2017, 128, 440–450. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Weimer, M.; Kelly, T.; Suzuki, A.; Subramanian, P.R. The Science, Technology, and Implementation of TiAl Alloys in Commercial Aircraft Engines. MRS Proc. 2013, 1516, 49–58. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, S.L. Advances in Gammalloy Materials–Processes–Application Technology: Successes, Dilemmas, and Future. JOM 2018, 70, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary aluminum-titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Stark, R.; Bartels, A.; Clemens, H.; Schimansky, F.P. On the formation of ordered ω-phase in high Nb containing TiAI based alloys. Adv. Eng. Mater. 2008, 10, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Oehring, M.; Pyczak, F.; Schreyer, A. In situ observation of various phase transformation paths in Nb-rich TiAl alloys during quenching with different rates. Adv. Eng. Mater. 2011, 13, 700–704. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Boettinger, W.J.; Burton, B.P.; Biancaniello, F.S.; Shoemaker, C.B. The formation of ordered ω-related phases in alloys of composition Ti4Al3Nb. Acta Metall. Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Song, L.; Xu, X.J.; You, L.; Liang, Y.F.; Lin, J.P. Phase transformation and decomposition mechanisms of the βo(ω) phase in cast high Nb containing TiAl alloy. J. Alloys Compd. 2014, 616, 483–491. [Google Scholar] [CrossRef]
- Schmoelzer, T.; Liss, K.D.; Zickler, G.A.; Watson, I.J.; Droessler, L.M.; Wallgram, W.; Buslaps, T.; Studer, A.; Clemens, H. Phase fractions, transition and ordering temperatures in TiAl-Nb-Mo alloys: An in- and ex situ study. Intermetallics 2010, 18, 1544–1552. [Google Scholar] [CrossRef]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of novel β-solidifying TiAl alloys with adjustable β/B2-phase fraction and excellent hot-workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Burgers, W. On the process of transition of the cubic-body-centered modification into the hexagonal-close-packed modification of zirconium. Physica 1934, 1, 561–586. [Google Scholar] [CrossRef]
- Blackburn, M.J. Some Aspects of Phase Transformations in Titanium Alloys. In The Science, Technology and Application of Titanium; Elsevier: Amsterdam, The Netherlands, 1970; pp. 633–643. [Google Scholar] [CrossRef]
- Kim, Y.W.; Dimiduk, D.M. Progress in the understanding of gamma titanium aluminides. JOM 1991, 43, 40–47. [Google Scholar] [CrossRef]
- Appel, F.; Wagner, R. Microstructure and deformation of two-phase γ-titanium aluminides. Mater. Sci. Eng. R Rep. 1998, 22, 187–268. [Google Scholar] [CrossRef]
- Clemens, H.; Kestler, H. Processing and applications of intermetallic γ-TiAl-based alloys. Adv. Eng. Mater. 2000, 2, 551–570. [Google Scholar] [CrossRef]
- Kothari, K.; Radhakrishnan, R.; Wereley, N.M. Advances in gamma titanium aluminides and their manufacturing techniques. Prog. Aerosp. Sci. 2012, 55, 1–16. [Google Scholar] [CrossRef]
- Kim, Y.W. Microstructural evolution and mechanical properties of a forged gamma titanium aluminide alloy. Acta Metall. Mater. 1992, 40, 1121–1134. [Google Scholar] [CrossRef]
- Clemens, H.; Bartels, A.; Bystrzanowski, S.; Chladil, H.; Leitner, H.; Dehm, G.; Gerling, R.; Schimansky, F.P. Grain refinement in γ-TiAl-based alloys by solid state phase transformations. Intermetallics 2006, 14, 1380–1385. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Kim, Y.W. Intermetallic alloys based on gamma titanium aluminide. JOM 1989, 41, 24–30. [Google Scholar] [CrossRef]
- Cui, N.; Wu, Q.; Bi, K.; Xu, T.; Kong, F. Effect of heat treatment on microstructures and mechanical properties of a novel β-solidifying TiAl alloy. Materials 2019, 12, 1672. [Google Scholar] [CrossRef] [Green Version]
- Schwaighofer, E.; Clemens, H.; Mayer, S.; Lindemann, J.; Klose, J.; Smarsly, W.; Güther, V. Microstructural design and mechanical properties of a cast and heat-treated intermetallic multi-phase γ-TiAl based alloy. Intermetallics 2014, 44, 128–140. [Google Scholar] [CrossRef]
- Inui, H.; Oh, M.H.; Nakamura, A.; Yamaguchi, M. Room-temperature tensile deformation of polysynthetically twinned (PST) crystals of TiAl. Acta Metall. Mater. 1992, 40, 3095–3104. [Google Scholar] [CrossRef]
- Dehm, G.; Motz, C.; Scheu, C.; Clemens, H.; Mayrhofer, P.H.; Mitterer, C. Mechanical size-effects in miniaturized and bulk materials. Adv. Eng. Mater. 2006, 8, 1033–1045. [Google Scholar] [CrossRef]
- Cha, L.; Clemens, H.; Dehm, G. Microstructure evolution and mechanical properties of an intermetallic Ti-43.5Al-4Nb-1Mo-0.1B alloy after ageing below the eutectoid temperature. Int. J. Mater. Res. 2011, 102, 703–708. [Google Scholar] [CrossRef]
- Cha, L.; Scheu, C.; Clemens, H.; Chladil, H.F.; Dehm, G.; Gerling, R.; Bartels, A. Nanometer-scaled lamellar microstructures in Ti-45Al-7.5Nb-(0; 0.5)C alloys and their influence on hardness. Intermetallics 2008, 16, 868–875. [Google Scholar] [CrossRef]
- Kim, Y.W. Strength and ductility in TiAl alloys. Intermetallics 1998, 6, 623–628. [Google Scholar] [CrossRef]
- Voice, W.E.; Henderson, M.; Shelton, E.F.J.; Wu, X. Gamma titanium aluminide, TNB. Proc. Intermet. 2005, 13, 959–964. [Google Scholar] [CrossRef]
- Huang, S.C. Structural Intermetallics; Darolia, R., Lewandowski, J.J., Liu, C.T., Martin, P.L., Miracle, D.P., Nathal, M.V., Eds.; TMS: Warrendale, PA, USA, 1993; pp. 299–308. [Google Scholar]
- Kainuma, R.; Fujita, Y.; Mitsui, H.; Ohnuma, I.; Ishida, K. Phase equilibria among α (hcp), β (bcc) and γ (L10) phases in Ti-Al base ternary alloys. Intermetallics 2000, 8, 855–867. [Google Scholar] [CrossRef]
- Gerling, R.; Clemens, H.; Schimansky, F.P. Powder metallurgical processing of intermetallic gamma titanium aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Imayev, R.M.; Imayev, V.M.; Oehring, M.; Appel, F. Alloy design concepts for refined gamma titanium aluminide based alloys. Intermetallics 2007, 15, 451–460. [Google Scholar] [CrossRef]
- Shaaban, A.; Signori, L.J.; Nakashima, H.; Takeyama, M. Effects of the addition of transition metals on phase equilibria and phase transformations in TiAl systems in between 1473 and 1073 K. J. Alloys Compd. 2021, 878, 160392. [Google Scholar] [CrossRef]
- Jabbar, H.; Monchoux, J.P.; Thomas, M.; Pyczak, F.; Couret, A. Improvement of the creep properties of TiAl alloys densified by Spark Plasma Sintering. Intermetallics 2014, 46, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Liu, Y.; Li, Y.P.; Zhang, W.; Chiba, A. Thermomechanical characterization of β-stabilized Ti-45Al-7Nb-0.4W-0. 15B alloy. Intermetallics 2011, 19, 1184–1190. [Google Scholar] [CrossRef]
- Watson, I.J.; Liss, K.D.; Clemens, H.; Wallgram, W.; Schmoelzer, T.; Hansen, T.C.; Reid, M. In situ characterization of a Nb and Mo containing γ-TiAl based alloy using neutron diffraction and high-temperature microscopy. Adv. Eng. Mater. 2009, 11, 932–937. [Google Scholar] [CrossRef]
- Qiang, F.; Kou, H.; Yang, G.; Tang, B.; Li, J. Multi-step heat treatment design for nano-scale lamellar structures of a cast Ti-45Al-8.5Nb-(W, B, Y) alloy. Intermetallics 2016, 79, 35–40. [Google Scholar] [CrossRef]
- Sun, F.S.; Cao, C.X.; Kim, S.E.; Lee, Y.T.; Yan, M.G. Alloying mechanism of beta stabilizers in a TiAl alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 1573–1589. [Google Scholar] [CrossRef]
- Ye, L.H.; Wang, H.; Zhou, G.; Hu, Q.M.; Yang, R. Phase stability of TiAl-X (X=V, Nb, Ta, Cr, Mo, W. and Mn) alloys. J. Alloys Compd. 2020, 819, 153291. [Google Scholar] [CrossRef]
- Schloffer, M.; Rashkova, B.; Schöberl, T.; Schwaighofer, E.; Zhang, Z.; Clemens, H.; Mayer, S. Evolution of the ωo phase in a β-stabilized multi-phase TiAl alloy and its effect on hardness. Acta Mater. 2014, 64, 241–252. [Google Scholar] [CrossRef]
- Oehring, M.; Stark, A.; Paul, J.D.H.; Lippmann, T.; Pyczak, F. Microstructural refinement of boron-containing β-solidifying γ-titanium aluminide alloys through heat treatments in the β phase field. Intermetallics 2013, 32, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Liu, Z.; Jia, Y.; Wang, T.; Zhao, L.; Guo, J.; Xiao, S.; Chen, Y. Effect of TiB2 addition on microstructure and fluidity of cast TiAl alloy. Vacuum 2020, 174, 109210. [Google Scholar] [CrossRef]
- Cheng, T.T. The mechanism of grain refinement in TiAl alloys by boron addition-an alternative hypothesis. Intermetallics 2000, 8, 29–37. [Google Scholar] [CrossRef]
- Hu, D. Role of boron in TiAl alloy development: A review. Rare Met. 2016, 35, 1–14. [Google Scholar] [CrossRef]
- Larson, D.J.; Liu, C.T.; Miller, M.K. Boron solubility and boride compositions in α2 + γ titanium aluminides. Intermetallics 1997, 5, 411–414. [Google Scholar] [CrossRef]
- Kartavykh, A.V.; Gorshenkov, M.V.; Podgorny, D.A. Grain refinement mechanism in advanced γ-TiAl boron-alloyed structural intermetallics: The direct observation. Mater. Lett. 2015, 142, 294–298. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Zhang, L.Q.; Su, Y.Q.; Chen, G.L. Microstructural control of TiAl-Nb alloys by directional solidification. Acta Mater. 2012, 60, 498–506. [Google Scholar] [CrossRef]
- Godfrey, A.B.; Loretto, M.H. The nature of complex precipitates associated with the addition of boron to a γ-based titanium aluminide. Intermetallics 1996, 4, 47–53. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Chen, Y.; Xu, L.; Tian, J. The effect of boron addition on the deformation behavior and microstructure of β-solidify TiAl alloys. Mater. Charact. 2018, 145, 312–322. [Google Scholar] [CrossRef]
- Kastenhuber, M.; Rashkova, B.; Clemens, H.; Mayer, S. Enhancement of creep properties and microstructural stability of intermetallic β-solidifying γ-TiAl based alloys. Intermetallics 2015, 63, 19–26. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Chen, Y.; Xu, L.; Tian, J. The effect of carbon addition on the high-temperature properties of β solidification TiAl alloys. J. Alloys Compd. 2019, 775, 441–448. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Chen, Y.; Xu, L.; Tian, J. The effect of boron addition on the high-temperature properties and microstructure evolution of high Nb containing TiAl alloys. Mater. Sci. Eng. A 2018, 733, 190–198. [Google Scholar] [CrossRef]
- Jiang, H.; Zhong-lei, W.; Wen-shuai, M.A.; Xiao-ran, F.; Zi-qiang, D.; Liang, Z.; Yong, L. Effects of Nb and Si on high temperature oxidation of TiAl. Trans. Nonferrous Met. Soc. China 2009, 18, 512–517. [Google Scholar] [CrossRef]
- Knaislováa, A.; Šimůnkováa, V.; Nováka, P.; Průšaa, F.; Cabibbob, M.; Jaworskac, L.; Vojtěch, D. Effect of alloying elements on the properties of Ti-Al-Si alloys prepared by powder metallurgy. J. Alloys Compd. 2021, 868. [Google Scholar] [CrossRef]
- Scheu, C.; Stergar, E.; Schober, M.; Cha, L.; Clemens, H.; Bartels, A.; Schimansky, F.P.; Cerezo, A. High carbon solubility in a γ-TiAl-based Ti-45Al-5Nb-0.5C alloy and its effect on hardening. Acta Mater. 2009, 57, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Christoph, U.; Appel, F.; Wagner, R. Dislocation dynamics in carbon-doped titanium aluminide alloys. Mater. Sci. Eng. A 1997, 239–240, 39–45. [Google Scholar] [CrossRef]
- Klein, T.; Schachermayer, M.; Mendez-Martin, F.; Schöberl, T.; Rashkova, B.; Clemens, H.; Mayer, S. Carbon distribution in multi-phase γ-TiAl based alloys and its influence on mechanical properties and phase formation. Acta Mater. 2015, 94, 205–213. [Google Scholar] [CrossRef]
- Appel, F.; Christoph, U.; Wagner, R. Solution and precipitation hardening in carbon-doped two-phase γ-titanium aluminides. In Proceedings of the Materials Research Society Symposium-Proceedings. Mater. Res. Soc. 1997, 460, 77–82. [Google Scholar] [CrossRef]
- Klein, T.; Rashkova, B.; Holec, D.; Clemens, H.; Mayer, S. Silicon distribution and silicide precipitation during annealing in an advanced multi-phase γ-TiAl based alloy. Acta Mater. 2016, 110, 236–245. [Google Scholar] [CrossRef]
- Noda, T.; Okabe, M.; Isobe, S.; Sayashi, M. Silicide precipitation strengthened TiAl. Mater. Sci. Eng. A 1995, 192–193, 774–779. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Rashkova, B.; Clemens, H.; Stark, A.; Mayer, S. Effect of carbon addition on solidification behavior, phase evolution and creep properties of an intermetallic β-stabilized γ-TiAl based alloy. Intermetallics 2014, 46, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Mayer, S.; Erdely, P.; Fischer, F.D.; Holec, D.; Kastenhuber, M.; Klein, T.; Clemens, H. Intermetallic β-Solidifying γ-TiAl Based Alloys − From Fundamental Research to Application. Adv. Eng. Mater. 2017, 19, 1–27. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Zhang, L.Q.; Wang, H.L.; Hao, G.J.; Chen, G.L. Microstructure development during directional solidification of Ti-45Al-8Nb alloy. J. Alloys Compd. 2010, 506, 115–119. [Google Scholar] [CrossRef]
- Yang, C.; Hu, D.; Huang, A.; Dixon, M. Solidification and grain refinement in Ti45Al2Mn2Nb1B subjected to fast cooling. Intermetallics 2013, 32, 64–71. [Google Scholar] [CrossRef]
- Chen, G.L.; Xu, X.J.; Teng, Z.K.; Wang, Y.L.; Lin, J.P. Microsegregation in high Nb containing TiAl alloy ingots beyond laboratory scale. Intermetallics 2007, 15, 625–631. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Y.; Gao, M.; Liu, K. Changes of Oxygen Content in Molten TiAl Alloys as a Function of Superheat during Vacuum Induction Melting. J. Mater. Sci. Technol. 2010, 26, 900–903. [Google Scholar] [CrossRef]
- Song, Y.; Dou, Z.; Zhang, T.; Liu, Y. A novel continuous and controllable method for fabrication of as-cast TiAl alloy. J. Alloys Compd. 2019, 789, 266–275. [Google Scholar] [CrossRef]
- Zollinger, J.; Lapin, J.; Daloz, D.; Combeau, H. Influence of oxygen on solidification behaviour of cast TiAl-based alloys. Intermetallics 2007, 15, 1343–1350. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Qi, H.; Zhang, L.Q.; Song, X.P.; Chen, G.L. Microstructure evolution of directionally solidified Ti-45Al-8.5Nb-(W, B, Y) alloys. J. Alloys Compd. 2011, 509, 4041–4046. [Google Scholar] [CrossRef]
- Lapin, J.; Gabalcová, Z.; Pelachová, T. Effect of Y2O3 crucible on contamination of directionally solidified intermetallic Ti-46Al-8Nb alloy. Intermetallics 2011, 19, 396–403. [Google Scholar] [CrossRef]
- Huang, A.; Loretto, M.; Hu, D.; Liu, K.; Wu, X. The role of oxygen content and cooling rate on transformations in TiAl-based alloys. Intermetallics 2006, 14, 838–847. [Google Scholar] [CrossRef]
- Liang, Y.F.; Xu, X.J.; Lin, J.P. Advances in phase relationship for high Nb-containing TiAl alloys. Rare Met. 2016, 35, 15–25. [Google Scholar] [CrossRef]
- Lapin, J.; Gabalcová, Z. Solidification behaviour of TiAl-based alloys studied by directional solidification technique. Intermetallics 2011, 19, 797–804. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Zhang, L.Q.; Su, Y.Q.; Hao, G.J.; Chen, G.L. A closely-complete peritectic transformation during directional solidification of a Ti-45Al-8.5Nb alloy. Intermetallics 2011, 19, 1115–1119. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, L.; He, J.; Huang, H.; Nan, H.; Lin, J.; Kim, Y.W. Numerical and experimental study on peritectic transition in TiAl-Nb alloys. Comput. Mater. Sci. 2019, 158, 333–339. [Google Scholar] [CrossRef]
- Küstner, V.; Oehring, M.; Chatterjee, A.; Güther, V.; Brokmeier, H.-G.; Clemens, H. Gamma Titanium Aluminides; Kim, Y.W., Clemens, H., Rosenberger, A.H., Eds.; TMS: Warrendale, PA, USA, 2003. [Google Scholar]
- Xu, Z.; Xu, X.; Lin, J.; Zhang, Y.; Wang, Y.; Lin, Z.; Chen, G. Homogenization treatment of high Nb containing TiAl alloys with as-cast and as-forged microstructures. Rare Met. 2008, 27, 181–186. [Google Scholar] [CrossRef]
- Xu, X.J.; Lin, J.P.; Wang, Y.L.; Gao, J.F.; Lin, Z.; Chen, G.L. Effect of forging on microstructure and tensile properties of Ti-45Al-(8-9)Nb-(W,B,Y) alloy. J. Alloys Compd. 2006, 414, 175–180. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, L.; He, J.; Zhang, F.; Feng, X.; Nan, H.; Lin, J.; Kim, Y.W. As-cast microstructure characteristics dependent on solidification mode in TiAl-Nb alloys. J. Alloys Compd. 2019, 809, 151862. [Google Scholar] [CrossRef]
- Huang, Z.W. Inhomogeneous microstructure in highly alloyed cast TiAl-based alloys, caused by microsegregation. Scr. Mater. 2005, 52, 1021–1025. [Google Scholar] [CrossRef]
- Xu, X.J.; Lin, J.P.; Teng, Z.K.; Wang, Y.L.; Chen, G.L. On the microsegregation of Ti-45Al-(8-9)Nb-(W, B, Y) alloy. Mater. Lett. 2007, 61, 369–373. [Google Scholar] [CrossRef]
- Chen, G.; Peng, Y.; Zheng, G.; Qi, Z.; Wang, M.; Yu, H.; Dong, C.; Liu, C.T. Polysynthetic twinned TiAl single crystals for higherature applications. Nat. Mater. 2016, 15, 876–881. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Johnson, D.R.; Lee, H.N.; Inui, H. Directional solidi® cation of TiAl-base alloys. Intermetallics 2000, 8, 511–517. [Google Scholar] [CrossRef]
- Klein, T.; Niknafs, S.; Dippenaar, R.; Clemens, H.; Mayer, S. Grain growth and β to α transformation behavior of a β-solidifying TiAl alloy. Adv. Eng. Mater. 2015, 17, 786–790. [Google Scholar] [CrossRef]
- Huang, H.; Ding, H.; Xu, X.; Chen, R.; Guo, J.; Fu, H. Phase transformation and microstructure evolution of a beta-solidified gamma-TiAl alloy. J. Alloys Compd. 2021, 860, 158082. [Google Scholar] [CrossRef]
- Wallgram, W.; Clemens, H.; Kremmer, S.; Otto, A.; Güther, V. Hot-die Forging of a β-stabilized γ-TiAl Based Alloy. MRS Online Proc. Libr. 2008, 1128, 109–114. [Google Scholar] [CrossRef]
- Schloffer, M.; Iqbal, F.; Gabrisch, H.; Schwaighofer, E.; Schimansky, F.P.; Mayer, S.; Stark, A.; Lippmann, T.; Göken, M.; Pyczak, F.; et al. Microstructure development and hardness of a powder metallurgical multi phase γ-TiAl based alloy. Intermetallics 2012, 22, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Lin, J.P.; Xu, X.J.; He, Y.H.; Wang, Y.L.; Chen, G.L. Effect of fabrication process on microstructure of high Nb containing TiAl alloy. J. Alloys Compd. 2008, 458, 313–317. [Google Scholar] [CrossRef]
- Wegmann, G.; Gerling, R.; Schimansky, F.P. Temperature induced porosity in hot isostatically pressed gamma titanium aluminide alloy powders. Acta Mater. 2003, 51, 741–752. [Google Scholar] [CrossRef]
- Haidar, J.; Gnanarajan, S.; Dunlop, J.B. Direct production of alloys based on titanium aluminides. Intermetallics 2009, 17, 651–656. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, N.; Wang, Y. Fabrication of Ti-Al intermetallics by a two-stage aluminothermic reduction process using Na2TiF6. Intermetallics 2017, 85, 156–162. [Google Scholar] [CrossRef]
- Stoephasius, J.C.; Friedrich, B. Production of γ-TiAl-Ingots by Aluminothermic Reduction of TiO2 and Refining by ESR. Proc.-Eur. Metall. Conf. EMC 2005 2005, 4, 1429–1444. [Google Scholar]
- Mphahlele, M.R.; Olevsky, E.A.; Olubambi, P.A. Spark plasma sintering of near net shape titanium aluminide: A review. Spark Plasma Sinter. 2019, 281–299. [Google Scholar] [CrossRef]
- Voisin, T.; Monchoux, J.P.; Hantcherli, M.; Mayer, S.; Clemens, H.; Couret, A. Microstructures and mechanical properties of a multi-phase β-solidifying TiAl alloy densified by spark plasma sintering. Acta Mater. 2014, 73, 107–115. [Google Scholar] [CrossRef]
- Terner, M.; Biamino, S.; Epicoco, P.; Penna, A.; Hedin, O.; Sabbadini, S.; Fino, P.; Pavese, M.; Ackelid, U.; Gennaro, P.; et al. Electron beam melting of high niobium containing TiAl alloy: Feasibility investigation. Steel Res. Int. 2012, 83, 943–949. [Google Scholar] [CrossRef]
- Biamino, S.; Penna, A.; Ackelid, U.; Sabbadini, S.; Tassa, O.; Fino, P.; Pavese, M.; Gennaro, P.; Badini, C. Electron beam melting of Ti-48Al-2Cr-2Nb alloy: Microstructure and mechanical properties investigation. Intermetallics 2011, 19, 776–781. [Google Scholar] [CrossRef]
- Qu, H.P.; Wang, H.M. Microstructure and mechanical properties of laser melting deposited γ-TiAl intermetallic alloys. Mater. Sci. Eng. A 2007, 466, 187–194. [Google Scholar] [CrossRef]
- Löber, L.; Schimansky, F.P.; Kühn, U.; Pyczak, F.; Eckert, J. Selective laser melting of a beta-solidifying TNM-B1 titanium aluminide alloy. J. Mater. Process. Technol. 2014, 214, 1852–1860. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Li, Z. Additive Manufacturing of Titanium Aluminides; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128140635. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Wallgram, W.; Schmölzer, T.; Cha, L.; Das, G.; Güther, V.; Clemens, H. Technology and mechanical properties of advanced γ-TiAl based alloys. Int. J. Mater. Res. 2009, 100, 1021–1030. [Google Scholar] [CrossRef]
- Yang, F.; Kong, F.; Chen, Y.; Xiao, S. Effect of heat treatment on microstructure and properties of as-forged TiAl alloy with β phase. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2011, 40, 1505–1509. [Google Scholar] [CrossRef]
- Bolz, S.; Oehring, M.; Lindemann, J.; Pyczak, F.; Paul, J.; Stark, A.; Lippmann, T.; Schrüfer, S.; Roth-Fagaraseanu, D.; Schreyer, A.; et al. Microstructure and mechanical properties of a forged β-solidifying γ TiAl alloy in different heat treatment conditions. Intermetallics 2015, 58, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Burtscher, M.; Klein, T.; Mayer, S.; Clemens, H.; Fischer, F.D. The creep behavior of a fully lamellar γ-TiAl based alloy. Intermetallics 2019, 114. [Google Scholar] [CrossRef]
- Droessler, L.M.; Schmoelzer, T.; Wallgram, W.; Cha, L.; Das, G.; Clemens, H. Microstructure and Tensile Ductility of a Ti-43Al-4Nb-1Mo-0.1B Alloy. Mater. Res. 2008, 1128. [Google Scholar] [CrossRef]
- Schillinger, W.; Clemens, H.; Dehm, G.; Bartels, A. Microstructural stability and creep behavior of a lamellar γ-TiAl based alloy with extremely fine lamellar spacing. Intermetallics 2002, 10, 459–466. [Google Scholar] [CrossRef]
- Bernal, D.; Chamorro, X.; Hurtado, I.; Madariaga, I. Evolution of lamellar microstructures in a cast TNM alloy modified with boron through single-step heat treatments. Intermetallics 2020, 124, 106842. [Google Scholar] [CrossRef]
- Kastenhuber, M.; Klein, T.; Clemens, H.; Mayer, S. Tailoring microstructure and chemical composition of advanced γ-TiAl based alloys for improved creep resistance. Intermetallics 2018, 97, 27–33. [Google Scholar] [CrossRef]
- Maruyama, K.; Yamamoto, R.; Nakakuki, H.; Fujitsuna, N. Effects of lamellar spacing, volume fraction and grain size on creep strength of fully lamellar TiAl alloys. Mater. Sci. Eng. A 1997, 239–240, 419–428. [Google Scholar] [CrossRef]
- Crofts, P.D.; Bowen, P.; Jones, I.P. The effect of lamella thickness on the creep behaviour of Ti-48Al-2Nb-2Mn. Scr. Mater. 1996, 35, 1391–1396. [Google Scholar] [CrossRef]
- Liu, X.; Song, L.; Stark, A.; Lazurenko, D.; Pyczak, F.; Zhang, T. Creep-induced ωo phase precipitation and cavity formation in a cast 45.5Ti-45Al-9Nb-0.5B alloy. J. Alloys Compd. 2021, 875, 160106. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Keller, M.; Mendiratta, M.G. The effect of lamellar lath spacing on the creep behavior of Ti-47 at% Al. Scr. Mater. 1998, 38, 1025–1031. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mecking, H.; Arzt, E.; Clemens, H. Creep behavior of γ-TiAl sheet material with differently spaced fully lamellar microstructure. Mater. Sci. Eng. A 2002, 329–331, 840–846. [Google Scholar] [CrossRef]
- Hu, D. Effect of boron addition on tensile ductility in lamellar TiAl alloys. Intermetallics 2002, 10, 851–858. [Google Scholar] [CrossRef]
- Zheng, R.T.; Zhang, Y.G.; Chen, C.Q.; Cheng, G.A. The ambient temperature tensile behavior of duplex γ-TiAl-based alloys. Mater. Sci. Eng. A 2003, 362, 192–199. [Google Scholar] [CrossRef]
- Chauniyal, A.; Janisch, R. Influence of lattice misfit on the deformation behaviour of α2/γ lamellae in TiAl alloys. Mater. Sci. Eng. A 2020, 796. [Google Scholar] [CrossRef]
- Liu, C.T.; Maziasz, P.J. Microstructural control and mechanical properties of dual-phase TiAl alloys. Intermetallics 1998, 6, 653–661. [Google Scholar] [CrossRef]
- Zhang, W.J.; Deevi, S.C.; Chen, G.L. On the origin of superior high strength of Ti-45Al-10Nb alloys. Intermetallics 2002, 10, 403–406. [Google Scholar] [CrossRef]
- Pavlina, E.J.; Van Tyne, C.J. Correlation of Yield Strength and Tensile Strength with Hardness for Steels. Mater. Eng Perform. 2008, 17, 888–893. [Google Scholar] [CrossRef]
- Appel, F.; Oehring, M.; Wagner, R. Novel design concepts for gamma-base titanium aluminide alloys. Intermetallics 2000, 8, 1283–1312. [Google Scholar] [CrossRef]


| Phase | Pearson Symbol | Space Group | Structure | Lattice Parameters |
|---|---|---|---|---|
| - | cI2 | Imm | A2 | a = 0.33065 |
| - | cP2 | Pmm | B2 | a = 0.3189 |
| - | hP2 | P63/mmc | A3 | a = 0.29504 c = 0.46833 c/a = 1.59 |
| - | hP8 | P63/mmc | D019 | a = 0.576 c = 0.4683 c/a = 0.81 |
| - | tP4 | P4/mmm | L10 | a = b = 0.399 c = 0.4062 |
| - | hP3 | P6/mmm | – | a = 0.463 c = 0.281 |
| - | hP6 | P63/mmc | B82 | a = 0.45803 c = 0.55204 |
| - | – | Pm1 | – | – |
| - | – | Pm1 | – | a = 0.45551 c = 0.5542 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lin, Q.; Zhang, W.; Horne, C.V.; Cha, L. Microstructure Design and Its Effect on Mechanical Properties in Gamma Titanium Aluminides. Metals 2021, 11, 1644. https://doi.org/10.3390/met11101644
Liu X, Lin Q, Zhang W, Horne CV, Cha L. Microstructure Design and Its Effect on Mechanical Properties in Gamma Titanium Aluminides. Metals. 2021; 11(10):1644. https://doi.org/10.3390/met11101644
Chicago/Turabian StyleLiu, Xuqi, Qia Lin, Wenjing Zhang, Constance Van Horne, and Limei Cha. 2021. "Microstructure Design and Its Effect on Mechanical Properties in Gamma Titanium Aluminides" Metals 11, no. 10: 1644. https://doi.org/10.3390/met11101644
APA StyleLiu, X., Lin, Q., Zhang, W., Horne, C. V., & Cha, L. (2021). Microstructure Design and Its Effect on Mechanical Properties in Gamma Titanium Aluminides. Metals, 11(10), 1644. https://doi.org/10.3390/met11101644







