Effect of Zr Additions on Non-Metallic Inclusions in X11CrNiMo12 Steel
Abstract
1. Introduction
2. Materials and Methods
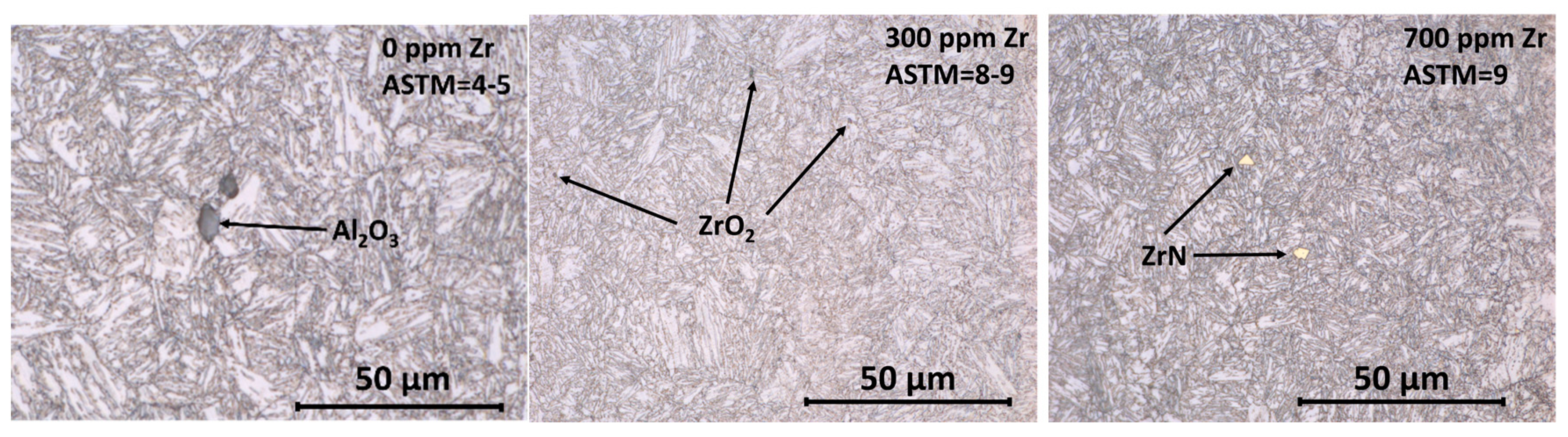
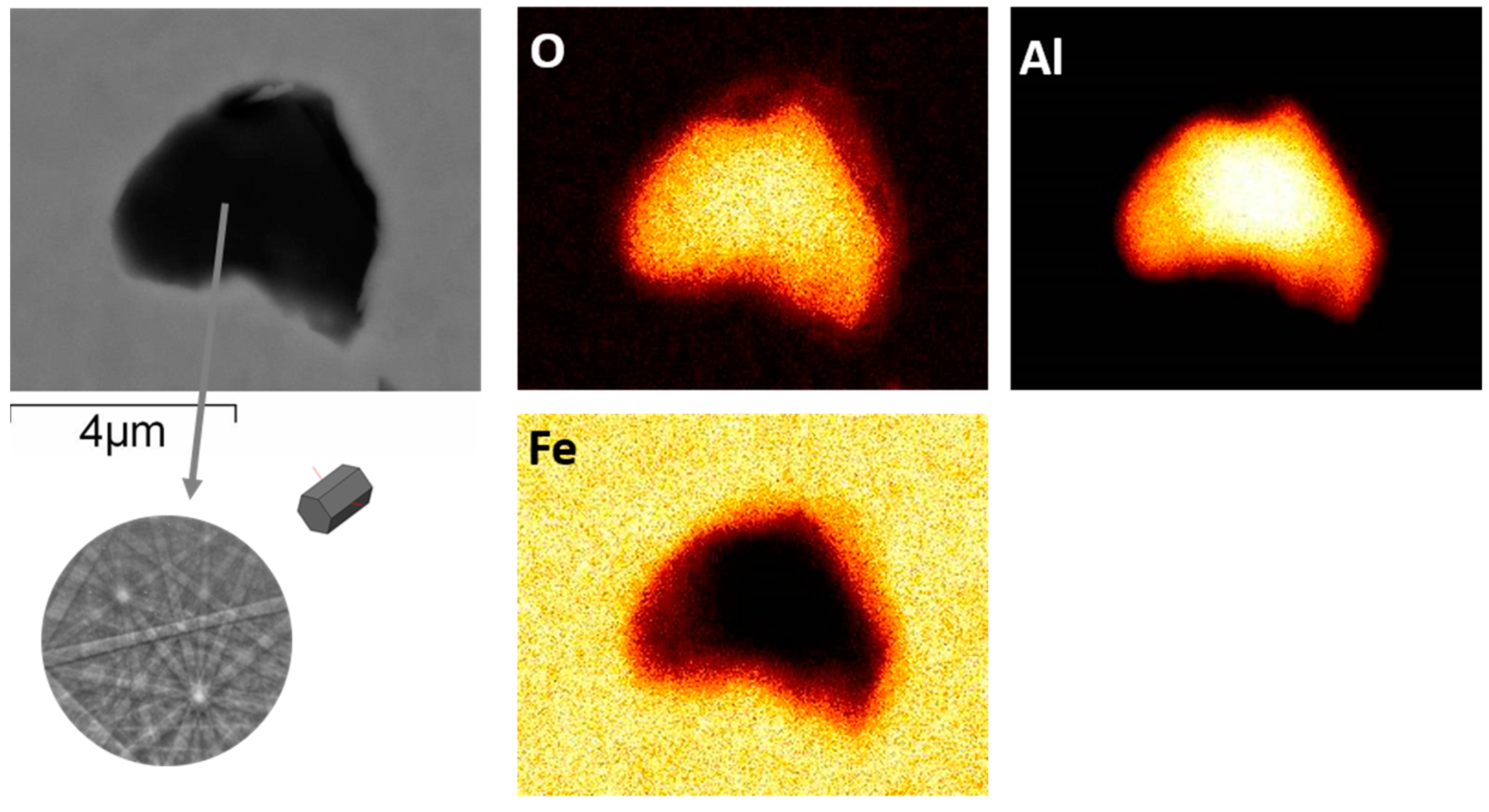
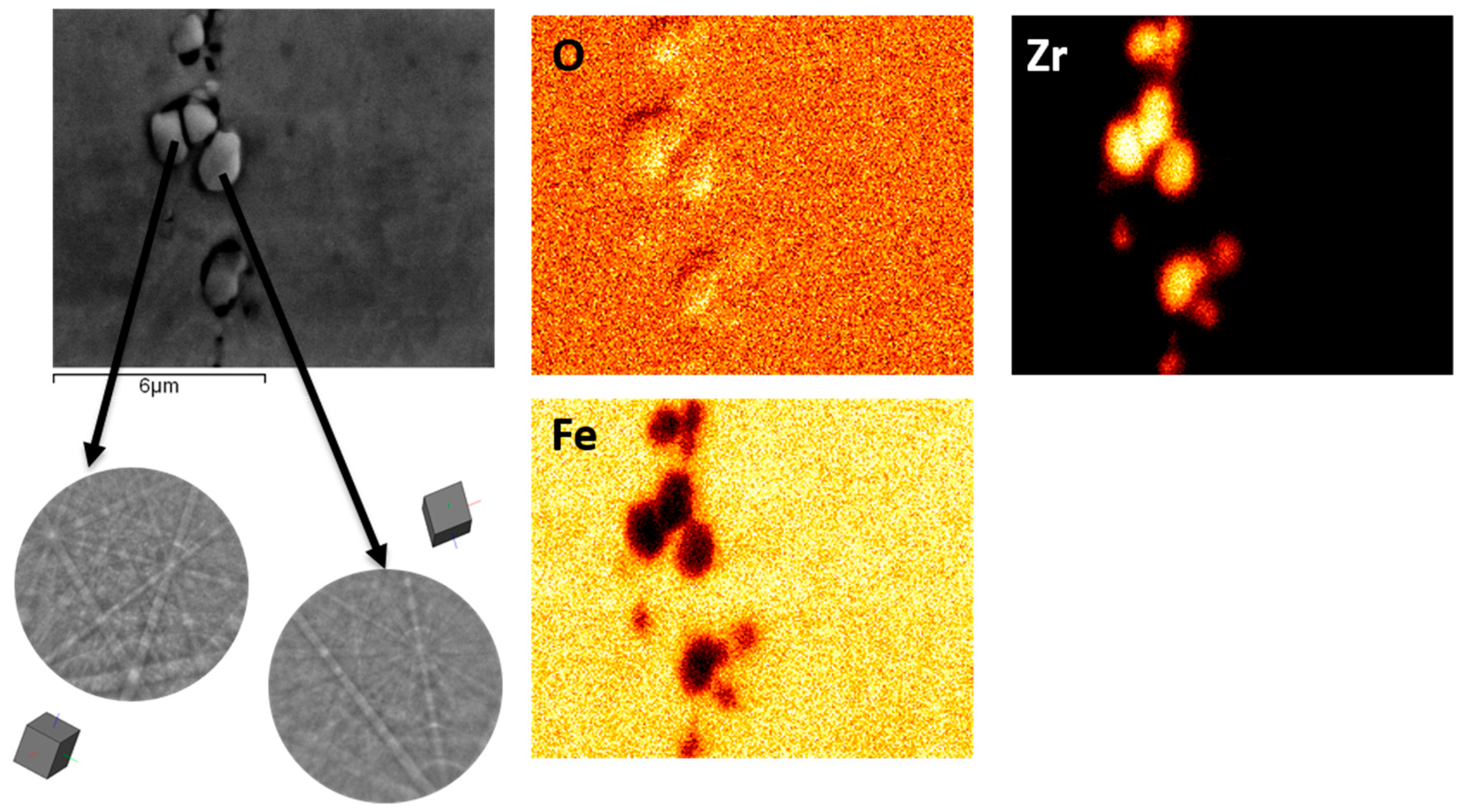
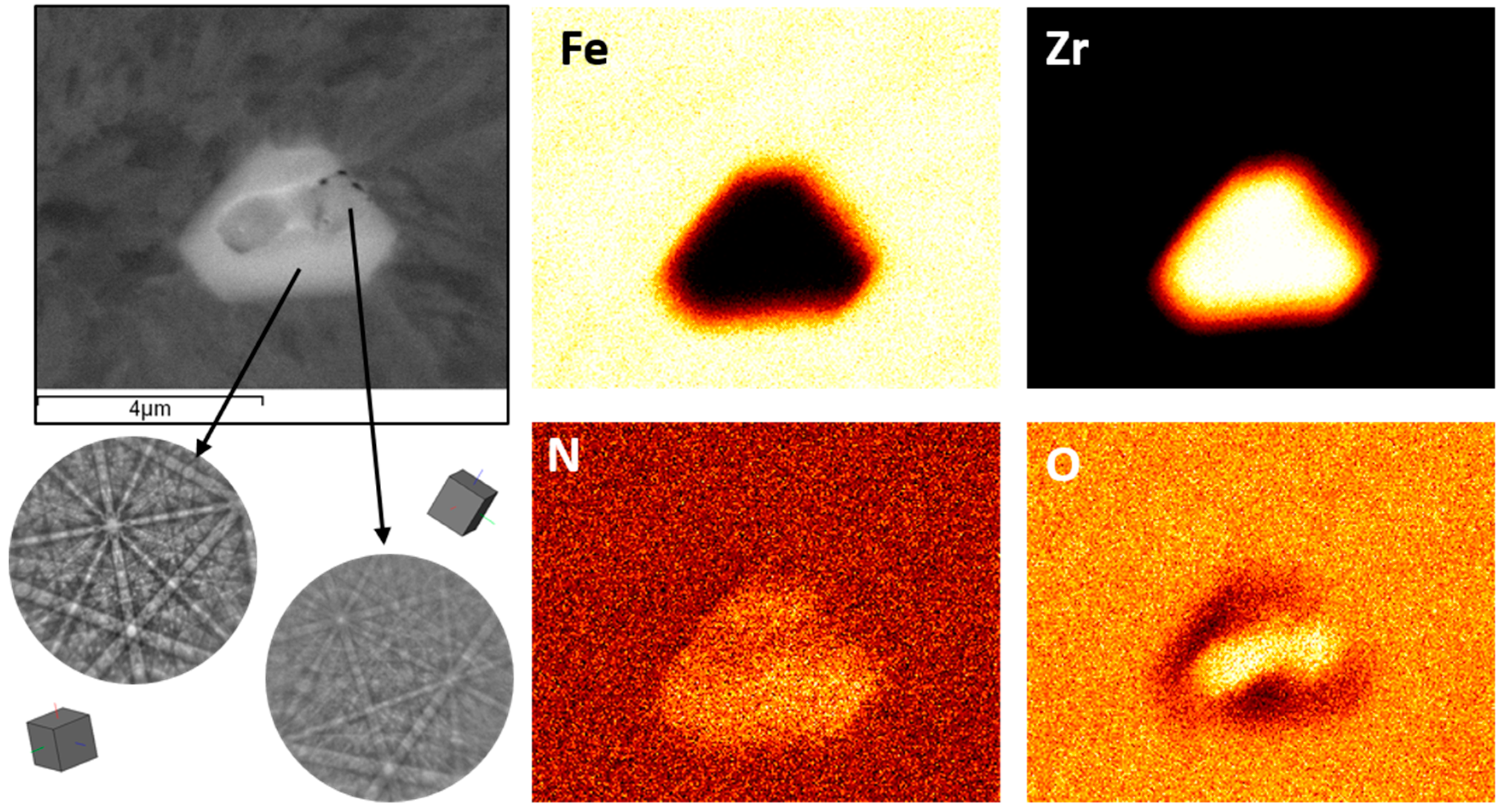
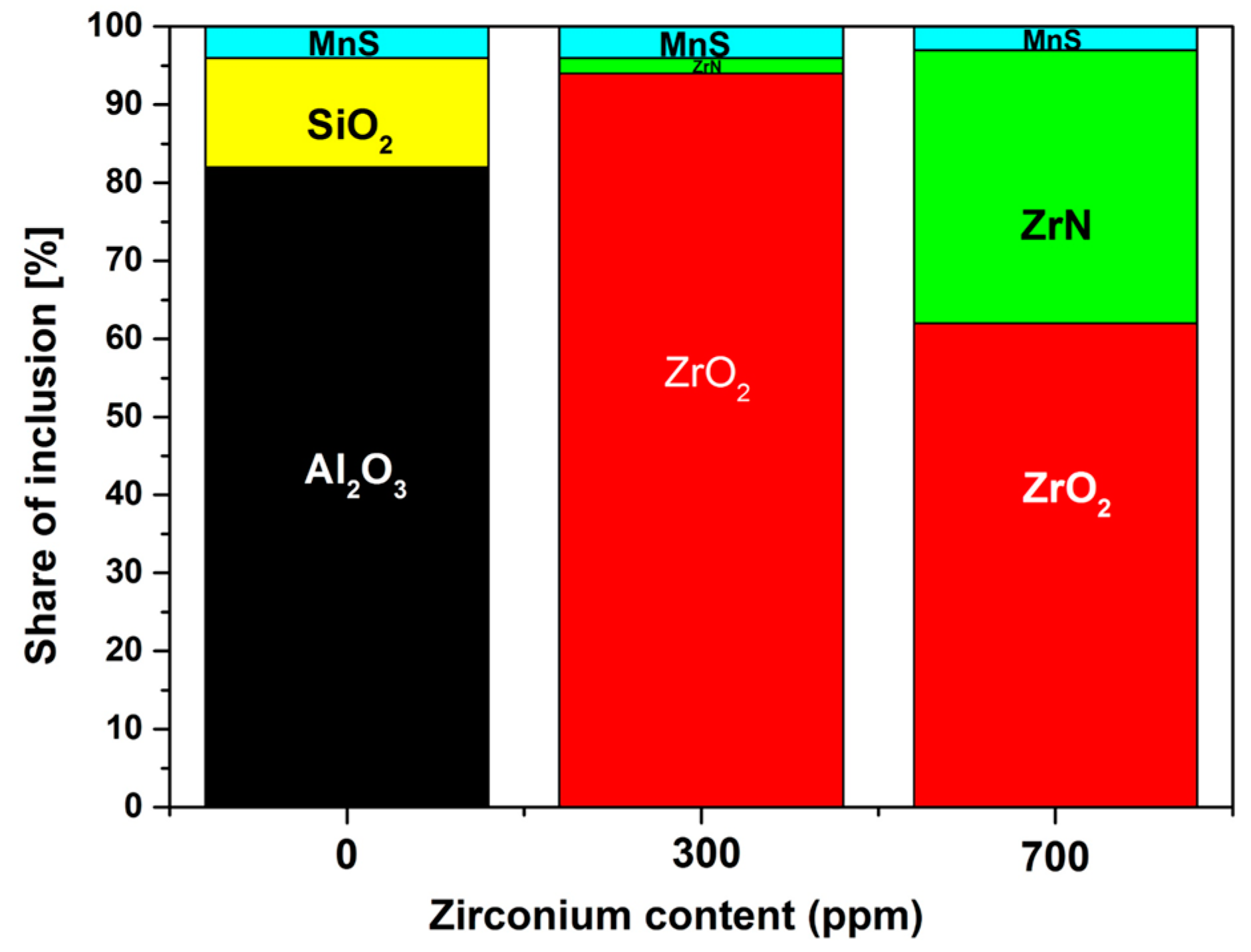
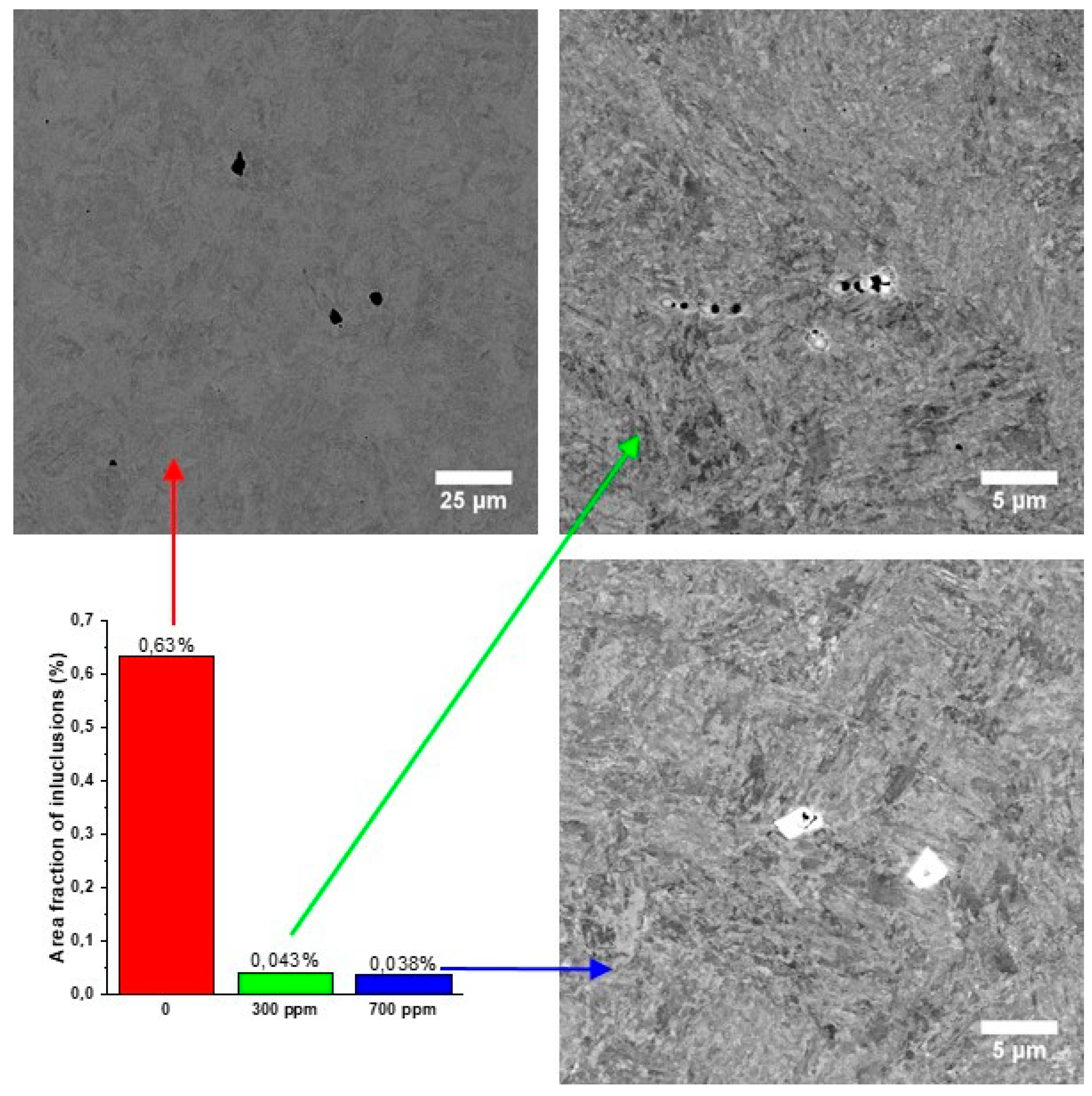
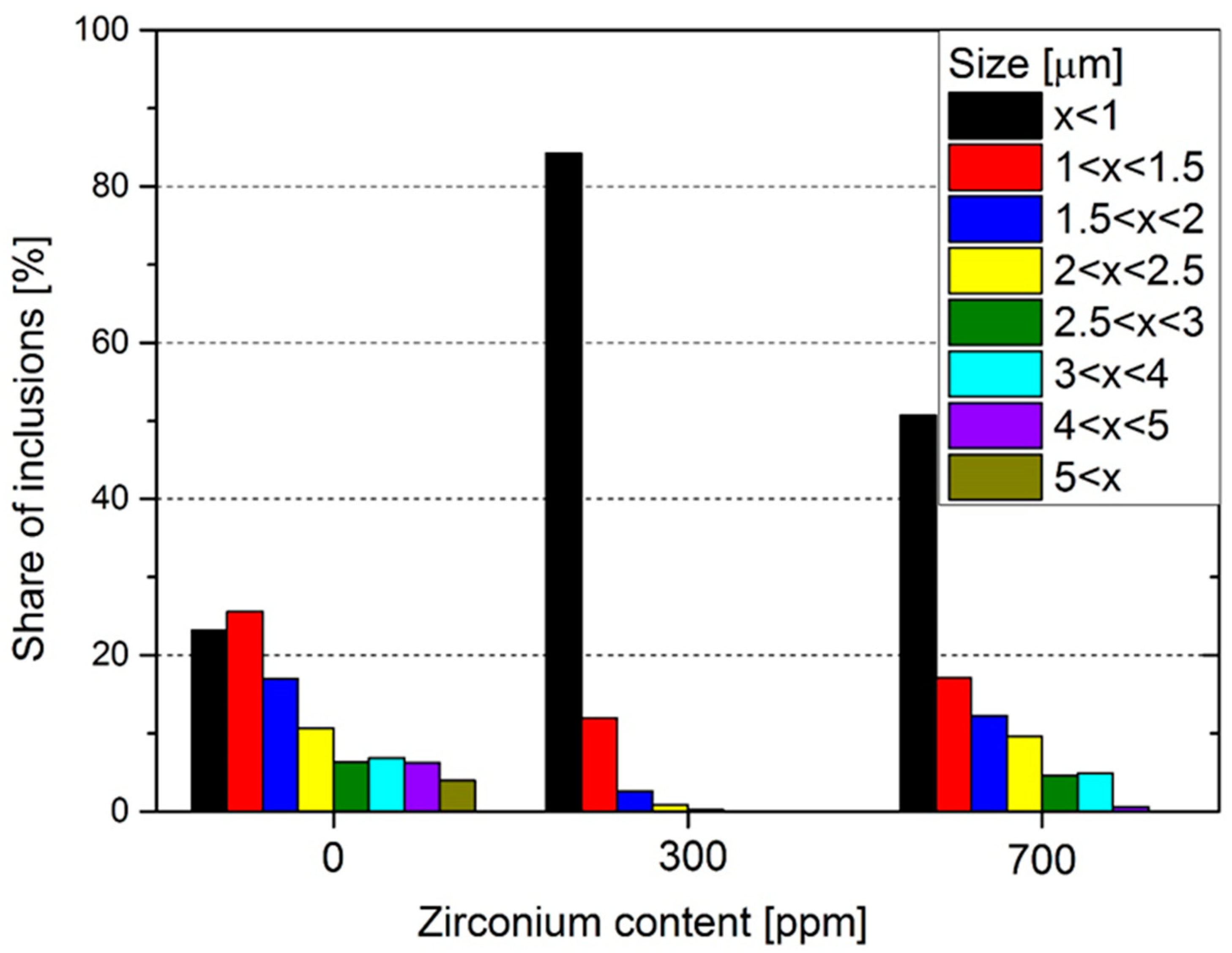
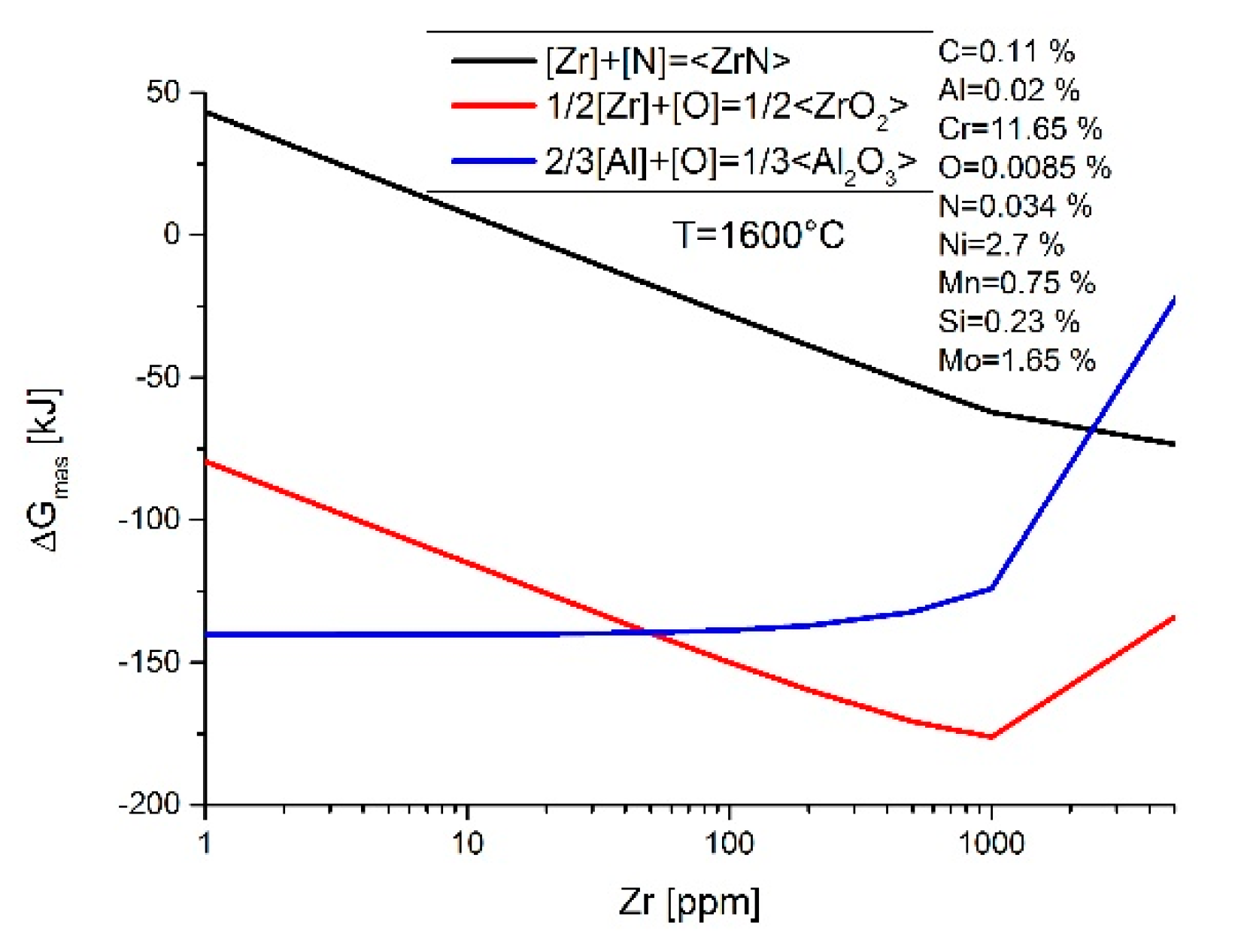
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhadeshia, H.K.D.H. Design of ferritic creep-resistant steels. ISIJ Int. 2001, 41, 626–640. [Google Scholar] [CrossRef]
- Žužek, B.; Vodopivec, F.; Podgornik, B.; Jenko, M.; Godec, M. Calculation of accelerated stationary creep rate activation energy for a steel microstructure with a uniform distribution of carbide particles. Mater. Tehnol. 2012, 46, 661–664. [Google Scholar]
- Žužek, B.; Vodopivec, F.; Jenko, M.; Podgornik, B. Effect of creep strain on creep rate in the temperature range 550–640 °C. Mater. Tehnol. 2014, 48, 545–548. [Google Scholar]
- Vodopivec, F.; Kafexhiu, F.; Žužek, B.; Podgornik, B. Glide Stress by Stationary Creep of Tempered Martensite with Polyhedral Particles. Steel Res. Int. 2017, 88. [Google Scholar] [CrossRef]
- Vodopivec, F.; Kafexhiu, F.; Žužek, B. Effect of Ferrite Lattice Vacancies on Creep Rate of the Steel X20CrMoV121 in the Range 763–913 K. Steel Res. Int. 2017, 88. [Google Scholar] [CrossRef]
- Abe, F. Analysis of creep rates of tempered martensitic 9% Cr steel based on microstructure evolution. Mater. Sci. Eng. A 2009, 510–511, 64–69. [Google Scholar] [CrossRef]
- Abe, F.; Kern, T.-U.; Visawanathan, R. Creep-Resistant Steels; Abe, F., Kern, T.-U., Visawanathan, R., Eds.; Woodhead Publishing and Maney Publishing: Cambridge, UK, 2008. [Google Scholar]
- Mapelli, C. Non-metallic inclusions and clean steel. Metall. Ital. 2008, 100, 43–52. [Google Scholar]
- Shin, J.H.; Park, J.H. Formation Mechanism of Oxide-Sulfide Complex Inclusions in High-Sulfur-Containing Steel Melts. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2018, 49, 311–324. [Google Scholar] [CrossRef]
- Da Costa E Silva, A.L.V. The effects of non-metallic inclusions on properties relevant to the performance of steel in structural and mechanical applications. J. Mater. Res. Technol. 2019, 8, 2408–2422. [Google Scholar] [CrossRef]
- Long, M.; Zuo, X.; Zhang, L.; Chen, D. Kinetic modeling on nozzle clogging during steel billet continuous casting. ISIJ Int. 2010, 50, 712–720. [Google Scholar] [CrossRef]
- Singh, S.N. Mechanism of Alumina Buildup in Tundish Nozzles During Continuous Casting of Aluminum-Killed Steels. Metall. Trans. 1974, 5, 2165–2178. [Google Scholar] [CrossRef]
- Li, Y.; Wan, X.L.; Lu, W.Y.; Shirzadi, A.A.; Isayev, O.; Hress, O.; Wu, K.M. Effect of Zr-Ti combined deoxidation on the microstructure and mechanical properties of high-strength low-alloy steels. Mater. Sci. Eng. A 2016, 659, 179–187. [Google Scholar] [CrossRef]
- Baker, T.N. Role of zirconium in microalloyed steels: A review. Mater. Sci. Technol. 2014, 31, 265–294. [Google Scholar] [CrossRef]
- Bizyukov, P.V.; Giese, S.R. Effects of Zr, Ti, and Al Additions on Nonmetallic Inclusions and Impact Toughness of Cast Low-Alloy Steel. J. Mater. Eng. Perform. 2017, 26, 1878–1889. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Wang, G. Effect of Hot Deformation and Controlled Cooling Process on Microstructures of Ti–Zr Deoxidized Low Carbon Steel. ISIJ Int. 2016, 56, 1800–1807. [Google Scholar] [CrossRef]
- Janis, J.; Karasev, A.; Nakajima, K.; Jönsson, P.G. Effect of Secondary Nitride Particles on Grain Growth in a Fe-20 mass% Cr Alloy Deoxidised with Ti and Zr. ISIJ Int. 2013, 53, 476–483. [Google Scholar] [CrossRef][Green Version]
- Suito, H.; Karasev, A.V.; Hamada, M.; Inoue, R.; Nakajima, K. Influence of Oxide Particles and Residual Elements on Microstructure and Toughness in the Heat-Affected Zone of Low-Carbon Steel Deoxidized with Ti and Zr. ISIJ Int. 2011, 51, 1151–1162. [Google Scholar] [CrossRef]
- Zhang, L.; Kannengiesser, T. Austenite grain growth and microstructure control in simulated heat affected zones of microalloyed HSLA steel. Mater. Sci. Eng. A 2014, 613, 326–335. [Google Scholar] [CrossRef]
- Du, Y.; Wu, K.M.; Cheng, L.; Li, Y.; Isayev, O.; Hress, O. Effect of Zr-Ti deoxidisation on the hydrogen-induced cracking of X65 pipeline steels. Mater. Sci. Technol. 2016, 32, 728–735. [Google Scholar] [CrossRef]
- Koležnik, M.; Burja, J.; Šetina Batič, B.; Nagode, A.; Medved, J. De-oxidation of Pk942 steel with Ti and Zr. Mater. Tehnol. 2017, 51, 1031–1036. [Google Scholar] [CrossRef]
- Suito, H.; Ohta, H.; Morioka, S. Refinement of Solidification Microstructure and Austenite Grain by Fine Inclusion Particles. ISIJ Int. 2006, 46, 840–846. [Google Scholar] [CrossRef]
- Wang, P.; Lu, S.P.; Xiao, N.M.; Li, D.Z.; Li, Y.Y. Effect of delta ferrite on impact properties of low carbon 13Cr-4Ni martensitic stainless steel. Mater. Sci. Eng. A 2010, 527, 3210–3216. [Google Scholar] [CrossRef]
- Burja, J.; Koležnik, M.; Župerl, Š.; Klančnik, G. Nitrogen and nitride non-metallic inclusions in steel. Mater. Tehnol. 2019, 53, 919–928. [Google Scholar] [CrossRef]
- Wasai, K.; Mukai, K. Thermodynamic analysis on metastable alumina formation in aluminum deoxidized iron based on Ostwald’s Step Rule and classical homogeneous nucleation theories. ISIJ Int. 2002, 42, 467–473. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Dispersion Behavior of MgO, ZrO2, Al2O3, CaO-Al2O3 and MnO-SiO2 Deoxidation Particles during Solidification of Fe-10mass%Ni Alloy. ISIJ Int. 2006, 46, 22–28. [Google Scholar] [CrossRef]
- Karasev, A.; Suito, H. Quantitative evaluation of inclusion in deoxidation of Fe-10 mass pct Ni alloy with Si, Ti, Al, Zr and Ce. Metall. Mater. Trans. B 1999, 30, 249–257. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Nitride Precipitation on Particles in Fe–10mass%Ni Alloy Deoxidized with Ti, M (M=Mg, Zr and Ce) and Ti/M. ISIJ Int. 2009, 49, 229–238. [Google Scholar] [CrossRef]
- Burja, J.; Šuler, B.; Nagode, A. Effect of ageing temperature on reverse austenite content in AISI 630 stainless steel. Materwiss. Werksttech. 2019, 50, 405–411. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Elliott, J.F. The Thermodynamics of Liquid Dilute Iron Alloys. Met. Sci. 1974, 8, 298–310. [Google Scholar] [CrossRef]
| Austenitization | Cooling | Tempering 1 | Cooling | Tempering 2 | Cooling |
|---|---|---|---|---|---|
| 1045 °C 4 h | oil | 660 °C 4.5 h | air | 640 °C 4.5 h | air |
| C | Si | Mn | S | Cr | Ni | Mo | V | Al | Zr | O | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Zr | 0.11 | 0.23 | 0.71 | 0.0055 | 11.65 | 2.70 | 1.63 | 0.30 | 0.021 | <0.001 | 0.0058 | 0.033 |
| 300 Zr | 0.11 | 0.22 | 0.73 | 0.0052 | 11.65 | 2.70 | 1.66 | 0.30 | 0.018 | 0.030 | 0.0088 | 0.034 |
| 700 Zr | 0.11 | 0.22 | 0.72 | 0.0053 | 11.62 | 2.70 | 1.64 | 0.30 | 0.019 | 0.070 | 0.0085 | 0.034 |
| Rp02/MPa | Rm/MPa | A/% | Hardness HB | |
|---|---|---|---|---|
| 0 Zr | 795 ± 7 | 1000 ± 8 | 15.4 ± 0.8 | 299 ± 3 |
| 300 Zr | 796 ± 6 | 1010 ± 6 | 15.7 ± 0.5 | 302 ± 2 |
| 700 Zr | 784 ± 8 | 985 ± 8 | 16.2 ± 0.9 | 298 ± 3 |
| Rp02/MPa | Rm/MPa | A/% | |
|---|---|---|---|
| 0 Zr | 585 ± 5 | 681 ± 7 | 21.3 ± 1.2 |
| 300 Zr | 605 ± 5 | 688 ± 6 | 22.7 ± 1.3 |
| 700 Zr | 584 ± 6 | 671 ± 5 | 23.0 ± 1.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burja, J.; Koležnik, M.; Batič, B.Š.; Medved, J. Effect of Zr Additions on Non-Metallic Inclusions in X11CrNiMo12 Steel. Metals 2020, 10, 1183. https://doi.org/10.3390/met10091183
Burja J, Koležnik M, Batič BŠ, Medved J. Effect of Zr Additions on Non-Metallic Inclusions in X11CrNiMo12 Steel. Metals. 2020; 10(9):1183. https://doi.org/10.3390/met10091183
Chicago/Turabian StyleBurja, Jaka, Mitja Koležnik, Barbara Šetina Batič, and Jožef Medved. 2020. "Effect of Zr Additions on Non-Metallic Inclusions in X11CrNiMo12 Steel" Metals 10, no. 9: 1183. https://doi.org/10.3390/met10091183
APA StyleBurja, J., Koležnik, M., Batič, B. Š., & Medved, J. (2020). Effect of Zr Additions on Non-Metallic Inclusions in X11CrNiMo12 Steel. Metals, 10(9), 1183. https://doi.org/10.3390/met10091183







