Abstract
With little success, researchers has been searching for alloys with elements such as tantalum to improve the long-term life of implants. The Ti–30Ta alloy presents an elastic modulus E = 69 GPa that is close to that of bone (E = 17–25 GPa) than Ti cp (E = 105 GPa). In addition, nanostructure surface modification influences cell behavior and antimicrobial activity. So, this study investigates the corrosion behavior of surface modification by TiO2 nanotube grown on Ti–30Ta alloy after anodization process in the electrolyte glycerol + NH4F 0.25% at 30 V, for nine hours without annealing and annealed in 450 °C, 530 °C and 600 °C (5 °C/min). The electrochemical behavior was evaluated by three electrodes cell. The counter-electrode of graphite, reference-electrode of saturated calomel electrode and working-electrode at electrolyte of 0.15 M NaCl + 0.03 M NaF, with pH = 6 for 8000 s. The scanned region ranged from −0.8 V to values up to 3.5 V with a sweep rate 0.166 mV/s. Potentiodynamic polarization curves were obtained with a potentiostat. The sample was characterized by scanning electron microscopy (SEM) imaging, X-ray diffraction analysis (XRD) and wettability with a contact angle goniometer. We concludes from the obtained results that all treatment surfaces are hydrophilic (<90°). The surface covered with TiO2 nanotube crystallinity showed anatase phase after annealing at 450 °C, 530 °C and 600 °C; the exceptions were the anodized-without-annealing treatment and without-surface-modification alloys. The electrochemical behavior of the five groups investigated showed similar high resistance to corrosion solution under all conditions.
1. Introduction
Among metallic biomaterials, titanium are the most popular as structural materials in the biomedical field due to their excellent mechanical and chemical properties, their good corrosion resistance and biocompatibility. The field of the application is wide, including hip joints, bone plates, screws, dental implants, stents and applications which are mainly used in implants that replace hard tissue [1,2,3,4].
However, researchers have been searching for alloys composed by elements such tantalum to improve the long-term life of the implant. Other improvements may apply in medical field by modifying Ti alloys such as weight reduction, corrosion resistance and good fracture resistance. The addiction of 30% of tantalum changes the elastics modulus to E = 69 GPa, being nearer to the bone (E = 17–25 GPa) than Ti commercially pure (E = 105 GPa) [5,6,7,8,9,10]. The elastic modulus is an important featuring for any hard implant. Once implanted in the body, different elastic modulus between metallic implant and the material adjacent bone leads to stress shielding phenomenon, causing lesions to the tissue. The studied alloy features E = 69 GPa. Being its elastic modulus nearer to the bone by comparing Ti cp. It is the main reason to development alloys with elastic modulus lower than 100 GPa [5,6,7,8,9,10].
Materials with an elastic modulus lower than 100 GPa are still presenting failures on long-term clinical application by bio-inert surface that cannot bind directly to living bone at the early stage after implantation into human body. Hence, in order to improve cellular response, the studies have modified the surface on nanoscale topography [11,12,13,14,15].
Our previous studies have investigated the nanostructure influence on cell behavior and antimicrobial activity on Ti–30Ta alloy. The biologic properties of Ti–30Ta alloy can be selectively improved by using simple anodization process associated with surface annealing process [7,9,16,17,18,19].
Corrosion stands for deterioration of the metals due to their reaction with surrounding corrosive elements. Corrosion damages can drive to metallic implant failure causing not just perjury in terms of economic, but also pain, productive work decrease and social effects [20,21,22,23,24,25]. Zhou investigated Ti–30Ta alloy surfaces and conclude that the alloy presents better corrosion behavior than Ti cp in the same test solution [6]. However, there is still a gap on corrosion research of the Ti–Ta alloys surface modification. It is essential the acknowledgment of how annealing temperature influences on corrosion behavior for biomedical application.
Hence, this study investigates the corrosion behavior of TiO2 nanotubes grown on Ti–30Ta alloy after anodization process in an electrolyte contained glycerol + NH4F 0.25% at 30 V, for 9 h without annealing and annealed in three different temperatures: 450 °C, 530 °C and 600 °C with a ramping rate of 5 °C/min.
2. Materials and Methods
2.1. Alloy Processing
The Ti–30Ta alloy was obtained from a titanium (Ti) and tantalum (Ta) pure metals in a high purity argon atmosphere. The ingot was homogenized in a vacuum at 1000 °C for 24 h. Then, the alloy was cold-worked by a rotary swaging process and solubilized at 950 °C for 2 h followed by water-cooling. The bars were cut into discs of 10 mm in diameter and 3 mm in thickness [10,26].
2.2. Nanotubes Growth
The nanotubes of TiO2 were obtained on the Ti substrate by anodization process in an electrolyte contained glycerol and NH4F 0.25% using a dual electrode system with platinum (counter electrode) and Ti–30Ta alloy (working electrode) fixed a 15 mm of distance. Then, applied 30 V at room temperature. The as-grown TiO2 nanotubes were then annealed in a furnace at different temperatures: 450 °C, 530 °C and 600 °C with a ramping rate of 5 °C/min, for 9 h [18,27].
Hence, the samples were divided into 5 groups: 1—Ti–30Ta alloy without surface modification, 2—anodized without (w/o) annealing-nanotubes of TiO2 without annealing, 3—450 °C-nanotubes of TiO2 annealed at 450 °C, 4—530 °C-nanotubes of TiO2 annealed at 530 °C annealed and 5—600 °C-nanotubes of TiO2 annealed at 600 °C.
2.3. Electrochemical Behavior
The electrochemical behavior of five groups: 1—Ti–30Ta alloy without surface modification, 2—anodized without (w/o) annealing-nanotubes of TiO2 without annealing, 3–450 °C-nanotubes of TiO2 annealed at 450 °C, 4–530 °C-nanotubes of TiO2 annealed at 530 °C annealed and 5–600 °C-nanotubes of TiO2 annealed at 600 °C was evaluated through corrosion resistance tests. The characterization was performed by three electrodes cell. The counter-electrode of graphite, reference-electrode of saturated calomel electrode and worked-electrode, with 1 cm2 exposed, samples of the 4 conditions The electrochemical measures were performed by having as electrolyte a solution of 0.15-M NaCl + 0.03-M NaF, with pH = 6 for 8000 s. The scanned region from −0.8 V until values close 3.5 V with sweep rate 0.166 mV/s. In addition, the potentiodynamic polarization curves were obtained with a potentiostat EG&G PAR 283 (PerkinElmer Instruments Inc., Guaratinguetá, Brazil) [28,29].
2.4. Sample Characterization
The surface topography was characterized by scanning electron microscopy (SEM) imaging (JEOL JSM 6100, Carl Zeiss, Guaratinguetá, Brazil). All substrates were coated with 15 nm layer of gold prior to imaging. The crystallinity of the samples was investigated by X-ray diffraction analysis (XRD), using X’ Pert Philips PMD (Philips, São Carlos, Brazil) with Panalytical X’Celerator detector (using a CuKα radiation, X = 1.5418 A, Philips, São Carlos, Brazil). The wettability of the substrate surfaces was investigated using sessile drop method (2 mL) with a contact angle goniometer (Kruss DSA 10, Kruss, Guaratinguetá, Brazil), equipped with video capture. The resulting images at the water–substrate interface were fit by the circle fitting profile.
3. Results and Discussion
Mechanical properties and surface are important criteria to choosing biomaterial for metal implants. Ti–30Ta alloy shows elastic modulus nearer to bone (E = 69 GPa) when comparing to Ti Cp (E = 105 GPa). The not commercial Ti–30Ta alloy smooth surface corrosion behavior was investigated and presented high corrosion resistance [6]. This word presents five condition named 5 Groups: 1—Ti–30Ta alloy without surface modification, 2—anodized w/o annealing-nanotubes of TiO2 without annealing, 3–450 °C-nanotubes of TiO2 annealed at 450 °C, 4–530 °C-nanotubes of TiO2 annealed at 530 °C annealed and 5–600 °C-nanotubes of TiO2 annealed at 600 °C. In addition, the chemical surface modification was performed to active an attractive surface to cell. The layers of nanotubes are studied in surface modifications because of their chemical stability and structural properties that mimic the human bone. The morphologies of SEM micrographs of Ti–30Ta alloy surface after anodization process in 0.25% NH4F + glycerol at 30 V for 9 h and the annealing process was performed at (a) anodized w/o annealing, (b) 450 °C, (c) 530 °C and (d) 600 °C (5 °C/min) for 1 h are shown in Figure 1.
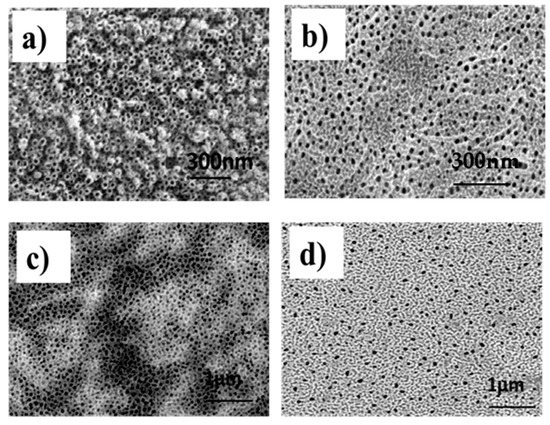
Figure 1.
SEM images of Ti–30Ta alloy surface after nanotubes of TiO2 growth in 0.25% NH4F + glycerol at 30 V for 9 h. (a) Group 2—anodized w/o annealing, (b) group 3—450 °C-nanotubes of TiO2 annealed at 450 °C, (c) group 4—530 °C-nanotubes of TiO2 annealed at 530 °C annealed and (d) group 5—600 °C-nanotubes of TiO2 annealed at 600 °C.
Figure 1a shows the anodized w/o annealing with nanotubes unorganized covered all surface of the alloy of Ti–30Ta after anodization process. The TiO2 layer presents nanotubes of average diameter about 80 to 100 nm. The effect the annealing temperature on the morphology of TiO2 nanotubes can be evaluated on Figure 1b–d. When the temperature was increased the diameter of the nanotube decreased. Likewise, the top of the nanotube seems to be covered of oxide at annealing temperature at 450 °C, 530 °C and 600 °C. The software ImageJ was used to determine the diameter h of the TiO2 nanotubes [30,31,32,33].
The crystallinity of the amorphous layer and nanotubular structure was investigated by X-ray diffraction. In Figure 2, the spectra obtained after anodizing in electrolyte glycerol +0.25% NH4F at 30 V for 9 h. The first spectrum show the Ti–30Ta alloy surface with titanium element. That anodized w/o annealing surface, and annealing at 450 °C, 530 °C and 600 °C showed anatase phase with the highest peaked around 25°. Tsurkan, 2020 found similar results when investigated the crystallinity of nanotubular topography on titanium [34,35]. The anatase phase is a desirable surface due to provides an improvement in the cellular response that is ideal for biomedical applications [20,34,36,37,38].
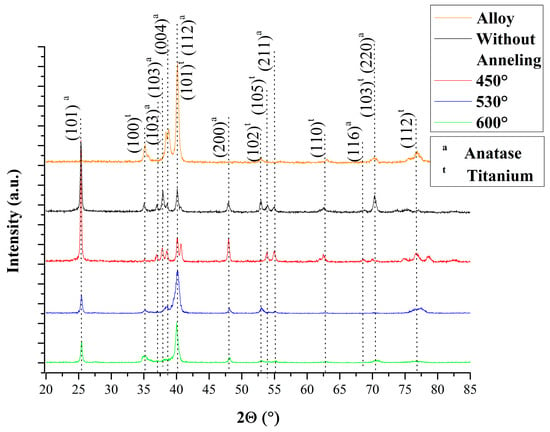
Figure 2.
XRD of Ti–30Ta alloy surface before and after nanotubes of TiO2 growth in 0.25% NH4F + glycerol at 30 V for 9 h. Group 1—Ti–30Ta alloy without surface modification, Group 2—anodized without annealing, Group 3—450 °C-nanotubes of TiO2 annealed at 450 °C, Group 4—530 °C-nanotubes of TiO2 annealed at 530 °C annealed and group 5—600 °C-nanotubes of TiO2 annealed at 600 °C.
The wettability of the surface is an important parameter to evaluating ideal biomaterial. Metallic biomaterial to biomedical application demands a hydrophilic surface due to cell behavior. The result shows all surfaces hydrophilic (<90°), as seen in Figure 3. The nanostructured topography favors the surface to interaction with cells. Previous studies have shown the antimicrobial effect on the TiO2 nanotubular surface [7,16,30,39].
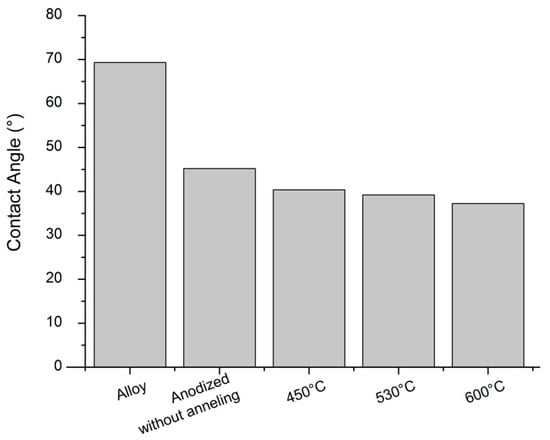
Figure 3.
Contact angle of Ti–30Ta alloy before and after nanotubes of TiO2 growth in 0.25% NH4 F + glycerol at 30 V for 9 h. Group 1—Ti–30Ta alloy without surface modification, Group 2—anodized without annealing, Group 3—450 °C-nanotubes of TiO2 annealed at 450 °C, Group 4—530 °C-nanotubes of TiO2 annealed at 530 °C annealed and group 5—600 °C-nanotubes of TiO2 annealed at 600 °C.
The electrochemical reactions at the interface of a material depend on the electrode potential. The reactions are verified in the applied potential and the current generated in the electrochemical reactions developed (anodic and cathodic). The scan potential and generated current present the electrochemical behavior of a biomaterial. It generates the polarization. Potentiodynamic polarization is the technique for obtaining polarization curves also provides continuous scanning of the potential. The corrosion potential is called open circuit potential which is established when the material is immersed in the solution [21,25,40,41].
Table 1 exhibit the impedance modulus, corrosion current density and corrosion potential of the Ti–30Ta alloy, amorphous layer, annealed surface at 450 °C, 530 °C and 600 °C. Table 1 shows the values of impedance modulus, corrosion current density and corrosion potential for the studied materials. The lowest value of corrosion potential is obtained for the Ti30 Ta alloy without surface modification. This indicates a higher reactivity of the alloy in the presence of the 0.15 M NaCl + 0.03 M NaF electrolyte. There is an increase in the corrosion potential for TiO2 nanotubes formed after anodizing and when it is subjected to annealing at 450 °C, 530 °C and 600 °C. This increase in the potential for corrosion is attributed to changes in the nature of the nanotube layer formed, as mentioned earlier in the X-ray diffraction analysis, which indicates the presence of the anatase phase, after heat treatment. The corrosion current density values obtained are very close and vary from 2.76 × 10−8 A/cm2 to 5.8 × 10−8 A/cm2. The small difference in this parameter does not allow ordering the different materials studied for corrosion resistance, but the extremely low values of current density indicate that they all have high resistance to corrosion. The lower impedance modulus values observed for annealed TiO2 nanotubes reveal that, in general, this treatment decreases corrosion resistance, which can be attributed to the change in properties such as porosity and conductivity of the TiO2 nanotube layer.

Table 1.
Impedance modulus, corrosion current density and corrosion potential of the Ti–30Ta alloy, amorphous layer, annealed surface at 450 °C, 530 °C and 600 °C.
Figure 4 shows the potentiodynamic polarization curves obtained from Ti–Ta alloy before and after anodization process in 0.25% NH4F + glycerol at 30 V for 9 h. Previous and post annealing process performed at, 450 °C, 530 °C and 600 °C (5 °C/min) for 1 h. The polarization measurement evaluates the stability and intensity for passive oxide film formation. All condition exhibited typical spontaneous passivation characteristics [15,42,43].
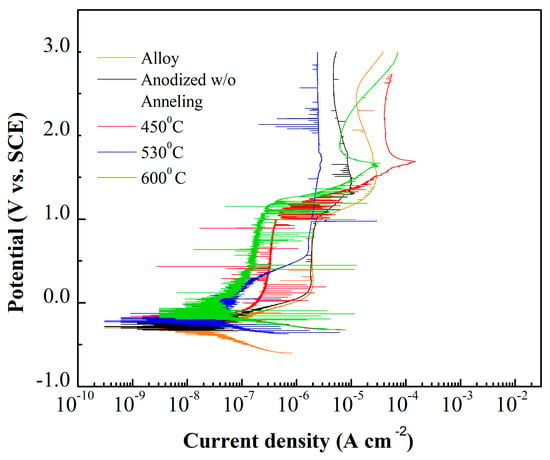
Figure 4.
Polarization curves in 0.15 M NaCl + 0.03 M NaF solution at 25 °C, and −0.8 V–3.5 V potential range and sweep rate 0.166 mV/s.
The open circuit potential (OCP) is the potential of working electrode relative to the reference electrode when no potential or current is being applied to the corrosion system. Therefore, OCP shows the tendency of metallic substrate to be subject in the electrochemical corrosion reactions. The electrolyte was 0.15 M NaCl + 0.03 M NaF, pH = 6, for 8000 s. OCP vs. time increases and reaches a stable value, consequently the investigated surface Ti–30Ta alloy, anodized without annealing, annealed at 450 °C, 530 °C and 600 °C had a noble behavior and it can resist to the electrolyte aggressive action, forming a protective film for the exposure time (Figure 5). The chemical surface analysis on the metallic substrates are useful to validate these conclusions that this material has great potential to biomedical application [23,30,43,44,45].
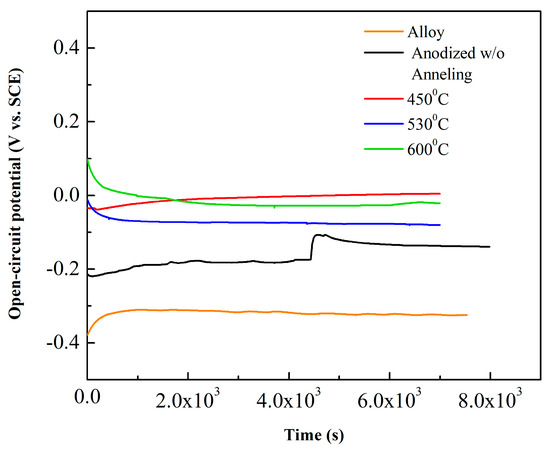
Figure 5.
Open circuit potential curves in 0.15 M NaCl + 0.03 M NaF solution at 25 °C.
Then, in a complimentary analysis electrochemical impedance spectroscopy (EIS) evaluated the effect of annealing temperature on the capacitive response of the oxide film. Figure 6 shows representative Bode plots from 4 samples. All four conditions exhibited capacitive behavior over a wide range of frequencies. In the high frequency range, the spectra showed a flat portion due to the response of the electrolyte resistance. In the lower frequency the spectra revealed a linear slope which is indicative of the capacitive characteristics of a passive film. The impedance response, described by the equivalent circuit (EC) model can be used to describe the capacitive behavior of Ti–30Ta alloys before and after anodization process and also on three different temperature of annealing; 450 °C, 530 °C and 600 °C [24,45,46].
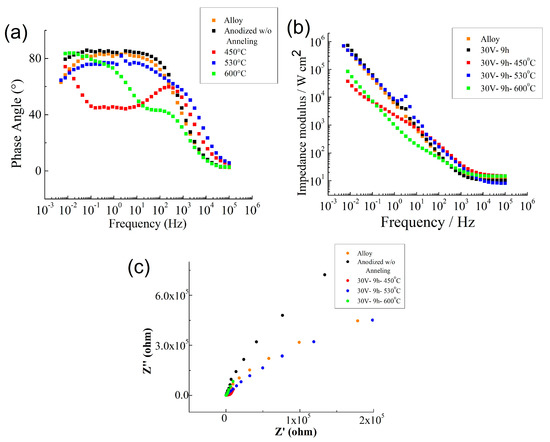
Figure 6.
Impedance modulus (a), impedance angle (b) and Nyquist plots (c) in 0.15 M NaCl + 0.03 M NaF solution at 25 °C.
4. Conclusions
Researchers have been searching for alloys with elements like tantalum to improve the long-term life of implant and nanostructure surface modifications to improve cell interactions in the implant and the human body.
Hence, in this study the corrosion behavior of TiO2 nanotube annealed at 450 °C, 530 °C and 600 °C for one hour was investigated.
The results obtained show TiO2 nanotube growth on the Ti–30Ta alloy and all surfaces are hydrophilic (<90°). Crystals covered the surface with TiO2 nanotubes showing anatase phase after annealed 450 °C, 530 °C and 600 °C in opposite of anodized without annealing and alloy without surface modification. The electrochemical behavior of the five groups investigated had good resistance to a corrosive solution of 0.15 M NaCl + 0.03 M NaF for 8000 s. In addition, all five groups presented the same corrosion features.
Author Contributions
Conceptualization, A.P.R.A.C.; methodology, A.P.R.A.C., P.C., R.Z.N.; software, P.C.; validation, P.C.; formal analysis, P.C.; investigation, P.C.; resources, C.A.d.C.Z., G.S., M.M.M., D.S.; data curation, P.C.; writing—original draft preparation, P.C.; writing—review and editing, F.B.V., P.C.; visualization, A.P.R.A.C.; supervision, A.P.R.A.C., C.A.d.C.Z.; project administration, A.P.R.A.C.; funding acquisition, G.R., C.A.d.C.Z., G.S., M.M.M., D.S., A.P.R.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazilian federal government and the National Council for Scientific, Technological Development (CNPq) and Capes. Grant number 201271/2010-9 and 486352/2013-7. Also, São Paulo Research Foundation (Fapesp). Grant number 2014/14533-3. Moreover, the APC was funded by Federal University of Itajubá (Unifei).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verma, R.P. Materials Today: Proceedings Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020, 2–5. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Wilson, J. Metallic biomaterials: State of the art and new challenges. Fundam. Biomater. Met. 2018, 1–33. [Google Scholar] [CrossRef]
- Pandey, A.; Awasthi, A.; Saxena, K.K. Metallic implants with properties and latest production techniques: A review. Adv. Mater. Process. Technol. 2020, 6, 405–440. [Google Scholar] [CrossRef]
- Domingues Goncalves, A.; Balestri, W.; Reinwald, Y. Biomedical Implants for Regenerative Therapies. In Biomaterials [Working Title]; IntechOpen: London, UK, 2020. [Google Scholar]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti-Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Capellato, P.; Claro, A.P.R.A.; Silva, G.; Zavaglia, C.A.C. Antimicrobial effect of TiO2 nanotubes coating for dental implant. Dent. Mater. 2018, 34, e21. [Google Scholar] [CrossRef]
- Kim, S.E.; Jeong, H.W.; Hyun, Y.T.; Lee, Y.T.; Jung, C.H.; Kim, S.K.; Song, J.S.; Lee, J.H. Elastic modulus and in vitro biocompatibility of Ti− xNb and Ti− xTa alloys. Met. Mater. Int. 2007, 13, 145–149. [Google Scholar] [CrossRef]
- Capellato, P.; Escada, A.L.A.; Popat, K.C.; Claro, A.P.R.A. Interaction between mesenchymal stem cells and Ti-30Ta alloy after surface treatment. J. Biomed. Mater. Res. Part A 2014, 102, 2147–2156. [Google Scholar] [CrossRef]
- Kulkarni, M.; Flašker, A.; Lokar, M.; Mrak-Poljšak, K.; Mazare, A.; Artenjak, A.; Čučnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar] [CrossRef]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef]
- Jedrzejowski, P.; Vetrone, F.; Zalzal, S.; Sarkissian, A.; Clair, S.; Richert, L.; Perepichka, D.F.; Wuest, J.D.; Yi, J.-H.; Variola, F.; et al. Improving Biocompatibility of Implantable Metals by Nanoscale Modification of Surfaces: An Overview of Strategies, Fabrication Methods, and Challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef]
- Fan, X.; Feng, B.; Liu, Z.; Tan, J.; Zhi, W.; Lu, X.; Wang, J.; Weng, J. Fabrication of TiO2 nanotubes on porous titanium scaffold and biocompatibility evaluation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2012, 100, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef]
- Capellato, P.; Sachs, D.; Claro, A.P.R.A.; Silva, G.; Zavaglia, C.A.C. Comparative research of bacteria gram-negative and positive on ti-30ta alloy. Dent. Mater. 2019, 35, e6–e7. [Google Scholar] [CrossRef]
- Capellato, P.; Smith, B.S.; Popat, K.C.; Alves Claro, A.P.R. Cellular Functionality on Nanotubes of Ti-30Ta Alloy; Materials Science Forum: Foz de Iguaçu, Brazil, 2015; Volume 805, ISBN 9783038352365. [Google Scholar]
- Capellato, P.; Smith, B.S.; Popat, K.C.; Claro, A.P.R.A. Fibroblast functionality on novel Ti30Ta nanotube array. Mater. Sci. Eng. C 2012, 32, 2060–2067. [Google Scholar] [CrossRef]
- Capellato, P.; Silva, G.; Popat, K.; Simon-Walker, R.; Alves Claro, A.P.; Zavaglia, C. Cell investigation of Adult Human dermal fibroblasts on PCL nanofibers/TiO2 nanotubes Ti-30Ta alloy for biomedical application. Artif. Organs 2020, 44, 877–882. [Google Scholar] [CrossRef]
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A review on the corrosion behaviour of nanocoatings on metallic substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Uwais, Z.A.; Hussein, M.A.; Samad, M.A.; Al-Aqeeli, N. Surface Modification of Metallic Biomaterials for Better Tribological Properties: A Review. Arab. J. Sci. Eng. 2017, 42, 4493–4512. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Trincă, L.C.; Mareci, D.; Solcan, C.; Fântânariu, M.; Burtan, L.; Vulpe, V.; Hriţcu, L.D.; Souto, R.M. In vitro corrosion resistance and in vivo osseointegration testing of new multifunctional beta-type quaternary TiMoZrTa alloys. Mater. Sci. Eng. C 2020, 108, 110485. [Google Scholar] [CrossRef]
- Mendis, S.; Xu, W.; Tang, H.P.; Jones, L.A.; Liang, D.; Thompson, R.; Choong, P.; Brandt, M.; Qian, M. Characteristics of oxide films on Ti-(10–75)Ta alloys and their corrosion performance in an aerated Hank’s balanced salt solution. Appl. Surf. Sci. 2020, 506, 145013. [Google Scholar] [CrossRef]
- Capellato, P.; Riedel, N.A.; Williams, J.D.; Machado, J.P.B.; Ketul Popat, K.C.; Alves Claro, A.P.R. Ion Bean Etching on ti-30ta Alloy for Biomedical Application; Materials Science Forum: Foz de Iguaçu, Brazil, 2015; Volume 805, ISBN 9783038352365. [Google Scholar]
- Eduardo Muzzio, N.; Azzaroni, O.; Moya, S.E.; Ángel Pasquale, M. Concepts for Designing Tailored Thin Film Surfaces with Potential Biological Applications. In Multilayer Thin Films—Versatile Applications for Materials Engineering; IntechOpen: London, UK, 2020. [Google Scholar]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.R.; Stevens, R. A microscopy study of the effect of heat treatment on the structure and properties of anodised TiO 2 nanotubes. Appl. Surf. Sci. 2010, 256, 2672–2679. [Google Scholar] [CrossRef]
- Regonini, D.; Jaroenworaluck, A.; Stevens, R.; Bowen, C.R. Effect of heat treatment on the properties and structure of TiO2 nanotubes: Phase composition and chemical composition. Surf. Interface Anal. 2010, 42, 139–144. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Mansourianfar, M.; Fathi, M.; Bonakdar, S.; Ebrahimi, M.; Zahrani, E.M.; Hojjati-Najafabadi, A.; Li, D. Surface modification of orthopedic implants by optimized fluorine-substituted hydroxyapatite coating: Enhancing corrosion behavior and cell function. Ceram. Int. 2020, 46, 2139–2146. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglic, A. Influence of anodization parameters on morphology of TiO2 nanostructured surfaces. Adv. Mater. Lett. 2016, 7, 23–28. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Decomposition of martensite α″ during aging treatments and resulting mechanical properties of Ti-Ta alloys. Mater. Sci. Eng. A 2004, 384, 92–101. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell–materials interaction. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 225–237. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Belikov, S.V.; Tsurkan, M.V.; Vyalykh, I.V.; Markaryan, A.Y.; Karabanalov, M.S.; Popov, A.A.; Wysokowski, M. Surface-Dependent Osteoblasts Response to TiO2 Nanotubes of Different Crystallinity. Nanomaterials 2020, 10, 320. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Bayram, C.; Demirbilek, M.; Yalçin, E.; Bozkurt, M.; Doǧan, M.; Denkbaş, E.B. Osteoblast response on co-modified titanium surfaces via anodization and electrospinning. Appl. Surf. Sci. 2014, 288, 143–148. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Ryan, S.; Popat, K.C. Biocompatibility and Mesenchymal Stem Cell Response to Poly(ɛ-Caprolactone) Nanowire Surfaces for Orthopedic Tissue Engineering. Tissue Eng. Part A 2009, 15, 2547–2559. [Google Scholar] [CrossRef]
- Devgan, S.; Sidhu, S.S. Evolution of surface modification trends in bone related biomaterials: A review. Mater. Chem. Phys. 2019, 233, 68–78. [Google Scholar] [CrossRef]
- LIU, X.; Chu, P.K.; DING, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4 V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Calderon Moreno, J.M.; Osiceanu, P.; Popa, M.; Vasilescu, E.; Marcu, M.; Drob, P. Long-term corrosion resistance of new Ti-Ta-Zr alloy in simulated physiological fluids by electrochemical and surface analysis methods. Corros. Sci. 2015, 93, 310–323. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. Anodic oxide nanotube layers on Ti–Ta alloys: Substrate composition, microstructure and self-organization on two-size scales. Corros. Sci. 2006, 12, 181–204. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Saji, V.S.; Choe, H.C. Electrochemical corrosion behaviour of nanotubular Ti–13Nb–13Zr alloy in Ringer’s solution. Corros. Sci. 2009, 51, 1658–1663. [Google Scholar] [CrossRef]
- Qadir, M.; Lin, J.; Biesiekierski, A.; Li, Y.; Wen, C. Effect of Anodized TiO2-Nb2O5-ZrO2 Nanotubes with Different Nanoscale Dimensions on the Biocompatibility of a Ti35Zr28Nb Alloy. ACS Appl. Mater. Interfaces 2020, 12, 6776–6787. [Google Scholar] [CrossRef] [PubMed]
- Chernozem, R.V.; Surmeneva, M.A.; Ignatov, V.P.; Peltek, O.O.; Goncharenko, A.A.; Muslimov, A.R.; Timin, A.S.; Tyurin, A.I.; Ivanov, Y.F.; Grandini, C.R.; et al. Comprehensive Characterization of Titania Nanotubes Fabricated on Ti-Nb Alloys: Surface Topography, Structure, Physicomechanical Behavior, and a Cell Culture Assay. ACS Biomater. Sci. Eng. 2020, 6, 1487–1499. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).