Abstract
Research for the recycling of lithium-ion batteries (LIBs) started about 15 years ago. In recent years, several processes have been realized in small-scale industrial plants in Europe, which can be classified into two major process routes. The first one combines pyrometallurgy with subsequent hydrometallurgy, while the second one combines mechanical processing, often after thermal pre-treatment, with metallurgical processing. Both process routes have a series of advantages and disadvantages with respect to legislative and health, safety and environmental requirements, possible recovery rates of the components, process robustness, and economic factors. This review critically discusses the current status of development, focusing on the metallurgical processing of LIB modules and cells. Although the main metallurgical process routes are defined, some issues remain unsolved. Most process routes achieve high yields for the valuable metals cobalt, copper, and nickel. In comparison, lithium is only recovered in few processes and with a lower yield, albeit a high economic value. The recovery of the low value components graphite, manganese, and electrolyte solvents is technically feasible but economically challenging. The handling of organic and halogenic components causes technical difficulties and high costs in all process routes. Therefore, further improvements need to be achieved to close the LIB loop before high amounts of LIB scrap return.
1. Introduction
Renewable energy sources have the potential to end the era of fossil fuels. Electrochemical storage systems, especially lithium-ion batteries (LIBs), are an important technology for the success of this transition. This is due to their sum of positive properties such as their high energy density and low self-discharge [1]. On the production site, the growing application of LIBs leads to high investments in research and development with a focus on questions such as optimized cell production or optimized active materials. These developments are accompanied by a critical discussion regarding the security of the necessary raw material supply, the environmental and social impact of the raw material production, and the responsible recycling of end-of-life batteries [2,3,4,5]. Beside base metals such as iron (Fe), aluminum (Al), copper (Cu), manganese (Mn), and nickel (Ni), LIBs also contain minor metals such as cobalt (Co) and lithium (Li) as well as graphite. The production of some of these raw materials can be connected to severe ecological and social impacts. Examples are the appearance of child labor in artisanal Co mining and the influence of Li production on the water balance in desert areas [6,7]. Furthermore, in the case of some raw materials, a small number of producers dominate the markets. This is especially true for Co and graphite [8]. In view of these developments, the recycling of LIBs is a key factor to manage the transition toward renewable energies in a sustainable way. However, LIB recycling is a challenging task due to their complex material composition and their electrical and chemical energy content leading to various health, safety, and environmental (HSE) risks [9].
In order to ensure a closed loop for LIBs (see Figure 1), extensive research activities started about 15 years ago. Several efforts led to the development of different recycling processes, which have been realized in a few pioneering industrial plants. All these process routes are characterized by long and complex process chains and use a combination of mechanical and/or thermal and/or pyrometallurgical and hydrometallurgical unit operations [10].
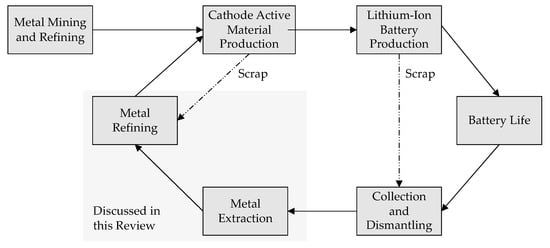
Figure 1.
Closed loop for battery materials.
This paper reviews and discusses the current status of industrial metallurgical processes from a European perspective. In contrast to existing reviews [11,12,13,14,15,16,17], which often focus on research at a lower technology readiness level and specific process technologies, this review emphasizes the complex dependencies between legal framework, economic conditions, and technical boundaries of industrial process routes. The focus here lays on the metal extraction and metal refining from battery cells/modules. Pre-treatment (discharging and dismantling of battery systems) is not covered in this review. For further details on this subject, see [10,11]. Products of metal refining are metal salts, compounds, or the respective metals, e.g., cathodes that can be used in different industries depending on the final quality.
In the following, after a background on current and future LIBs as well as legislation is given in Section 2, the currently pursued industrial recycling routes are presented in Section 3. In Section 4, the recycling processes are critically discussed with respect to legislation, recovery rate, process robustness, economics, and HSE. Section 5 summarizes the main conclusions and gives an outlook regarding the upcoming developments.
2. Background
The LIB technology has reached a wide acceptance since its introduction and is applied in a growing number of applications. First, it was widely applied in batteries for mobile phones and laptops, followed by pedelecs and power tools. Nowadays, LIBs capture the markets for stationary storage systems and electric vehicles (xEVs) [1]. Table 1 summarizes the characteristics of LIBs in different applications.

Table 1.
Characteristics of lithium-ion batteries (LIBs) used in battery electric vehicles (BEVs), plug-in hybrid electric vehicles (PHEVs), electric bikes (pedelecs), and mobile phones [1,11,16,18].
LIBs are configured in cells, modules, and systems. Battery modules and especially systems need peripheral units such as a temperature and a battery management system. Depending on the field of application, the design of cells and modules varies considerably. Whereas applications with a smaller battery size often use cylindrical cells due to their dimensions, prismatic cells are primarily used for bigger battery systems, e.g., traction batteries. Pouch cells with an Al composite foil as casing instead of a rigid Al or steel casing are used among a wide range of applications in order to increase the energy density. In addition, battery systems without module levels are currently under development [1,19,20,21].
Despite the wide range of applications and different designs for cells, modules, and systems, the chemical composition of LIBs is similar. A typical composition of a battery system is given in Figure 2. Here, cells form 56% of the battery system [22]. In newer LIB systems, an increase of the cell fraction, especially when using pouch cells, is observed. The cells consist of five main components: the positive and negative electrodes, the ion-permeable separator, the electrolyte, and a cell casing. For a detailed explanation concerning the functionality and production of battery cells, modules, and systems, see [1,20].
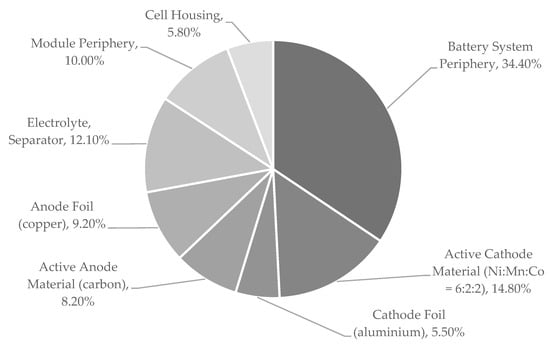
Figure 2.
Typical composition of a generic traction battery system based on data from [22].
Table 2 gives concentrations of the metals of interest and graphite for recycling in a typical xEV battery module and their origin.

Table 2.
Fraction of specific metals and graphite in a generic battery module with an NMC (LiNixMnyCozO2) chemistry of 6:2:2 [10].
2.1. Current Lithium-Ion Battery Composition
In this section, the main battery cell components, cathode, anode, electrolyte, and separator of current LIBs are presented, since these components have the biggest influence on the recycling processes.
2.1.1. Positive Electrode (Cathode)
The positive electrode consists of the active material, which is coated on the current collector foil, usually Al. In most cases, as binder, polyvinylidene fluoride (PVDF) with the addition of carbons as conducting agents is used. The active material must be able to deintercalate Li ions and oxidize the transition metals for charge balance [1]. There are three different types of cathode active materials: layered oxides (LiMO2 with M = Co, Ni, Mn, Al), spinels (LiM2O4 with M = Mn, Ni), and phosphates (LiMPO4 with M = Fe, Mn, Co, Ni). Commercially available active materials are presented in Table 3.

Table 3.
Summary of the properties of the main commercially available cathode active materials [1,23,24,25].
In most markets, layered oxides are the foremost commercially used cathode active materials, especially in traction batteries [25]. For optimized specific and reversible capacity compared to NMC 1:1:1 and a higher thermal stability compared to NCA, the Ni-content of NMC is currently maximized from 1:1:1 to Ni-rich compositions up to 8:1:1 [25,26,27]. In contrast, particularly in China, LiFePO4 (LFP)-based batteries have been widely used in buses and xEVs, which means that in China, significant return flows of this battery chemistry have to be expected [10].
2.1.2. Negative Electrode (Anode)
On the anodic side of the battery, the current collector foil consists of Cu and the active material is graphite in most cases. Typically, as binder, styrene–butadiene rubber (SBR) in combination with the polymeric thickener carboxymethyl cellulose (CMC) and carbons as conducting agents is used [28]. Consumer applications usually use natural graphite because of the satisfactory properties at an acceptable price. For high-energy or high-power applications, artificial graphite is used [1,25]. To combine the benefits of both, a combination is possible as well. A limited but increasing number of cell producers include small fractions of about 5% silicon (Si) or SiO2 to increase cell energy in their anode active material [25,29]. Furthermore, lithium titanate (LTO) is applied as anode active material in high-power applications. In this case, Al is used as a current collector foil instead of Cu [30].
Artificial and natural graphite have a market share of 89% while amorphous carbon has a share of 7%, which is in total a 96% market share of solely carbon-based anodic materials. C/Six composites and LTO share the remaining 4% market share equally [25].
2.1.3. Electrolyte and Separator
This section shortly summarizes the typically used electrolytes and separators. Electrolytes consist of the conducting salt, solvents, and additives. They need to provide high and stable conductivity and a manageable safety level. As electrolyte solvents carbonates, esters as well as ethers are used commercially. State of the art are mixtures of cyclic carbonates such as ethylene carbonate (EC) or propylene carbonate (PC) with open chained carbonates such as dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), and/or diethyl carbonate (DEC). Usually, these mixtures strongly depend on the application of the battery [1]. Currently, new solvent components, that could for example substitute EC, are evaluated [29].
The conducting salt in all commercially available batteries is lithium hexafluorophosphate (LiPF6). Other possible conducting salts are lithium bis(trifluormethyl)sulfonylimid (LiTFSI) and its derivates (e.g., lithium bis(fluorosulfonyl) imide (LiFSI)) or lithium [tris(pentafluorethyl)-trifluorphosphate] (LiFAP), lithium 4,5-dicyano-2-trifluoromethyl-imidazolide (LiTDI), and lithium bis(oxalate)borate (LiBOB) [1,29,31]. LiFSI and LiTDI are already commercially available [32].
Separators divide the space between the electrodes and are only permeable for ions. Four different separator types are existent: microporous membranes, ceramic-coated separators, non-woven mats, and solid inorganic and polymeric electrolytes. Polyolefin-based membranes currently dominate the market for separators [1,25]. Currently, coated separators (e.g., with PVDF or ceramics) experience increased application [26,32].
2.2. Future Lithium-Ion Battery Composition
For effective planning of recycling plants, knowledge about possible developments in the LIB technology is crucial. Consequently, this section presents the main possible future chemical compositions of LIB cells.
2.2.1. Positive Electrode (Cathode)
A short-term goal is to further improve NMC materials. Especially, the formation of spherical NMC particles that have a Ni-rich core for high capacity and a Mn-rich shell for stability seems interesting [27]. In addition, currently, a growing interest in LFP chemistries can be observed due to their low raw material costs and intrinsic safety [21]. Li- and Mn-rich oxides are discussed in research because of their high theoretical specific capacity and low costs. However, they are considered a medium- to long-term development because of the need for a different electrolyte system [27,33,34] also mentioned possible positive effects of doping or coating active material particles, which can also be considered a long-term optimization approach. Doping can promote high specific capacity and good long-term stability, which are two properties that usually do not appear together. Possible dopants are cations such as Ag+, Al3+, Cr3+, Fe3+, Mg2+, Mo6+, Ru2+, Ti4+ and Zr4+, as well as anions such as F−. Coatings such as Al2O3, AlPO4, AlF3, PrPO4, TiO2, and V2O5 are also discussed, especially for protection measures of the particles against components of the electrolyte [27,33].
2.2.2. Negative Electrode (Anode)
Anode active materials with larger Si fractions of up to 40% or the use of tin (Sn) over Si are in discussion [35]. Li metal is a promising anode active material in the medium term, especially for all-solid-state batteries [1,25,29].
2.2.3. Electrolyte and Separator
The application of standard electrolyte systems with LiPF6 will continue in the upcoming years. According to [25], even high voltage cathode materials are suitable for that system. Solid electrolytes that combine the functions of the separator and the electrolyte may gain importance in the long term. It is still unclear if the advantages of solid electrolytes are strong enough compared to already commercially functioning separators, since major technological breakthroughs are pending [25].
2.3. Legislative Framework
The recycling of batteries in Europe is regulated in the Batteries Directive (2006/66/EG) [36]. It came into force in 2006 and is implemented in national laws by each member of the EU. In terms of LIB recycling, the producer carries an extended responsibility and must bear the cost of collecting, treating, and recycling. Furthermore, the directive requires a minimum recycling efficiency. All collected and identifiable spent batteries must be treated and recycled according to the state of the art. For LIBs, a recycling efficiency of at least 50% by weight must be achieved. Since 2006, the directive has been amended several times, most recently in 2013 [37].
The Batteries Directive is currently under major revision, and the presentation of a draft version of the new directive is expected in the fourth quarter of 2020 [38]. Although details have not been published yet, increasing collection targets for consumer batteries, the introduction of element-specific recycling quotas for Li, Co, and Ni, more specific regulations for traction batteries, as well as uniform regulations for the calculation of recycling quotas are expected by most stakeholders. In China, similar regulations, especially regarding element-specific recycling targets, are in force [39,40].
3. Process Chains for the Recycling of Lithium-Ion Batteries
Section 2 described the current composition of LIBs and possible future developments. From a recycling point of view, the main challenges derive, on the one hand, from the complex material composition including halogenic and organic compounds as well as the high energy content and, on the other hand, from the requirements regarding high recycling rates, HSE, and economics. This results in comparatively long and complex process chains in comparison to earlier battery generations. In the last years, several different recycling processes have been developed, which can be classified into two general process routes, as shown in Figure 3. The first group of processes combines pyrometallurgy with hydrometallurgy and the second group consists of mechanical treatment prior to metallurgy.
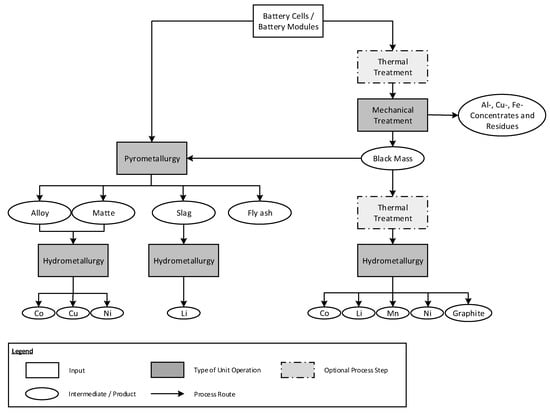
Figure 3.
Possible process routes in the recycling of lithium-ion batteries.
In case of a pyrometallurgical treatment, a Co-, Cu-, and Ni-containing alloy (metallic phase) or matte (sulfidic phase), an Al-, Mn- and Li-containing slag (oxidic phase), and a fly ash are produced. Alloy/matte and slag can be treated by hydrometallurgy to recover the individual metals. The fly ash is usually used as an outlet for undesirable elements such as fluorine and hence, it is landfilled.
In the second group of processes, LIBs are treated mechanically, often after a thermal treatment step. Typical products of the mechanical process are ferrous and non-ferrous metal concentrates including Al and Cu concentrates as well as a fraction containing the active electrode materials, which is called black mass. The black mass can be either fed in pyrometallurgy or treated directly in hydrometallurgy. Depending on the overall process design, the black mass requires a thermal treatment prior to hydrometallurgy to remove the organic components and to concentrate the metal content. In hydrometallurgy, Co, Li, Mn, Ni, and, if applicable, graphite can be recovered.
In the following, both process routes are described in further detail, see Section 3.2 and Section 3.3. For those not familiar with the applied unit operations, Section 3.1 gives a short introduction for a better understanding.
3.1. Background on Processes Applied in Lithium-Ion Battery Recycling
Mechanical processes typically start with comminution aiming at liberation of the materials/components. Afterwards, the materials/components are sorted by their physical properties such as particle size, form, density, and electric and magnetic properties. Usually, in mechanical processing, concentrates for further metallurgical treatment are produced [41,42,43].
Pyrometallurgy includes high-temperature processes such as roasting or smelting for winning and refining metals. Roasting is the term for processes consisting of a gas–solid reaction, e.g., oxidizing roasting, with the goal of purifying the ore or secondary raw material. Smelting means reactions that are aiming at extracting a metal from an ore or secondary raw material. Smelting uses heat and a chemical reducing agent to decompose the ore/secondary raw material, driving off other elements as gases or slag and leaving the metal base behind. The reducing agent is commonly a source of carbon [41,44]. Pyrometallurgical processes have been used for a long time in history. Pyrometallurgy has different advantages such as high reaction rates, small plant size for a given throughput, and a high overall efficiency. On the downside, these processes often only produce intermediates that require further hydrometallurgical refining, require extensive off-gas treatment, and are uneconomic for low-grade concentrates [41].
In contrast, hydrometallurgy is based on aqueous chemistry, typically at low temperatures. Hydrometallurgical processes include three major process steps. The first one is leaching, which describes the dissolution of the metals, in most cases with the help of an acid, base, or salt. The second step is purification, which separates the metals via selective chemical reactions. This includes solid–liquid reactions, e.g., ion exchange, precipitation, and liquid–liquid reactions, e.g., solvent extraction. In the last step, the metals of interest need to be recovered from solution as a solid product, i.e., a metal, a metal salt, or a compound, by crystallization, ionic precipitation, reduction with gas, electrochemical reduction, or electrolytic reduction. Hydrometallurgical unit operations often occur as refining steps at the end of a process chain because of their ability to produce high-quality products. Their ability to produce high-quality products is opposed by comparatively large plant sizes for a given throughput, the need for waste water treatment, and a costlier residue management in case of sludges in comparison to slags [41,45,46].
3.2. Pyrometallurgy with Subsequent Hydrometallurgical Treatment
The first industrially implemented process routes for LIB recycling combine pyrometallurgical unit operations with hydrometallurgical unit operations. The processes can be divided into the co-processing of LIBs in existing primary or secondary Co, Cu, and Ni smelters and into dedicated plants specifically designed for LIB recycling. In the following, examples for the different process routes are given.
3.2.1. Co-Processing in Primary and Secondary Co-, Cu-, Ni-Smelters
Due to the high fluorine, Al, Li, and organic content, LIBs are a difficult feed for conventional Co, Cu, and Ni smelters with respect to corrosion, slag properties, energy, and mass balance. Fluorine and Li can severely attack the refractories, and the first one is also an issue in the off-gas treatment. Al increases the viscosity of the slag and is therefore only acceptable to a certain extent, which depends on the applied slag system and operating temperature of the furnace. Furthermore, in addition to the organic and graphite content, its participation in redox reactions adds a high amount of energy to the system, which also needs to be considered. Nevertheless, some smelters accept a certain amount of LIBs as feed material. Examples are Nickelhütte Aue GmbH (Aue, Germany) and Glencore Sudbury INO (Greater Sudbury, ON, Canada) [47,48]. The first smelter is a comparatively small smelter with an annual smelting capacity of 20,000 t for secondary raw materials such as Co-, Cu-, and Ni-bearing spent catalysts, electroplating sludges, filter dusts, and ashes [49]. The second one is capable of producing 95,000 t of Ni, Cu, and Co in matte annually, primarily from sulfidic ore concentrates. In this case, the batteries are calcined before smelting presumably in order to reduce the organic, graphite, and fluorine content and to avoid explosions of battery cells within the smelter. Afterwards, the calcined batteries are fed into an electric furnace together with calcine from the primary concentrates. Before granulation, the Fe content is reduced to about 2% by converting [48].
Both plants concentrate Co, Cu, and Ni in a matte, which is a typical intermediate in these processes. The Al, Li, and Mn content of LIBs is mainly transferred to the slag phase. As a result of the low LIB fraction in the feed, Li is highly diluted in the slag and therefore difficult to recover. The organic content and graphite are utilized as fuel and reductants. Halogens are captured in the off-gas treatment [48,49]. The matte is further refined by hydrometallurgy.
For the matte treatment, three main routes exist, which are based on oxygen–sulfuric acid, chlorine, and ammonia–air leaching [50]. The first two options are presented exemplary in the following, since they are currently applied by Nickelhütte Aue GmbH (oxygen–sulfuric acid leaching [49]) and Glencore Nikkelverk AS (Kristiansand, Norway) (chlorine leaching [50]).
Matte Processing at Nickelhütte Aue GmbH (Aue, Germany)
Nickelhütte Aue GmbH operates a comparatively small hydrometallurgical plant and produces approximately 3900 t Ni per year and smaller amounts of Co and Cu [49]. A simplified flowsheet is given in Figure 4.
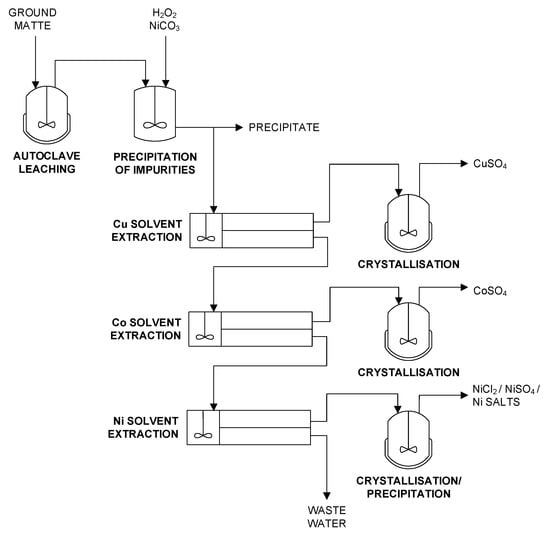
Figure 4.
Simplified flowsheet of Nickelhütte Aue GmbH based on [49].
Matte processing starts with comminution followed by pressure oxidation leaching at 6 to 8 bar. Afterwards, impurities are removed prior to solvent extraction. For example, Fe is precipitated as goethite (FeOOH) by using H2O2 as an oxidizing agent and basic nickel carbonate for pH adjustment. Leaching and precipitation residues are recirculated into the smelter. Co, Cu, and Ni are separated and purified by several solvent extraction circuits. Depending on the process configuration, cobalt sulfate, nickel sulfate, nickel carbonate, nickel chloride, and copper sulfate are produced in electroplating grade or similar [47,49].
Matte processing at Glencore Nikkelverk AS (Kristiansand, Norway)
The Nikkelverk refinery [50,51] produces Cu, Co, and Ni electrodes, H2SO4 as well as by-products such as platinum group metals (PGMs). It treats matte from the Sudbury smelter (Canada) and other sources and has an annual capacity of 4700 t Co, 39,000 t Cu, 92,000 t Ni, and 115,000 t H2SO4. Table 4 gives the general composition of the matte. A simplified flow sheet of the complex process is shown in Figure 5.

Table 4.
Composition of mattes entering Glencore Nikkelverk AS [50].
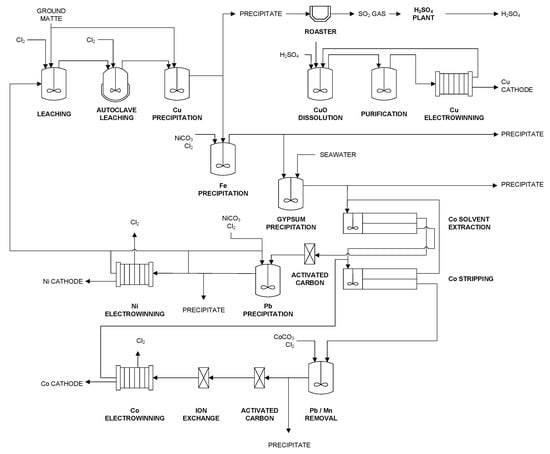
Figure 5.
Simplified flowsheet of Glencore Nikkelverk AS refinery adapted from [50,51].
In the Nikkelverk process, the ground matte is pulped with solution from Ni electrowinning and fed to a multistage leaching operation. In the first stages, chlorine gas is injected. At this stage, the principal reactions are the dissolution of Ni3S2 and Cu2S to yield Ni and Cu in solution and elemental sulfur in the residue. The reactions are exothermic and maintained at the boiling point. Afterwards, the slurry is transferred to autoclave leaching, which is operated at 150 °C. These conditions favor the following metathesis reaction
to separate Cu from Ni and Co. A further reduction of the Cu concentration to less than 0.5 g/L is achieved by precipitation using fresh matte as precipitant. The occurring chemical reactions are similar to Equation (1).
Subsequently, the slurry is washed and filtered. The residue, which contains about 50% Cu, 5% Ni, 40% S, 1% Co, and 1% Fe is transferred to the Cu roaster. The solution, which contains about 220 g/L Ni, 11 g/L Co, 7 g/L Fe, and 0.5 g/L Cu, is pumped to Fe removal.
Fe is precipitated from the solution as Fe(OH)3 by using chlorine to oxidize Fe2+ to Fe3+ and NiCO3 to raise the pH. After filtration, cooling, and gypsum removal, Co is extracted from the solution by solvent extraction. Before Co is recovered by electrowinning from the strip solution, the solution is purified by ion exchange and activated carbon to remove Zn and trace amounts of Cu as well as entrained organics.
The extraction raffinate, which contains 220 g/L Ni, 0.25 g/L lead (Pb), and 0.2 g/L Mn, is pumped through carbon columns to remove entrained organics from solvent extraction and is further purified by the precipitation of undesired metals such as Pb and Mn with NiCO3 and chlorine gas. The purified solution is diluted to 65 g/L and fed to Ni electrowinning. The solution from electrowinning contains about 54 g/L Ni and is recycled.
The residue from Cu precipitation is roasted in a fluidized bed reactor to produce a calcine of CuO and SO2 gas, which is sent to a double-adsorption H2SO4 plant after cleaning. The calcine is mixed with solution from Cu electrowinning and fresh acid to yield Cu into solution. After purification of the leach solution, Cu is recovered by electrowinning. A bleed is withdrawn from this stream and returned to chlorine leaching to remove Ni from the Cu circuit. PGMs are recovered from the residue of Cu leaching. Further details are given in [50,51].
3.2.2. Dedicated Processes
In contrast to co-processing, dedicated pyrometallurgical processes for LIB recycling are specifically developed for the treatment of LIBs. The processes enable the enrichment of Li in the slag, and the furnaces are designed to handle the highly corrosive feed material. Furthermore, the off-gas treatment is dimensioned to capture the high amounts of halogenic and organic compounds. Nevertheless, also in dedicated processes, LIBs are often co-processed with other feed materials in order to meet a suitable energy and mass balance as well as to enable sufficient plant utilization in this emerging market.
One of the most prominent examples is Umicore SA (Belgium), which operates a semi-industrial furnace at Hoboken (Belgium) with a capacity of 7000 t/year [52]. Umicore announced an increase of its recycling capacity by a factor of approximately 10 by the mid-2020s [53]. During the last 15 years, Umicore has published several patents regarding LIB recycling. Although little is known about the exact current direction, the history of patents indicates various changes regarding process design as well as furnace technology. In principal, all process options produce one or two alloys containing Co, Cu, and Ni, a Li-bearing slag, and a fly ash phase. Regarding process design, two principal flow sheets were published in the patents, which are shown in Figure 6.
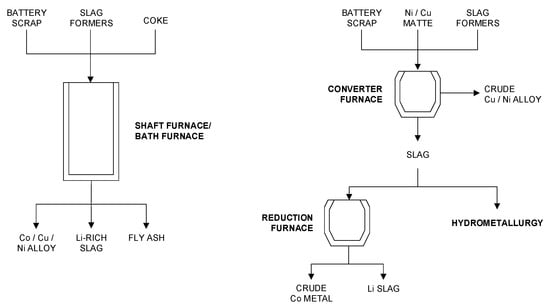
Figure 6.
Simplified flowsheets of the two different processes published by Umicore SA based on [54,55,56,57].
According to the patent history, the first approach was to produce a Co/Cu/Ni alloy and a Li-bearing slag using a single-furnace process (Figure 6, left). The first patents, e.g., [54,55], describe the use of a shaft furnace, in which Co/Ni-bearing batteries (LIB and NiMH batteries) and other materials are fed together with slag formers, mainly CaO and SiO2, and coke as a reductant. A shaft furnace was chosen in order to take advantage of the temperature gradient over the packed bed. The temperature of the batteries is slowly increased by the rising counter current gas generated in the smelting and reducing zone. Therefore, the electrolyte is slowly evaporated in the upper part of the bed (pre-heating zone), which lowers the risk of battery explosions. The furnace is operated at a comparatively high bath temperature of about 1450 °C, which is necessary to keep the viscosity of the Al-rich slag system sufficiently low.
According to [56], the drawbacks of the process are a very high coke consumption, which is necessary to carry out the reduction and to keep the packed bed sufficiently porous. Furthermore, the occurrence of size segregation in the shaft leads to an increased pressure drop over the packed bed, and large quantities of fines are carried over with gases, resulting in problems at the bag house. In order to solve these problems, [56] suggests the use of a bath smelting process instead of a shaft furnace. Due to the rapid heating of the battery cells in the molten bath, the batteries are susceptible to violent explosions, which can damage the lining. Therefore, and as protection against chemical attack, the bath smelter is equipped with freeze lining at the bath level. The operating temperature is again about 1450 °C, and SiO2 and CaO are added to the feed in order to flux the Al.
The latest patent family indicates a change in direction (Figure 6, right). Instead of a fully dedicated process for Co/Ni-containing batteries, [57] suggests the processing of Co-bearing batteries and their scraps in a converter furnace together with Cu or Cu/Ni matte in order to produce a crude Cu/Ni alloy and a Co- and Li-bearing slag. Co can be recovered from the slag either by hydrometallurgy or by deep reduction, producing a crude Co metal phase and a Li-bearing slag.
The advantages of this approach are the ability to treat variable amounts of battery scrap in large-scale installations, an increased flexibility to meet a suitable energy and mass balance, as well as the pyrometallurgical separation of Co from Ni, which probably allows the hydrometallurgical refining of Co in existing plants designed for the refining of cobalt hydroxide produced from the African Cu/Co belt.
Processing of the Alloy
The further hydrometallurgical processing of the Co-, Cu-, and Ni-containing alloy is conducted in plants similar to those described in Section 3.2.1. Umicore produces a variety of Co and Ni products, including battery grade chemicals and cathode materials for LIBs [52,58].
Processing of the Slag
The Li content of the slags is comparable to the Li content of spodumene concentrates, which are beside Li-containing brines the major commercial source for Li [59]. In addition, the chemical composition shows similarities; see Table 5. Besides the listed oxides, minor amounts of Fe and Mn oxides occur. Therefore, an economic extraction might be feasible in comparison to slags with lower Li concentrations from co-processing plants.

Table 5.
Composition of Li-rich slag and spodumene concentrate [59,60,61,62,63].
The extraction of Li from different slag compositions was investigated in the research project Lithium-Ion Battery Initiative (LiBRi) [64] as well as by [58]. A general flow sheet of the developed process is given in Figure 7.
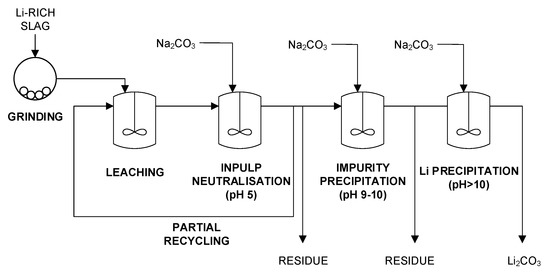
Figure 7.
Simplified flowsheet for the production of Li2CO3 adapted from [58].
In a first step, the slag is milled to obtain a micrometer-sized powder. Then, this powder is leached in H2SO4 or HCl at 80 °C. Good Li yields are obtained with both media at a free acid concentration of approximately 10 g/L. In order to ensure good filtration, the leach solution is neutralized to obtain a solution at pH 5. Al, the main impurity in solution, is removed under these conditions. Several neutralization agents can be used. In a sulfate medium, CaO has the advantage that CaSO4 is formed, which reduces the salt load of the solution. In a chloride medium, NaOH is preferred over CaO because Ca is more difficult to separate from Li. Na2CO3 can be used as a neutralization agent in both media but could decrease the Li yield of the process if it is already used in this stage of the flowsheet. After the removal of Al by filtration, it is advantageous to increase the Li concentration in solution by using the filtrate again in the next leach operation a few times until the Li concentration is high enough for the next steps. When the desired Li concentration is reached, the leach solution can be essentially purified of other metals by precipitation between pH 9 and pH 10 with Na2CO3. This is an efficient way to remove Ca and Mg by forming their carbonate salts. Ca and Mg carbonate salts are less soluble than Li2CO3. Finally, pure Li2CO3 is precipitated at high temperature (between 80 and 100 °C) and pH > 10. The solubility of Li2CO3 decreases at higher temperature and higher Na2CO3 content. Moreover, the Li yield in this step increases at higher initial Li concentration. Therefore, the yield can be improved if water is removed from the solution prior to precipitation, e.g., by evaporation [58,64].
Investigations by [64] showed that silica management is of paramount importance. Under certain conditions, the dissolution of silica leads to gel formation, resulting in severe filtration problems and low yields. Overall Li yields under favorable conditions were reported to be between 60% and 70%.
Another critical issue is residue management. Due to the low Li content of the slags, the amount of residue from the leaching and precipitation of impurities is high. The disposal of such residues is restricted in Europe and is associated with high costs.
A possible solution to this problem is the application of the slags as a neutralization agent instead of conventional Ca-based agents in spodumene processing as described in [65]. Furthermore, this bypasses the need to establish a specific process for comparatively low amounts of slag. Spodumene is a pyroxene mineral consisting of lithium aluminium inosilicate (LiAl(SiO3)2). A simplified spodumene processing flow sheet is given in Figure 8.
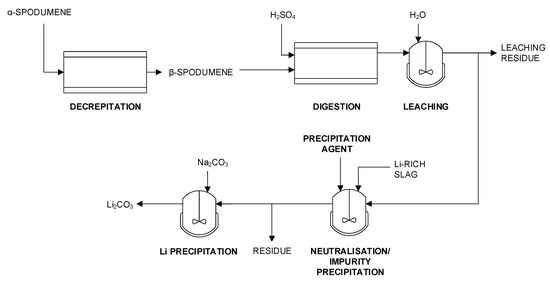
Figure 8.
Simplified flowsheet of spodumene processing with partial replacement of Ca-based neutralizing agent based on [59,65].
After spodumene ores have been mined, concentrated, and comminuted, the finely divided material is submitted to a first high-temperature treatment step during which α-spodumene is converted into β-spodumene. Following the phase transformation, the material is mixed with H2SO4 and submitted to a roasting step that aims to liberate the Li from the mineral. This step is performed at 250–300 °C with an excess of acid with respect to Li.
The roasted material is subsequently mixed with water, upon which the Li2SO4 dissolves, together with the free H2SO4. Next, a conventional neutralizing agent such as CaCO3, CaO, or Ca(OH)2 is added to neutralize the free acid and to precipitate a number of impurities.
Typically, the neutralization step is performed at a pH of 5 to 6 to remove impurities such as Al, Si, and Fe from the solution. A solid–liquid separation step is applied to separate the crude Li2SO4 solution from the residue that mostly contains aluminum silicates, gypsum, and precipitated impurities. Then, further purification steps are applied for the removal of Ca, Mg, and other impurities before Li precipitation as carbonate or hydroxide.
In the described process, the Li-bearing metallurgical slag is used to substitute part of the conventional neutralizing agent. In this neutralization step, most of the Li in the slag is released and supplements the Li liberated from the spodumene. To ensure the optimum release of the Li from the slag, it is preferred to neutralize with Li-bearing slag up to a pH of less than 4. Above, it is recommended to proceed with a conventional neutralization agent to reach a pH between 5 and 7. So far, no information has been published about the industrial implementation. According to information published in the patent, overall Li yields around 90% from the slag are achievable, which is comparable to Li yields from spodumene concentrates [59].
3.3. Mechanical Processing with Subsequent Metallurgical Treatment of Black Mass
Apart from the process chains presented in Section 3.2, LIBs can be processed using a combination of mechanical treatment with pyrometallurgical and/or hydrometallurgical treatment of a certain fraction, which is called black mass. Specialized hydrometallurgical treatment of the black mass can either lead to intermediate products that can be fed into the hydrometallurgical processes described in Section 3.2 or directly to high-grade products. In the following, examples for the different process routes are given.
3.3.1. Mechanical Treatment
In most cases, LIBs undergo a thermal treatment, e.g., pyrolysis, before mechanical treatment in order to reduce the energy content in a controlled way, to eliminate the organic components and to reduce the halogenic content [19,66,67,68]. This process usually takes place at around 500 °C and is limited by the melting point of Al (660 °C) [69,70]. Afterwards, the pyrolyzed batteries can be treated mechanically without fire hazards.
Mechanical treatment starts with comminution. Afterwards, the crushed material is sorted by its physical properties using unit operations such as sieving, sifting, magnetic, and eddy current separation. Common fractions are an Al/Cu foil fraction (conducting foils), coarse non-ferrous metals (Al from casings, Cu), coarse ferrous metals (casings, screws), and a fine fraction called black mass (active electrode materials) [10,22,71].
Alternatively, a few companies follow different approaches without thermal treatment in order to avoid the complex thermal pre-treatment, which requires comparatively high throughputs to be economic. In this case, specific safety measures are mandatory to prevent explosion and ignition during the mechanical treatment [72]. One possibility is crushing under inert atmosphere, e.g., N2, CO2, or Ar, and a subsequent removal/recovery of the volatile components, e.g., by vacuum distillation [73,74] or drying at moderate temperatures. However, possible drawbacks are high costs for volatile organic component (VOC) abatement, low black mass yields, and high levels of cross-contamination with black mass and organic components. The latter is mainly because of an insufficient detachment of the active materials from the foils resulting from the strong bonding, which cannot be destroyed by purely mechanical processes [70,75].
Another possibility is comminution in a solution, e.g., in a slightly alkaline medium [76,77]. These processes have to deal with organic wastewater pollution, corrosion, wet product fractions, and also low black mass yields.
With the exception of black mass, all product fractions can be fed into established industrial recycling processes. Black mass is a relatively new intermediate product on the market and the most valuable fraction of the mechanical processing due to the Co and Ni content. It contains mainly the active electrode materials, i.e., graphite and Li transition metal compounds containing Co, Ni, and Mn. Further components are the fluorine containing conducting salt or its degradation products and several impurities such as Cu, Al, and Fe. Concentrations ranges of major black mass components produced from layered oxide chemistries are given in Table 6.

Table 6.
Concentration ranges of major black mass components produced from layered oxides chemistries (see Section 2), adapted from [69,78,79]. LiPF6: lithium hexafluorophosphate, PVDF: polyvinylidene fluoride.
In case of LFP, Fe und P can be significantly higher than shown in Table 6. Without thermal treatment, the concentrations will be lower due to dilution by the organic content (solvent residues, binder and separator residue).
3.3.2. Metallurgical Processing of Black Mass
The black mass can either be fed into pyrometallurgical routes, described in the previous Section 3.2, or directly treated by hydrometallurgical methods. Both approaches are pursued industrially. Due to its unique composition and highly variable chemistry, the black mass does not fit into most available metallurgical processes. Furthermore, besides Co and Ni, the recycling of most other elements is under research and development, and an industrial implementation still pending.
Compared to batteries, the black mass fits better in most pyrometallurgical processes due to the significantly reduced Al and organic content and higher Co and Ni concentrations. Nevertheless, the fluorine and Li content lead to corrosion problems, and the efficient recovery of Li is still an issue.
In hydrometallurgy, a distinction can be made between two alternatives. The first one is the production of intermediates by leaching and precipitation with the aim of producing a Co/Ni and a separate Li product for further processing in existing refineries. The second one is the direct production of high-grade products by more complex processes. Compared to pyrometallurgy, the hydrometallurgical treatment of black mass can enable the recovery of more materials, i.e., Mn, graphite (and Li). However, it faces challenges regarding fluorine, Mn control, an efficient recovery of Li, and the production of a marketable graphite product. Additionally, the processes are sensitive to organics because of the resulting contamination of process water and possible interference with the organic phase of solvent extraction processes. Therefore, typically only black mass from processes, which include a thermal treatment, is used as feed material.
Production of Intermediates
Due to the limited amount of black mass in Europe, currently, no specialized processes to produce high-grade products from black mass are in operation. Instead, the production of intermediates in pilot plants or treatment in existing variable plants can be observed.
These processes are typically based on leaching and precipitation and focus on the separation of Co and Ni from Mn, as most Co and Ni refineries can tolerate only limited amounts of Mn [80]. If the black mass still contains graphite after roasting, graphite can be recovered after leaching [12]. In addition, the production of Mn and Li intermediates is possible but only carried out in a few cases due to economic reasons. Possible flowsheets based on common precipitation processes are presented in Figure 9.
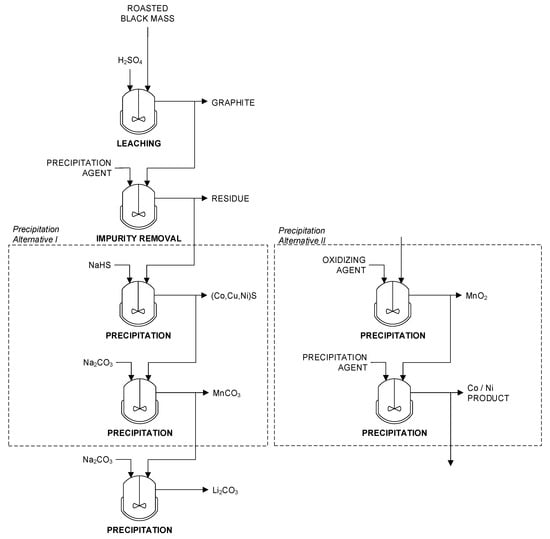
Figure 9.
Simplified flowsheet for the hydrometallurgical route for the production of intermediates based on [12,80].
The leaching of active electrode material can be done with various acids [12]. In most cases, H2SO4 at elevated temperature assisted by H2O2 is used. Due to the fluorine content of most black masses, the formation of hydrofluoric acid takes place. This issue is discussed in the next section. Impurities such as Al and Fe can be precipitated as hydroxides. The separation of Co and Ni from Mn is a common challenge in the primary production of non-ferrous metals. In case of high Mn content, sulfide precipitation of Co and Ni is often performed utilizing the high selectivity of the reaction. Afterwards, Mn can be precipitated for example as carbonate (Figure 9, left) [66,80].
Alternatively, a selective precipitation of Mn as MnO2 might be applied after leaching (Figure 9, right). Typically, the reaction requires a strong oxidizing agent such as ozone, Caro’s acid, or hypochlorite. However, Co losses in the Mn precipitate can be very high: up to 25% according to the literature [80]. According to [80], better results can be achieved using SO2/air mixtures. Co losses below 0.5% were reported. Co and Ni can then be precipitated, e.g., as carbonates. For the precipitation of Li, Li2CO3 is the preferred compound in case of intermediates [59].
Direct Production of High-Grade Products
Currently, the production of high-grade products from black mass and similar production waste only takes place in Asia due to the sufficient amount of available feed material, especially production wastes, as most battery producers are located in Asia [71,81]. Nevertheless, several European companies work on comparable processes, as European battery cell production is expected to increase significantly within the next years [82]. Most companies follow a similar process structure given by the chemistry of the elements and commercially available extractants for solvent extraction. A typical example with possible variants is shown in Figure 10.
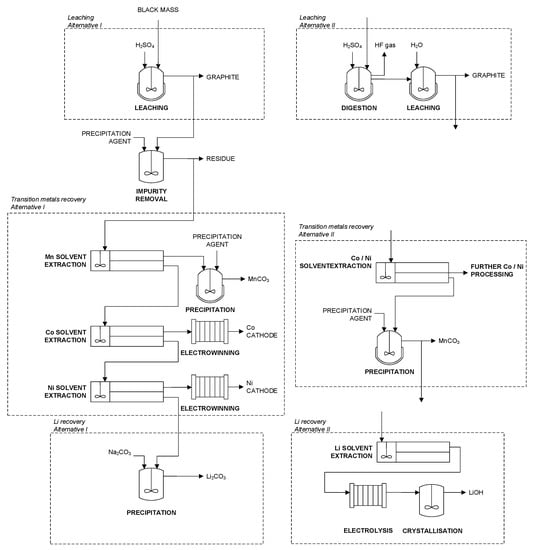
Figure 10.
Simplified flowsheet of the process at JX Nippon Mining & Metals Corporation (left) based on [66] and process alternatives for leaching, transition metal recovery, and Li recovery (right) based on [83,84].
In comparison to the production of intermediates, these processes typically include solvent extraction to separate and purify the metals of interest. Therefore, the necessary investment and process complexity are high, but the processes enable a much higher product quality and added value.
For the production of high-grade products, Figure 10 shows simplified flowsheets. The left side shows the proposed process of JX Nippon Mining & Metals Corp. (Tokyo, Japan). It consists of leaching, precipitation, solvent extraction, and electrowinning. The metals are leached in H2SO4. In the following, impurities such as Al and Fe are removed via precipitation. Then, the purified leach liquor is fed into solvent extraction circuits to recover each transition metal individually. First, Mn is extracted with di-(2-ethylhexyl)phosphoric acid (DEHPA) and precipitated as manganese carbonate. After that, Co extraction is performed with a dialkyl phosphinic acid and Ni extraction is performed with a carboxylic acid. Co and Ni are recovered by electrowinning as pure metals. At last, Li is precipitated as Li2CO3 [66].
One concern when processing black mass is the formation of hydrofluoric acid during leaching due to the significant amount of fluorine in the black mass; see Table 6. The hydrofluoric acid requires measures concerning HSE. Moreover, it can cause corrosion and act as a complexing agent for certain metals such as Al, which leads to different chemical behaviors of these elements. However, so far, little has been published about the exact behavior of fluorine in such processes and possible measures. [66] suggests addressing this problem by precipitation, presumably as calcium fluoride (CaF). However, according to [85], the precipitation of fluoride as CaF is pH dependent and only takes place to a certain extent below pH 7. Furthermore, the co-precipitation of gypsum can be expected, and another cation is added to the system, leading to a higher impurity profile. The German battery recycling company Duesenfeld GmbH developed and patented an alternative approach, in which the black mass is digested with concentrated H2SO4 at elevated temperatures. In this process step, the fluorine is removed as hydrogen fluoride via the gas phase, and the metals are converted to water-soluble sulfates. The gas phase is scrubbed to remove hydrogen fluoride, and the dry digestion product is subsequently leached with water [83]. The advantages of an early-stage fluorine removal are opposed by higher requirements regarding reactor design and materials of construction. Another option to handle the fluorine content might be selective washing of the black mass under mildly alkaline conditions. The removal of halogens by selective washing is industrial practice, e.g., for Waelz oxide and fly ashes [86,87].
Another concern in the hydrometallurgical processing of black mass is the handling of Mn. From a circular economy point of view, the recovery of Mn is desirable, but it is challenging from an economic point of view. In the left flowsheet, the solvent extraction of Mn is a necessary purification step prior to the extraction of Co and Ni for chemical reasons. However, the revenues of the Mn product do not cover the costs of the solvent extraction process, which is therefore subsidized by the Co and Ni revenues. An alternative is the use of a dialkyl dithiophosphinic acid such as bis(2,4,4-trimethylpentyl)dithiophosphinic acid as extractant [80,88]. This configuration enables a selective Co and Ni extraction in the presence of Mn. After the extraction, Mn can be recovered by precipitation. However, the extractant is difficult to handle, as it is highly sensitive to oxidation and metal poisoning [89]. Therefore, its industrial use is limited.
In Figure 10, Co and Ni are won from the strip solution by electrowinning. Alternatively, the direct crystallisation of metal salts is possible. However, the starting materials for high-purity metal salts are often metal cathodes, as the electrowinning process provides an additional refining step [90].
In the left flowsheet, Li is directly recovered by precipitation as a carbonate. Due to the low Li concentration and an unfavorable Na:Li ratio, which results from the use of caustic soda as a neutralizing agent in solvent extraction, low yields and product quality can be expected. A possible solution to this problem might be the extraction of Li, using the newly developed phosphorus-based extractant Cyanex 936P (Solvay SA, Brussels, Belgium), which shows a high selectivity for Li over other alkaline metals [91]. From the strip solution, the Li can be precipitated as carbonate or directly converted into a LiOH product using electrolysis and crystallization [84,92]. This technology has been developed to extract Li from brines, and its application in recycling has not been proven so far. Due to the discussed difficulties, an earlier extraction of Li from the black mass is of high interest and is addressed in various research and development projects. Examples are the selective carbonation of Li [93,94] and the application of ion-selective membranes [95,96].
So far, graphite is not recovered in industrial processes, although it is often present in the black mass and can principally be recovered after leaching. Currently, several companies and research groups are working on the material recycling of graphite. Prerequisites for a graphite recovery are appropriate temperatures/atmosphere during the thermal treatment to prevent the loss of graphite and a sufficient removal of impurities to meet the specifications of established graphite products [12].
4. Discussion
In the following, the processes presented in Section 3 are going to be critically discussed from a European perspective based on the categories legislation, recovery rate, robustness, economics, and HSE. Compared to Asia, the battery production capacity in Europe is still small, but it is characterized by high growth rates. Hence, currently, only low amounts of production scrap are available. Due to projected production capacities of up to 2000 GWh in 2029 worldwide, thereof 500 GWh in Europe, sharply increasing quantities of production scraps can be expected within the next years. As a result of the complex production process, 5% to 10% of the production capacity end up as production scrap [97,98]. Within the next years, production scrap will be the main feed for LIB recycling plants. The available amount of end-of-life batteries is also currently low and dominated by consumer batteries (8200 t in 2020 according to [99]). The return flow of traction batteries is slowly increasing due to their long lifespan and is estimated to reach about 50,000 t/a in 2025 in Europe [100].
Consequently, the recycling in Europe currently takes place in small plants or co-processing plants. Therefore, the costs for recycling are still high, but they are expected to decrease significantly when higher recycling capacities are installed.
4.1. Legislation
In Europe, the legislative framework for the LIB recycling is defined by the Batteries Directive (2006/66/EG) from 2006. Due to the limited application of LIBs in the early 2000s, the recycling of LIBs from xEVs is not specifically addressed. Therefore, the Batteries Directive is currently under revision. From a processing point of view, an increased mass specific recovery rate as well as the introduction of specific recovery rates for individual metals would have the biggest influence. The current required recovery rate of 50 wt % is easily achieved in case of battery systems due to a relatively high fraction of peripheral materials such as the Al casing; see Figure 2.
In general, pyrometallurgy with subsequent hydrometallurgy will be more affected by increasing requirements regarding mass specific recovery rates due to their focus on Co, Cu, and Ni and the loss of metallic Al, Mn, non-metallic components, and Li in some cases (see Section 4.2.1). In contrast, a mechanical treatment enables a higher material recycling rate. Therefore, new process combinations, e.g., the separation of Al and Fe casing prior to pyrometallurgy, might be necessary in the future to balance the individual advantages and disadvantages.
One further legislative framework, which might influence the recycling of LIBs, is the European Union Emission Trading Scheme. Currently, the influence is low but it is expected to grow with increasing CO2 prices. However, it is unclear which price level will be necessary for a significant steering effect, which processes will have a competitive advantage, and how the CO2 price will influence the competitiveness of the European LIB recycling industry.
4.2. Recovery Rate
As presented in Section 2, batteries contain various metallic and non-metallic components. Currently, due to economic and thermodynamic reasons, many processes focus on the high recovery rates of Co, Cu, and Ni. Li recovery is still a challenge due to thermodynamic reasons despite its economic value and political interest. There is less focus on Al and Mn, but the first one is partly recovered in mechanical processes. So far, non-metallic components such as graphite and the solvents of the electrolyte are not recovered with a few exceptions. Depending on future legislations, the recovery of non-metallic components might be of interest to comply with required mass specific recovery rates, especially in case of battery chemistries with low Co and Ni contents such as LFP.
4.2.1. Pyrometallurgical with Hydrometallurgical Processing
Pyrometallurgical processes achieve high yields for Co, Cu, and Ni (>95%) [55,101]. Li can only be recovered in dedicated processes, in which a concentration of Li in the slag is realized. Consequently, co-processing plants have major disadvantages if a specific Li recovery rate is required by law. Al and non-metallic components are utilized as reductant and fuel and therefore substitute primary energy sources. Mn mainly reports to the slag, which can be used as a construction material or dumped.
In hydrometallurgy, metal losses for Co, Cu, and Ni are very low (<5%), which results in high overall recovery rates for these process routes. For Li recovery from the slag, no industrial process data are available. The literature indicates possible recovery rates for Li from the slag of around 90% [65]. However, the overall Li recovery is presumably lower as Li is partially fumed in pyrometallurgy according to [56].
4.2.2. Mechanical with Metallurgical Processing
Mechanical processes with subsequent hydrometallurgy enable the recovery of more elements and materials. Typically, mechanical processes produce different ferrous and non-ferrous metal concentrates. A high black mass yield is of paramount importance for economic reasons and in order to minimize the cross-contamination of other product fractions with carcinogenic Co/Ni-containing dusts. According to [75], processes with thermal pre-treatment of LIBs prior to mechanical processing reach black mass yields of up to 95%, whereas the yield in processes without thermal pre-treatment is significantly lower.
Whereas Al and Cu concentrates can be fed into established recycling processes, the black mass is a new type of concentrate, which requires at least the adaptation of existing processes due to the unique combination of metals and the fluorine content.
If the black mass is fed into pyrometallurgy, treatment follows the above (see Section 4.2.1) described processes with its respective advantages and disadvantages. In contrast, black mass processing in hydrometallurgy offers the possibility to recover more materials, especially graphite, Mn, and Li. However, the recovery of graphite and Mn are not established industrially due to economic reasons. Li recovery takes place in some plants, especially in Asia [102]. Industrial recovery rates of Li are not known but presumably lower than those of Ni or Co.
4.3. Robustness
High process robustness is a prerequisite for the long-term economic success of large-scale installations. As a result of the constant development of LIBs, the processes must be able to deal with changes in LIB design, chemistry, and size. Furthermore, especially in the case of consumer batteries, recycling processes need to be robust toward missorting. Another aspect concerning the process robustness is the required pre-treatment, especially discharging, as some batteries cannot be discharged for safety reasons, e.g., damaged batteries, or economic reasons, e.g., consumer batteries.
4.3.1. Pyrometallurgical with Hydrometallurgical Processing
Pyrometallurgical processes are generally robust. They are usually able to treat all kinds of LIB scraps within certain limitations, coming from LIB production as well as end-of-life. Discharging is not mandatory and only pursued by some battery recyclers in case of battery systems to enable a safe dismantling. Exceptions are LIB chemistries with low Co and Ni content, as these processes are primarily designed for the recovery of Co, Ni, and Cu and battery modules, which exceed a certain size and weight limit, depending on the applied furnace technology. In this case, a mechanical pre-treatment is necessary in order to achieve a size reduction, which allows feeding without damaging the furnace. Most critical elements are safely removed via the gas phase—for example, halogens or volatile toxic heavy metals such as mercury (Hg) or cadmium (Cd) from missorted batteries. Other metals of low economic value and high affinity to oxygen such as Mn or Ti are transferred to the slag phase. Nevertheless, the halogenic and Li content are challenging with respect to corrosion. Due to the high energy content, the share of LIBs in the feed is limited. However, mixing different feed materials allows the production of a homogeneous alloy or matte phase for the subsequent hydrometallurgical processing. Due to the above-mentioned pyrometallurgical separation, changes in LIB chemistry are less likely to affect the hydrometallurgical treatment.
4.3.2. Mechanical with Metallurgical Processing
Mechanical processes combined with metallurgical processes are generally less robust. In these kinds of process routes, the higher recovery rates, especially of non-metallic materials, often lead to a high process sensitivity. Therefore, processes with a thermal treatment prior to mechanical treatment are predominant.
If current LIBs undergo thermal treatment prior to mechanical processing, sufficient yields and product qualities are achieved. Future LIB generations might require process adaptations. Without thermal treatment, the organic content leads to a more complex mechanical treatment with respect to the risks of fire and explosion. In the case of dry processing, discharging of the LIBs is mandatory. Furthermore, product yield and quality might suffer from organic contamination and insufficient detachment of the electrode coatings. In both cases, the missorting of batteries leads to the contamination of products, which is especially critical in case of Cd, Hg, and Pb.
The direct treatment of the black mass in hydrometallurgy is more challenging than the treatment of an alloy or a matte. The reasons are higher levels of contaminants and a less homogenous feed material. Organic contaminants are typically removed via thermal treatment as organic components can interfere with solvent extraction and require additional wastewater treatment.
In contrast to pyrometallurgical processing, mechanical processing achieves a lower separation of elements, leading to higher separation effort in hydrometallurgy. Especially, fluorine, Mn, and trace elements lead to additional process steps to achieve high product qualities. Heterogenic feed material makes process control more challenging, especially in solvent extraction and with respect to impurity control. Due to the changing chemistry of batteries and various doping elements (see Section 2), there is an elevated risk for the enrichment of contaminants in the solvent extraction circuits.
4.4. Ecomonics
Currently, in Europe, most LIBs have a negative market value due to new and complex processes, high research and development expenses, and small plants. In the upcoming years, the market value of LIBs is expected to increase in case of Co- and Ni-containing chemistries because of the growing number of competitors operating larger plants. LFP batteries will presumably continue to have a negative market value.
Possible revenues mainly result from Co, Ni, Cu, and Li while other materials are of minor importance; see Table 7.

Table 7.
Prices of materials based on an average 03/2019–02/2020 [103,104].
Figure 11 shows exemplarily possible revenues from one ton of black mass assuming a NMC 6:2:2 chemistry and 100% yield of each element based on the prices given in Table 7. For graphite, a low-quality product was assumed. It can be clearly seen that Co, Ni, and Li dominate the revenues under current market conditions. Other elements and components are only of minor importance or even generate costs, which is the case with fluorine and organics.
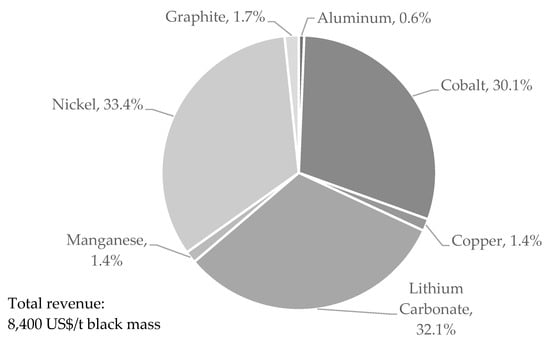
Figure 11.
Theoretical revenue distribution of black mass with NMC 622.
4.4.1. Pyrometallurgical with Hydrometallurgical Processing
Generally, large-scale pyrometallurgical processes are highly cost efficient to treat complex secondary raw materials. In case of LIBs, the available quantities are presently low. Therefore, co-processing has a larger market share than the treatment in dedicated plants. In the future, this is expected to change because dedicated plants are able to enrich Li in the slag in addition to Co, Ni, and Cu in the alloy/matte and are better adapted to the specific challenges. Nevertheless, the economic Ni and Co dependency of such processes is high. Under current market conditions, the use of carbon and organics as reductants and fuel as well as the separation of Mn from the other transition metals seems to have economic advantages.
In the subsequent hydrometallurgical treatment of the intermediates, Co, Ni, and Cu can be recovered in established plants. At present, Li recovery from the slag is not established industrially but it is expected to be introduced with increasing available amounts. The costs for Li recovery are unknown, but based on the described processes, it can be assumed that the costs will be in the range of spodumene processing or even lower. According to [105], the processing costs from spodumene concentrates are around 4500 US$/t lithium carbonate equivalent (LCE).
4.4.2. Mechanical with Metallurgical Processing
Compared to the process described above, a higher number of materials can be recovered in mechanical processes in principle. However, it is unclear whether this translates to economic advantages under future market conditions.
In most cases, a cost-intensive thermal removal of organics takes place before comminution. Compared to smelting, the metals are concentrated but not separated.
The main advantages of this approach are the (partial) recovery of Al and Fe, which leads to additional revenues. In addition, the separation of Cu from the transition metals in the black mass might have economic advantages. However, Mn reports to the black mass and is currently considered as an impurity in hydrometallurgy due to its low value (see Table 7) and expensive separation from Co and Ni. Solvent and graphite recovery have been demonstrated at the pilot scale, but so far, there are no established markets for these products, and the large-scale economic viability is unknown.
The black mass is still a comparatively new intermediate product, which requires dedicated hydrometallurgical treatment due to its complex composition and to recover all components. The installation of these plants requires high investments and sufficient feed material. Currently, such plants are only available in Asia due to the high amounts of production scraps in this region [102].
4.5. Health, Safety, and Environment
In most countries, scrap LIBs are classified as hazardous waste. The main reasons are the electrical and chemical energy content, the carcinogenic Co- and Ni-containing cathode active material, as well as the toxic, corrosive, and hazardous conducting salt [9]. All of these hazards need to be handled in LIB recycling.
Regarding life-cycle assessment, available studies indicate positive results of LIB recycling in comparison to the primary production in most impact categories [106]. However, most of the results are based on laboratory or pilot-scale process data and need verification at an industrial scale. Moreover, comparative studies would be of interest but are difficult to generate due to the data sensitivity of industrial processes, complex process routes, and co-processing with other primary or secondary materials.
4.5.1. Pyrometallurgical with Hydrometallurgical Processing
As pyrometallurgical processes are high-temperature processes, the high energy content of LIBs does not pose specific risks from an HSE point of view. Halogens and Co- and Ni-containing dusts are handled by an extensive off-gas treatment.
In the further treatment of the alloy/matte, dust formation needs to be addressed during comminution. In the design of hydrometallurgical plants, the high water hazard class of Co and Ni salts has to be considered.
4.5.2. Mechanical with Metallurgical Processing
In most cases, thermal deactivation is used prior to mechanical processing in order to remove the high energy and organic content of LIBs in a controlled way to avoid fire and explosion during further processing. If thermal deactivation is not applied, special measures during the mechanical processing of the LIBs are necessary, see Section 3.3, including the capture of VOCs.
During mechanical processing, dust control is of paramount importance to handle the carcinogenic dusts. In addition, cross-contamination of Ni- and Co-containing black mass in other fractions can be an issue.
Fluorine is concentrated in the black mass and therefore transferred to the metallurgical treatment, where it needs to be addressed. Otherwise, for the hydrometallurgical treatment, the same measures apply as described above (see Section 4.5.1).
5. Conclusions and Outlook
In the industrial recycling of LIBs, there are two main process routes pursued. The first plants are in operation with typical annual capacities of a few thousand tons. Within the next decade, recycling capacities are expected to increase significantly to meet the growing demand, which will probably lead to decreasing processing costs as already observed in Asia [102].
Although the principal process routes are already defined, there are still open questions regarding the next generation of plants.
Currently, battery modules are often directly fed into the pyrometallurgical routes. However, a mechanical conditioning might be beneficial to reduce the Al and Fe content of the feed material and to simplify the feeding process by a size reduction of the modules. Al and Fe can be recycled, and their reduction is beneficial to the energy and mass balance of most pyrometallurgical processes.
The optimal processing of the black mass is still subject to ongoing discussions. Both process routes have their challenges and opportunities with respect to fluorine control and the handling of graphite, Li, and Mn. The further development will be strongly influenced by economic and regulatory incentives.
Efficient Li recovery is still a challenge in all process routes, and further research and development is required. Besides Co and Ni, Li is one of the main value carriers, and a solution for efficient Li recovery could be a competitive advantage, especially in case of battery chemistries with low Co and/or Ni contents.
The recovery of non-metallic components is in an early stage, and the technical and economic feasibility is still uncertain. Challenges derive from inhomogeneous feed, product quality requirements, and currently low revenues.
A widely ignored problem is the recycling of LFP batteries. Although their market share is comparatively low in Europe, they are present on the market and consequently appear in recycling. The current industrial practice is to co-process them with layered oxide chemistries to a limited extend (<20%). However, if their share increases and/or with more stringent legislative requirements, the introduction of specific processes might become necessary despite their low value.
Due to the complex process chains, most companies do not cover all process steps. Instead, the formation of consortia is observed. Typically, pre-treatment, metallurgy, refining, and cathode material production are operated by different companies. At present, mainly activities of European companies are observed in Europe. However, Asian companies might enter the market as currently seen in LIB production. Due to the experience advantage of some companies, they have to be considered as serious competitors.
Especially in the metallurgical processing, co-processing is pursued at various stages to take advantage of large-scale installations and to homogenize the feed material. A further advantage of using established process routes is an easier entry of recycling material into the loop, as no additional certification of the products is necessary. However, co-processing in pyrometallurgy without Li-enrichment in the slag phase might lose in importance with increasing significance of Li recovery. Furthermore, more dedicated installations can be expected with the growing availability of LIB scrap.
In conclusion, within the last years, significant progress has been achieved in industrial LIB recycling in Europe. Nevertheless, the developments are very dynamic, and further progress in many areas can be expected in the upcoming years.
Author Contributions
The authors conceived and wrote the article together. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge support by Open Access Publishing Fund of Clausthal University of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Korthauer, R. Handbuch Lithium-Ionen-Batterien; Imprint: Springer Vieweg: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-30652-5. [Google Scholar]
- Petroff, A. Carmakers and Big Tech Struggle to Keep Batteries Free from Child Labor. Available online: https://money.cnn.com/2018/05/01/technology/cobalt-congo-child-labor-car-smartphone-batteries/index.html (accessed on 22 May 2020).
- Frankel, T.C. The Cobalt Pipeline: Tracing the path from deadly hand-dug mines in Congo to consumers’ phones and laptops. The Washington Post [Online]. 30 September 2016. Available online: https://www.washingtonpost.com/graphics/business/batteries/congo-cobalt-mining-for-lithium-ion-battery/ (accessed on 22 May 2020).
- Mau, K. Dreckige Rohstoffe für saubere Autos. Zeit Online [Online]. 11 December 2019. Available online: https://www.zeit.de/mobilitaet/2019-11/elektroautos-kobalt-lithium-batterie-akkus-rohstoffe-umweltschutz/komplettansicht (accessed on 22 May 2020).
- Draper, R. This metal is powering today’s technology—At what price?: As demand soars for powerful batteries, Bolivia dreams of striking it rich by tapping its huge lithium deposit. But will its people benefit? National Geographic Magazine [Online]. Available online: https://www.nationalgeographic.com/magazine/2019/02/lithium-is-fueling-technology-today-at-what-cost/ (accessed on 22 May 2020).
- CBS News. CBS News finds children mining cobalt for batteries in the Congo. CBC Interactive Corporation [Online]. 5 March 2018. Available online: https://www.cbsnews.com/news/cobalt-children-mining-democratic-republic-congo-cbs-news-investigation/ (accessed on 22 May 2020).
- Katwala, A. The spiralling environmental cost of our lithium battery addiction: As the world scrambles to replace fossil fuels with clean energy, the environmental impact of finding all the lithium required could become a major issue in its own right. Wired on Energy [Online]. 5 August 2018. Available online: https://www.wired.co.uk/article/lithium-batteries-environment-impact/ (accessed on 22 March 2020).
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process. Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Elwert, T.; Römer, F.; Schneider, K.; Hua, Q.; Buchert, M. Recycling of Batteries from Electric Vehicles. In Behaviour of Lithium-Ion Batteries in Electric Vehicles: Battery Health, Performance, Safety, and Cost; Pistoia, G., Liaw, B., Eds.; Springer: Cham, Switzerland, 2018; pp. 289–321. ISBN 978-3-319-69950-9. [Google Scholar]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials (Basel) 2020, 13, 801. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The Recycling of Spent Lithium-Ion Batteries: A Review of Current Processes and Technologies. Electrochem. Energ. Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Velázquez-Martínez; Valio; Santasalo-Aarnio; Reuter; Serna-Guerrero. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries. In Lithium Batteries; Julien, C., Mauger, A., Vijh, A., Zaghib, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–68. ISBN 978-3-319-19107-2. [Google Scholar]
- Lazuen, J. Batteries: The true drivers behind LFP demand – new safety standards, costs, IP rights, ESG & simplified battery pack designs. Roskill News [Online]. 25 June 2020. Available online: https://roskill.com/news/batteries-the-true-drivers-behind-lfp-demand-new-safety-standards-costs-ip-rights-esg-simplified-battery-pack-designs/ (accessed on 8 July 2020).
- Kwade, A.; Diekmann, J. Recycling of Lithium-Ion Batteries: The LithoRec Way; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319705712. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Omenya, F.; Chernova, N.A.; Zhou, H.; Siu, C.; Whittingham, M.S. Comparative Study Nickel Rich Layered Oxides: NMC 622, NMC 811 and NCA Cathode Materials for Lithium Ion Battery. Meet. Abstr. 2018, MA2018-01, 531. [Google Scholar]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- LG Chem Europe GmbH. Website 2019. Available online: https://www.lgchem.com/product/PD00000066 (accessed on 28 November 2019).
- Susai, F.A.; Sclar, H.; Shilina, Y.; Penki, T.R.; Raman, R.; Maddukuri, S.; Maiti, S.; Halalay, I.C.; Luski, S.; Markovsky, B.; et al. Horizons for Li-Ion Batteries Relevant to Electro-Mobility: High-Specific-Energy Cathodes and Chemically Active Separators. Adv. Mater. 2018, 30, e1801348. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; Lanceros-Méndez, S. Printed Batteries: Materials, Technologies and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-111-928-7889. [Google Scholar]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium titanate as anode material for lithium-ion cells: A review. Ionics 2014, 20, 601–620. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Porion, P.; Timperman, L.; Schmidt, G.; Lemordant, D.; Anouti, M. LiTDI as electrolyte salt for Li-ion batteries: Transport properties in EC/DMC. Electrochim. Acta 2015, 180, 778–787. [Google Scholar] [CrossRef]
- Arkema Group. PVDF Electrode Binders & Separator Coatings. 2019. Available online: https://www.extremematerials-arkema.com/en/markets-and-applications/renewable-energy/lithium-ion-battery/?_ga=2.35991904.1158937935.1575031365-829431959.1575030587 (accessed on 29 November 2019).
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on Challenges and Recent Advances in the Electrochemical Performance of High Capacity Li- and Mn-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—A critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Otero, M.; Heim, C.; Leiva, E.P.M.; Wagner, N.; Friedrich, A. Design-Considerations regarding Silicon/Graphite and Tin/Graphite Composite Electrodes for Lithium-Ion Batteries. Sci. Rep. 2018, 8, 15851. [Google Scholar] [CrossRef] [PubMed]
- Europäische Union. Richtlinie 2006/66/EG des Europäischen Parlaments und des Rates: L 266/1. 2006. Available online: http://gww.de/wp-content/uploads/Batterierichtlinie.pdf (accessed on 4 July 2018).
- European Commission. Batterie & Accumulators Waste Streams. Legislation. Available online: https://ec.europa.eu/environment/waste/batteries/legislation.htm (accessed on 23 April 2020).
- EUWID Europäischer Wirtschaftsdienst. EU Battery Directive to be revised in Q4 2020. Available online: https://www.euwid-recycling.com/news/policy/single/Artikel/eu-battery-directive-to-be-revised-in-q4-2020.html (accessed on 22 May 2020).
- Industry Standard Conditions for Comprehensive Utilization of Waste Power Batteries of New Energy veHicles (2019 Edition). Available online: http://www.miit.gov.cn/n1146295/n1652858/n1652930/n4509607/c7595282/content.html (accessed on 2 January 2020).
- Interim Measures for Management of Industry Standard Announcement for Comprehensive Utilization of Waste Power Batteries of New Energy Vehicles (2019 Edition). Available online: http://www.miit.gov.cn/n1146295/n1652858/n1652930/n4509607/c7595282/content.html (accessed on 2 January 2020).
- Hiskey, B. Metallurgy, Survey. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2000; ISBN 0471238961. [Google Scholar]
- Schubert, H. Aufbereitung Fester Mineralischer Rohstoffe; Dt. Verl. für Grundstoffindustrie: Leipzig, Germany, 1989; ISBN 3-342-00289-1. [Google Scholar]
- Schubert, H. Sortierprozesse: Dichtesortierung, Sortierung in Magnetfeldern, Sortierung in Elektrischen Feldern, Flotation, Klauben, Sortierung nach Mechanischen und nach Thermischen Eigenschaften; Dt. Verl. für Grundstoffindustrie: Stuttgart, Germany, 1996; ISBN 3342005556. [Google Scholar]
- Habashi, F. Principles of Extractive Metallurgy: Volume 1: General Principles; Gordon and Breach Science Publishers Inc.: New York, NY, USA, 1969; ISBN 978-0677017709. [Google Scholar]
- Habashi, F. A Textbook of Hydrometallurgy; Librairie des Presses de l’Université Laval: Québec, QC, Canada, 1993; ISBN 2980324701. [Google Scholar]
- Gupta, C.K.; Mukherjee, T.K. Hydrometallurgy in Extraction Processes, Volume I; Routledge: Boca Raton, FL, USA, 1990; ISBN 9780849368042. [Google Scholar]
- Nickelhuette Aue GmbH. Website. 2019. Available online: http://www.nickelhuette-aue.de/ (accessed on 20 August 2019).
- Glencore. Sudbury Integrated Nickel Operations Website. 2019. Available online: https://www.sudburyino.ca/en/Pages/home.aspx (accessed on 18 December 2019).
- Neumann, M.; Kuhnert, C. State-of-the-Art Recycling of Ni, Co, Cu and V. In Production and Recycling of Non-Ferrous Metals: Saving Resources for a Sustainable Future: European Metallurgical Conference EMC 2017: June 25-28, Leipzig, Germany: Proceedings of EMC 2017; GDMB Verlag GmbH: Clausthal-Zellerfeld, Germany, 2017; pp. 115–122. ISBN 978-3-940276-72-8. [Google Scholar]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Hydrometallurgical Production of High-Purity Nickel and Cobalt. In Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherlands, 2011; pp. 281–299. ISBN 9780080968094. [Google Scholar]
- Nikkelverk. Nikkelverk A Glencore Company Website. 2019. Available online: https://www.nikkelverk.no/en/about-us/production/Pages/flowchart.aspx (accessed on 12 December 2019).
- Umicore AG & Co. KG. Umicore Website. 2019. Available online: https://www.umicore.de/de/industries/automotive/umicore-battery-recycling/ (accessed on 24 May 2019).
- Manthey, N. Umicore to Ramp up Recycling of Electric Car Batteries. 2018. Available online: https://www.electrive.com/2018/08/07/umicore-to-ramp-up-recycling-of-electric-car-batteries/ (accessed on 7 August 2020).
- Cheret, D.; Santen, S. Battery Recycling. Patent US000007169206A2, 30 January 2007. [Google Scholar]
- Cheret, D.; Santén, S. Battery Recycling. Patent EP000001589121A1, 31 December 2018. [Google Scholar]
- Verscheure, K.; Campforts, M.; van Camp, M. Process for the Valorization of Metals from Li-Ion Batteries. U.S. Patent 8,840,702, 23 September 2014. [Google Scholar]
- Suetens, T.; van Hoerebeek, D. Process for Recycling Cobalt-Bearing Materials. Patent WO002011035915A1, 26 April 2018. [Google Scholar]
- Verhaeghe, F.; Goubin, F.; Yazicioglu, B.; Schurmans, M.; Thijs, B.; Haesebroek, G.; Tytgat, J.; van Camp, M. Valorisation of battery recycling slags. In Proceedings of the Second International Slag Valorisation Symposium; The Transitition To Sustainable Materials Management, Katholieke Universiteit Leuven, 18.-20.04.2011; Jones, P.T., Ed.; Umicore: Brussels, Belgium, 2011. [Google Scholar]
- Wietelmann, U.; Steinbild, M. Lithium and Lithium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Chichester, UK, 2010; ISBN 9783527306732. [Google Scholar]
- Treffer, F. Entwicklung eines Realisierbaren Recyclingkonzeptes für die Hochleistungsbatterien zukünftiger Elektrofahrzeuge-Lithium-Ionen Batterierecycling Initiative-LiBRi: Verbundprojekt; Gemeinsamer Abschlussbericht des Konsortiums; Berichtszeitraum: 01.09.2009 bis 30.09.2011; Technische Informationsbibliothek u. Universitätsbibliothek: Hanover, Germany; Umicore AG & Co.KG: Hanau, Germany, 2011. [Google Scholar]
- Quix, M.; van Horebeek, D.; Suetens, T. Lithium-rich Metallurgical Slag. Patent WO002017121663A1, 20 July 2017. [Google Scholar]
- Albermarle. Spodumene Concentrate, SC 6.8: Technical Data Sheet. Available online: https://www.albemarle.com/storage/components/T402211.PDF (accessed on 26 January 2017).
- Albermarle. Spodumene Concentrate, SC 7.2 premium: Technical Data Sheet. Available online: https://www.albemarle.com/storage/components/T402209.PDF (accessed on 26 January 2017).
- Elwert, T.; Goldmann, D.; Schirmer, T.; Strauß, K. Recycling von Li-Ionen-Traktionsbatterien: Das Projekt LiBRi. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; TK-Verl.: Neuruppin, Germany, 2012; pp. 679–690. ISBN 9783935317818. [Google Scholar]
- Oosterhof, H.; Dupont, D.; Drouard, W. Process for the Recovery of Lithium. Patent WO201818 4876A1, 2018.
- Haga, Y.; Saito, K.; Hatano, K. Waste Lithium-Ion Battery Recycling in JX Nippon Mining & Metals Corporation. In Materials Processing Fundamentals 2018; Lambotte, G., Lee, J., Allanore, A., Wagstaff, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 143–147. ISBN 978-3-319-72130-9. [Google Scholar]
- Nottez, E. RecLionBat Project: Lithium-ION Battery Recycling; Viviez. Available online: http://ec.europa.eu/environment/life/project/Projects/index.cfm?fuseaction=home.showFile&rep=file&fil=LIFE05_ENV_F_000080_LAYMAN.pdf (accessed on 25 October 2019).
- Arnberger, A. Recycling von Lithium-Ionen-Batterien. Available online: https://www.ffg.at/sites/default/files/allgemeine_downloads/thematische%20programme/Produktion/6_arnberger_recycling_von_lithium-ionen-batterien.pdf (accessed on 8 July 2020).
- Wang, H.; Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li--Ion Batteries. J. Sustain. Metall. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhang, T.; Xie, W.; Zhu, X. Enhancement in liberation of electrode materials derived from spent lithium-ion battery by pyrolysis. J. Clean. Prod. 2018, 199, 62–68. [Google Scholar] [CrossRef]
- Vezzini, A. Manufacturers, Materials and Recycling Technologies. Lithium-Ion Batteries; Elsevier: Amsterdam, The Netherlands, 2014; pp. 529–551. ISBN 9780444595133. [Google Scholar]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Hanisch, C. Recycling Method for Treating Used Batteries, in Particular Rechargeable Batteries, and Battery Processing Installation. Patent WO002018073101A1, 26 April 2018. [Google Scholar]
- Hanisch, C. Recycling Method for Treating Used Batteries, in Particular Rechargeable Batteries, and Battery Processing Installation. Patent EP000003312922A1, 25 April 2018. [Google Scholar]
- Sojka, R.T. Sichere Aufbereitung von Lithium-Ionen-basierten Batterien durch thermische Konditionierung. In Recycling und Sekundärrohstoffe, Band 13; Thomé-Kozmiensky, E., Holm, O., Friedrich, B., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Nietwerder, Germany, 2020; pp. 506–523. ISBN 978-3-944310-51-0. [Google Scholar]
- Retriev Technologies Inc. Retriev Website. Available online: https://www.retrievtech.com/ (accessed on 15 June 2020).
- PROMESA GmbH & Co. KG. PROMESA Website. Available online: http://promesa-tec.de/ (accessed on 15 June 2020).
- Vest, M.; Georgi-Maschler, T.; Friedrich, B.; Weyhe, R. Rückgewinnung von Wertmetallen aus Batterieschrott. Chemie Ingenieur Technik-CIT 2010, 82, 1985–1990. [Google Scholar] [CrossRef]
- Kwade, A.; Diekmann, J.; Hanisch, C.; Spengler, T.; Thies, C.; Herrmann, C.; Dröder, K.; Cerdas, J.F.; Gerbers, R.; Scholl, S.; et al. Recycling von Lithium-Ionen-Batterien-LithoRec II: Abschlussberichte der Beteiligten Verbundpartner; Technische Universität Braunschweig: Braunschweig, Germany, 2016. [Google Scholar]
- Ferron, C.J. The Control of Mangenese in Acidic Leach Liquors, with Special Emphasis to Laterite Leach Liquors. 2002. Available online: https://pdfs.semanticscholar.org/2224/690b72a8adc61c531ab5e87ef2d4c6e7c795.pdf (accessed on 1 April 2020).
- Rapier, R. Why China Is Dominating Lithium-Ion Battery Production. 2019. Available online: https://www.forbes.com/sites/rrapier/2019/08/04/why-china-is-dominating-lithium-ion-battery-production/#6f1f4a893786 (accessed on 1 April 2020).
- Infinity Lithium Industry News. March News-European Li-ion Battery Supply Chain. Available online: https://www.linkedin.com/feed/update/urn:li:activity:6650314043599003649/ (accessed on 15 June 2020).
- Hanisch, C.; Elwert, T.; Brückner, L. Verfahren zum Verwerten von Lithium-Batterien. Patent WO002019149698A1, 29 January 2019. [Google Scholar]
- Lipp, J. Lithium Solvent Extraction (LiSX TM) Process Evaluation using Tenova Pulsed Columns (TPC). In Proceedings of the ISEC 2017-The 21st International Solvent Extraction Conference, Miyazaki City, Japan, 5–9 November 2017. [Google Scholar]
- Dietrich, G. Hartinger Handbuch Abwasser- und Recyclingtechnik; 3.; Vollständig Überarbeitete Auflage; Hanser: Munich, Germany, 2017; ISBN 9783446449015. [Google Scholar]
- Schwab, B.; Ruh, A.; Manthey, J.; Drosik, M. Zinc. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Chichester, UK, 2010; pp. 1–25. ISBN 9783527306732. [Google Scholar]
- Thomé-Kozmiensky, K.J.; Amsoneit, N.; Baerns, M.; Majunke, F. Waste, 6. Treatment. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Chichester, UK, 2010; p. 484. ISBN 9783527306732. [Google Scholar]
- Solvay AS. CYANEX® 301: Product Information. Available online: https://www.solvay.com/en/product/cyanex-301#product-documents (accessed on 2 April 2020).
- Peek, E.; Åkre, T.; Asselin, E. Technical and business considerations of cobalt hydrometallurgy. JOM 2009, 61, 43–53. [Google Scholar] [CrossRef]
- Lascelles, K.; Morgan, L.G.; Nicholls, D.; Beyersmann, D.; Institute, N. Nickel Compounds. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1–17. ISBN 9783527306732. [Google Scholar]
- Solvay SA. Solvay Website: CYANEX® 936P Product Information. Available online: https://www.solvay.com/en/product/cyanex-936p (accessed on 15 June 2020).
- Tenova S.p.A. Tenova Website: Lithium Processing. Available online: https://www.tenova.com/product/lithium-processing/ (accessed on 15 June 2020).
- Bertau, M.; Martin, G. Integrated Direct Carbonation Process for Lithium Recovery from Primary and Secondary Resources. MSF 2019, 959, 69–73. [Google Scholar] [CrossRef]
- Sabarny, P.; Peters, L.; Sommerfeld, M.; Stallmeister, C.; Schier, C.; Friedrich, B. Early-Stage Lithium Recovery (ESLR) for Enhancing Efficiency in Battery Recycling. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.H.; Moon, S.J.; Jung, J.T.; Wang, H.H.; Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E.; Lee, Y.M. Lithium recovery from artificial brine using energy-efficient membrane distillation and nanofiltration. J. Membr. Sci. 2020, 598, 117683. [Google Scholar] [CrossRef]
- Zenn, R. About 500 GWh/a Li-Ion Battery Cell Production Capacity Announced in Europe. Available online: https://www.linkedin.com/posts/roland-zenn_gigafactories-battery-europe-activity-6673867930679218176-Hebm (accessed on 30 June 2020).
- Green Car Congress Website. Roskill Forecasts Li-Ion Battery Demand to Increase more than Ten-Fold by 2029 to >1,800GWh. Available online: https://www.greencarcongress.com/2020/06/20200609-roskill.html (accessed on 30 June 2020).
- Weyhe, R.; Yang, X. Investigations about Lithium-Ion Battery Market Evolution and Future Potential of Secondary Raw Material from Recycling. 2018. Available online: https://accurec.de/wp-content/uploads/2018/04/0-2Market-Research_YXF_3.0.pdf (accessed on 13 July 2020).
- Reimer, M.V.; Schenk-Mathes, H.Y. Systemdynamische Modellierung und Prognose der Rückflüsse von Lithium-Ionen-Batterien in der Circular Economy. In Forschungsfeldkolloquium 2020; Langefeld, O., Mrotzek-Blöß, A., Eds.; Papierflieger: San Francisco, CA, USA, 2020; pp. 45–55. ISBN 978-3-86948-767-0. [Google Scholar]
- Industry Expert. Lithium-Ion Battery Recycling in Asia. Oral Communication. 2019.
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Deutsche Rohstoffagentur (DERA) in der Bundesanstalt für Geowissenschaften und Rohstoffe. DERA Preismonitor. February 2020. Available online: https://www.bgr.bund.de/DE/Themen/Min_rohstoffe/Produkte/Preisliste/pm_20_02.pdf?__blob=publicationFile (accessed on 24 April 2020).
- Reuter, B.; Hendrich, A.; Hengstler, J.; Kupferschmid, S.; Schwenk, M. Rohstoffe für innovative Fahrzeugtechnologien-Herausforderungen und Lösungsansätze. 2019. Available online: https://www.e-mobilbw.de/fileadmin/media/e-mobilbw/Publikationen/Studien/Material-Studie_e-mobilBW.pdf (accessed on 24 April 2020).
- Buchert, M.; Sutter, J. Aktualisierte Ökobilanzen zum Recyclingverfahren EcoBatRec für Lihtium-Ionen-Batterien (Stand 09/206). 2016. Available online: https://www.erneuerbar-mobil.de/sites/default/files/2017-01/EcoBatRec-LCA-Update%202016.pdf (accessed on 30 June 2020).
- Buchert, M.; Sutter, J. Aktualisierte Ökobilanzen zum Recyclingverfahren LithoRec II für Lithium-Ionen-Batterien (Stand 09/2016). 2016. Available online: https://www.erneuerbar-mobil.de/sites/default/files/2017-01/LithoRec%20II-LCA-Update%202016.pdf (accessed on 30 June 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).