Effect of Weld and Surface Defects on the Corrosion Behavior of Nickel Aluminum Bronze in 3.5% NaCl Solution
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Specimens
2.3. Measurement and Characterization
2.3.1. Electrochemical Test
2.3.2. Weight Loss
2.3.3. Scanning Electron Microscope (SEM) and X-ray Diffraction (XRD)
3. Result and Discussion
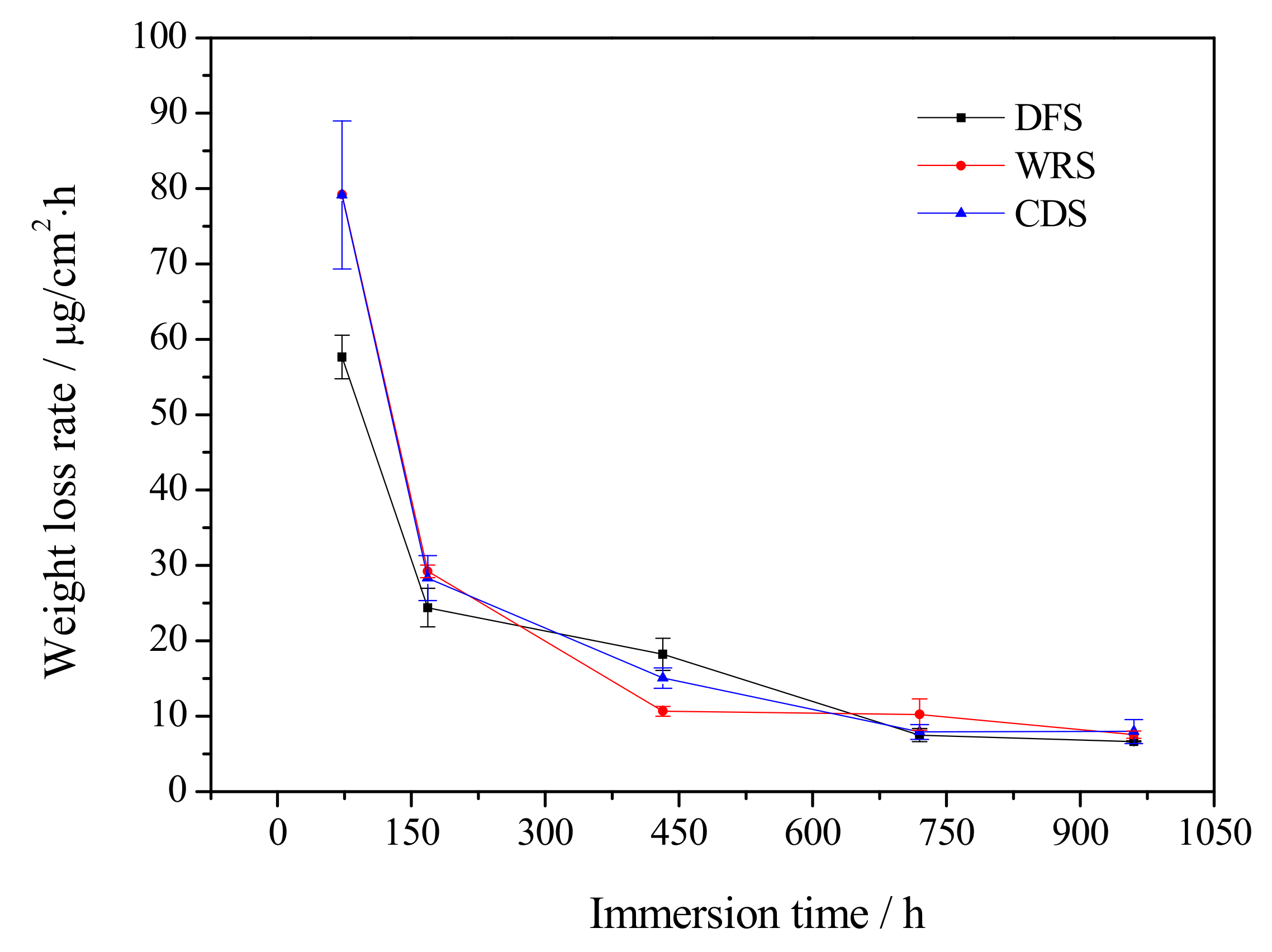
3.1. Weight Loss Analysis
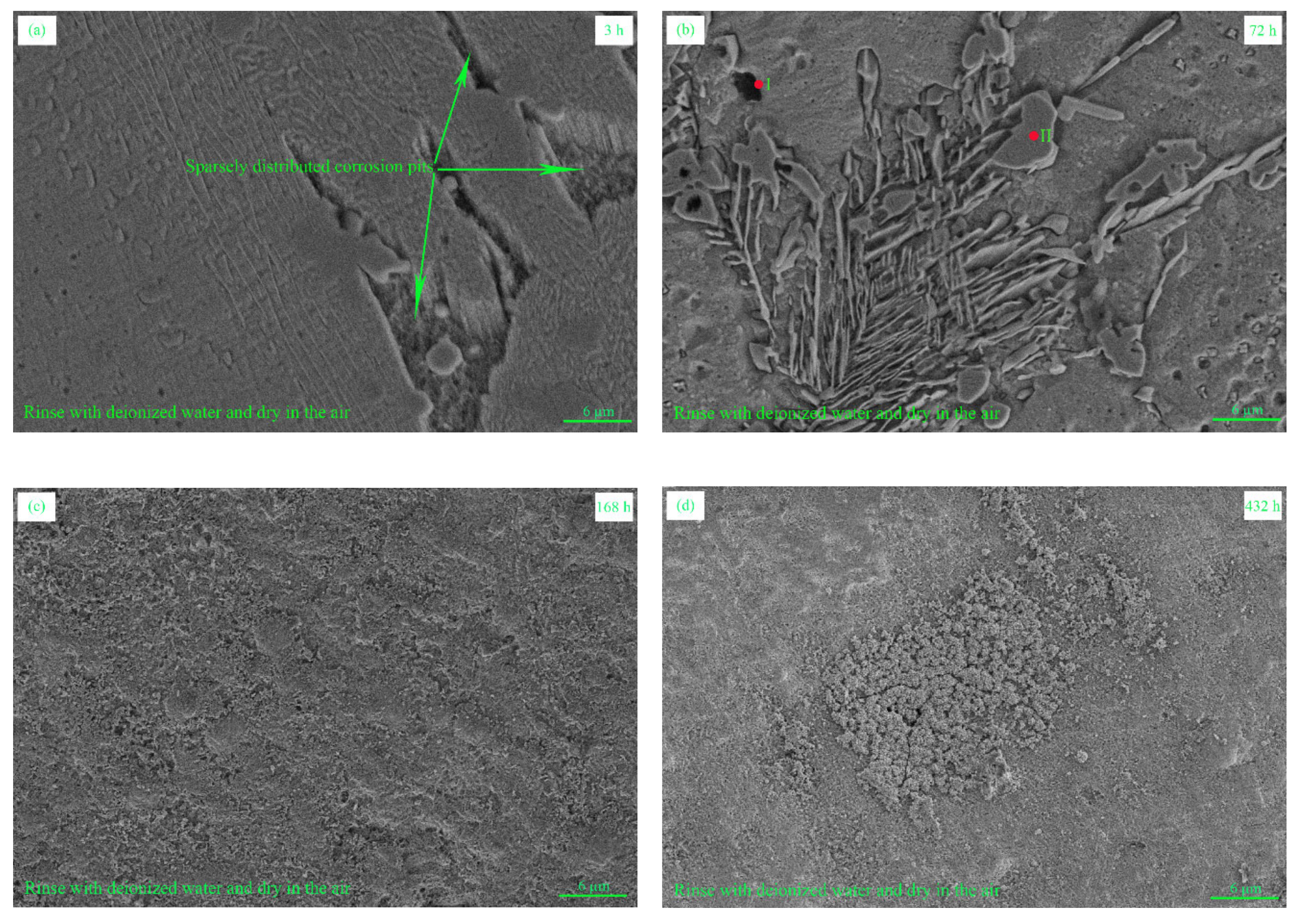
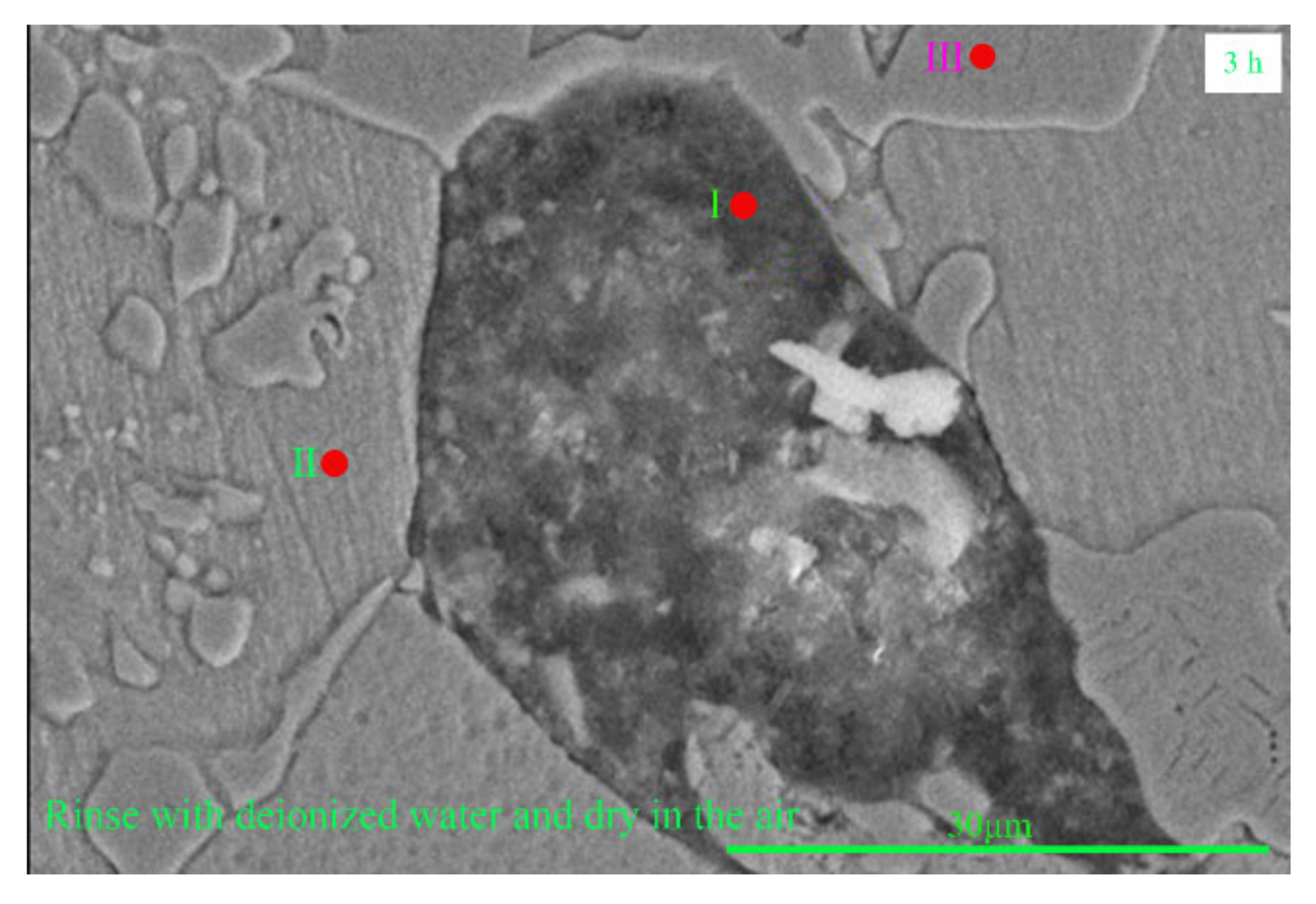
3.2. Corrosion Morphology and Microstructure
3.3. Corrosion Product Film Structure
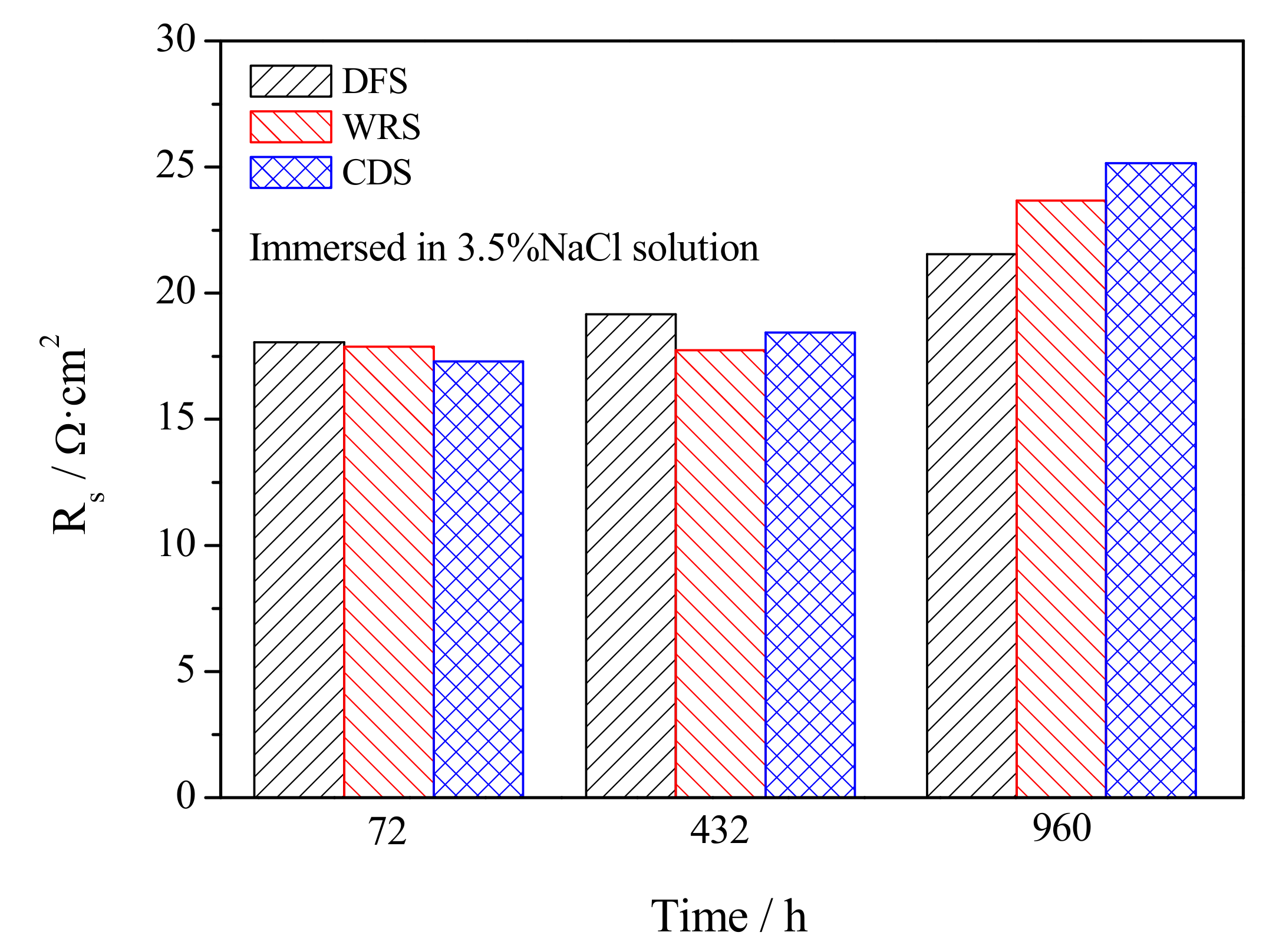
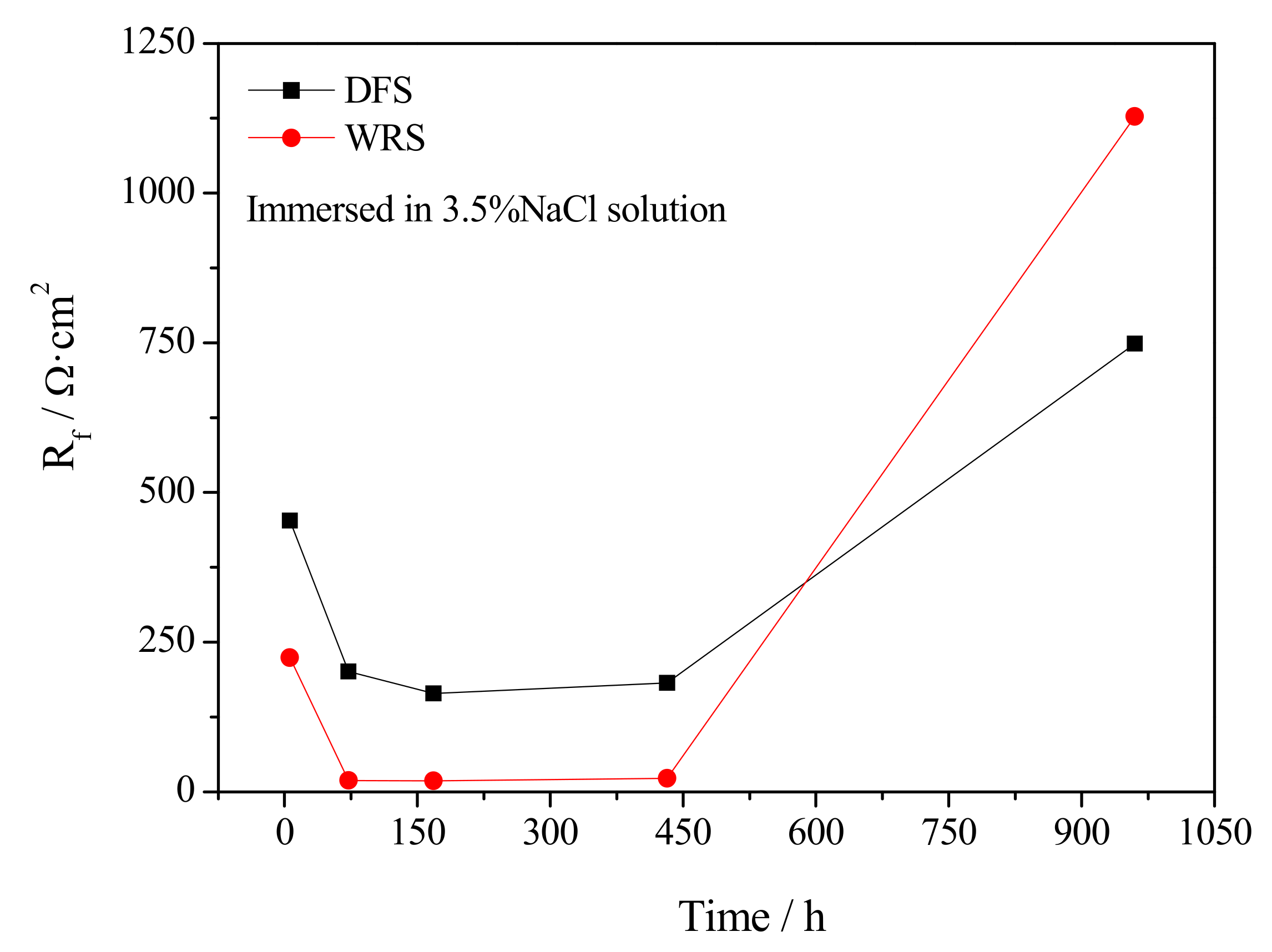
3.4. Electrochemical Corrosion Mechanism
4. Conclusions
- (1)
- Both welding repair and casting defects increase the corrosion rate of UNS C95810.
- (2)
- Welding repair does not affect the composition of the corrosion product of UNS C95810, but it has an effect on the amount of the specific composition. Casting defects affect the composition of the corrosion product of UNS C95810, making it lack the outermost layer of Cu2(OH)3Cl.
- (3)
- Welding repair makes the Rf and Rt in the early stage of corrosion smaller while making the Rf and Rt in the later stage of corrosion larger. Casting defects always produce an increase in the dissolution rate of UNS C95810.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabbaghzadeh, B.; Parvizi, R.; Davoodi, A.; Moayed, M.H. Corrosion evaluation of multi-pass welded nickel–aluminum bronze alloy in 3.5% sodium chloride solution: A restorative application of gas tungsten arc welding process. Mater. Des. 2014, 58, 346–356. [Google Scholar] [CrossRef]
- Wharton, J.A.; Barik, R.C.; Kear, G.; Wood, R.J.K.; Stokes, K.R.; Walsh, F.C. The corrosion of nickel–aluminium bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Alfantazi, A.M.; Ahmed, T.M.; Tromans, D. Corrosion behavior of copper alloys in chloride media. Mater. Des. 2009, 30, 2425–2430. [Google Scholar] [CrossRef]
- Schüssler, A.; Exner, H.E. The corrosion of nickel-aluminium bronzes in seawater—II. The corrosion mechanism in the presence of sulphide pollution. Corros. Sci. 1993, 34, 1803–1811. [Google Scholar] [CrossRef]
- Young-Gab, C.; Su-Il, P.; Chang-Ha, K. Effect of aluminium content on the anodic behaviour of copper-aluminium alloys in 3.5 wt% NaCl solution. Mater. Lett. 1994, 20, 265–270. [Google Scholar] [CrossRef]
- Al-Hashem, A.; Riad, W. The role of microstructure of nickel–aluminium–bronze alloy on its cavitation corrosion behavior in natural seawater. Mater. Charact. 2002, 48, 37–41. [Google Scholar] [CrossRef]
- Fonlupt, S.; Bayle, B.; Delafosse, D.; Heuze, J.L. Role of second phases in the stress corrosion cracking of a nickel–aluminium bronze in saline water. Corros. Sci. 2005, 47, 2792–2806. [Google Scholar] [CrossRef]
- Pidaparti, R.M.; Aghazadeh, B.S.; Whitfield, A.; Rao, A.S.; Merrier, G.P. Classification of corrosion defects in NiAl bronze through image analysis. Corros. Sci. 2010, 52, 3661–3666. [Google Scholar] [CrossRef]
- Barik, R.C.; Wharton, J.A.; Wood, R.J.K.; Tan, K.S.; Stokes, K.R. Erosion and erosion–corrosion performance of cast and thermally sprayed nickel–aluminium bronze. Wear 2005, 259, 230–242. [Google Scholar] [CrossRef]
- Olszewski, A.M. Dealloying of a Nickel–Aluminum Bronze Impeller. J. Fail. Anal. Prev. 2008, 8, 505–508. [Google Scholar] [CrossRef]
- Ji, W.X.; Sun, X.F.; Li, Z.M.; Song, W. Repair Feasibility Research of Nickel Aluminum Bronze Surface Corrosion Damage with Precision Pulsed Plasma Powder Surfacing. Hot Work. Technol. 2018, 47, 218–226. [Google Scholar]
- Abbasi-Khazaei, B.; Keshavarz, S. Nickel-aluminum-bronze/Al2O3 surface nanocomposite produced by friction-stir processing: Corrosion properties and microstructure. Mater. Corros. 2017, 68, 883–891. [Google Scholar] [CrossRef]
- Tang, C.H.; Cheng, F.T.; Man, H.C. Effect of laser surface melting on the corrosion and cavitation erosion behaviors of a manganese-nickel-aluminium bronze. Mater. Sci. Eng. A 2004, 373, 195–203. [Google Scholar] [CrossRef]
- Tang, C.H.; Cheng, F.T.; Man, H.C. Laser surface alloying of a marine propeller bronze using aluminium powder Part II: Corrosion and erosion-corrosion synergism. Surf. Coat. Technol. 2006, 200, 2594–2601. [Google Scholar] [CrossRef]
- Li, X.Y.; Yan, Y.G.; Ma, L.; Xu, Z.M.; Li, J.G. Cavitation erosion and corrosion resistance of as-welded nickel aluminum bronze. J. Shanghai Jiaotong Univ. 2004, 38, 1464–1467. [Google Scholar]
- Kear, G.; Barker, B.D.; Stokes, K.; Walsh, F.C. Flow influenced electrochemical corrosion of nickel aluminium bronze–Part I. Cathodic polarisation. J. Appl. Electrochem. 2004, 34, 1235–1240. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.H.; Wang, J.T.; Zhang, Z.P.; Zhu, J.; Quan, L.L.; He, D.C. Effect of cast defects on corrosion behavior and mechanism of UNS C95810 in artificial seawater. Materials 2020, 13, 1790. [Google Scholar] [CrossRef]
- Qi, Y.H.; Zhang, Z.P.; Miao, M.; Zhang, X.Z. Studies on estimating methods of polarization performance for coated steel in seawater. Mater. Sci. Forum 2010, 654, 2418–2421. [Google Scholar] [CrossRef]
- Du, C.Y. Research on Corrosion Resistance of the Nickel-Aluminum Bronze and the Surface Treatment on It. Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2014. [Google Scholar]
- Song, Q.N. Effect of Friction Stir Processing on the Cavitation Erosion and Corrosion Behaviors of As-Cast Ni-Al Bronze. Ph.D. Thesis, Institute of metal research, Chinese Academy of Sciences, Shenyang, China, 2015. [Google Scholar]
- Cao, C.N.; Zhang, J.Q. Introduction to Electrochemical Impedance Spectroscopy; Science Press: Beijing, China, 2002. [Google Scholar]
- Cao, C.N. Principles of Electrochemistry of Corrosion, 3rd ed.; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Li, X.G. Corrosion Behaviors and Mechanisms of Marine Engineering Materials; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
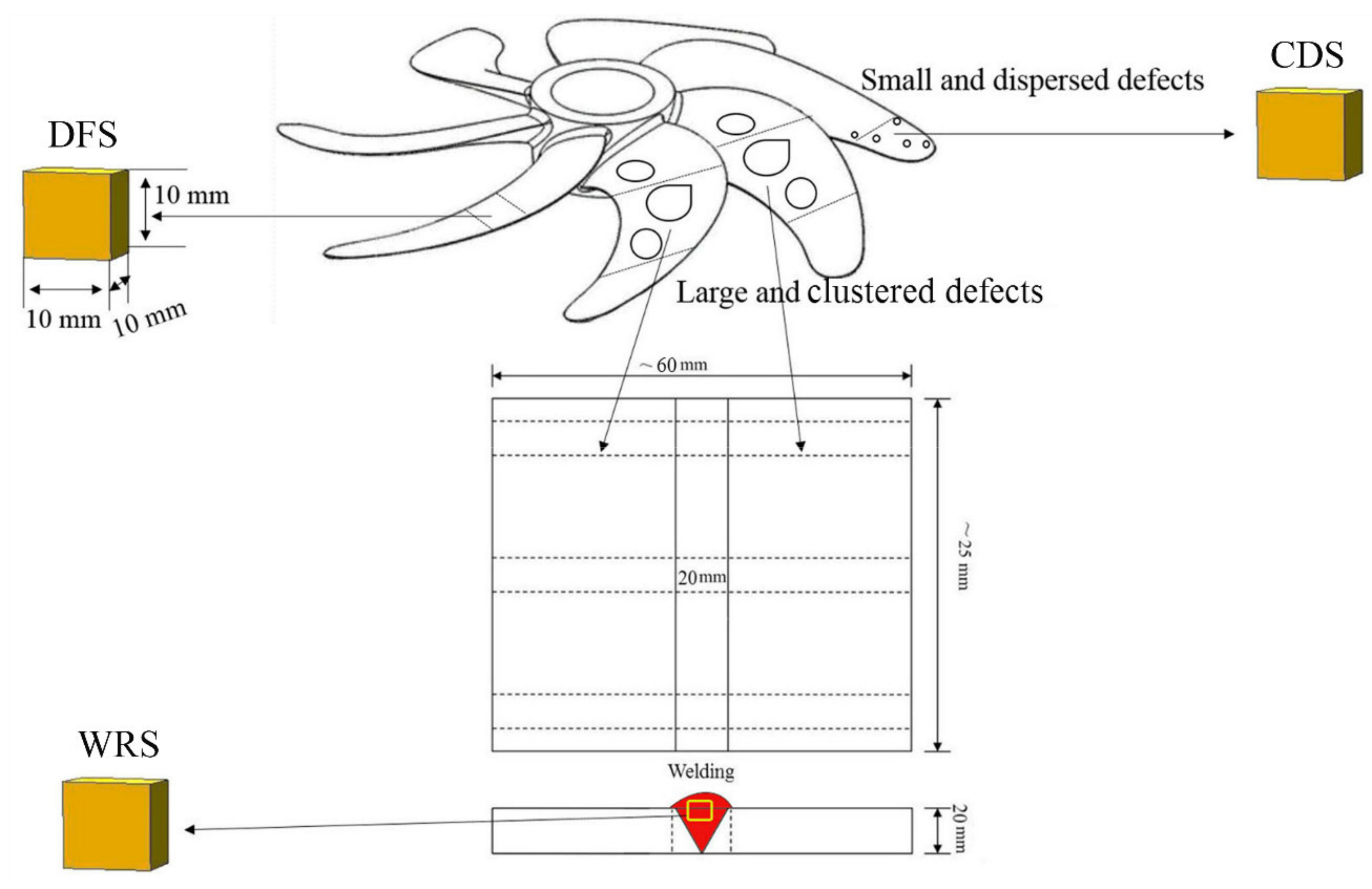





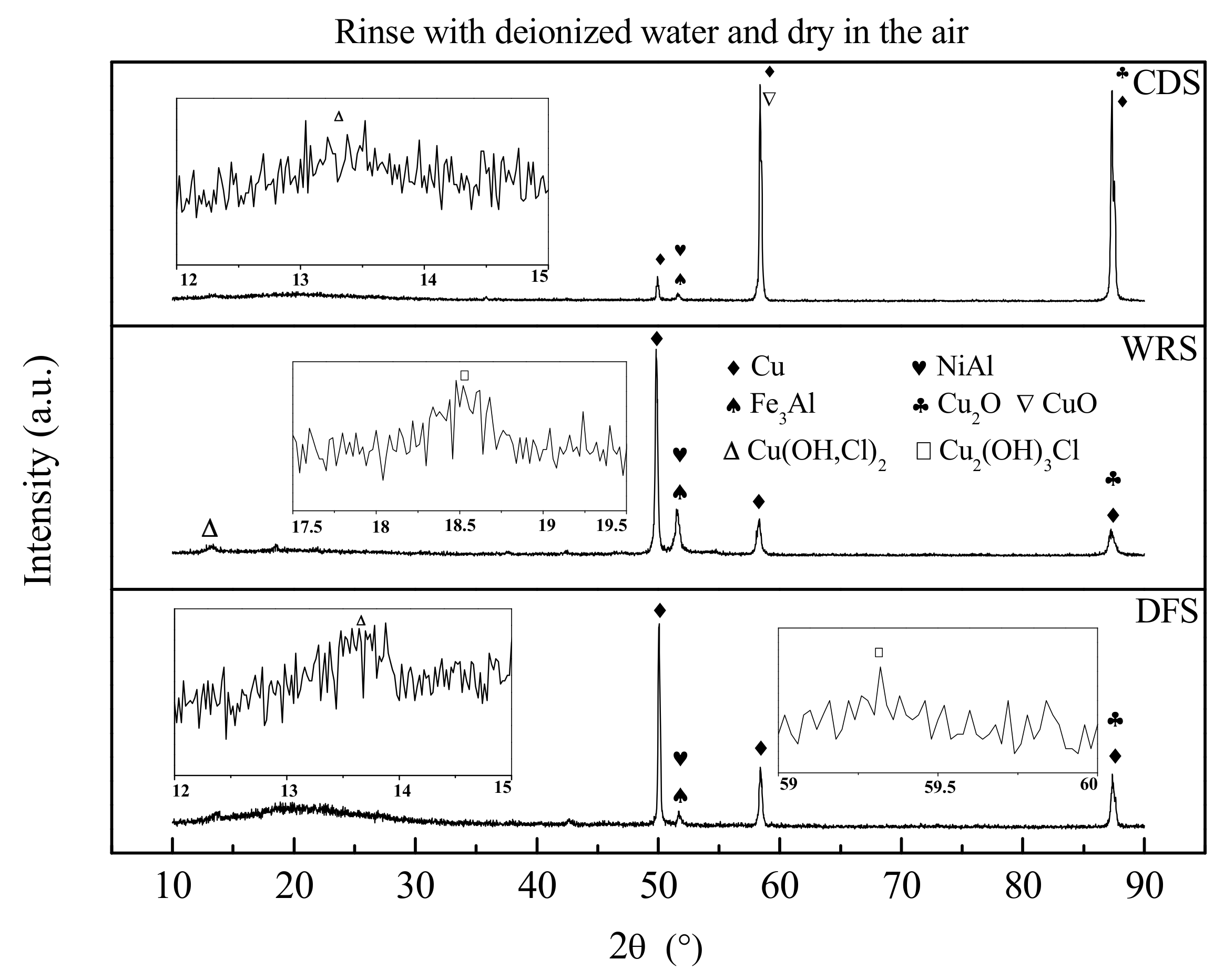

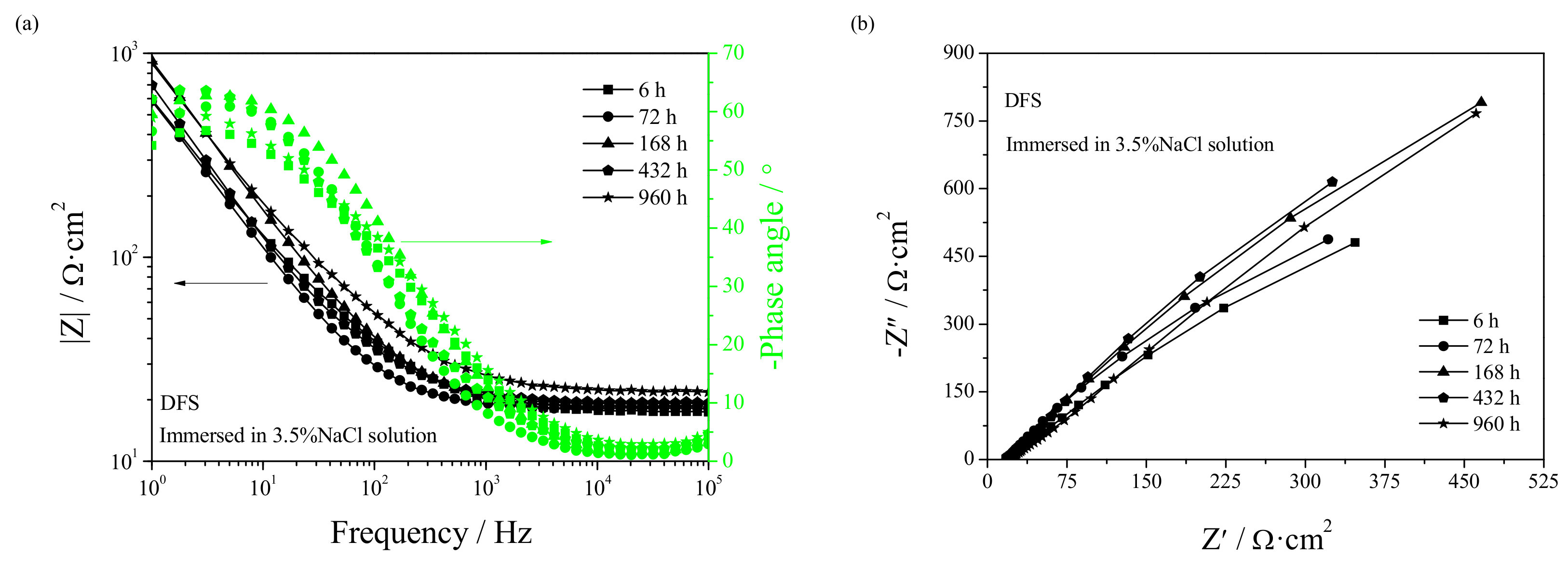
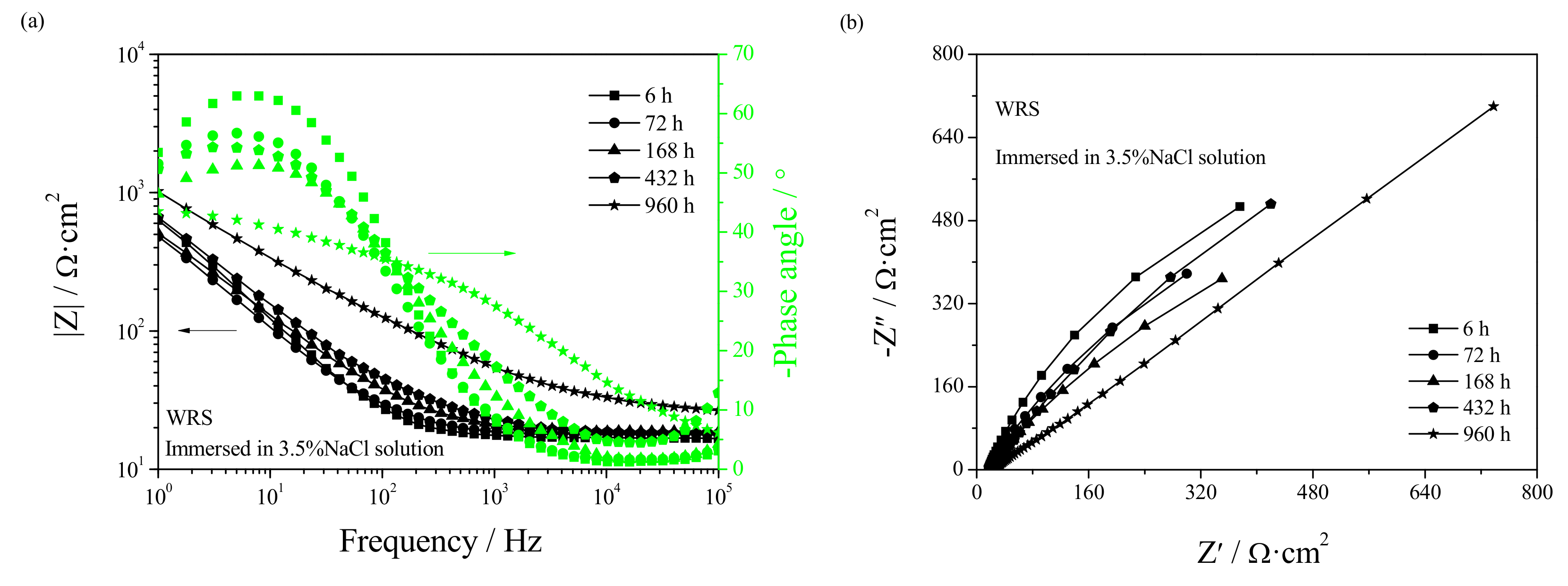
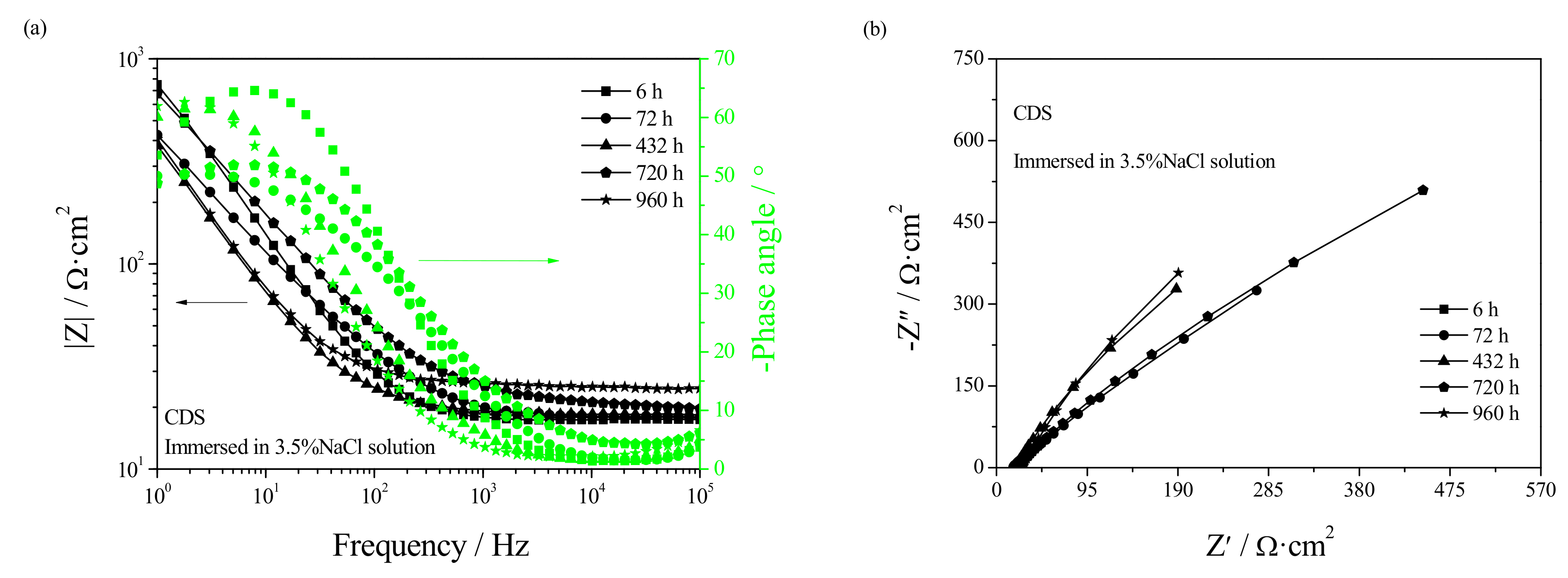


| Elements | Cu | Mn | Fe | Al | Ni | Zn | Sn | Pb | C | Si |
|---|---|---|---|---|---|---|---|---|---|---|
| DFS | 79.9 | 1.39 | 4.8 | 9.24 | 4.43 | 0.005 | 0.023 | 0.018 | <0.0005 | 0.002 |
| WRS | 81.9 | 1.52 | 3.4 | 8.66 | 4.2 | 0.019 | 0.011 | 0.015 | <0.0005 | 0.062 |
| CDS | 79.8 | 1.75 | 4.6 | 9.25 | 4.4 | 0.075 | 0.021 | 0.017 | 0.029 | |
| Welding Wire | 85.66 | 1.85 | 1.9 | 8.5 | 2.06 | 0.003 | <0.0025 | <0.0025 | <0.0025 | 0.045 |
| Position | Element Content (atom.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | Mn | Cl | Fe | Ni | Cu | ||
| I (II) | DFS | 45.96 (0) | 9.45 (34.12) | 0 (2.61) | 13.78 (0) | 10.78 (26.77) | 0 (19.73) | 20.03 (16.77) |
| WRS | 53.05 (0) | 11.68 (0) | 0.71 (0) | 3.14 (0) | 4.06 (1.62) | 3.8 (0) | 23.56 (98.38) | |
| CDS | 60.71 (0) | 8.87 (35.61) | 0.49 (2.11) | 2.71 (0) | 3.76 (28.82) | 1.49 (22.21) | 21.97 (11.25) | |
| Element Content (atom.%) | |||||||
|---|---|---|---|---|---|---|---|
| CDS | O | Al | Mn | Cl | Fe | Ni | Cu |
| Position I | 73.30 | 3.33 | - | 2.13 | 2.90 | 1.44 | 16.90 |
| Position II | - | 15.07 | 1.41 | - | 2.65 | 2.33 | 78.54 |
| Position III | - | 29.27 | 2.6 | - | 46.35 | 12.96 | 8.82 |
| Element | O | Al | Cl | Mn | Fe | Ni | Cu |
|---|---|---|---|---|---|---|---|
| DFS | 41.05 | 12.19 | 3.51 | 1.5 | 12.05 | 8.12 | 21.58 |
| WRS | 18.29 | 14.42 | 0 | 0.96 | 4.14 | 4.03 | 58.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Qi, Y.; Wang, J.; Peng, T.; Zhang, Z.; Li, K. Effect of Weld and Surface Defects on the Corrosion Behavior of Nickel Aluminum Bronze in 3.5% NaCl Solution. Metals 2020, 10, 1227. https://doi.org/10.3390/met10091227
Zhao X, Qi Y, Wang J, Peng T, Zhang Z, Li K. Effect of Weld and Surface Defects on the Corrosion Behavior of Nickel Aluminum Bronze in 3.5% NaCl Solution. Metals. 2020; 10(9):1227. https://doi.org/10.3390/met10091227
Chicago/Turabian StyleZhao, Xu, Yuhong Qi, Jintao Wang, Tianxiang Peng, Zhanping Zhang, and Kejiao Li. 2020. "Effect of Weld and Surface Defects on the Corrosion Behavior of Nickel Aluminum Bronze in 3.5% NaCl Solution" Metals 10, no. 9: 1227. https://doi.org/10.3390/met10091227
APA StyleZhao, X., Qi, Y., Wang, J., Peng, T., Zhang, Z., & Li, K. (2020). Effect of Weld and Surface Defects on the Corrosion Behavior of Nickel Aluminum Bronze in 3.5% NaCl Solution. Metals, 10(9), 1227. https://doi.org/10.3390/met10091227






