Effect of Solute Redistribution on Seeding Process of TiAl Alloys with Limited Convection in a Float Zone
Abstract
:1. Introduction
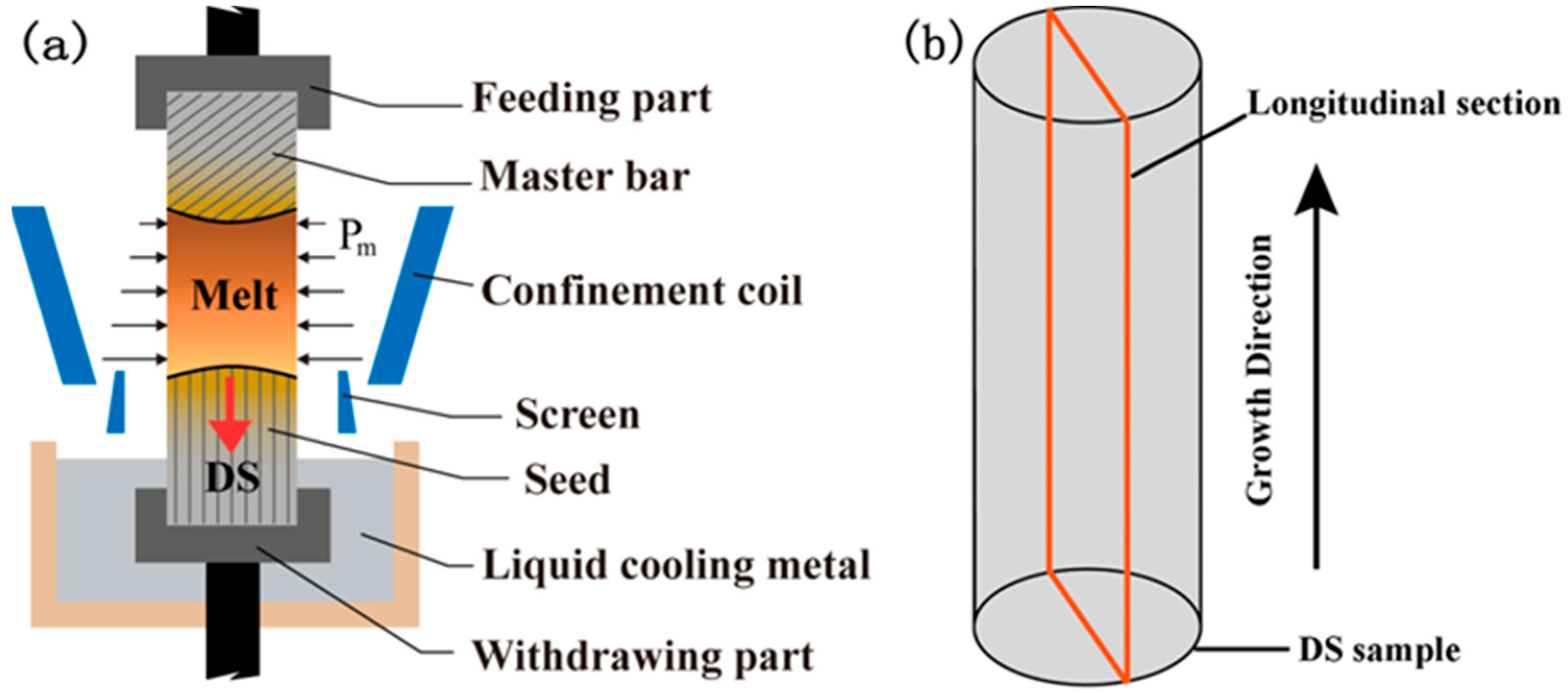
2. Materials and Methods
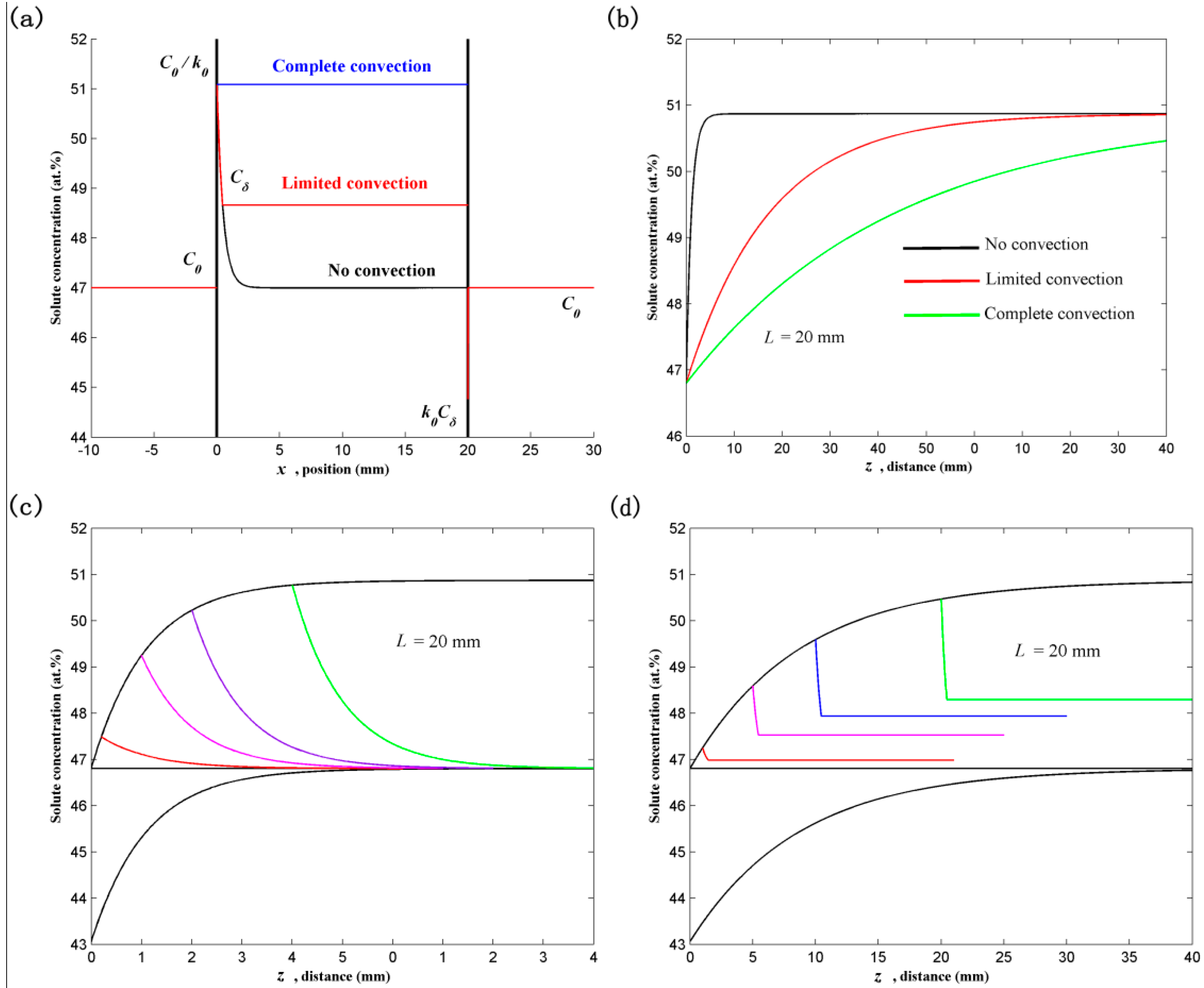
2.1. Solute Redistribution in the Steady State
- (i)
- Solidifying crystal (−∞ < x ≤ 0)
- (ii)
- Melt (0 ≤ x ≤ L)
- (iii)
- Feed crystal (L ≤ x < ∞)where Cs, Cl and Cm are the solute distributions in the solidifying crystal, melt and feed crystal, Cl* the solidifying interface concentration in the melt, Cδ the solute concentration at the terminal of diffusion boundary, Dl and Ds the solute diffusion coefficients in the melt and feed crystal, L the length of the float zone, k0 the solute partition coefficient, V the growth velocity, x the distance from the solidifying interface.
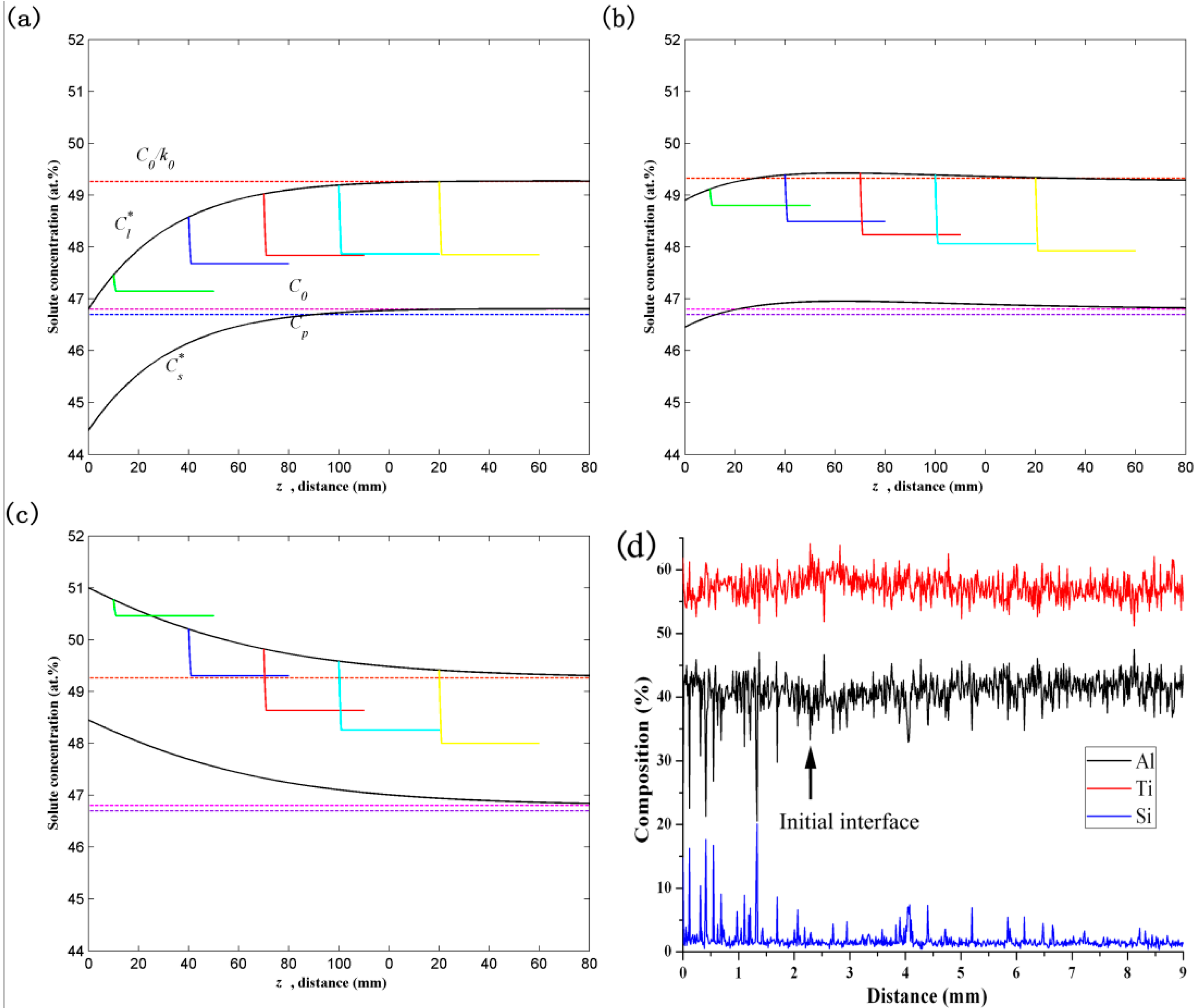
2.2. Solute Redistribution in the Initial Transient
2.3. Solute Redistribution during the General Seeding Process
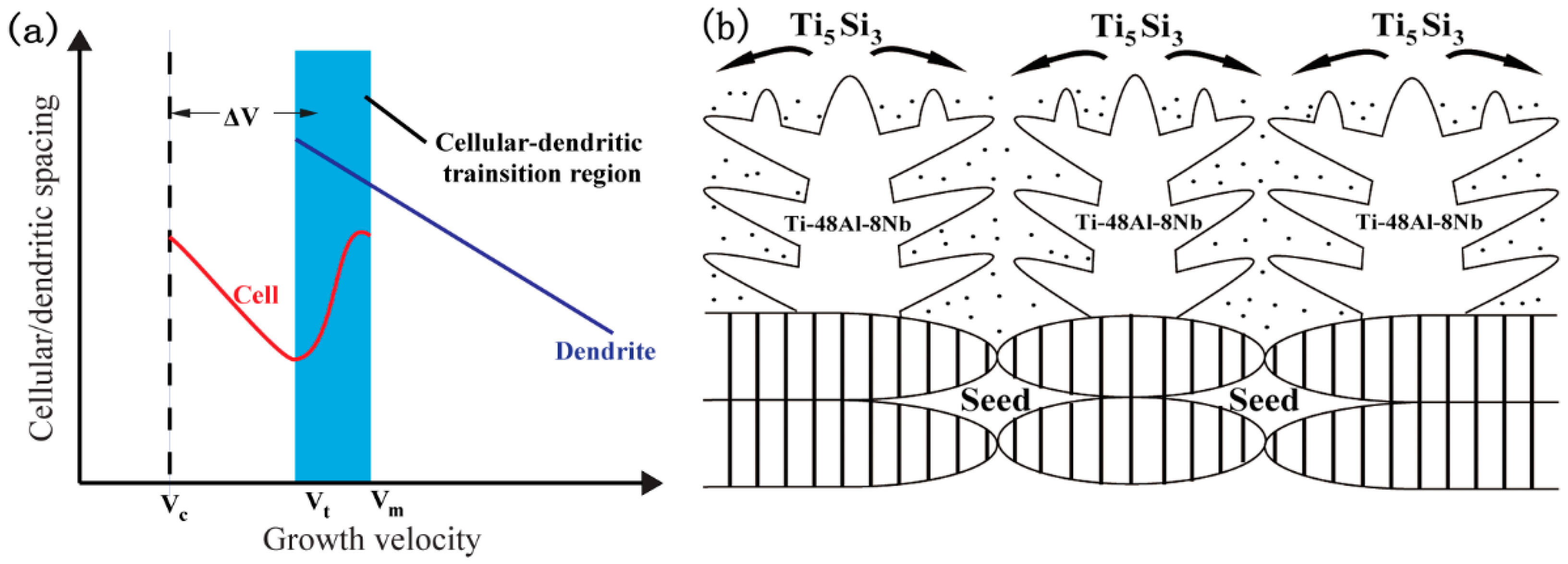
3. Results and Discussion
3.1. Microstructural Development during the Quasi-Seeding Process
3.2. Microstructural Development during the General Seeding Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.N.; Johnson, D.R.; Inui, H.; Oh, M.H.; Wee, D.M.; Yamaguchi, M. Microstructural control through seeding and directional solidification of TiAl alloys containing Mo and C. Acta Mater. 2000, 48, 3221–3233. [Google Scholar] [CrossRef]
- Lee, H.N.; Johnson, D.R.; Inui, H.; Oh, M.H.; Wee, D.M.; Yamaguchi, M. A composition window in the TiAl–Mo–Si system suitable for lamellar structure control through seeding and directional solidification. Mater. Sci. Eng. A 2002, 329–331, 19–24. [Google Scholar] [CrossRef]
- Takeyama, M.; Yamamoto, Y.; Morishima, H.; Koike, K.; Chang, S.Y.; Matsuo, T. Lamellar orientation control of Ti-48Al PST crystal by unidirectional solidification. Mater. Sci. Eng. A 2002, 329–331, 7–12. [Google Scholar] [CrossRef]
- Du, Y.; Shen, J.; Xiong, Y.; Shang, Z.; Wang, L.; Fu, H. Lamellar microstructure alignment and fracture toughness in Ti–47Al alloy by electromagnetic confinement and directional solidification. Mater. Sci. Eng. A 2015, 621, 94–99. [Google Scholar] [CrossRef]
- Du, Y.; Shen, J.; Xiong, Y.; Shang, Z.; Fu, H. Stability of lamellar microstructures in a Ti–48Al–2Nb–2Cr alloy during heat treatment and its application to lamellae alignment as a quasi-seed. Intermetallics 2015, 61, 80–84. [Google Scholar] [CrossRef]
- Kim, M.C.; Oh, M.H.; Lee, J.H.; Inui, H.; Yamaguchi, M.; Wee, D.M. Composition and growth rate effects in directionally solidified TiAl alloys. Mater. Sci. Eng. A 1997, 239–240, 570–576. [Google Scholar] [CrossRef]
- Johnson, D.R.; Inui, H.; Muto, S.; Omiya, Y.; Yamanaka, T. Microstructural development during directional solidification of α-seeded TiAl alloys. Acta Mater. 2006, 54, 1077–1085. [Google Scholar] [CrossRef]
- Johnson, D.R.; Masuda, Y.; Inui, H.; Yamaguchi, M. Alignment of the TiAl/Ti3Al lamellar microstructure in TiAl alloys by directional solidification. Mater. Sci. Eng. A 1997, 239–240, 577–583. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Xu, L.; Tian, J.; Chen, Y. Microscale investigation of perovskite-Ti3AlC strengthening and plastic deformation in high niobium containing TiAl alloys. J. Alloys Compd. 2021, 857, 157563. [Google Scholar] [CrossRef]
- Rackel, M.W.; Stark, A.; Gabrisch, H.; Pyczak, F. Screening for O phase in advanced γ–TiAl alloys. Intermetallics 2021, 131, 107086. [Google Scholar] [CrossRef]
- Tiller, W.A.; Jackson, K.A.; Rutter, J.W.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Metall. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Smith, V.G.; Tiller, W.A.; Rutter, J.W. A Mathematical Analysis of Solute Redistribution During Solidification. Can. J. Phys. 1955, 33, 723–745. [Google Scholar] [CrossRef]
- Jackson, K.A.; Pfann, W.G. Producing a linear gradient of solute concentration by changing the volume of a molten zone. J. Appl. Phys. 1965, 36, 320. [Google Scholar] [CrossRef]
- Humphreys, L.B.; Heminger, J.A.; Young, G.W. Morphological instability in a float zone. J. Cryst. Growth 1990, 100, 31–50. [Google Scholar] [CrossRef]
- Louchev, O.A.; Otani, S. Morphological instability of the solid-liquid interface and the supersaturation gradient in crystal growth from a high-temperature solution. Phys. Rev. E 1996, 54, 6372–6380. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, H.; Huang, H.; Liang, H.; Chen, R.; Guo, J.; Fu, H. Microstructure formation and columnar to equiaxed transition during cold crucible directional solidification of a high-Nb TiAl alloy. J. Mater. Res. Technol. 2021, 11, 2221–2234. [Google Scholar] [CrossRef]
- Xu, X.; Ding, H.; Li, W.; Huang, H.; Liang, H.; Chen, R.; Guo, J.; Fu, H. The microstructure and high cycle fatigue performance of as-cast and directionally solidified Ti46Al7Nb alloy under the three-point bending loading. Mater. Sci. Eng. A 2021, 822, 141633. [Google Scholar] [CrossRef]
- Burton, J.A.; Prim, R.C.; Slichter, W.P. The Distribution of Solute in Crystals Grown from the Melt. Part I. Theoretical. J. Chem. Phys. 1953, 21, 1987–1991. [Google Scholar] [CrossRef]
- Seidensticker, R.G. Kinetic Effects in Temperature Gradient Zone Melting. J. Electrochem. Soc. 1966, 113, 152. [Google Scholar] [CrossRef]
- Pfann, W.G. Zone melting. Metall. Rev. 1957, 2, 29–76. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Diffusion in the Ti–Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- Jung, I.-S.; Kim, M.-C.; Lee, J.-H.; Oh, M.-H.; Wee, D.-M. High temperature phase equilibria near Ti–50 at% Al composition in Ti–Al system studied by directional solidification. Intermetallics 1999, 7, 1247–1253. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary aluminum-titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Trivedi, R.; Somboonsuk, K. Pattern formation during the directional solidification of binary systems. Acta Metall. 1985, 33, 1061–1068. [Google Scholar] [CrossRef]
- Eshelman, M.; Seetharaman, V.; Trivedi, R. Cellular spacings—I. Steady-state growth. Acta Metall. 1988, 36, 1165–1174. [Google Scholar] [CrossRef]




| Property | Symbol | Value | Unit |
|---|---|---|---|
| Diffusion coefficient of Al in liquid [8] | Dl | 5 × 10−9 | m2/s |
| Diffusion coefficient of Al in solid [21] | Ds | 1 × 10−11 | m2/s |
| Peritectic temperature [22,23] | Tp | 1491.8 | °C |
| Liquid concentration at Tp [22,23] | CL | 49.5 | at.% |
| Partition coefficient of α phase [8] | kα | 0.94 | - |
| Liquidus slope of α phase [8] | mα | −2.4 | K/at.% |
| Liquidus slope of β phase [8] | mβ | −8.5 | K/at.% |
| Length of the float zone | L | 20 | mm |
| Thickness of the diffusion boundary | δ | 0.45 | mm |
| Growth velocity | V | 10 | µm/s |
| Temperature gradient | G | 2 × 104 | K/m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.-L.; Shen, J.; Du, Y.-J. Effect of Solute Redistribution on Seeding Process of TiAl Alloys with Limited Convection in a Float Zone. Metals 2021, 11, 1525. https://doi.org/10.3390/met11101525
Xiong Y-L, Shen J, Du Y-J. Effect of Solute Redistribution on Seeding Process of TiAl Alloys with Limited Convection in a Float Zone. Metals. 2021; 11(10):1525. https://doi.org/10.3390/met11101525
Chicago/Turabian StyleXiong, Yi-Long, Jun Shen, and Yu-Jun Du. 2021. "Effect of Solute Redistribution on Seeding Process of TiAl Alloys with Limited Convection in a Float Zone" Metals 11, no. 10: 1525. https://doi.org/10.3390/met11101525
APA StyleXiong, Y.-L., Shen, J., & Du, Y.-J. (2021). Effect of Solute Redistribution on Seeding Process of TiAl Alloys with Limited Convection in a Float Zone. Metals, 11(10), 1525. https://doi.org/10.3390/met11101525






