Influence of Exposure to Environment on Degradation of a Friction Stir Welded Aluminum Alloy
Abstract
1. Introduction
- The nugget zone (NZ).
- Thermo-mechanical affected zone (TMAZ).
- Heat-affected zone (HAZ).
- Since pitting corrosion occurred both at and along the edges of the intermetallic compounds present in the microstructure, an increase in both the presence and distribution of intermetallic compounds in the NZ contributes to a gradual increase in the corrosion galvanic couple.
- A refinement in grain structure of the NZ results in an increase in corrosion sensitivity.
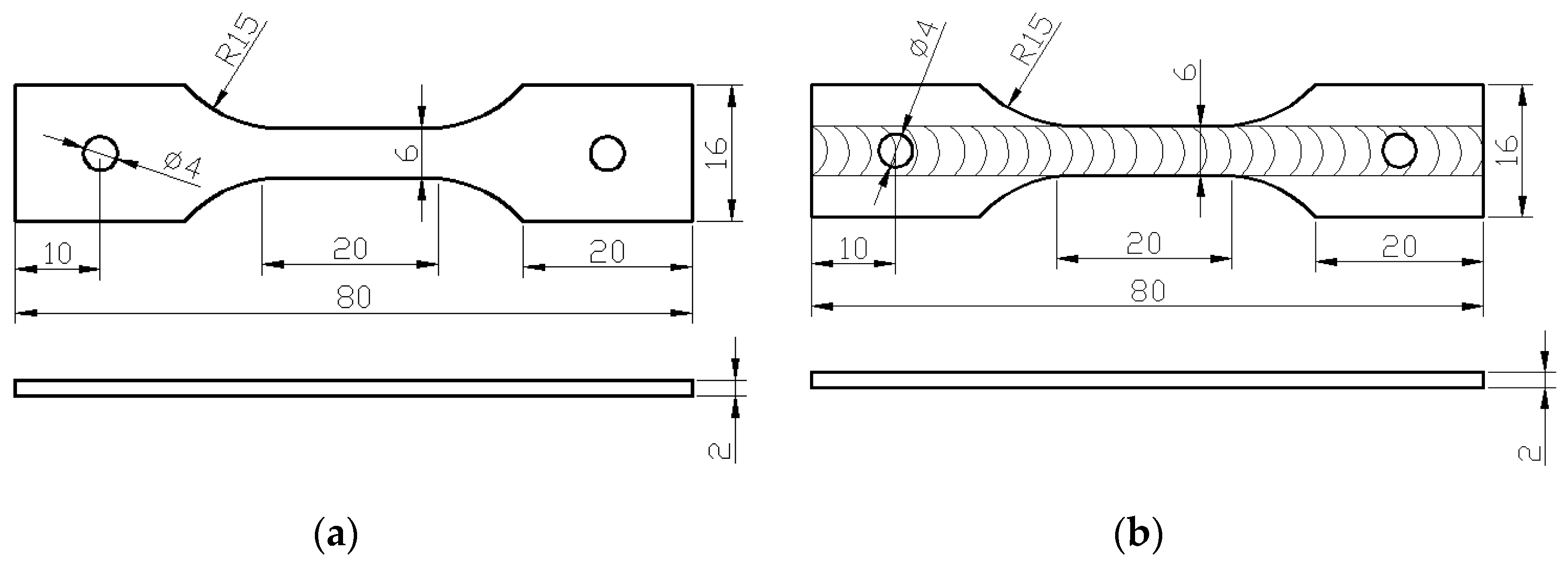
2. Materials and Methods
- A loading device for the stress corrosion test was used to apply a constant tensile stress on the test specimen while being exposed to the environment during loading;
- A continuous zoom video microscope (Model: UNION DZ3; Union.Co., Chuo-ku, Kobe, Japan) was used to observe the morphology, nature, extent and severity of environment-induced damage, or corrosion experienced by the test specimens taken from both the base metal and the FSW joint.
- A low magnification scanner (Model: HP 8200, Hewlett-Packard, Palo Alto, CA, USA) used to establish the macroscopic corrosion morphology both at and surrounding the zone of corrosion.
- A laser displacement sensor (Model: Keyence LK-G30, Keyence (China) Co. Ltd., Shanghai, China) to measure the depth of pits on the surface of test specimens exposed to the aggressive aqueous solution (i.e., EXCO solution) while concurrently obtaining distribution data specific to depth of the pit. The measurement accuracy of the instrument can reach 0.02 μm.
3. Results and Discussion
3.1. Analysis of Morphology of Electrochemical Corrosion
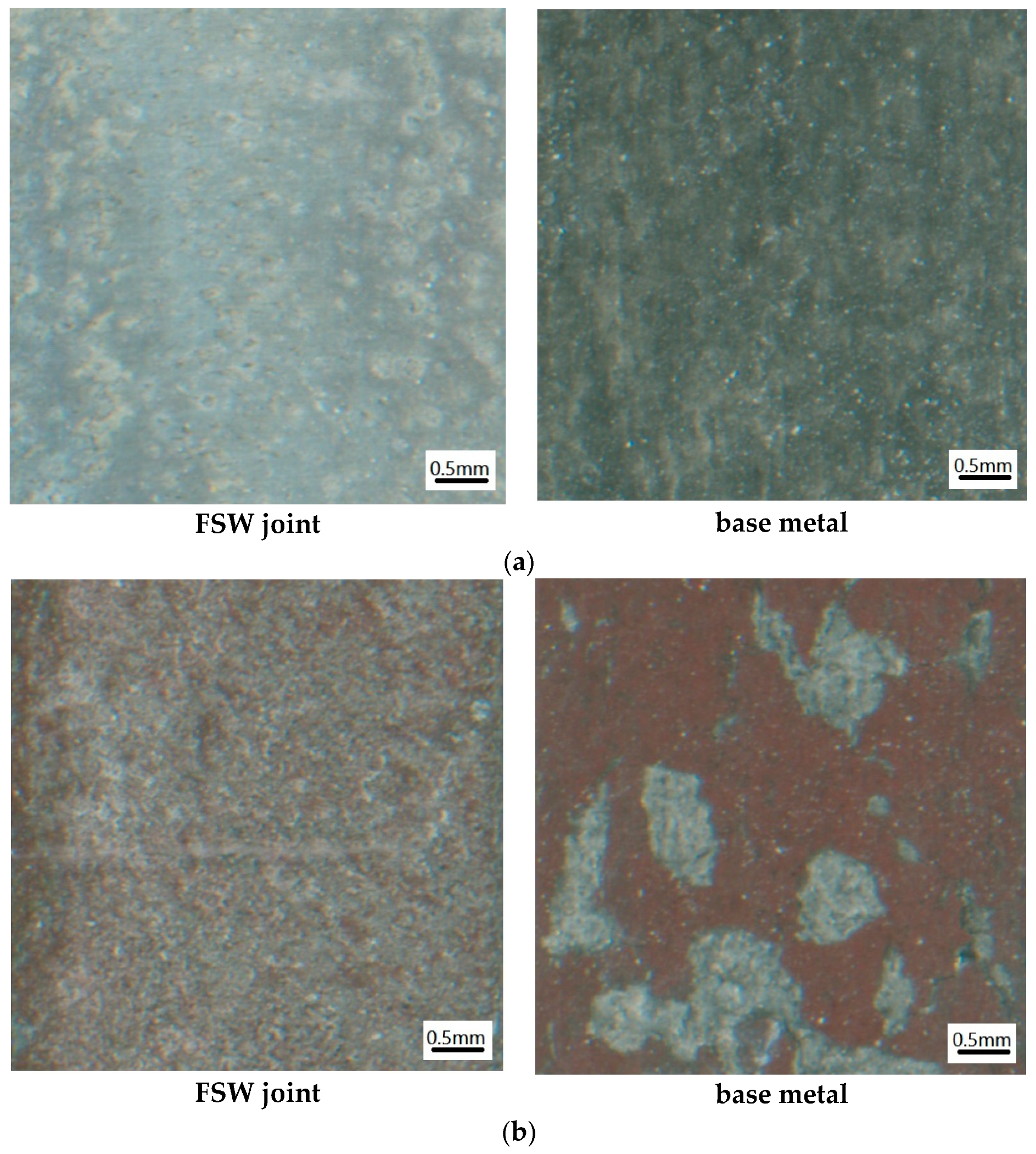
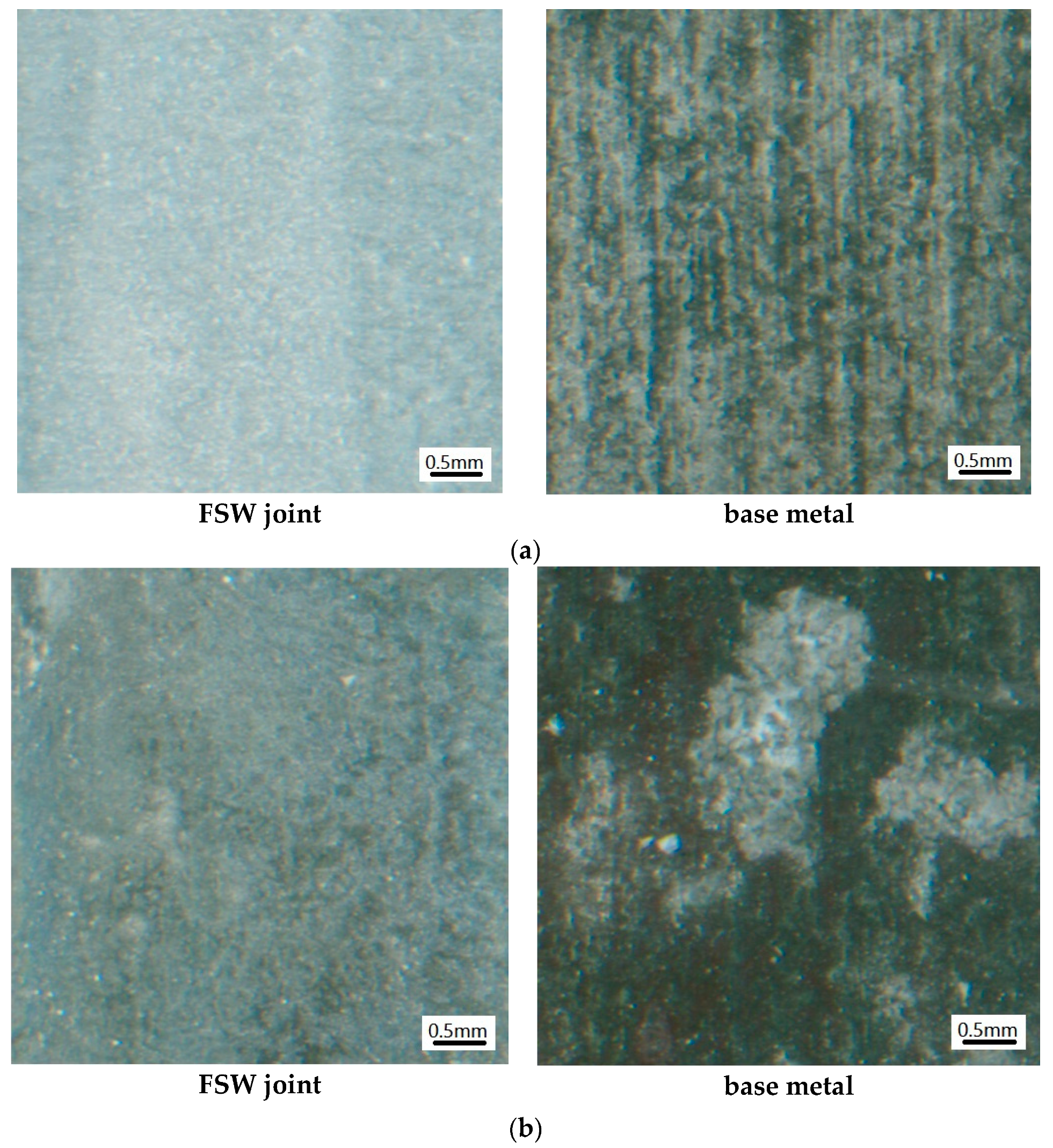
3.1.1. Morphology at the Macroscopic Level
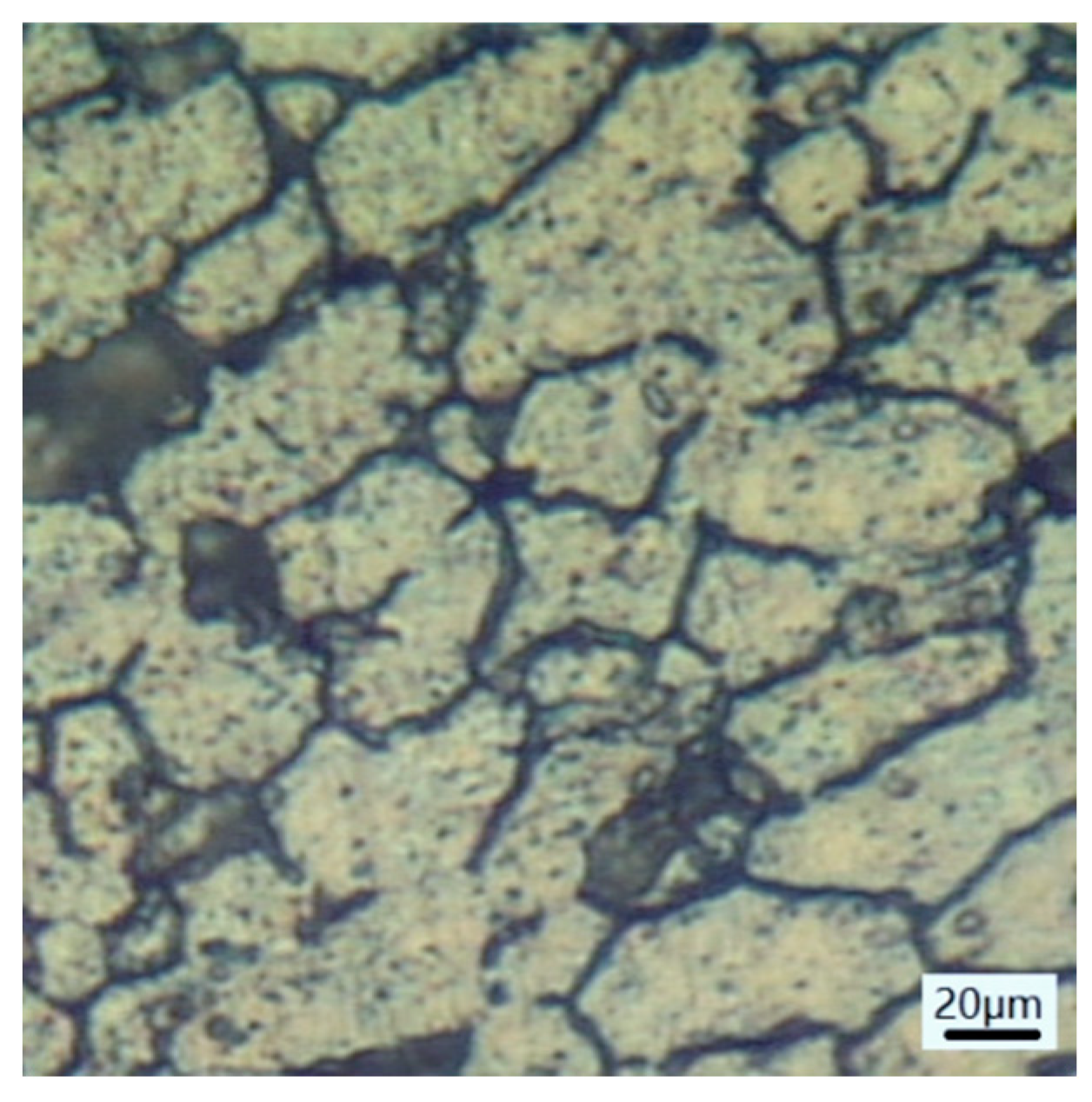
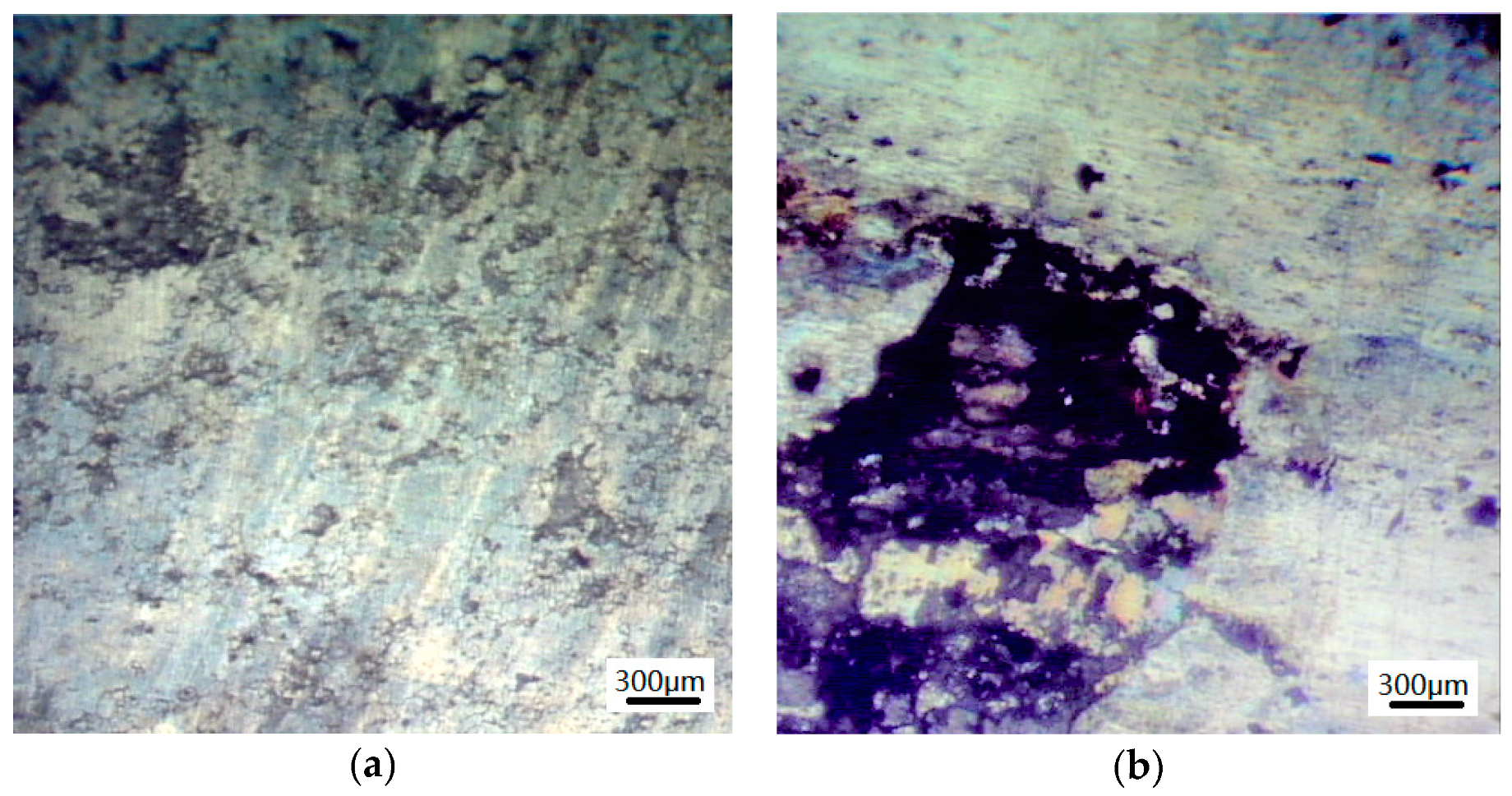
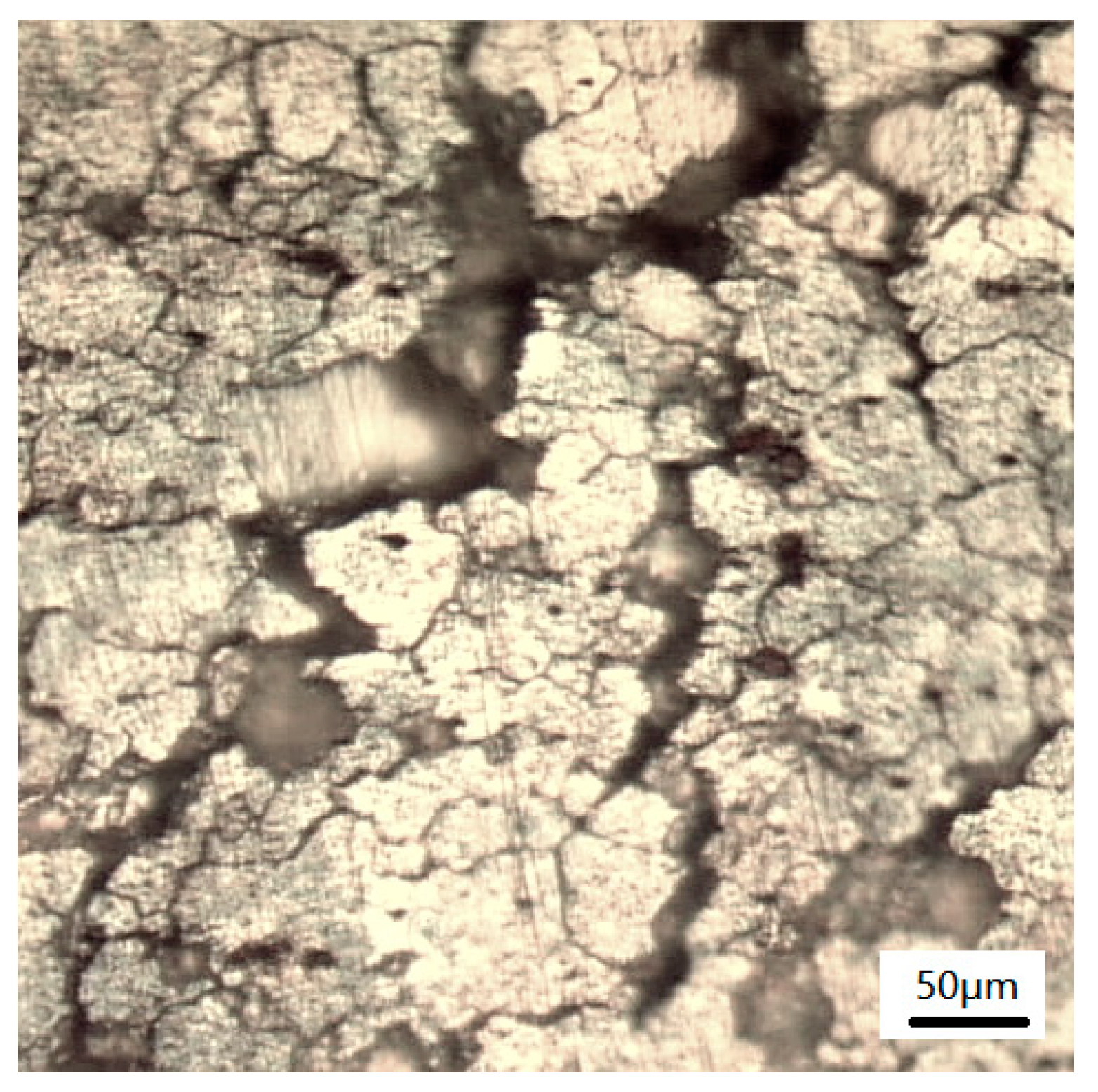
3.1.2. Microscopic Morphology of Pure Electrochemical Corrosion
- The magnitude and severity of corrosion experienced by the test specimens increases.
- The density of pits increases with the smaller pits gradually growing and developing to become larger pits.
- The grain boundaries tend to gradually dissolve indicating the occurrence of intergranular corrosion.
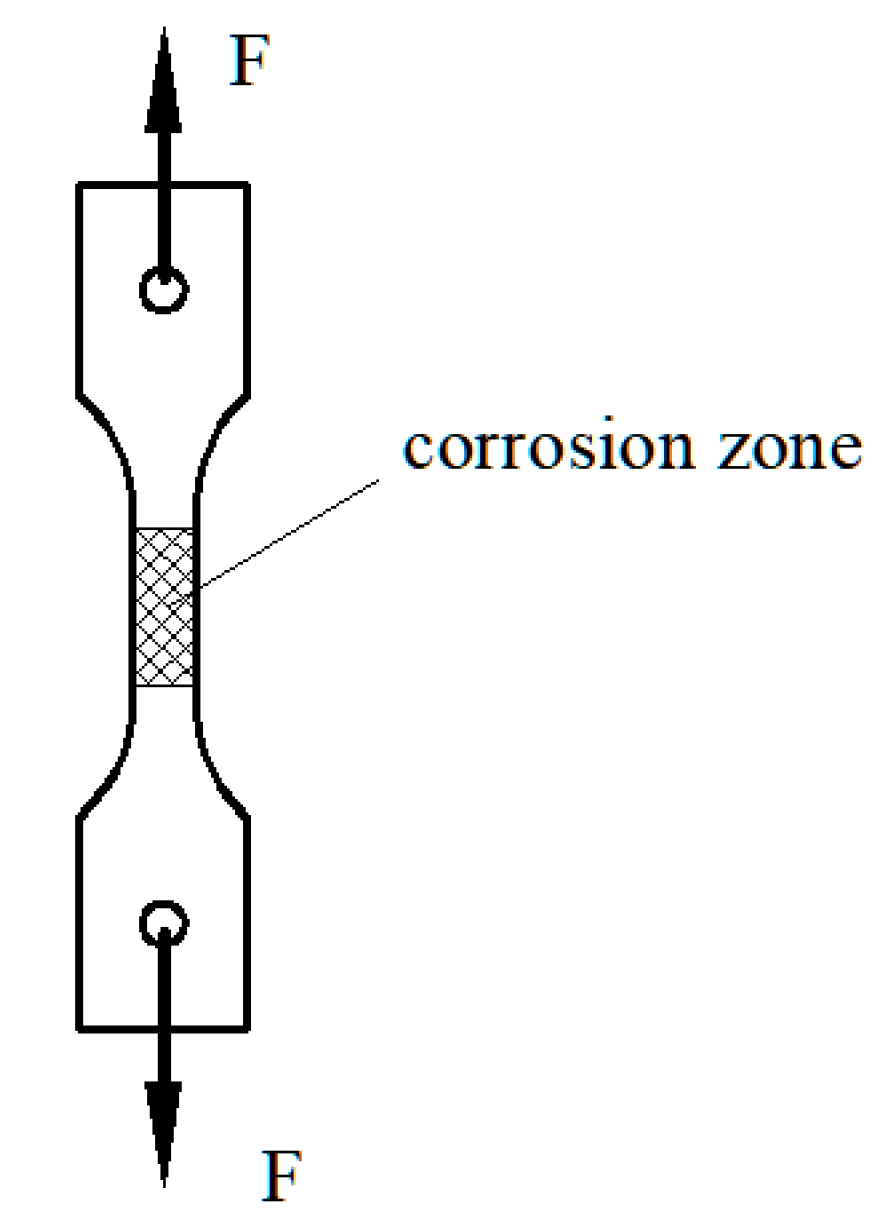
3.2. Analysis of Morphology of Stress Corrosion
3.2.1. Morphology of Stress Corrosion at the Macroscopic Level
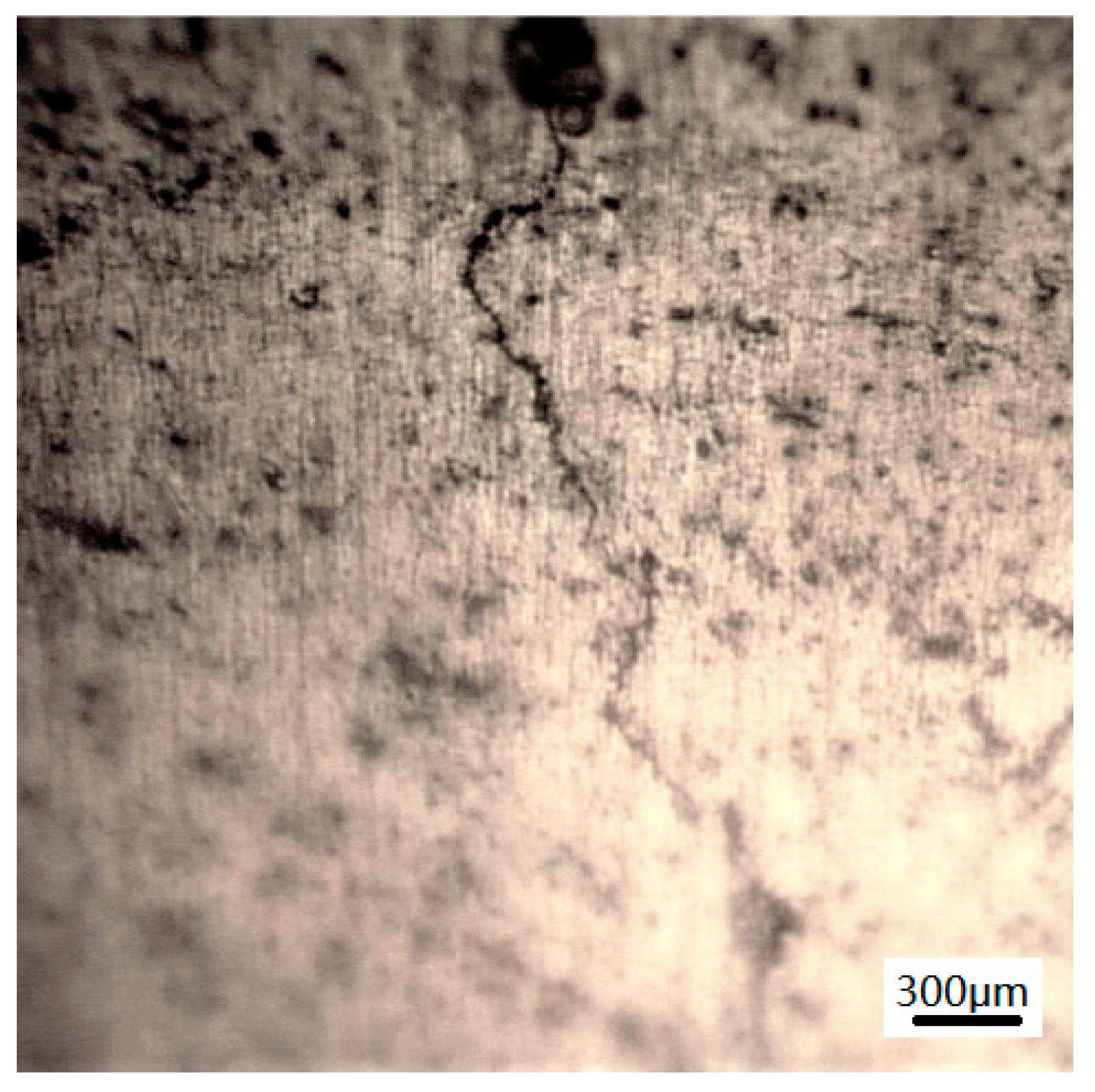
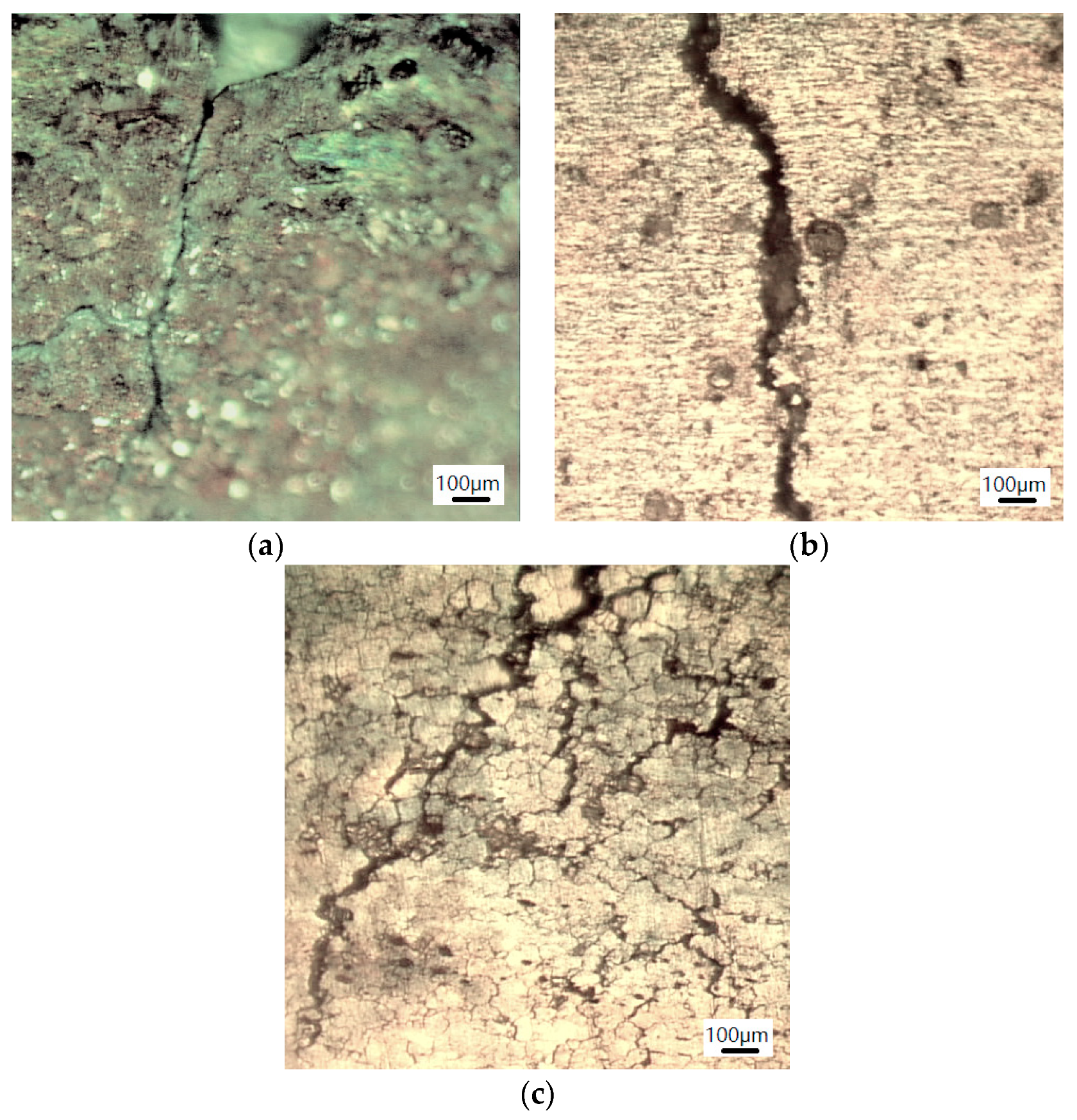
3.2.2. Morphology of Stress Corrosion at the Microscopic Level
3.3. Analysis of Damage Due to Corrosion
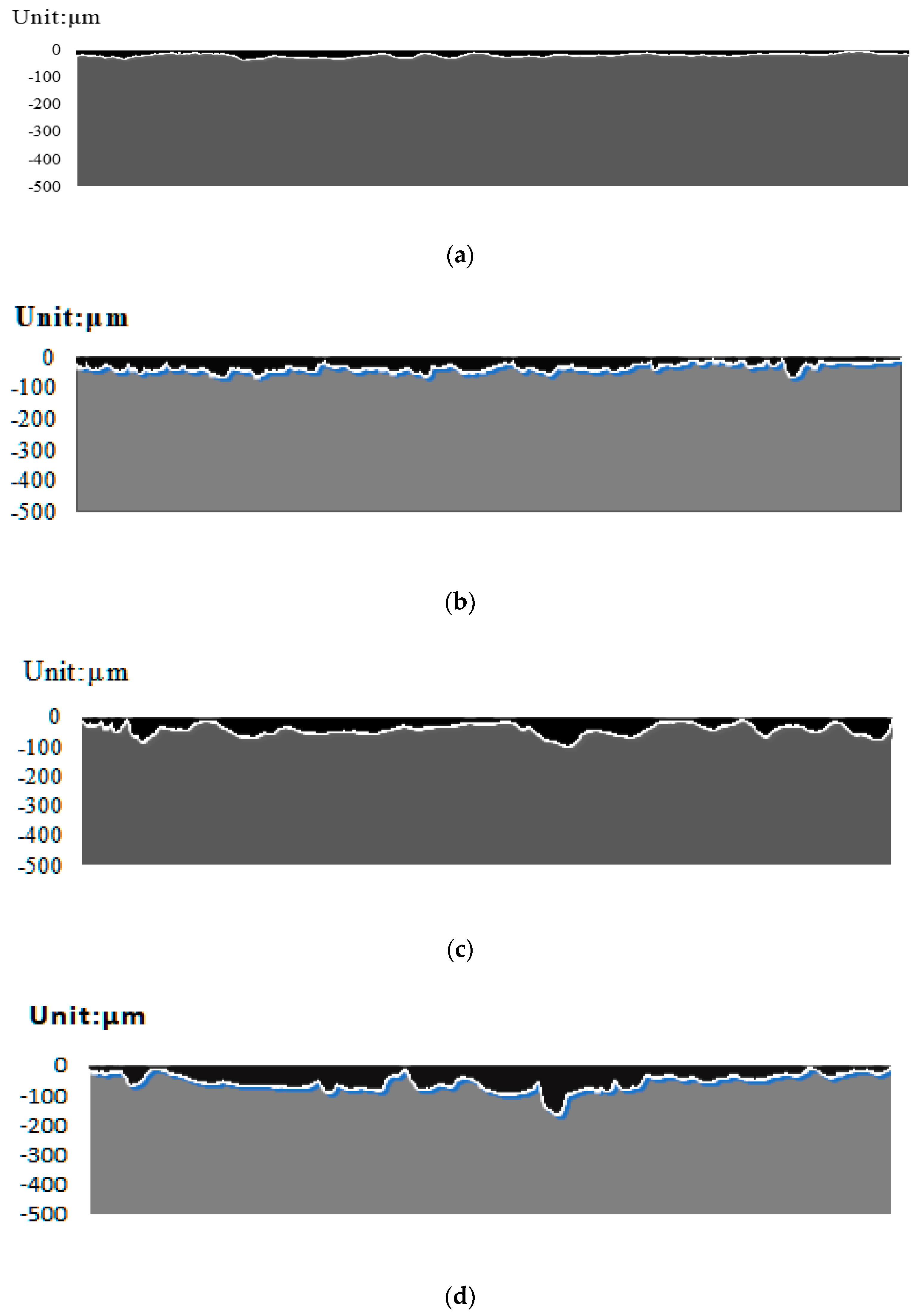
3.3.1. Model for Corrosion-Induced Damage
3.3.2. An Analysis of Damage Due to Electrochemical Corrosion
3.3.3. A Comparative Analysis of Damage Due to Stress Corrosion
3.3.4. Relationship between Extent of Damage and Applied Stress
4. Conclusions
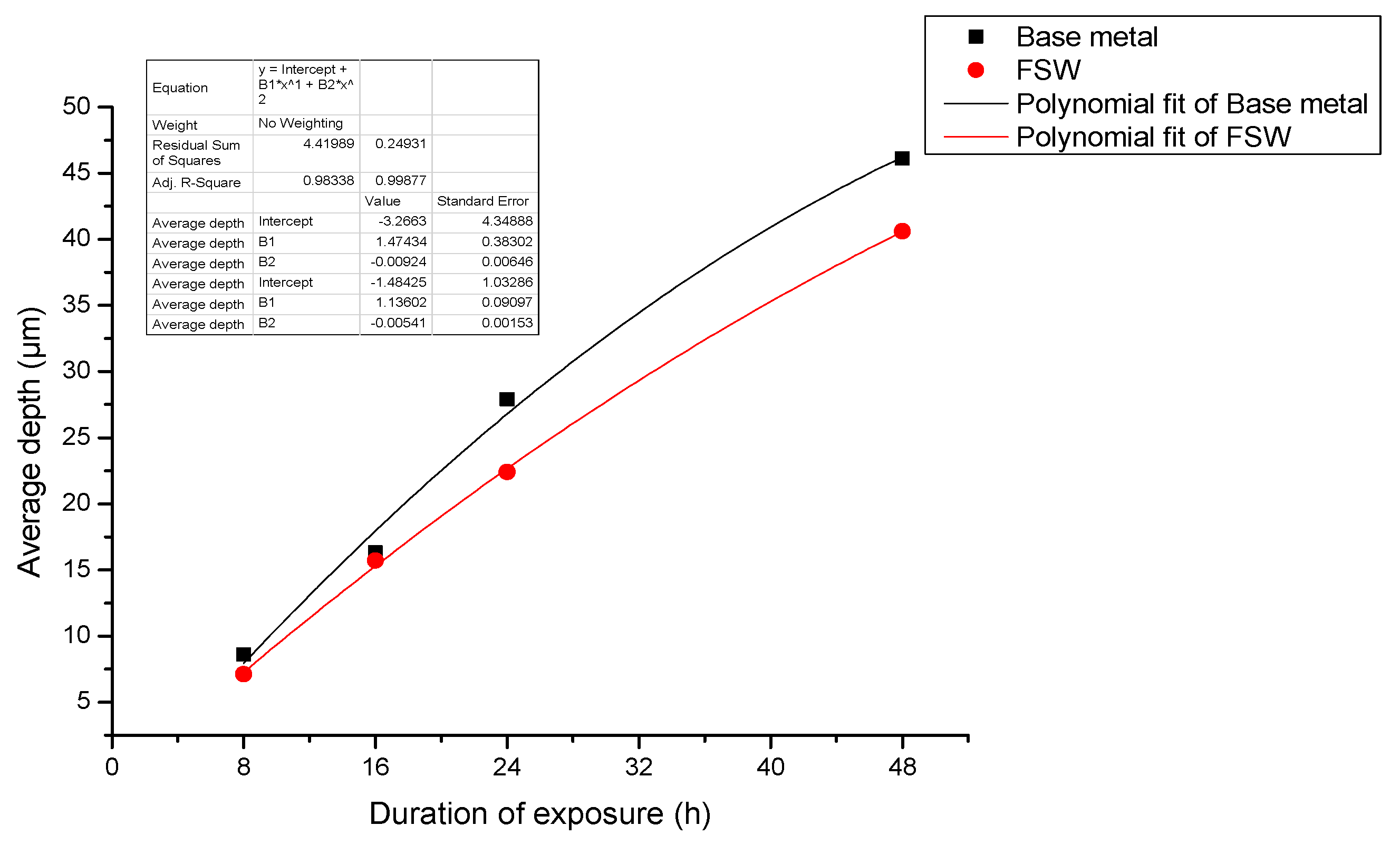
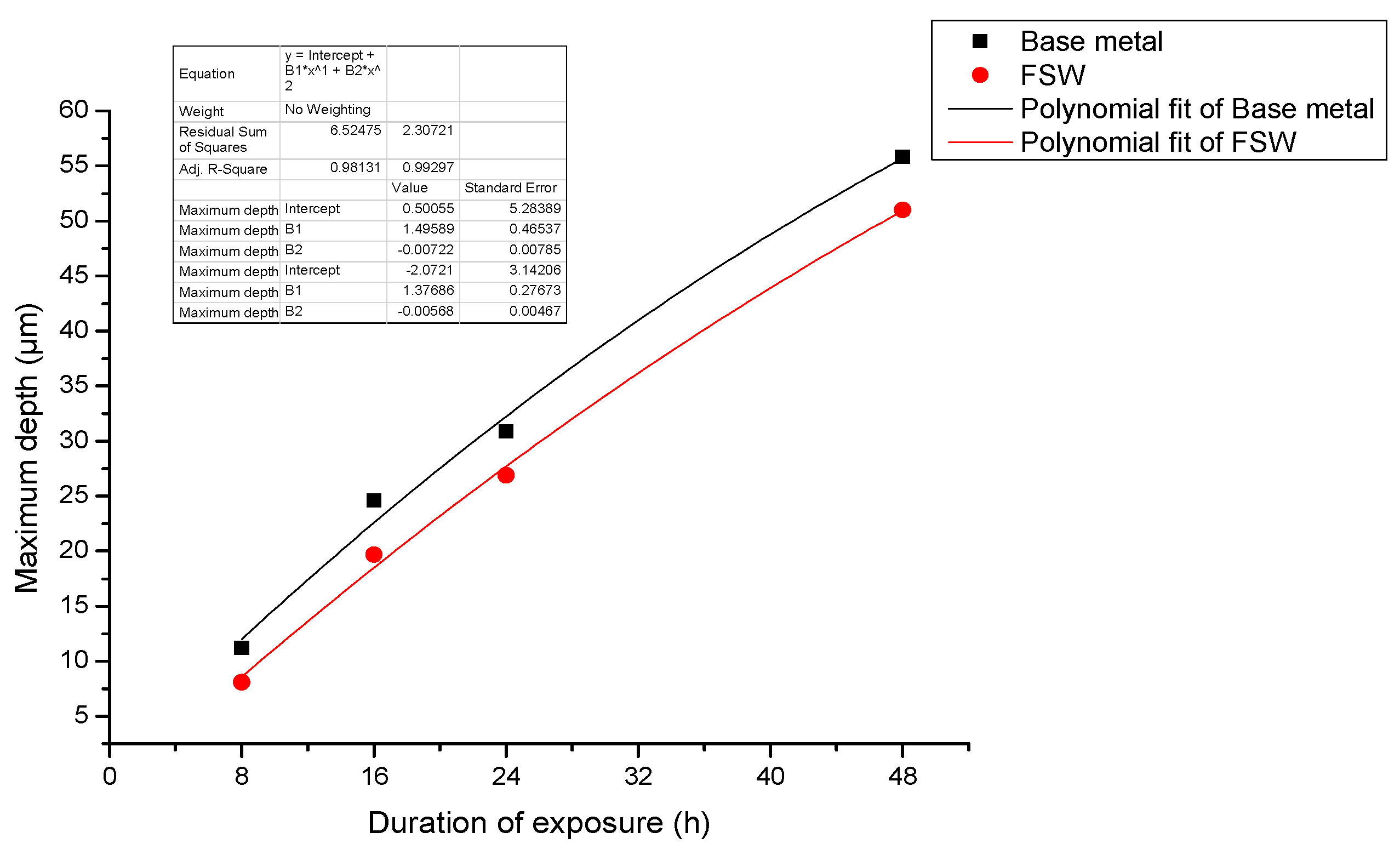
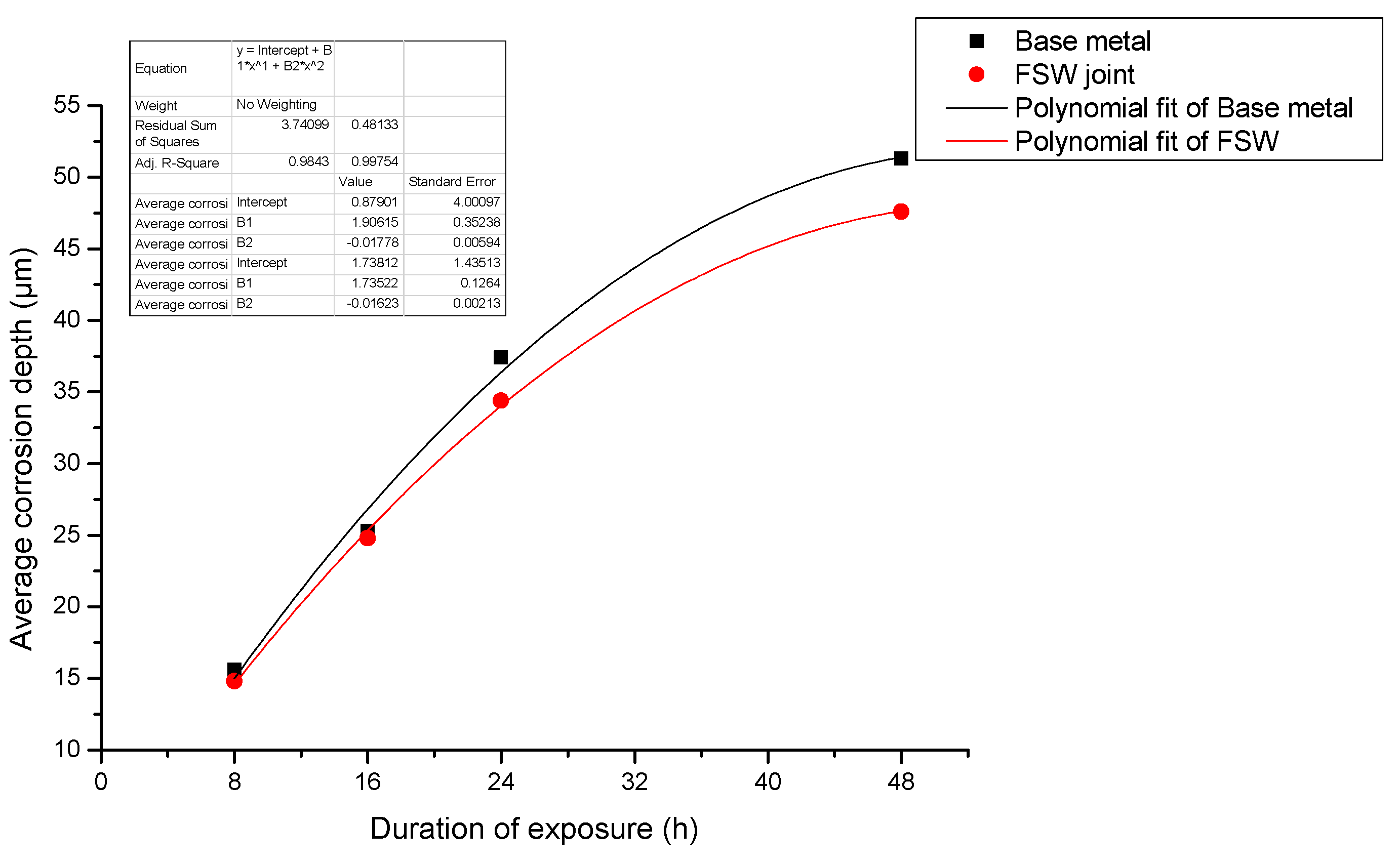
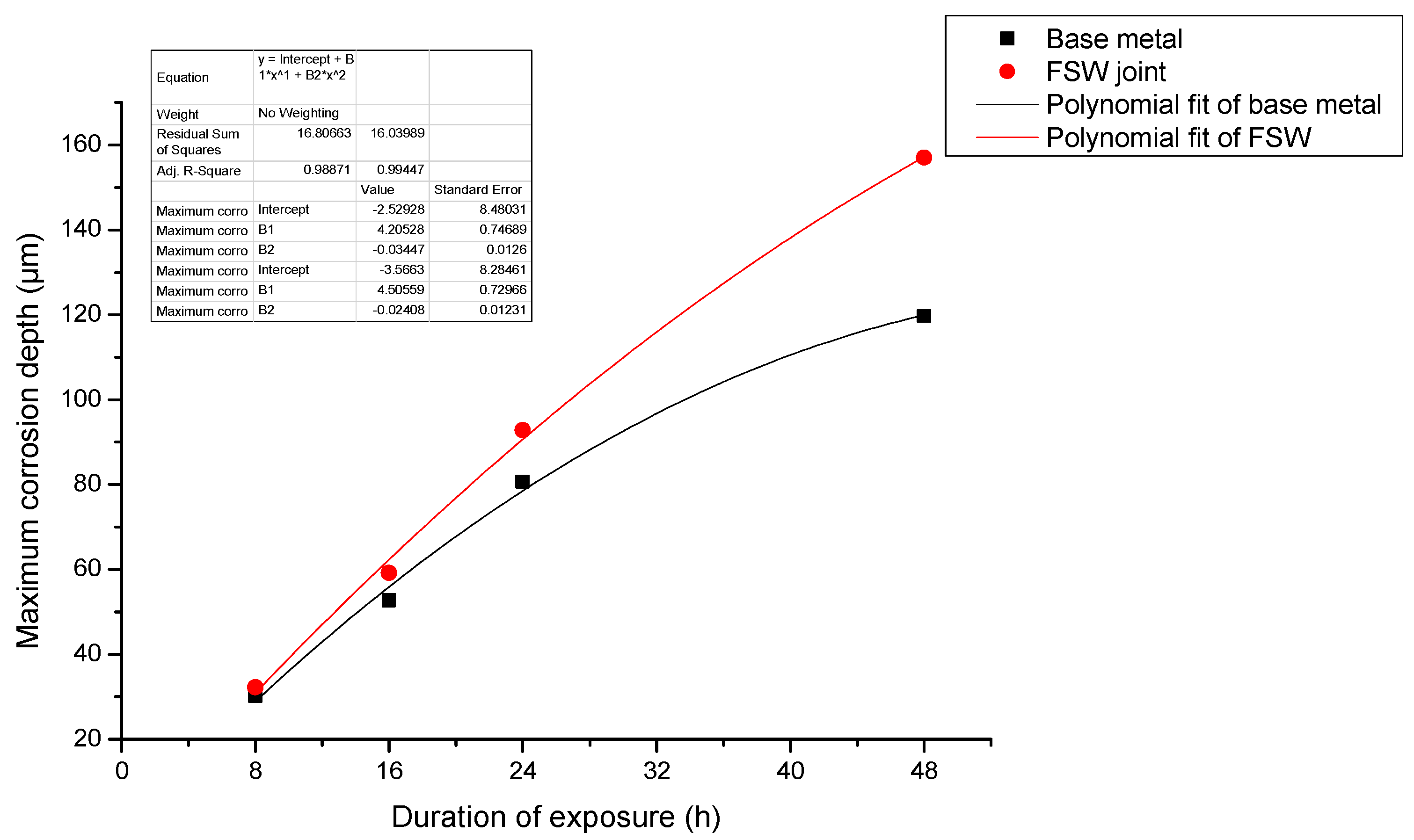
- When pure electrochemical corrosion occurs, the average corrosion depth and maximum corrosion depth of test specimen containing the joint are less than that of the test specimen of the base metal for four different durations of exposure to the aqueous environment. This suggests that the overall corrosion resistance of test specimens containing an FSW joint is better than that of the base metal.
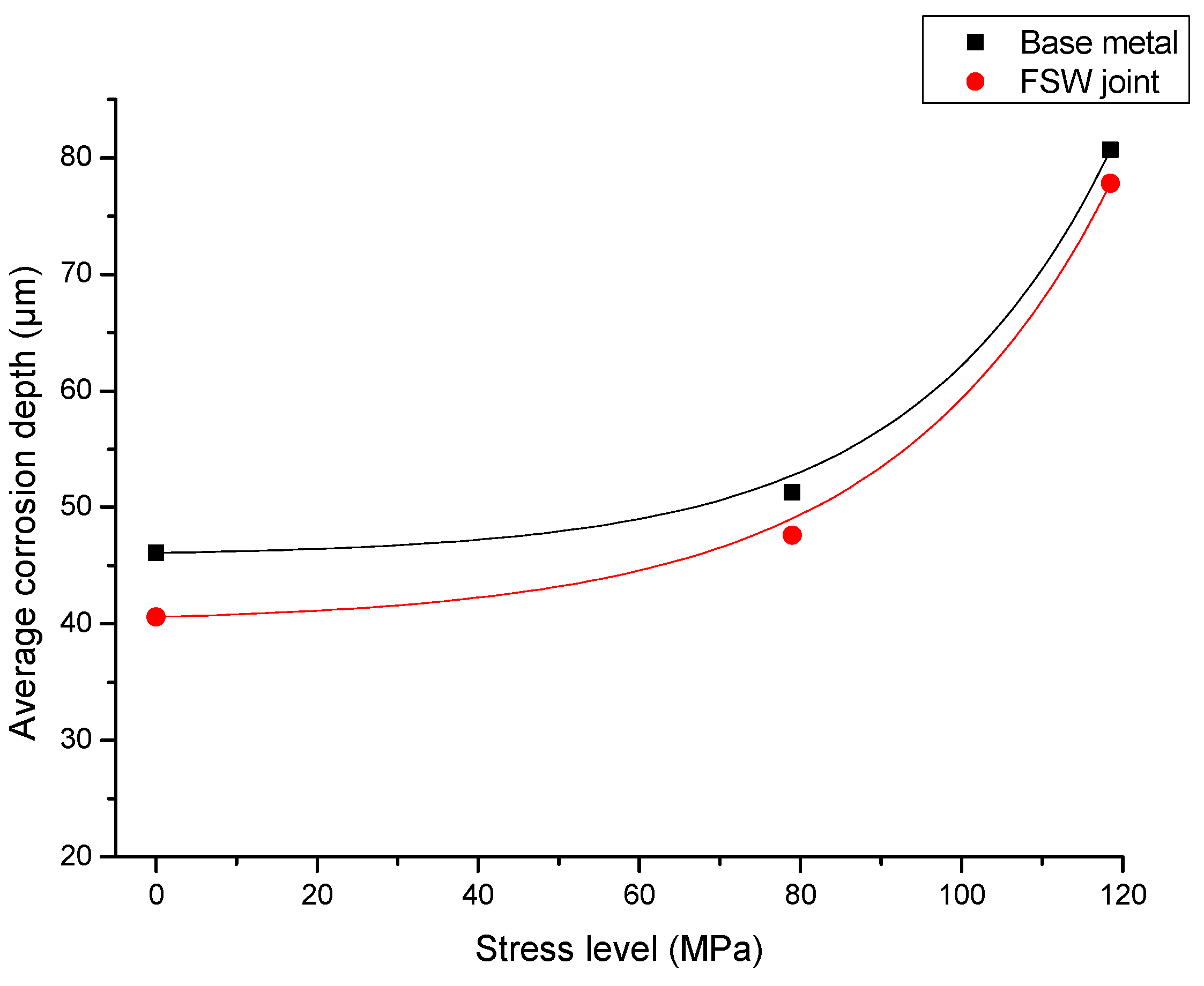
- When stress corrosion occurs, due to reduced mechanical properties of the test specimen containing the FSW joint, the stress corrosion resistance is inferior to that of the base metal, i.e., aluminum alloy 2219, and fine microscopic cracks are favored to occur.
- When stress corrosion occurs, the average corrosion depth of test specimen of the alloy containing an FSW joint is less than that of the base metal. The maximum depth of corrosion is greater than that of the base metal. This indicates that the test specimen containing the FSW joint is more susceptible to damage and degradation than the base metal upon exposure to the same aqueous environment.
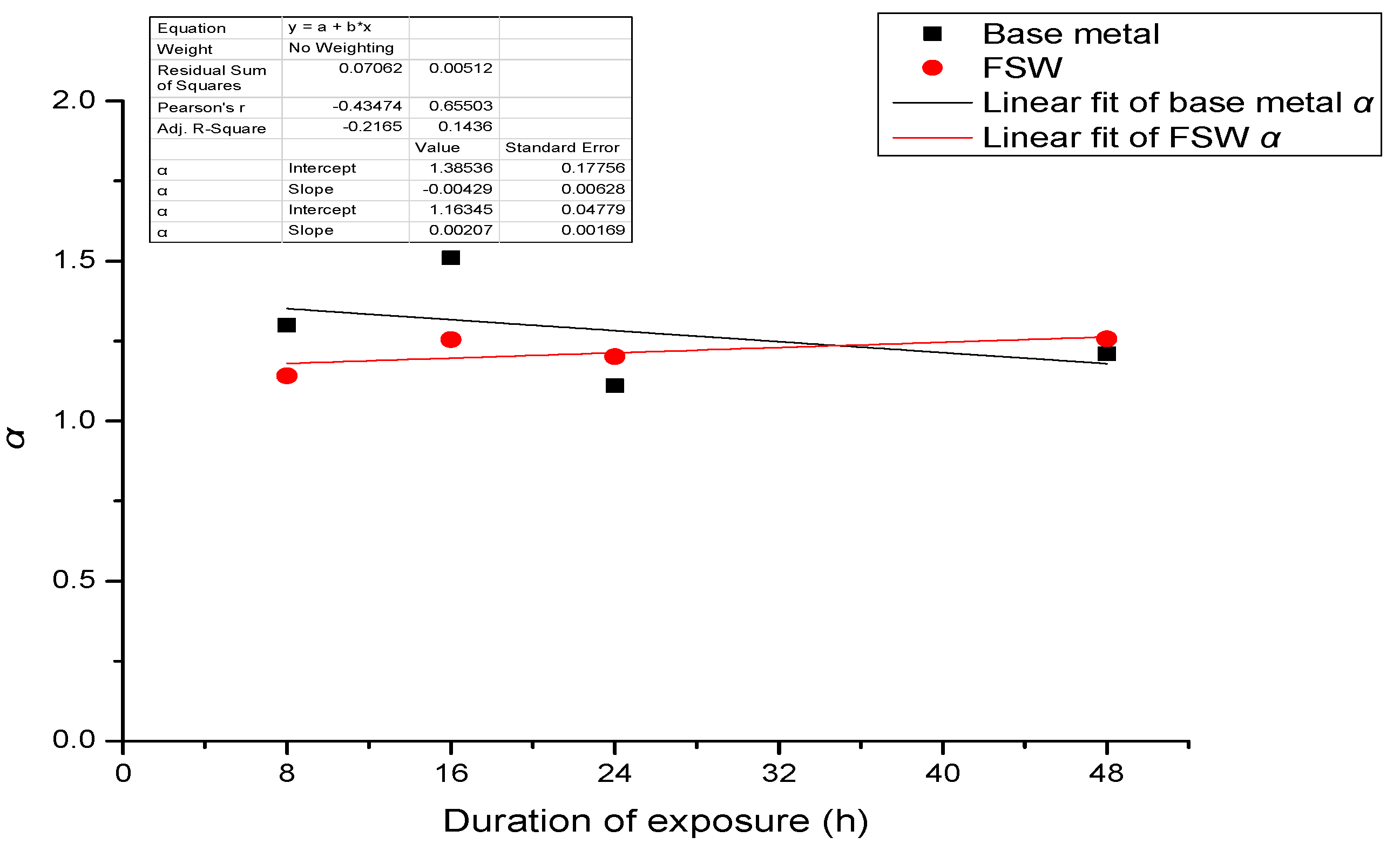
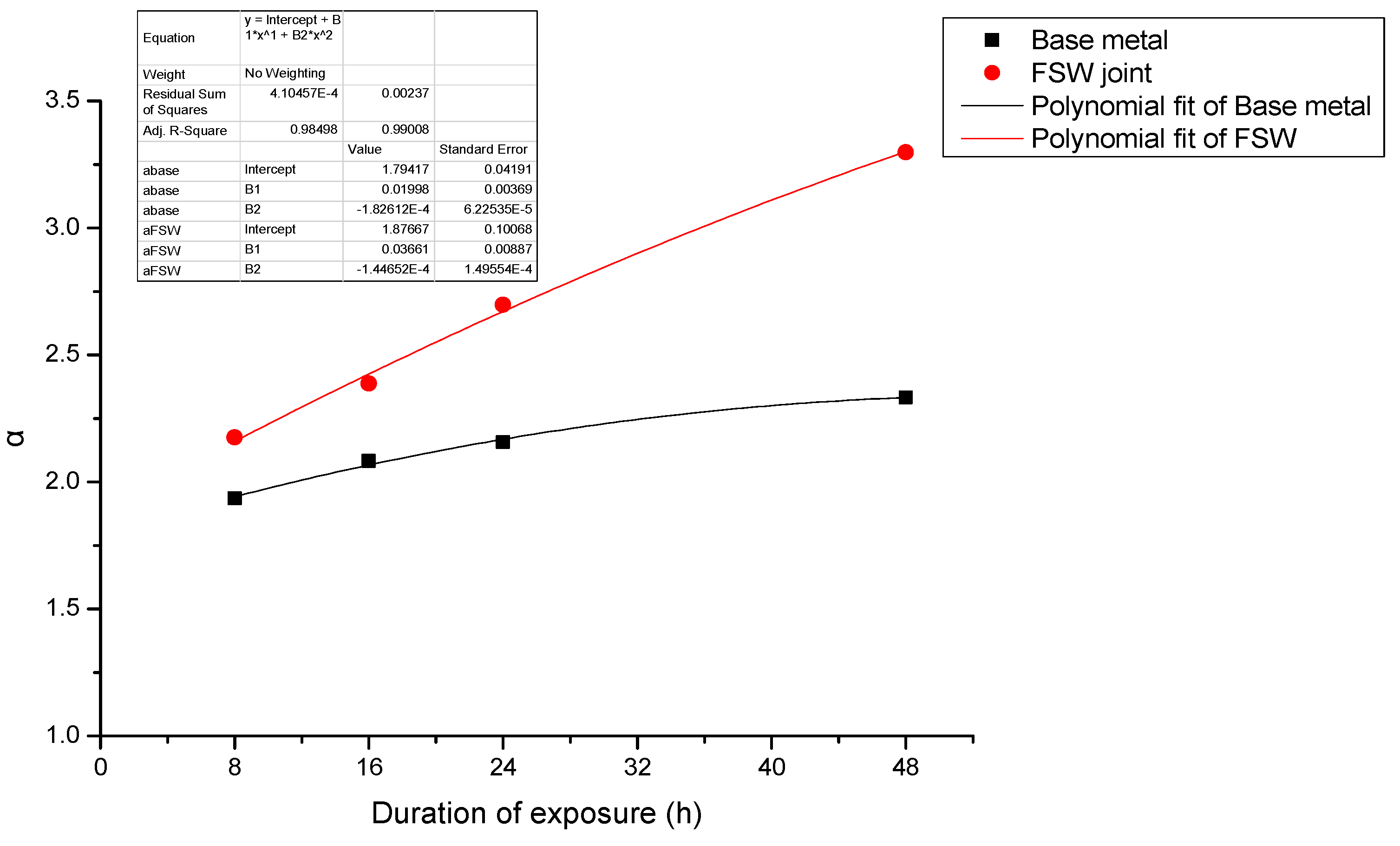
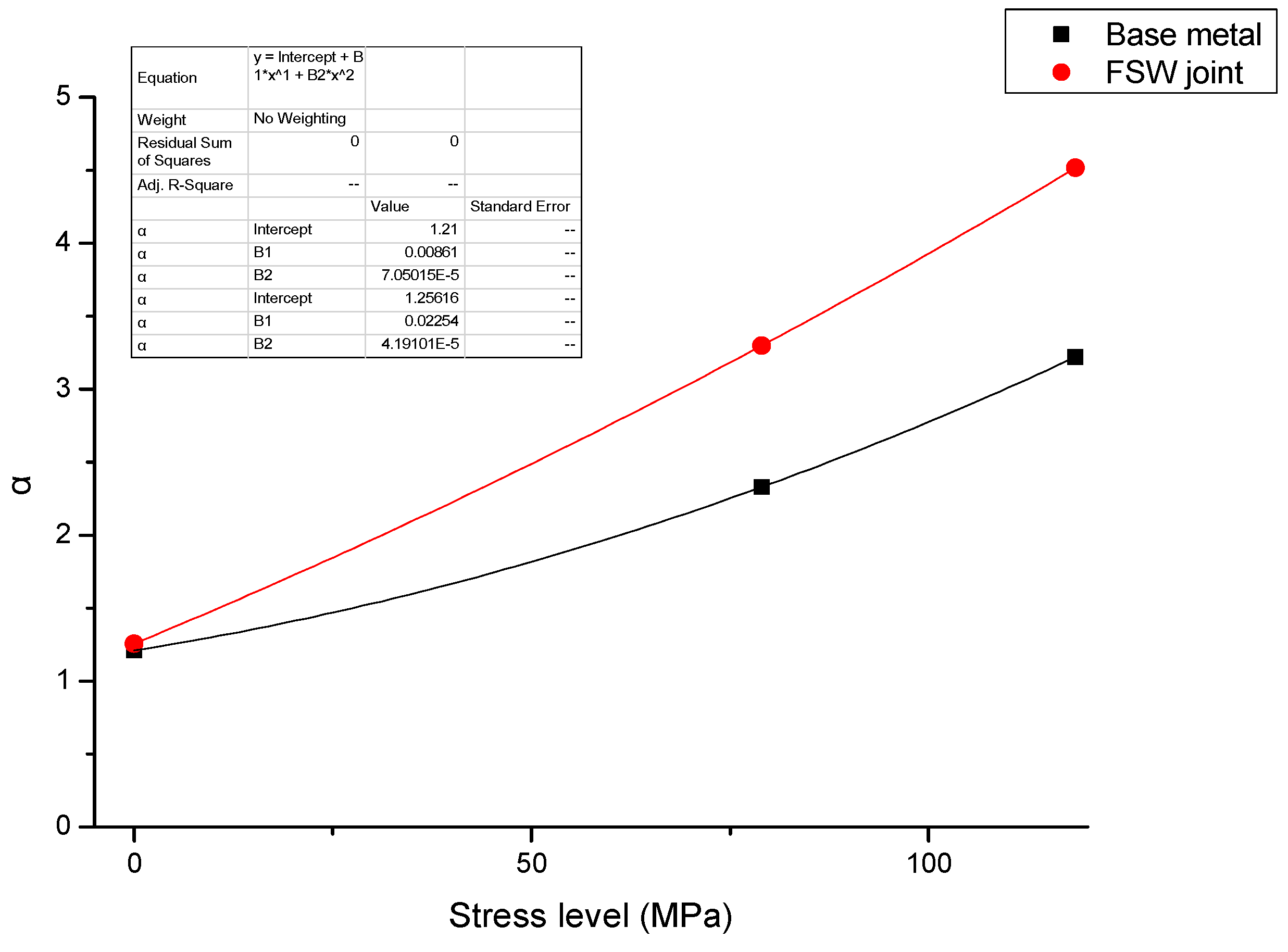
- When stress corrosion occurs, the ratio of maximum depth to average depth (denoted as α) increases with an increase in applied stress and duration of exposure to the aqueous environment (EXCO solution).
- The ratio (α) for test specimens containing the FSW joint is significantly higher than that for specimens of the base metal. Further, the observed increase in α with applied stress is faster for the test specimens containing an FSW joint than for test specimens of the base metal.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Threadgill, P.L.; Leonard, A.J.; Shercliff, H.R.; Withers, P.J. Friction stir welding of aluminium alloys. Int. Mater. Rev. 2009, 54, 49–93. [Google Scholar] [CrossRef]
- Surekha, K.; Murty, B.S.; Prasad, R.K. Comparison of corrosion behaviour of friction stir processed and laser melted AA 2219 aluminium alloy. Mater. Des. 2011, 32, 4502–4508. [Google Scholar] [CrossRef]
- Williston, D.H. Comparison of joining processes for Haynes 230 nickel based super alloy. Ph.D. Thesis, Mississippi State University, Starkville, MI, USA, 2013. [Google Scholar]
- Fratini, L.; Buffa, G.; Palmeri, D. Material flow in FSW of AA7075-T6 butt joints: Continuous dynamic recrystallization phenomena. J. Eng. Mater. Technol. 2006, 128, 2857–2863. [Google Scholar] [CrossRef]
- Gan, W.; Okamoto, K.; Hirano, S.; Chung, K.; Kim, C.; Wagoner, R.H. Properties of friction-stir welded aluminum alloys 6111 and 5083. J. Eng. Mater. Technol. 2008, 130, 389–392. [Google Scholar] [CrossRef]
- Nandan, R.; Debroy, T.; Bhadeshia, H.K.D.H. Recent Advances in Friction-Stir Welding-Process, Weldment Structure and Properties. Prog. Mater. Sci. 2008, 53, 980–1023. [Google Scholar] [CrossRef]
- Sullivan, A.; Robson, J. Microstructural Properties of Friction Stir Welded and Post-Weld Heat-Treated 7449 Aluminum Alloy Thick Plate. Mater. Sci. Eng. A 2008, 478, 351–360. [Google Scholar] [CrossRef]
- Wang, Q. Corrosion behavior of spray formed 7055 aluminum alloy joint welded by underwater friction stir welding. Mater. Des. 2015, 68, 97–103. [Google Scholar] [CrossRef]
- Gharavi, F.; Matori, K.A.; Yunus, R.; Othman, N.K.; Fadaeifard, F. Corrosion evaluation of friction stir welded lap joints of AA6061-T6 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 684–696. [Google Scholar] [CrossRef]
- Gharavi, F.; Matori, K.; Yunus, R.; Othman, N.; Fadaeifard, F. Corrosion behavior of Al6061 alloy weldment produced by friction stir welding process. J. Mater. Res. Technol. 2015, 4, 314–322. [Google Scholar] [CrossRef]
- Lumsden, J.B.; Mahoney, M.W.; Poliock, G.; Rhodes, C.G. Intergranular Corrosion Following Friction Stir Welding of Aluminum Alloy 7075-T651. Corrosion (Houston) 1999, 55, 1127–1135. [Google Scholar] [CrossRef]
- Lumsden, J.B.; Pollock, G.; Mahoney, M.W. Corrosion behavior of friction-stir-welded AA7075-T7651. Corrosion 2003, 59, 212–219. [Google Scholar] [CrossRef]
- Chen, Z. Microstructure characterization, mechanical properties, and corrosion behaviors of friction stir welded AA5086 and AA6061. Master’s Thesis, . University of HAWAI’I at MĀNO, Honolulu, HI, USA, 2014. [Google Scholar]
- Esmaily, M.; Mortazavi, N.; Osikouicz, W.; Hindsefelt, H.; Svensson, J.E.; Halvarsson, M.; Martin, J.; Johansson, L.G. Bobbin and conventional friction stir welding of thick extruded AA6005-T6 profiles. Mater. Des. 2016, 108, 114–125. [Google Scholar] [CrossRef]
- Venugopal, T.; Rao, K.S.; Rao, K.P. Studies on Friction Stir Welded AA7075 Aluminum Alloy. Trans. Indian Inst. Met. 2004, 57, 659–663. [Google Scholar]
- Wadeson, D.A.; Zhou, X.; Thompson, G.E.; Skeldon, P.; Djapic, O.L.; Scamans, G. Corrosion Behaviour of Friction Stir Welded AA7108 T79 Aluminium Alloy. Corros. Sci. 2006, 48, 887–897. [Google Scholar] [CrossRef]
- Paglia, C.S.; Buchheit, R.G. A Look in the Corrosion of Aluminum Alloy Friction Stir Welds. Scr. Mater. 2008, 58, 383–387. [Google Scholar] [CrossRef]
- Paglia, C.S.; Carroll, M.C.; Pitts, B.C.; Reynolds, T.; Buchheit, R.G. Strength, Corrosion, and Environmentally Assisted Cracking of A 7075-T6 Friction Stir Weld. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2002; Volume 396, pp. 1677–1684. [Google Scholar]
- Bousquet, E.; Poulon-Quintin, A.; Puiggali, M.; Devos, O.; Touzet, M. Relationship between microstructure, microhardness and corrosion sensitivity of an AA 2024-T3 friction stir welded joint. Corros. Sci. 2011, 53, 3026–3034. [Google Scholar] [CrossRef]
- Litwinski, E. Effect of FSW Process and Parameters on Corrosion Susceptibility. In Proceedings of the Physical Sciences Symposia—Falilure Analysis and Prevention. Microscopy and Microanalysis, Honolulu, HI, USA, 31 July–4 August 2005. [Google Scholar]
- Barbara, N.P. Investigation into the Stress Corrosion Cracking Properties of AA2099, an Al-Li-Cu Alloy. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2008. [Google Scholar]
- Astarita, A.; Bitondo, C.; Squillace, A.; Armentani, E.; Bellucci, F. Stress corrosion cracking behavior of conventional and innovative Aluminium alloys for aeronautic applications. Surf. Interface Anal. 2013, 45, 1610–1618. [Google Scholar] [CrossRef]
- ASTM G34-01. Standard Test Method for Exfoliation Corrosion Susceptibility in 2××× and 7××× Series Aluminum Alloys; IHS Markit: Englewood, CO, USA, 2014. [Google Scholar]



| Cu | Si | Zr | Fe | Zn | V | Ti | Mn | Mg | Al |
|---|---|---|---|---|---|---|---|---|---|
| 5.8 ~ 6.5 | ≤0.2 | 0.1 ~ 0.25 | ≤0.3 | 0.1 | 0.05 ~ 0.15 | 0.01 ~ 0.1 | 0.2 ~ 0.4 | ≤0.02 | Balance |
| Density (g/cm3) | Heat Treatment State | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Hardness (Hv) |
|---|---|---|---|---|---|
| 2.84 | C10S | 475 | 395 | 10 | 105 |
| Composition | NaCl | KNO3 | HNO3(70%) |
|---|---|---|---|
| Content | 234 g/L | 50 g/L | 63 ml/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Li, Z.; Gao, X.; Srivatsan, T.S. Influence of Exposure to Environment on Degradation of a Friction Stir Welded Aluminum Alloy. Metals 2020, 10, 1437. https://doi.org/10.3390/met10111437
Lv S, Li Z, Gao X, Srivatsan TS. Influence of Exposure to Environment on Degradation of a Friction Stir Welded Aluminum Alloy. Metals. 2020; 10(11):1437. https://doi.org/10.3390/met10111437
Chicago/Turabian StyleLv, Shengli, Zhi Li, Xiaosheng Gao, and Tirumalai S. Srivatsan. 2020. "Influence of Exposure to Environment on Degradation of a Friction Stir Welded Aluminum Alloy" Metals 10, no. 11: 1437. https://doi.org/10.3390/met10111437
APA StyleLv, S., Li, Z., Gao, X., & Srivatsan, T. S. (2020). Influence of Exposure to Environment on Degradation of a Friction Stir Welded Aluminum Alloy. Metals, 10(11), 1437. https://doi.org/10.3390/met10111437





