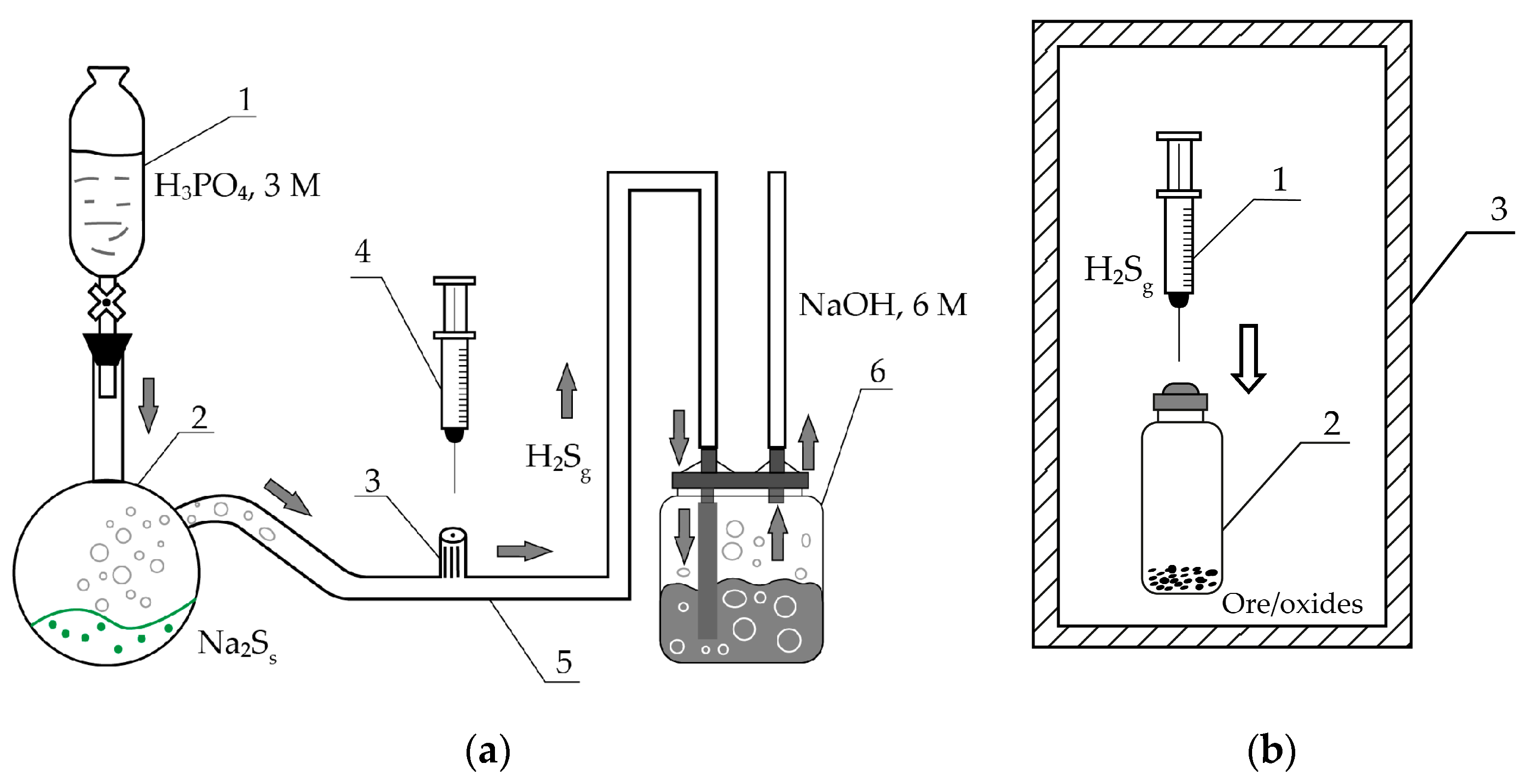
1. Introduction
The emission of waste gases containing toxic substances, including sulfur-containing compounds, is a serious environmental problem of the metallurgical industry [
1,
2,
3,
4,
5,
6]. Sorption methods are still effective in the field of the air environment cleaning process. Considering the volume of evolved gaseous substances, porous inorganic materials containing substances possessed with oxidative properties to a number of gaseous compounds can be used as promising and inexpensive sorbents [
7]. Manganese oxides are widely used in gas cleaning technologies as oxidizing agents. There exist various artificial sorption materials with the manganese oxide film coated surface. Oxidative destruction of phenols and cyanide compounds, sorption of hydrogen sulfide and sulfur (IV) oxide, as well as organic compounds, is taking place on the surface of manganese oxide.
The use of both products and raw materials of metallurgy plants during the gas cleaning process can be cost-effective. Their application will ensure low or even waste-free main production technologies due to the full engagement of the mentioned materials. Reducing the ecological impact on the environment caused by metallurgical enterprises is possible by involving unrefined ferromanganese ores in the technological cycle. Providing high sorption characteristics comparable to the properties of synthesized sorbents based on manganese oxide [
8], ferromanganese ores can be used in the process of sulfur-containing gas clearing.
The authors of this work investigate the sorption properties of ferromanganese material—unrefined ferromanganese ore samples of the Ulu-Telyaksky deposit (Republic of Bashkortostan), which is the raw material for manganese and iron oxide production. The presence of manganese (IV) and iron (III) compounds in ore samples provides oxidizing properties in relation to gaseous compounds, which contain reducing elements. Iron and manganese oxides can also exhibit catalytic properties. Accordingly, in the process of heterogeneous catalysis, the interaction of the reactant with the surface of the solid catalyst will have a dominant role. Consequently, the nature of physical and chemical interactions with the surface of the ferromanganese material will determine the kinetics of the hydrogen sulfide sorption process, the study of which is the subject of this work.
Since catalysis is a specific phenomenon, only a specific (individual) catalyst can be used for each chemical reaction or process. Zhuravskiy et al. investigated the process of hydrogen sulfide sorption on active coals [
9], which comes with the oxidation of hydrogen sulfide to elemental sulfur and water due to the presence of nitrogen and oxygen atoms in the coals. Oxidized nitrogen-modified activated carbons were used as a sorbent. As a result of the study, the authors determined the presence of elemental sulfur traces and made a conclusion about the mechanism of electronic catalysis.
Chung Lau L. et al. studied the absorption of H
2S on activated carbon, which was impregnated with a solution of cerium (III) nitrate, washed with sodium hydroxide, filtered, and dried at 80 °C [
10]. Kinetic studies were carried out in the temperature range of 30–70 °C. The calculation of kinetic parameters was carried out using pseudo-first and pseudo-second order models according to the equations:
Pseudo-second order
where
Qt—adsorption capacity at time
t;
Qe—full sorbent capacity, calculated based on thermodynamic data;
k1—pseudo-first order reaction rate constant;
k2—pseudo-second order reaction rate constant.
Based on the results of linear dependencies plotting, the authors have chosen a pseudo-second-order calculation model. The calculated values of the reaction rate constants at temperatures of 30, 40, 50, 60, and 70 °C are: (2.39; 2.73; 3.98; 3.64 and 4.07) × 10−6 g/mg × min, the activation energy value was 11.7 kJ/mol.
Aslam Z. et al. investigated the oil fly ash (OFA) collected during the utilization of power plants, which was processed by the method of physicochemical activation to improve the surface properties [
11]. The synthesized activated carbon from oil fly ash was used for the adsorption of hydrogen sulfide. The sorbent was obtained by adding a mixture of acids (20% nitric acid and 80% phosphoric acid) to OFA, after which the sorbent was treated with 2 M potassium hydroxide to increase the surface affinity. As a result of the treatment, the sorption capacity of the material in relation to H
2S was increased.
To describe the kinetics of H2S adsorption on the alkali-modified activated carbon, Thomas, Yoon–Nelson, and Clark models were used, which made it possible to establish the partial dependencies of the flow rate and concentration of H2S on the adsorption value.
The desulfurization kinetics of hot coal gas (H
2S 0.25%, H
2 10.6%, CO 18%, and carrier gas N
2) is described by an improved deactivation kinetic model based on the use of the chemical stoichiometric Equation (3) by the authors of [
12]:
The mesoporous zeolite (M41) based sorbent containing LaFeO3 with a molar ratio of elements La/Fe = 1:2 (LF2) and their different percentages (LF2 40, 50, 60 and 100%) was used in the mentioned research. According to the percentage, the obtained reaction rate constants were k × 103 min−1 × g−1 8.89; 8.52; 7.61 and 2.52. Despite the obvious chemical (redox) interactions between hydrogen sulfide and LaFeO3, the calculated value of the activation energy did not exceed 25.1 kJ/mol.
Thus, the kinetic dependencies of sorption processes on zeolite and carbonaceous materials impregnated with substances exhibiting oxidizing properties are approximated by the equations of formal kinetics [
13]. However, the contribution of physical and chemisorption to the main sorption process, as well as the effect of desorption of reaction products, remains unclear. A quantitative assessment of the catalytic effect of sorption of hydrogen sulfide on the covalent or ionic surfaces of solids has not been given. There is no understanding of the amount of the active surface of the solid phase responsible for the physical sorption effects. This work was carried out in order to determine the kinetic dependencies of the hydrogen sulfide sorption by solid sorbents exhibiting obvious oxidizing properties. Another purpose of this study was to determine the mechanism and to reveal a number of specific features of the heterogeneous process sorption.
3. Results and Discussion
As a result of the experiment, the kinetic dependencies of the hydrogen sulfide concentration (C) change on the sorption time were obtained, as shown in
Figure 3.
The kinetic parameters were calculated using the laws of formal kinetics. The reaction order was determined by a graphical method of plotting linear forms of kinetic dependencies. The rate constants of the sorption process were calculated using linear forms of the dependence of the H
2S concentration logarithm on the sorption time on ferromanganese ore at temperatures of 252, 280, 298 K, which is presented in
Figure 4.
Approximation equations, correlation values, and rate constants of the sorption process are presented in
Table 2.
To confirm the first order of the reaction, the periods of half-conversion (sorption) of hydrogen sulfide were determined at various initial concentrations. The obtained kinetic dependencies of the H
2S concentration on time are shown in
Figure 5.
According to the calculations based on Equation (3):
where
,
—half-conversion time at initial concentrations, sec,
C 1 = 1.06∙10
−2 mol/L,
C 2 = 1.35∙10
−2 mol/L and
C 3= 2.89∙10
−3 mol/L;
,
—half-conversion concentration at initial concentrations
C 1,
C 2 and
C 3, the reaction order within the error tolerance was 1.
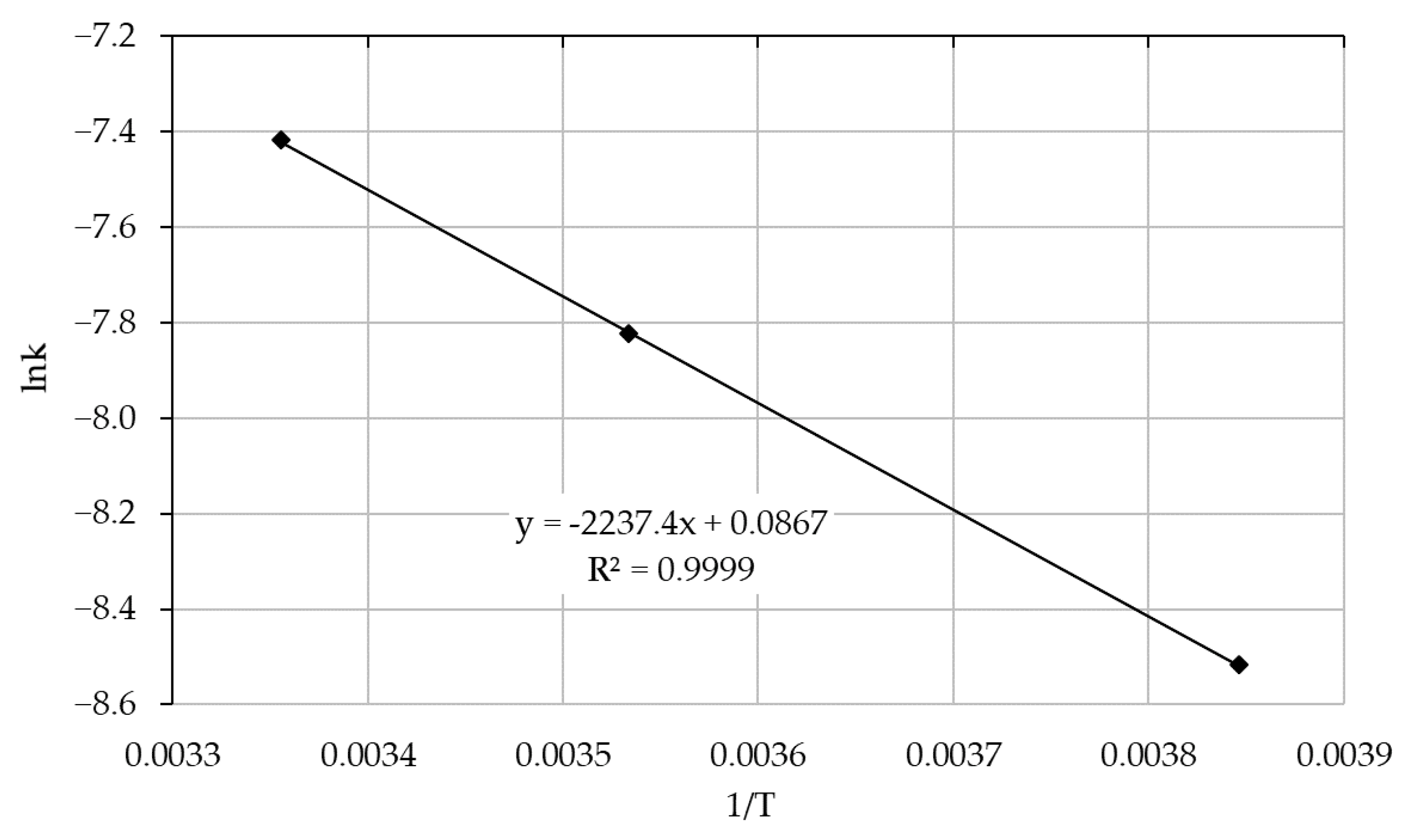
Based on the graphical dependence of the reaction rate constant logarithm value on the reciprocal temperature (lnk = −470.55 (1/T) − 4.1644, R
2 = 0.99) the value of the activation energy of the sorption process was calculated, which was E
a = 3.9 ± 0.2 kJ/mol (
Figure 6).
The results of the chemical analysis of the studied ore samples after contact with H
2S, obtained by the X-ray fluorescence analysis method (
Table 3), indicate a significant increase in the sulfur content in the ore, and, as a consequence, a decrease in the relative content of manganese, iron and other elements.
The data on the elemental composition before and after sorption are presented in
Table 4.
According to the results of X-ray phase analysis of ore samples, after contact with gas, there are no crystalline sulfur-containing phases, while along with quartz hydrated silicate H
2Si
2O
5 and iron (III) oxide, a new phase MnO (OH) appears, which is a hydrated form of manganese (III) oxide Mn
2O
3 2H
2O. Takanelite (Mn,Ca)Mn
4O
9 × 3H
2O was not found in the ore (
Figure 7).
The formation of elemental sulfur in the composition of ore samples is indicated by the chromatographic analysis results of samples obtained by dissolving with toluene a sulfur-containing substance, which was removed from the surface of the ore after contact with H
2S. Based on the phase analysis, it can be assumed that, along with the process of physical adsorption of gas, a chemical redox reaction occurs:
Physical sorption is caused by the presence of uncompensated interatomic interaction forces on the surface, due to which hydrogen sulfide molecules are attracted. Generally, physical adsorption proceeds at a high rate without activation energy and leads to equilibrium coverage of the active surface of the solid substance.
Temperature range of physical sorption cannot significantly exceed the hydrogen sulfide condensation temperature equal to 212.7. Therefore, with the increasing temperature, the equilibrium coverage will decrease, while there is no such limitation for chemisorption, it can occur both at low (less than 273 K) and at higher temperatures (more than 273 K). Chemisorption of the reactant, opposed to physical sorption, is proceeding with the involvement of an activated complex characterized by a certain activation energy. The presence of iron (III) oxide in the ore composition can affect the chemical sorption process, contributing to its acceleration due to the generated catalytic effect [
15,
16].
To determine the effect of iron (III) oxide on the chemical reaction rate, a series of hydrogen sulfide sorption experiments were carried out on iron (III) oxide with a specific surface area of 31 m
2/g and a pore size of 4.84 × 10
−8 m and on a model sample consisting of SiO
2, Al
2O
3 and MnO
2 oxides mixture, which does not contain Fe
3O
4 and quantitatively represents the composition of the studied ore. The specific surface area of 75 m
2/g and the pore size of 3.33 × 10
−8 m of the model sample are practically commensurable with those of real ore samples, which makes it possible to compare their kinetic sorption characteristics. According to the experimental results, iron (III) oxide showed no sorption tendency to hydrogen sulfide. During the experiment phase and H
2S concentration changes were not observed. The dependencies of the H
2S concentration change on time during sorption on model samples containing manganese (IV) oxide and the rate constant logarithm on reciprocal temperature are shown in
Figure 8 and
Figure 9.
The calculated value of the hydrogen sulfide sorption activation energy on model samples containing manganese (IV) oxide was 18.6 ± 0.9 kJ/mol. The increase in the activation energy is probably caused by the absence of iron (III) oxide in the composition of the sorption material.
According to the Bronsted–Polanyi equation for catalytic processes, the difference between the activation energy of the process in the absence (E
0) and in the presence (E) of the catalyst is determined by the heat effect of H
2S chemisorption by the catalyst, where α is a constant coefficient for a given reaction and catalyst, varying from 0 to 1:
The catalytic conversion of H
2S includes the following stages: physical adsorption, chemisorption, chemisorption of reaction products, physical adsorption of reaction products and proceeds according to the scheme:
| (1) H2S + [] → H2Sads. |
| (2) H2Sads. + 2[MnO2] → [Mn2O3] + Sads. + H2Oads. |
| (3) Sads. + H2Oads. → S + H2O + [], |
where []—free for chemisorption area on the active surface. Therefore, the energy path of the hydrogen sulfide sorption and oxidation process will include three peaks corresponding to three stages of the process. Consequently, for each individual process, the values of the activation energies can be expressed in accordance with the Bronsted–Polanyi rule:
where E
1,2,3—is the activation energy of the first, second and third stages of the process with the catalyst participation, ΔH
1 and ΔH
2—chemisorption heats of the initial material H
2S
ads. and reaction products S
ads. + H
2O
ads, α—coefficients for the corresponding reaction (5) and catalyst.
According to the Bronsted–Polanyi rule, the activation energy of the first stage will decrease by the hydrogen sulfide chemisorption heat amount of ΔH
1, while the activation energy of the second stage should increase by the chemisorption heat value of the initial substance H
2S due to the formed bonds of adsorbed H
2S with the catalyst and decrease with an increase in the chemisorption heat of the reaction products S
ads. + H
2O
ads. The energy of the third stage will increase with an increase in the products chemisorption heat. Therefore, according to the first reaction the hydrogen sulfide sorption rate can be expressed by the equation:
and the chemical reaction rate is expressed by Equation (11):
and thus, the hydrogen sulfide desorption rate is expressed by Equation (12):
where
and
are the sections of Fe
3O
4 surface, occupied by the chemisorption components of H
2S, S
ads and H
2O
ads;
,
,
—rate constants of first, second and third process stages;
,
,
—coefficients independent of H
2S, S
ads and H
2O
ads chemisorption heat.
The system will remain a stationary state if some of its essential characteristics do not change over time or if the formation rate of a system component is equal to its decay rate. When the stationary state of the hydrogen sulfide sorption process is reached, the total speed of the system will be equal to the speed of each of the conjugate stages:
from the equality of which we find the values of
and
by Equations (14) and (15):
and express the overall process rate:
From the conditions
at
and
at
we find the solutions of this equation:
the substitution of which in Equations (14) and (15) allows to obtain expressions for the section of the catalyst surface
and
, optimally covered with chemisorbed substances:
If we assume that the free surface of the catalyst is evenly distributed between H2Sads, Sads and H2Oads, then the values of and are equal, which is observed at α = 0.5 and corresponds to the maximum catalytic reaction rate. To assess the uniform distribution degree of the reactants and reaction products on the Fe3O4 surface, the heat of hydrogen sulfide sorption on the surface of ferromanganese ore was measured. The heat value of the sorption process was: ΔH = −69.44 ± 1.39 kJ/mol.
If the calculated activation energy of sorption on ore samples is taken as the total value of the activation energies of the chemisorption stage, chemical transformation stage, and desorption stage, then the coverage degree of the iron (III) oxide surface with chemisorbed substances can be estimated. According to the calculations by Formulas (6) and (17), the coverage degree values were: = 0.23 and = 0.07. With these values of the coverage degree, the rate constant ratio of physical sorption and chemical reaction was estimated, which was 1.25 × 10−14, which characterizes chemisorption (redox reaction) as the limiting stage of the process. The Fe3O4 role as a catalyst resides in the electronic conductivity presence due to the element existence in two different valence states, in equivalent positions of the crystal lattice, and the possibility of electron exchange between iron ions (2+) and (3+). The general mechanism of catalyst action in oxidation reactions reside in the simplification of electronic transitions in reacting molecules due to their own electrons. The process begins with the interaction of the solid body electrons with the reacting molecules of hydrogen sulfide, which leads to deformation of the adsorbed molecule and weakening of intramolecular bonds.
The catalytic oxidation of hydrogen sulfide is of interest, first of all, as an effective method for purifying industrial gas emissions from it. In addition, the study of this process is important for the development of the catalysts selective action theory, since the formation of three sulfur-containing products is possible during the oxidation of hydrogen sulfide: S, SO2 and SO3. The use of ferromanganese materials for the sulfur-containing gases purification, for which unrefined ferromanganese ore was used in this work, makes it possible to eliminate any losses of H2S and provide the waste-free process. The adsorbed sulfur is recovered during ore reprocessing into iron and manganese compounds. The method, based on selective catalytic oxidation of hydrogen sulfide from hydrocarbon and metallurgical industrial gases, makes preliminary gas purification from hydrogen sulfide, its concentration and oxidation to sulfurous anhydride unnecessary. The use of manganese and iron (III) oxides has a technological advantage over the currently used titanium and aluminum oxides, which require a constant composition and high specific surface area: the oxidation process of H2S with manganese oxide is characterized by one-stage and continuous operation, unlike the known Claus method, and mild temperature conditions for the implementation of the process.
The obtained quantitative regularities of the direct catalytic oxidation process of hydrogen sulfide made it possible to estimate the contribution of physical and chemical sorption to the main process of the elemental sulfur formation. The mathematical description of the desorption process, taking into account the conjugated stages of sorption and desorption of all participants of the hydrogen sulfide oxidation process, based on the Bronsted–Polyani theory, can be used to describe any catalytic processes in order to determine the catalytic activity of the catalyst and the fraction of its active surface. Foresight of the catalytic action is the most important task of the catalysis theory.