Hydrophobic Modification of Graphene Oxide and Its Effect on the Corrosion Resistance of Silicone-Modified Epoxy Resin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
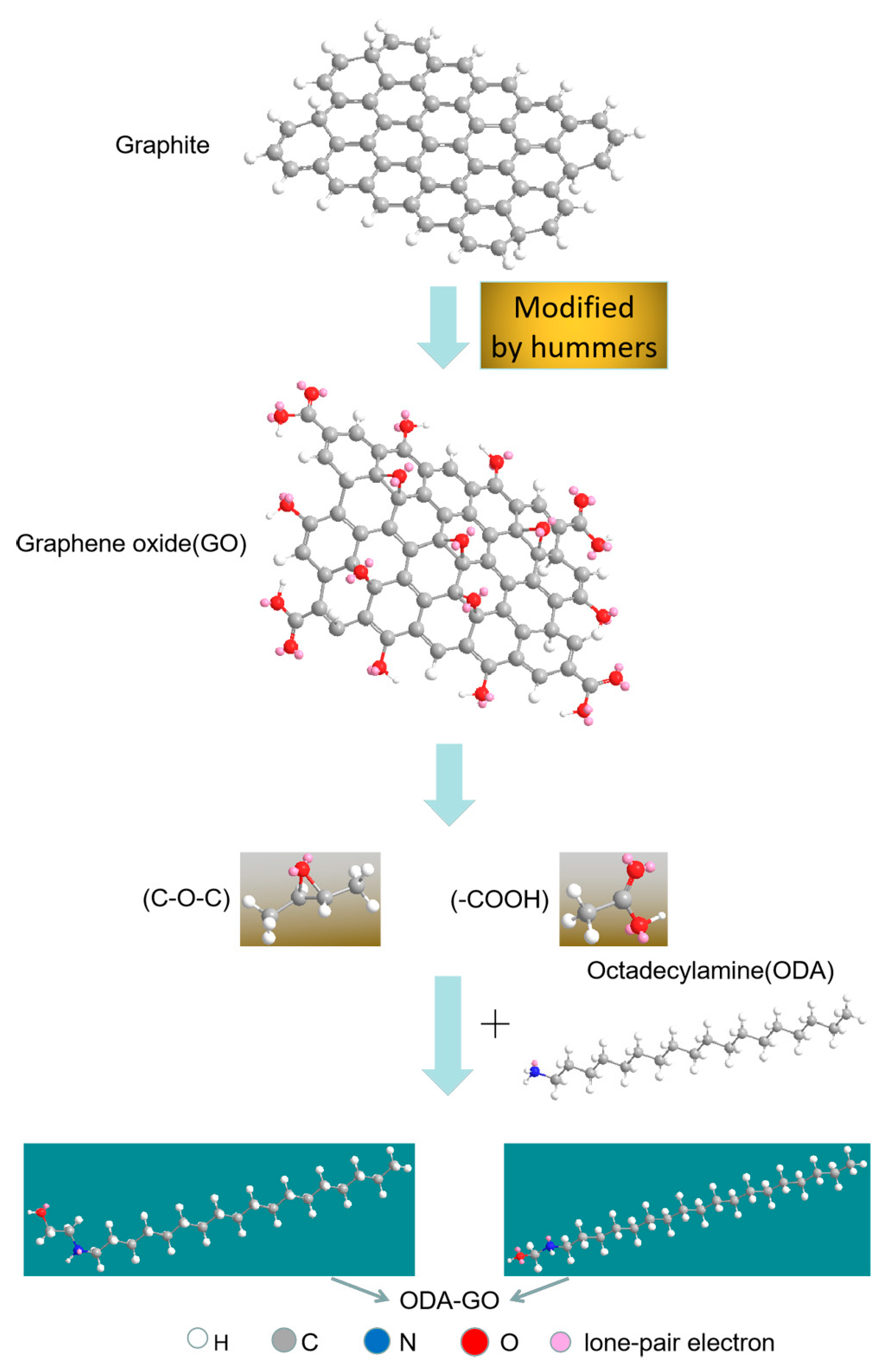
2.2. Modification of GO with ODA
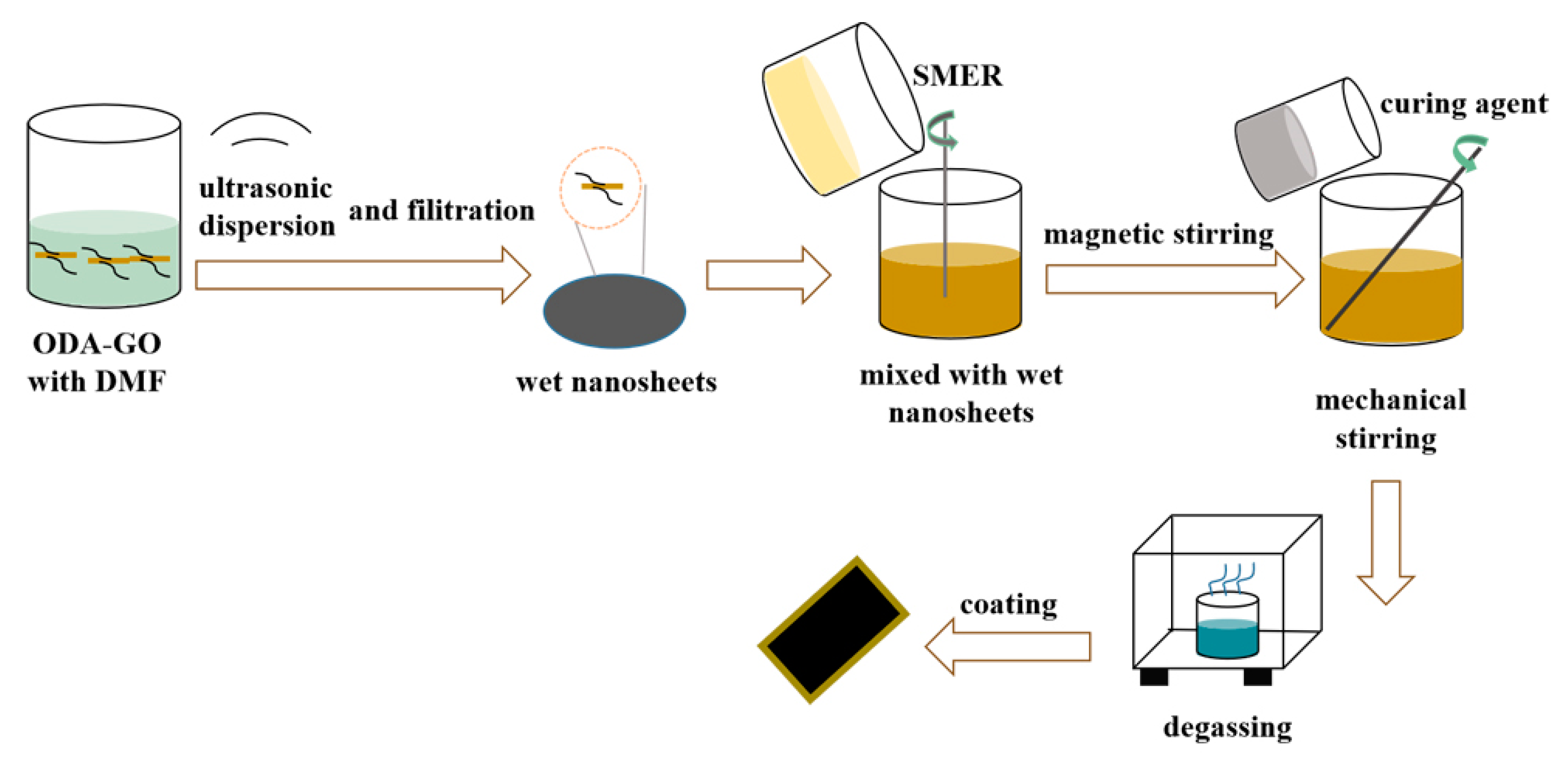
2.3. Preparation of ODA/SMER Coating
2.4. Characterization and Measurement
2.4.1. Characterization of ODA-GO Nanosheets and ODA-GO SMER Coating
2.4.2. Salt Spray Test
2.4.3. Electrochemical Measurements
3. Results and Discussion
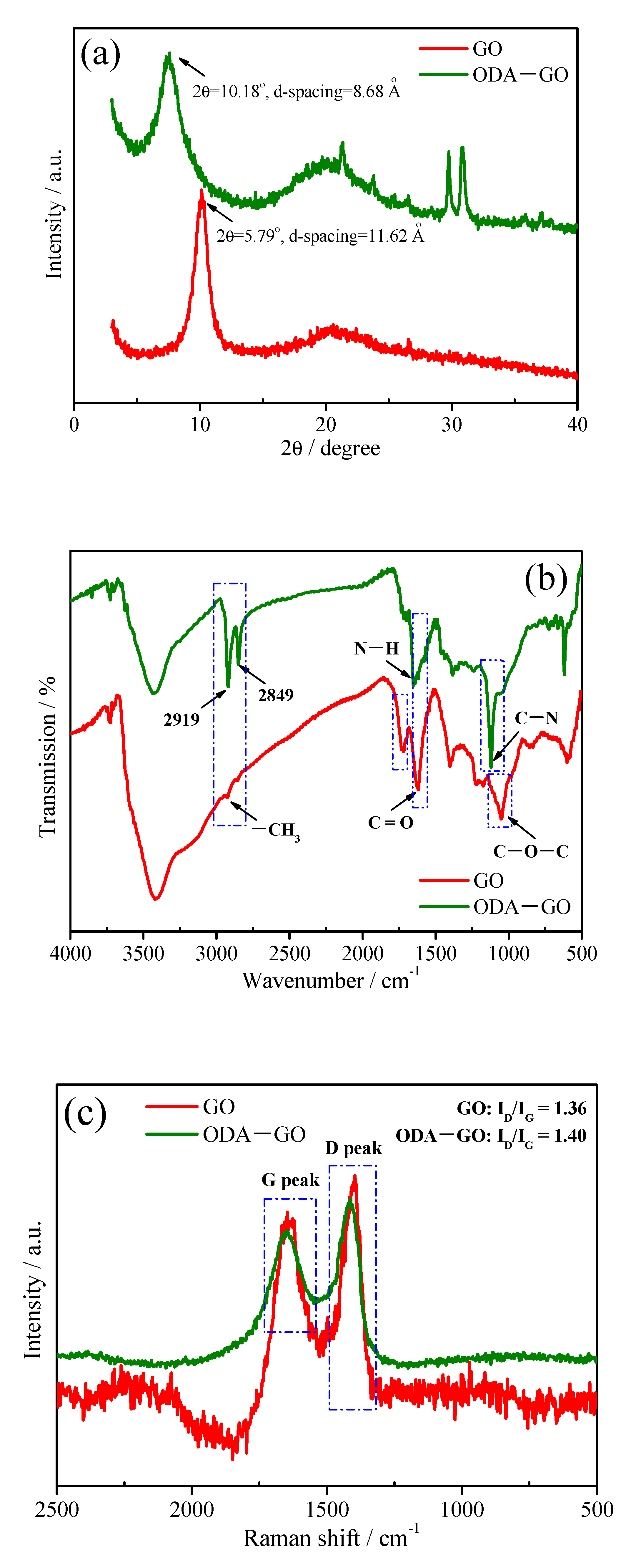
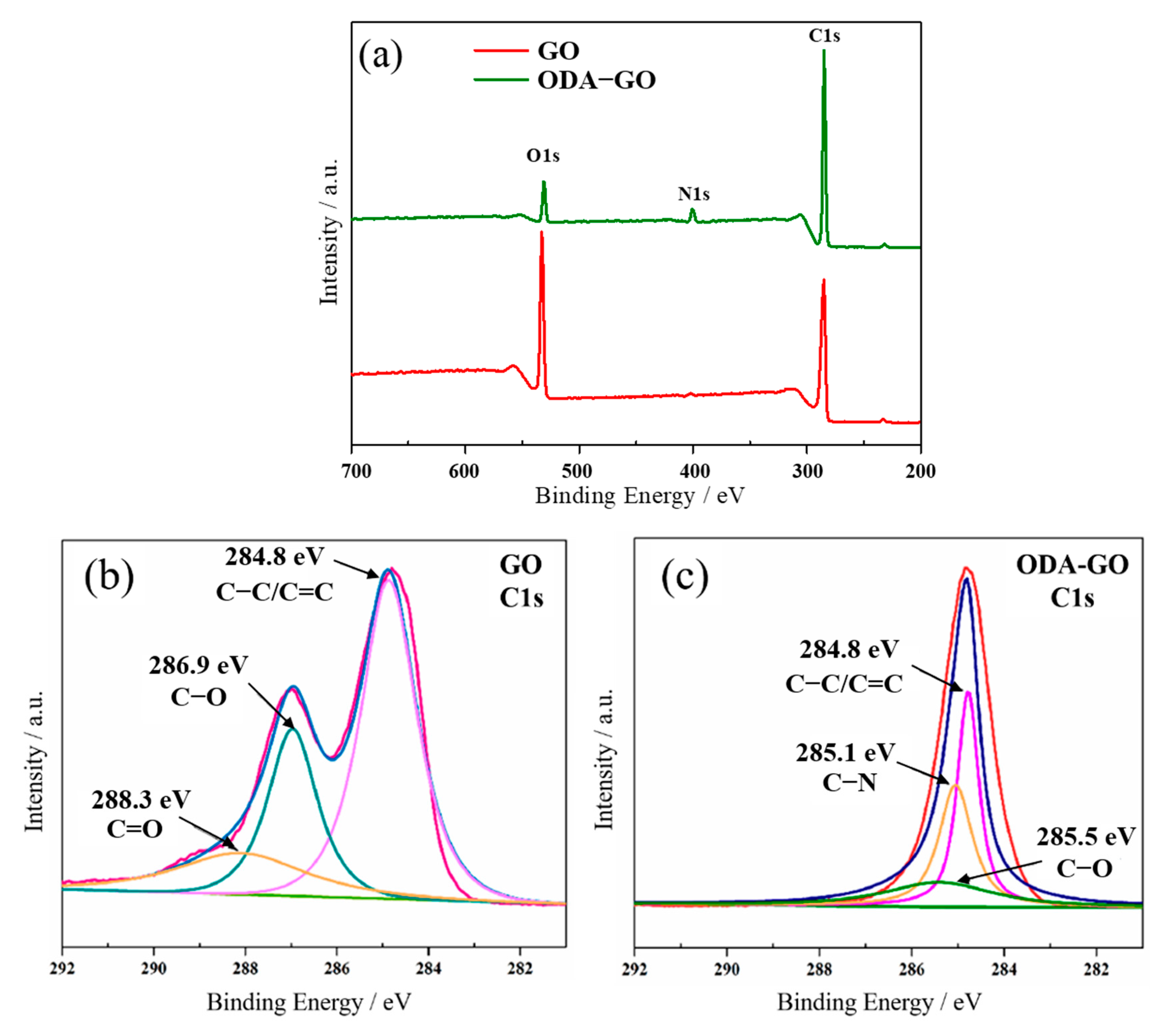
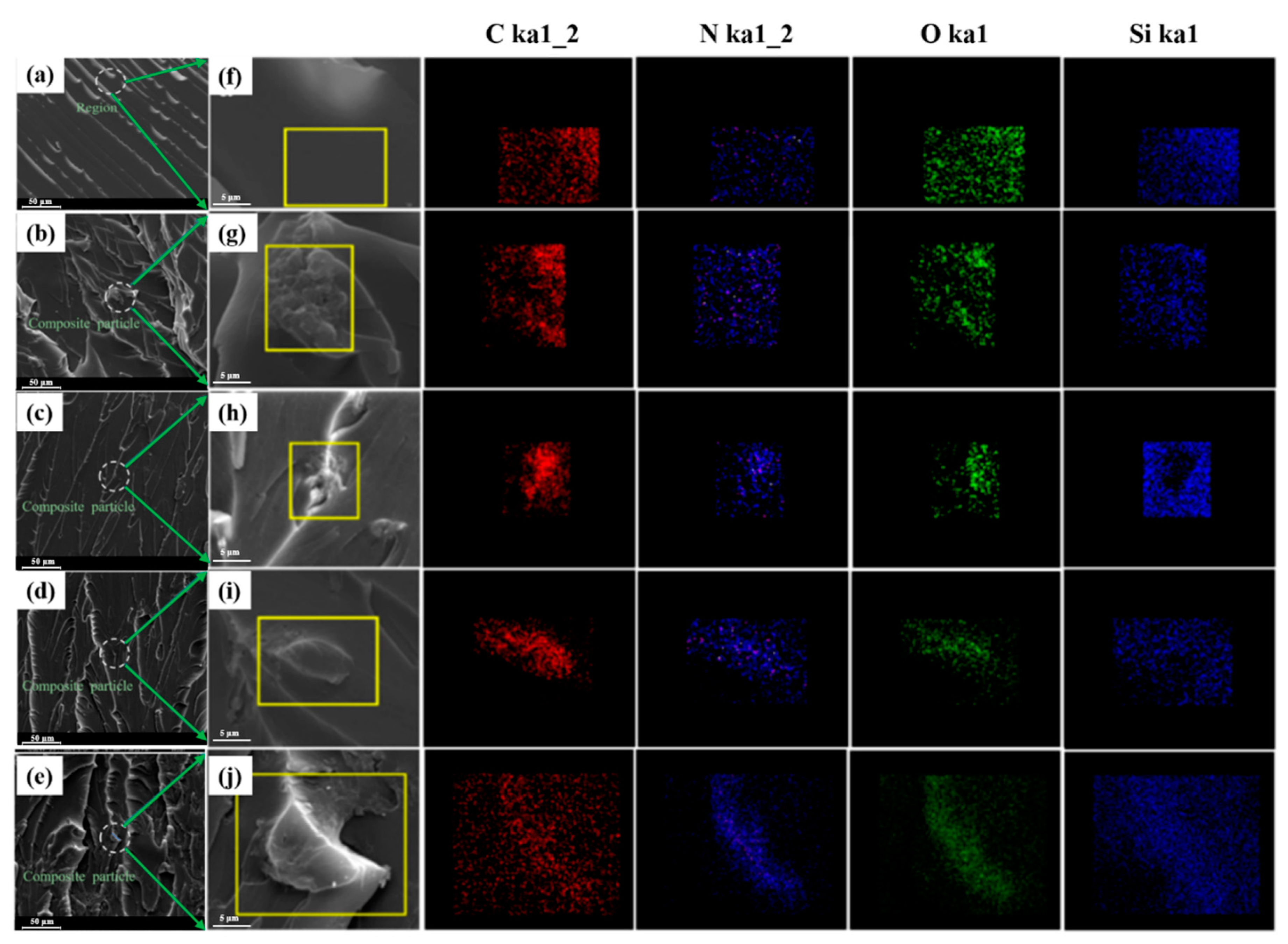
3.1. Characterization of ODA-GO Nanosheets
3.2. Physical Performance Measurements of the ODA-GO Nanosheets
3.3. Interfacial Adhesion Properties of ODA-GO/SMER Coatings
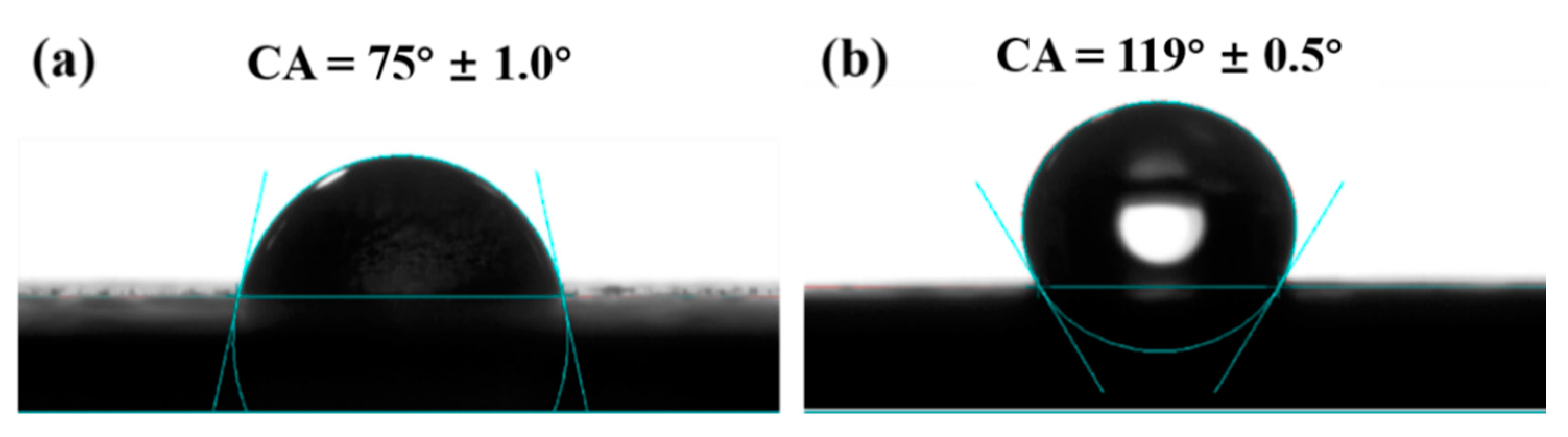
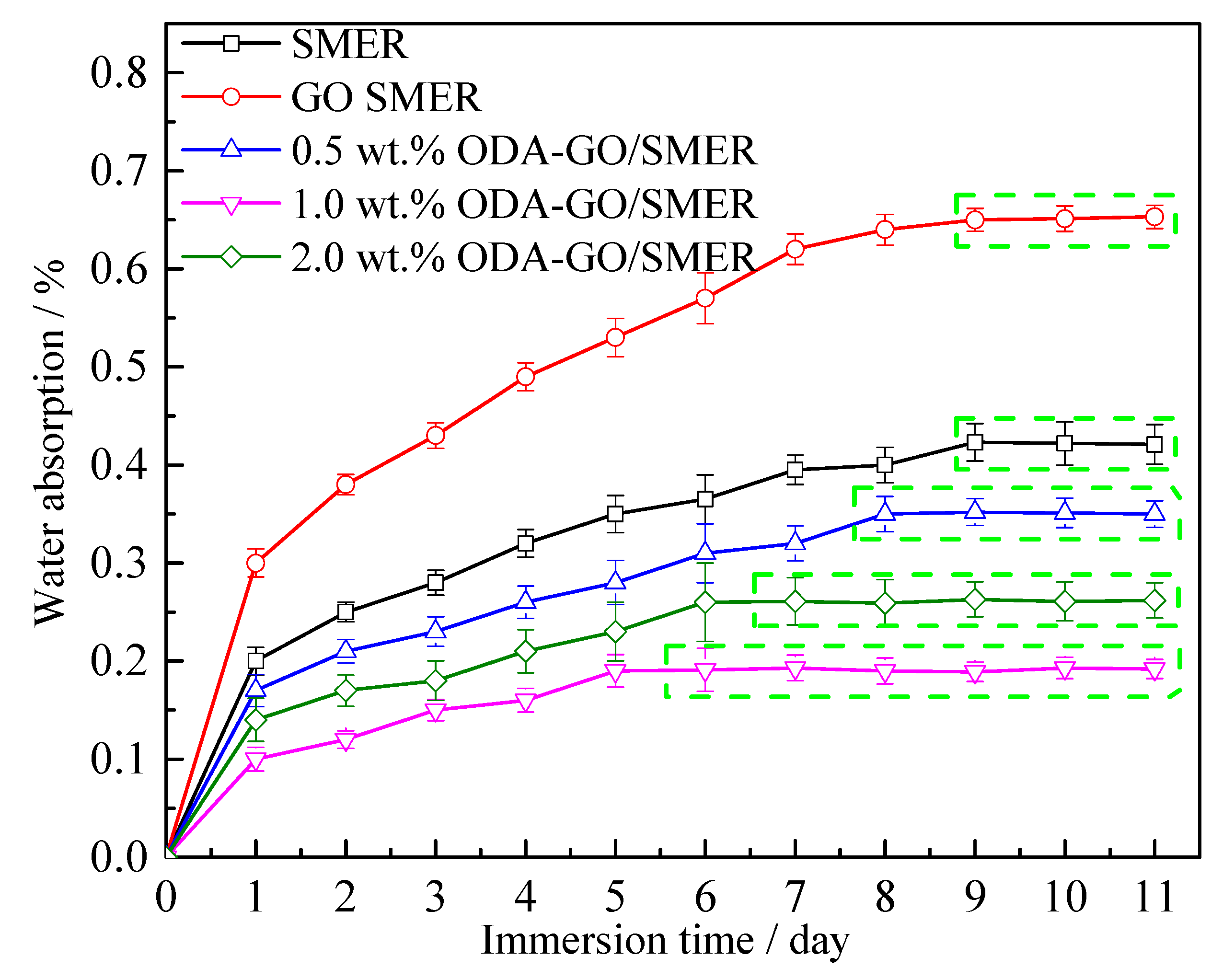
3.4. Hydrophobicity and Water Absorption Properties of the ODA-GO/SMER Coating
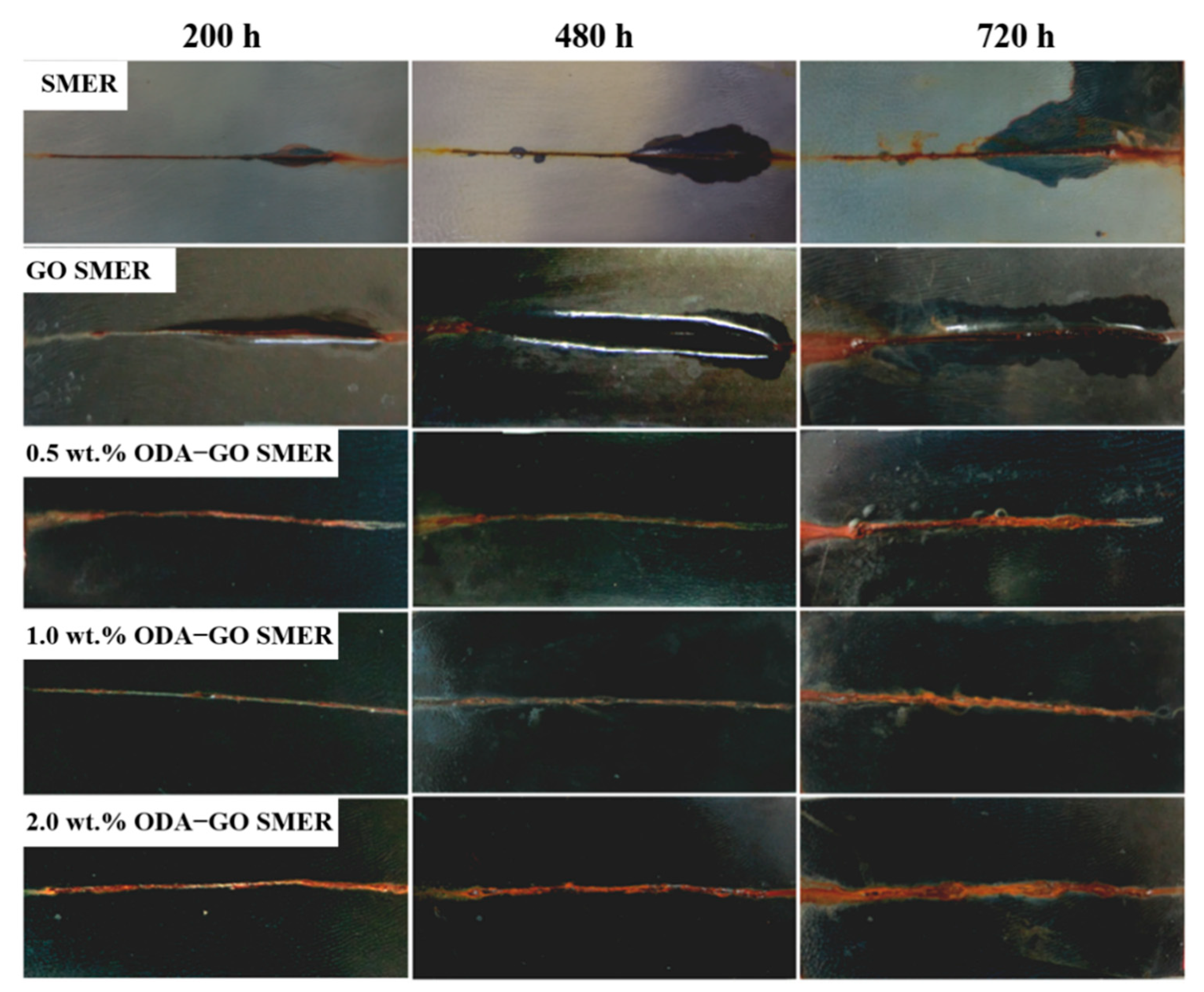
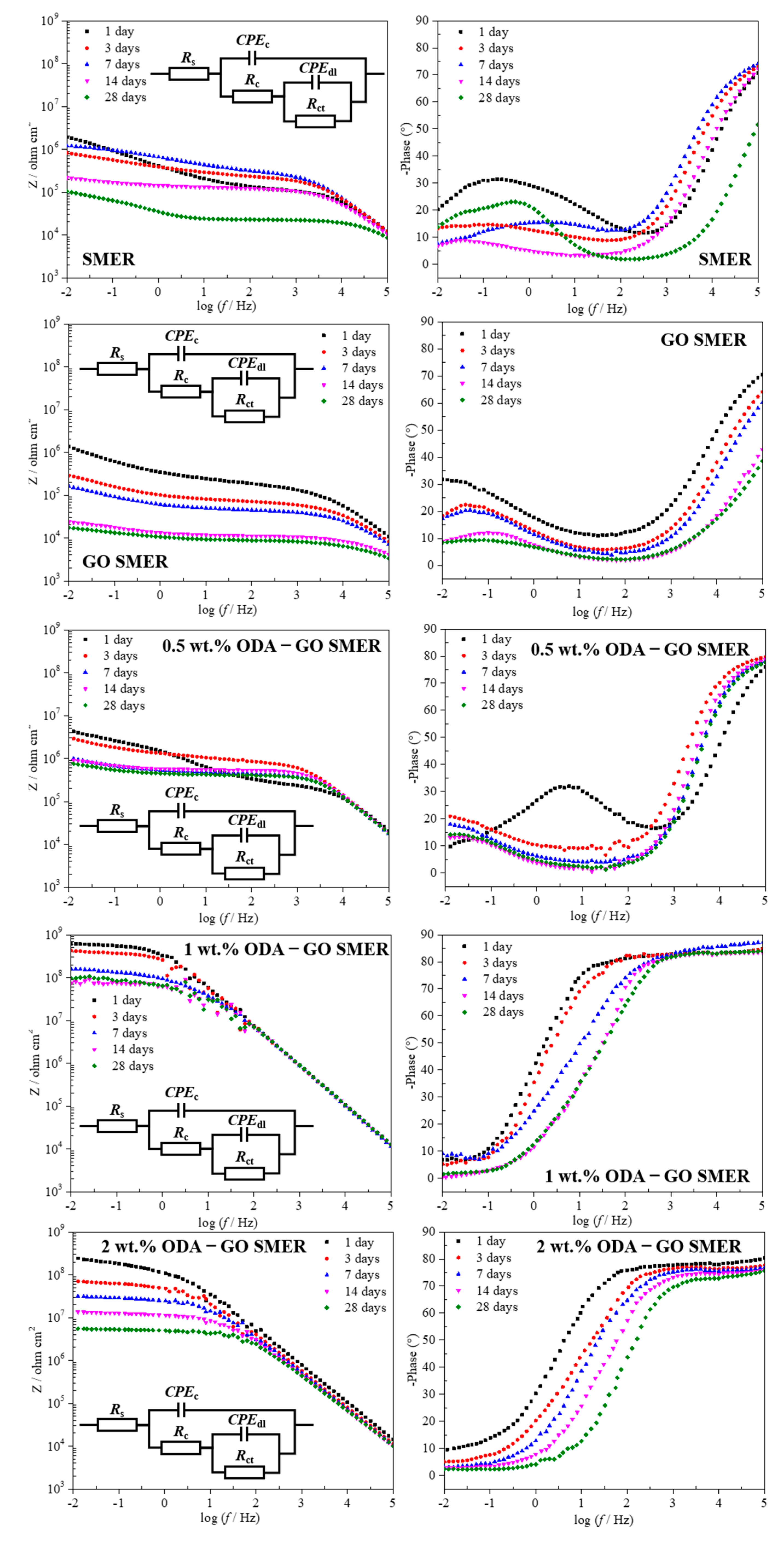
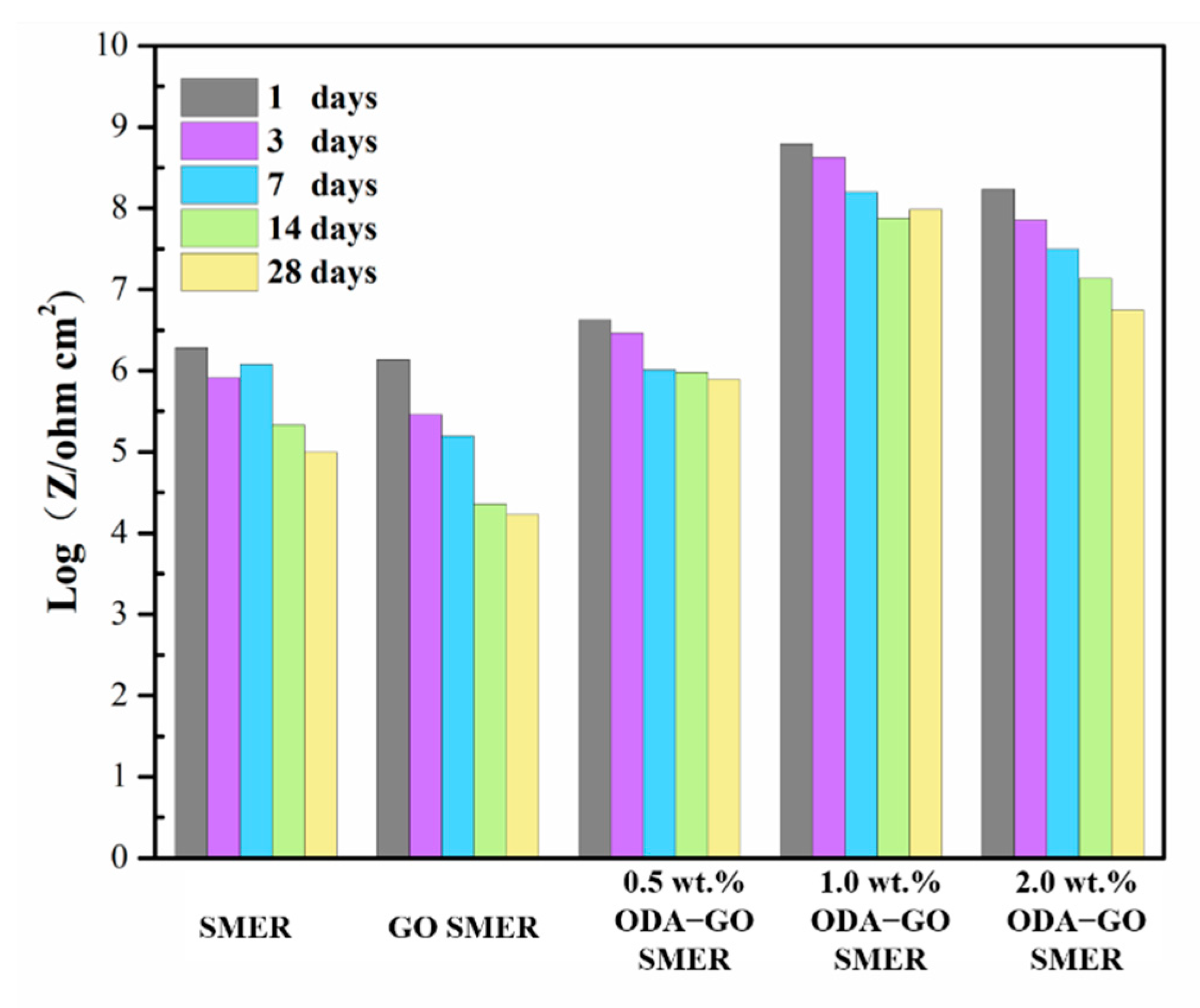
3.5. Effect of ODA-GO on SMER Coating Corrosion Resistance
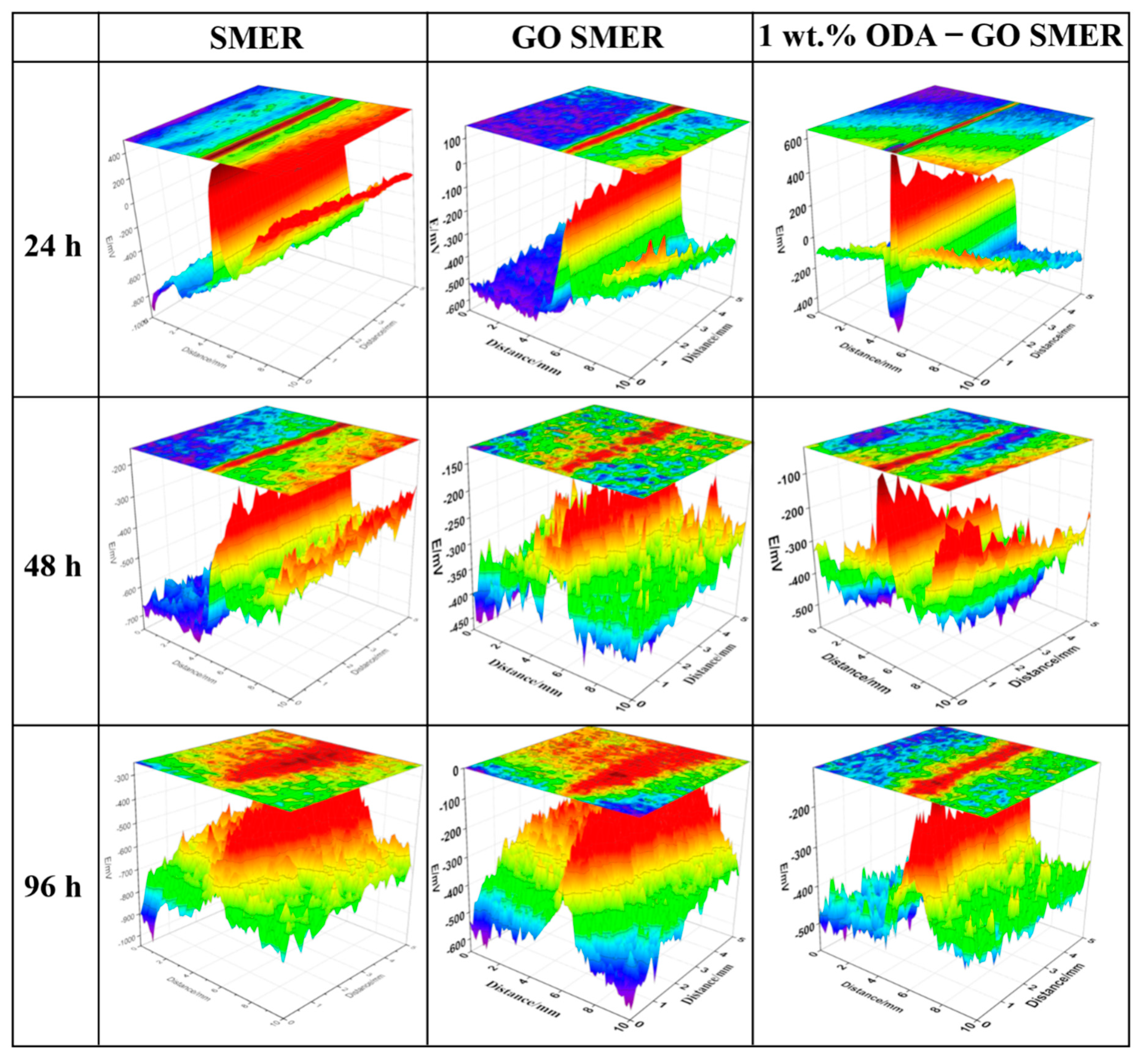
3.6. Cathodic Anti-Peeling Performance of the ODA-GO/SMER Composite Coatings
- (1)
- Anodic reaction: Fe—2e → Fe2+
- (2)
- Cathodic reaction: O2 + 2H2O + 4e → 4OH−
- (3)
- Secondary corrosion reaction: Fe + 2OH− → Fe(OH)2The Fe(OH)2 formed at the steel matrix is easily oxidized to Fe2O3 and FeOOH.
- (4)
- 2Fe(OH)2 + 1/2O2 → 2FeOOH + H2O
- (5)
- 4Fe(OH)2 + O2 → 2Fe2O3 + 4H2O
- (6)
- Anodic reaction: Fe—2e → Fe2+
- (7)
- Cathodic reaction: 8FeOOH + Fe2+2e → 3Fe3O4 + 4H2O
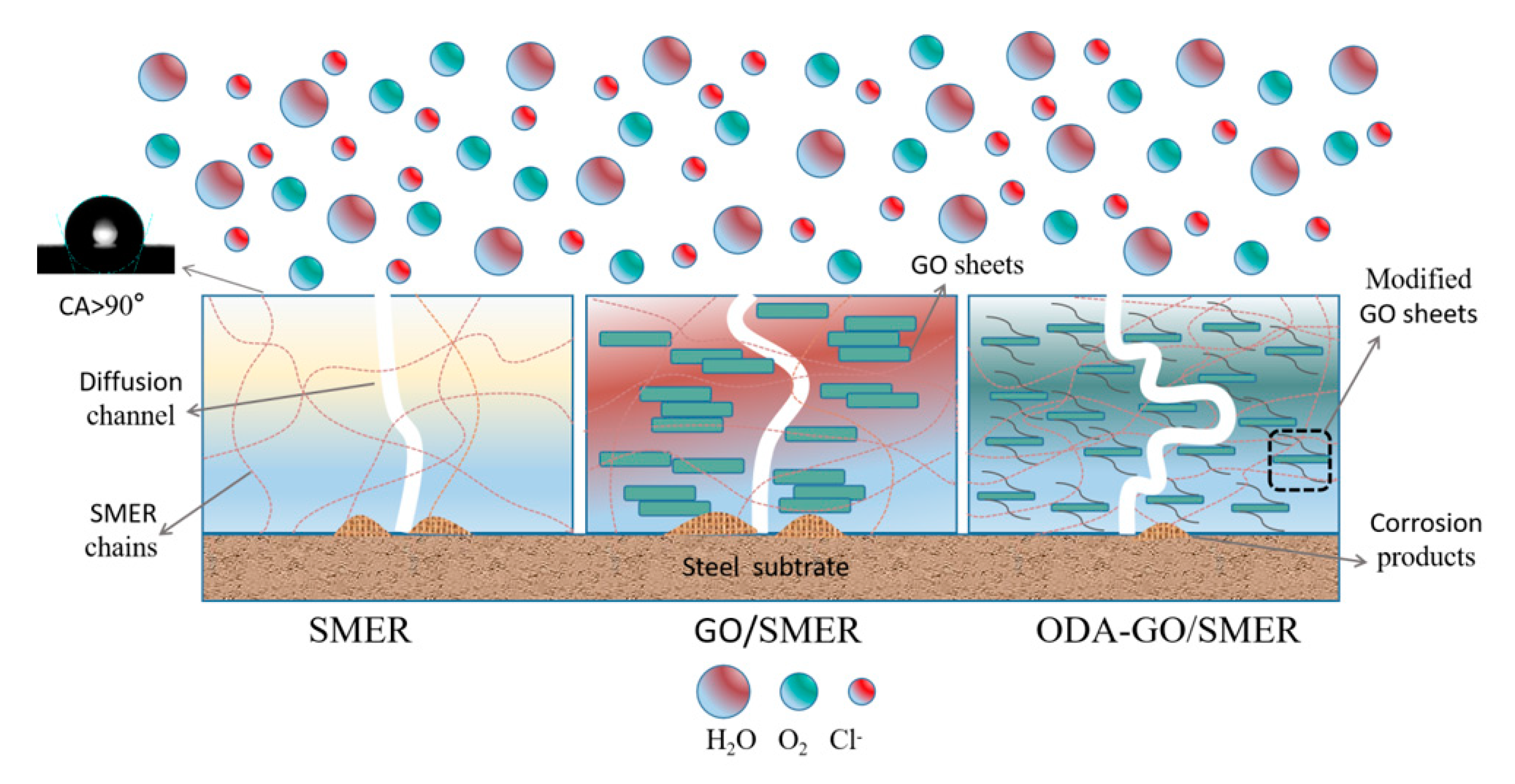
3.7. Mechanism by Which ODA-GO Improves Corrosion Resistance in SMER Coatings
4. Conclusions
- (1)
- XRD, FT-IR, Raman, XPS, SEM, and other characterization methods confirmed that the ODA successfully grafted onto the GO surfaces by chemical bonding with oxygen-containing groups of the GO, which gave the ODA-GO material high hydrophobicity and good dispersibility in SMER coating.
- (2)
- The good compatibility of ODA-GO with the SMER coating effectively enhanced their interfacial adhesion, reduced the water absorption of the coating, prolonged the diffusion path of the corrosive medium inside the coating and strengthened the anti-peeling performance of the coating applied on the steel. Hence, the physical barrier effect and corrosion resistance of the SMER coating were comprehensively improved.
- (3)
- With 1 wt.% addition of ODA-GO to SMER, the composite coating on the carbon steel matrix gave the best anticorrosive effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, M.J.; Ren, S.M.; Zhao, H.C.; Xue, Q.J.; Wang, L.P. Polydopamine coated graphene oxide for anticorrosive rein-forcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Kumar, S.A.; Sasikumar, A. Studies on novel silicone/phosphorus/sulphur containing nano-hybrid epoxy anticorro-sive and antifouling coatings. Prog. Org. Coat. 2010, 68, 189–200. [Google Scholar] [CrossRef]
- Mert, B.D. Corrosion protection of aluminum by electrochemically synthesized composite organic coating. Corros. Sci. 2016, 103, 88–94. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide–alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Zheng, H.P.; Shao, Y.W.; Wang, Y.Q.; Meng, G.Z.; Liu, B. Reinforcing the corrosion protection property of epoxy coat-ing by using graphene oxide–poly(urea–formaldehyde) composites. Corros. Sci. 2017, 123, 267–277. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Zhang, Y.; Meng, G.; Zhang, T.; Wang, F. Using high-temperature mechanochemistry treatment to modify iron oxide and improve the corrosion performance of epoxy coating -II. Effect of grinding temperature. Corros. Sci. 2015, 90, 463–471. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Zhang, Y.; Meng, G.; Zhang, T.; Wang, F. Using high-temperature mechanochemistry treatment to modify iron oxide and improve the corrosion performance of epoxy coating -I. High-temperature ball milling treat-ment. Corros. Sci. 2015, 90, 451–462. [Google Scholar] [CrossRef]
- Luo, H.L.; Ao, H.Y.; Peng, M.X.; Yao, F.L.; Yang, Z.Y.; Wan, Y.Z. Effect of highly dispersed graphene and graphene ox-ide in 3D nanofibrous bacterial cellulose scaffold on cell response: A comparative study. Mater. Chem. Phys. 2019, 235, 121774. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Padhiari, S.; Biswal, S.K.; Panda, B.B.; Hota, G. Fe3O4 nanoparticles functionalized GO/g-C3N4 nanocompo-site: An efficient magnetic nanoadsorbent for adsorptive removal of organic pollutants. Mater. Chem. Phys. 2020, 244, 122710. [Google Scholar] [CrossRef]
- Sari, M.J.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an epoxy composite coating with enhanced corrosion re-sistance through impregnation of functionalized graphene oxide-co-montmorillonite Nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild stee. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Y.; Wong, C.-P. Facile Fabrication of Superhydrophobic Octadecylamine-Functionalized Graphite Oxide Film. Langmuir 2010, 26, 16110–16114. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, H.; Zhong, Q. Synthesis and characterization of MOF-aminated graphite oxide composites for CO2 capture. Appl. Surf. Sci. 2013, 284, 138–144. [Google Scholar] [CrossRef]
- Jahandideh, S.; Shirazi, M.J.S.; Tavakoli, M. Mechanical and thermal properties of octadecylamine-functionalized graphene oxide reinforced epoxy nanocomposites. Fibers Polym. 2017, 18, 1995–2004. [Google Scholar] [CrossRef]
- Cao, N.; Zhan, Y. Study of Reduced Graphene Oxide Preparation by Hummers’ Method and Related Characteriza-tion. J. Nanomater. 2015, 2, 1–5. [Google Scholar]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Das, A.K.; Srivastav, M.; Layek, R.K.; Uddin, M.E.; Jung, D.; Kim, N.H.; Lee, J.H. Iodidemediated room temperature re-duction of graphene oxide: A rapid chemical route for the synthesis of a bifunctional electrocatalyst. J. Mater. Chem. A 2014, 2, 1332–1340. [Google Scholar] [CrossRef]
- Wang, C.; Lan, Y.; Yu, W.; Li, X.; Qian, Y.; Liu, H. Preparation of amino-functionalized graphene oxide/polyimide composite films with improved mechanical, thermal and hydrophobic properties. Appl. Surf. Sci. 2016, 362, 11–19. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Syn-thesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.M. Characterization of covalently-grafted polyisocyanate chains onto graphene oxide for polyurethane composites with improved mechanical properties. Chem. Eng. J. 2015, 281, 869–883. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wan, Y.; Wu, J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Bi, H.; Sykes, J. Cathodic disbonding of an unpigmented epoxy coating on mild steel under semi- and full-immersion conditions. Corros. Sci. 2011, 53, 3416–3425. [Google Scholar] [CrossRef]
- Xiao, K.; Dong, C.; Zhang, X.; Wu, J.; Xu, L.; Lin, Y.-C. Corrosion of carbon steel under epoxy-varnish coating studied by scanning Kelvin probe. J. Wuhan Univ. Technol. Sci. Ed. 2012, 27, 825–829. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.M. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Cao, X.K.; Huang, F.; Huang, C.; Liu, J.; Cheng, F. Preparation of grahene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Golru, S.S.; Attar, M.; Ramezanzadeh, B. Studying the influence of nano-Al2O3 particles on the corrosion performance and hydrolytic degradation resistance of an epoxy/polyamide coating on AA-1050. Prog. Org. Coat. 2014, 77, 1391–1399. [Google Scholar] [CrossRef]
- Qi, K.; Sun, Y.M.; Duan, H.W.; Guo, X.P. A corrosion -protective coating based on a solution-processable poly-mer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Hu, Q.; Zhang, J.; Huang, F.; Liu, J. Hydrophobic Modification of Graphene Oxide and Its Effect on the Corrosion Resistance of Silicone-Modified Epoxy Resin. Metals 2021, 11, 89. https://doi.org/10.3390/met11010089
Yuan W, Hu Q, Zhang J, Huang F, Liu J. Hydrophobic Modification of Graphene Oxide and Its Effect on the Corrosion Resistance of Silicone-Modified Epoxy Resin. Metals. 2021; 11(1):89. https://doi.org/10.3390/met11010089
Chicago/Turabian StyleYuan, Wei, Qian Hu, Jiao Zhang, Feng Huang, and Jing Liu. 2021. "Hydrophobic Modification of Graphene Oxide and Its Effect on the Corrosion Resistance of Silicone-Modified Epoxy Resin" Metals 11, no. 1: 89. https://doi.org/10.3390/met11010089
APA StyleYuan, W., Hu, Q., Zhang, J., Huang, F., & Liu, J. (2021). Hydrophobic Modification of Graphene Oxide and Its Effect on the Corrosion Resistance of Silicone-Modified Epoxy Resin. Metals, 11(1), 89. https://doi.org/10.3390/met11010089



