Applicability of the Electrochemical Oxygen Sensor for In-Situ Evaluation of MgO Solubility in the MgF2–LiF Molten Salt Electrolysis System
Abstract
1. Introduction
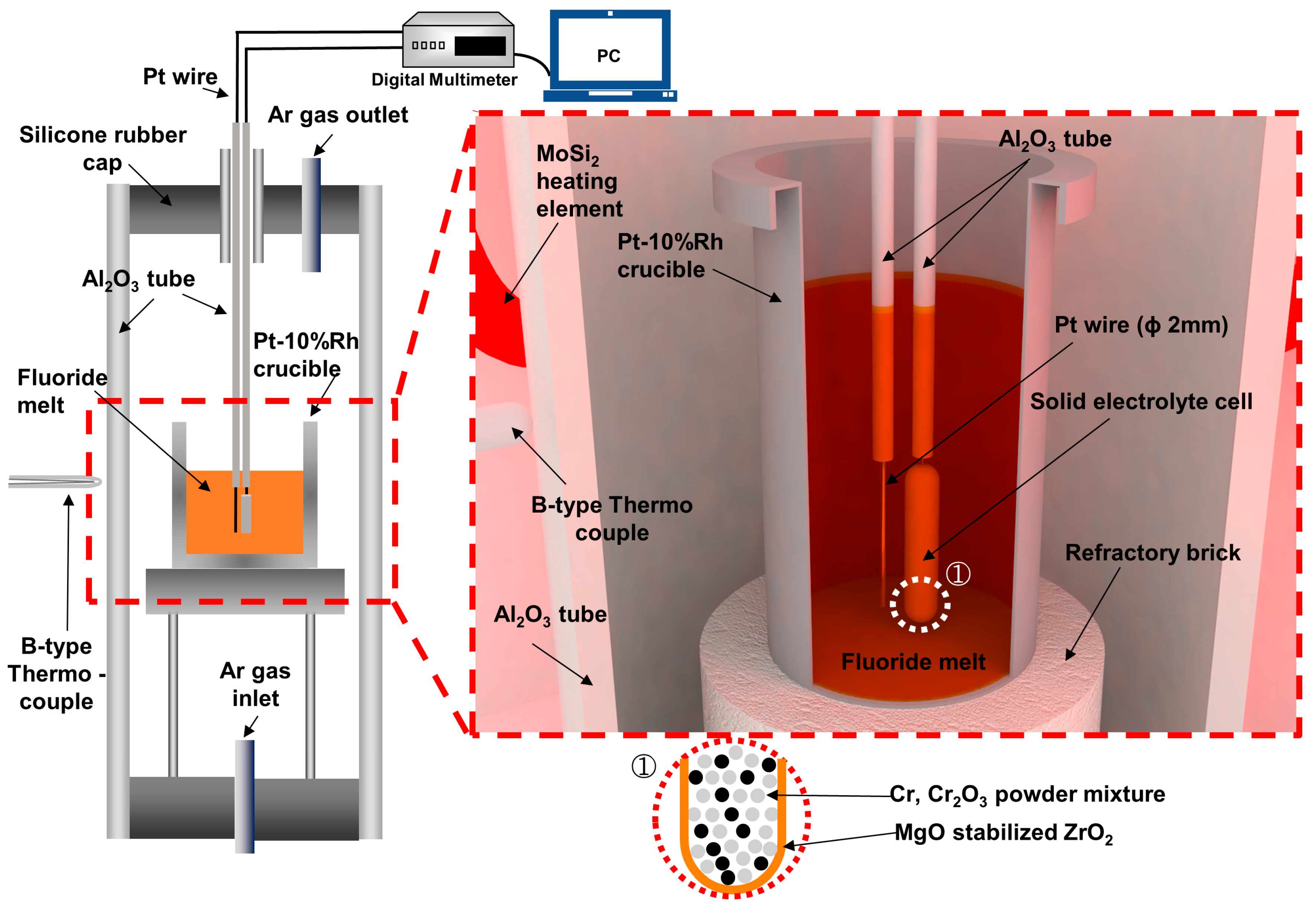
2. Experimental Procedures
2.1. Sample Preparation and MgO Solubility Analysis
2.2. Electromotive Force (EMF) Measurement
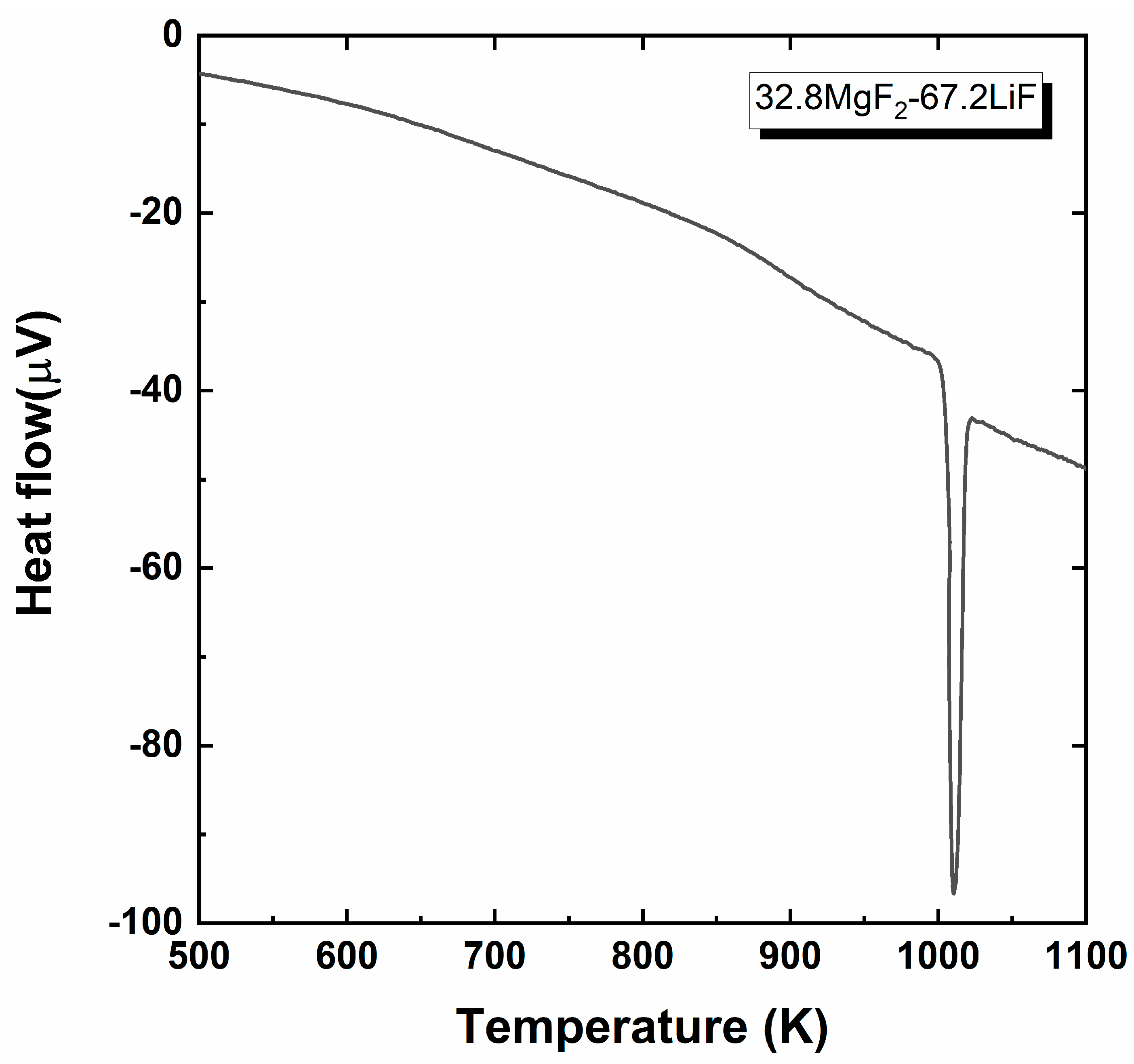
2.3. Thermal Property Analysis
3. Results and Discussion
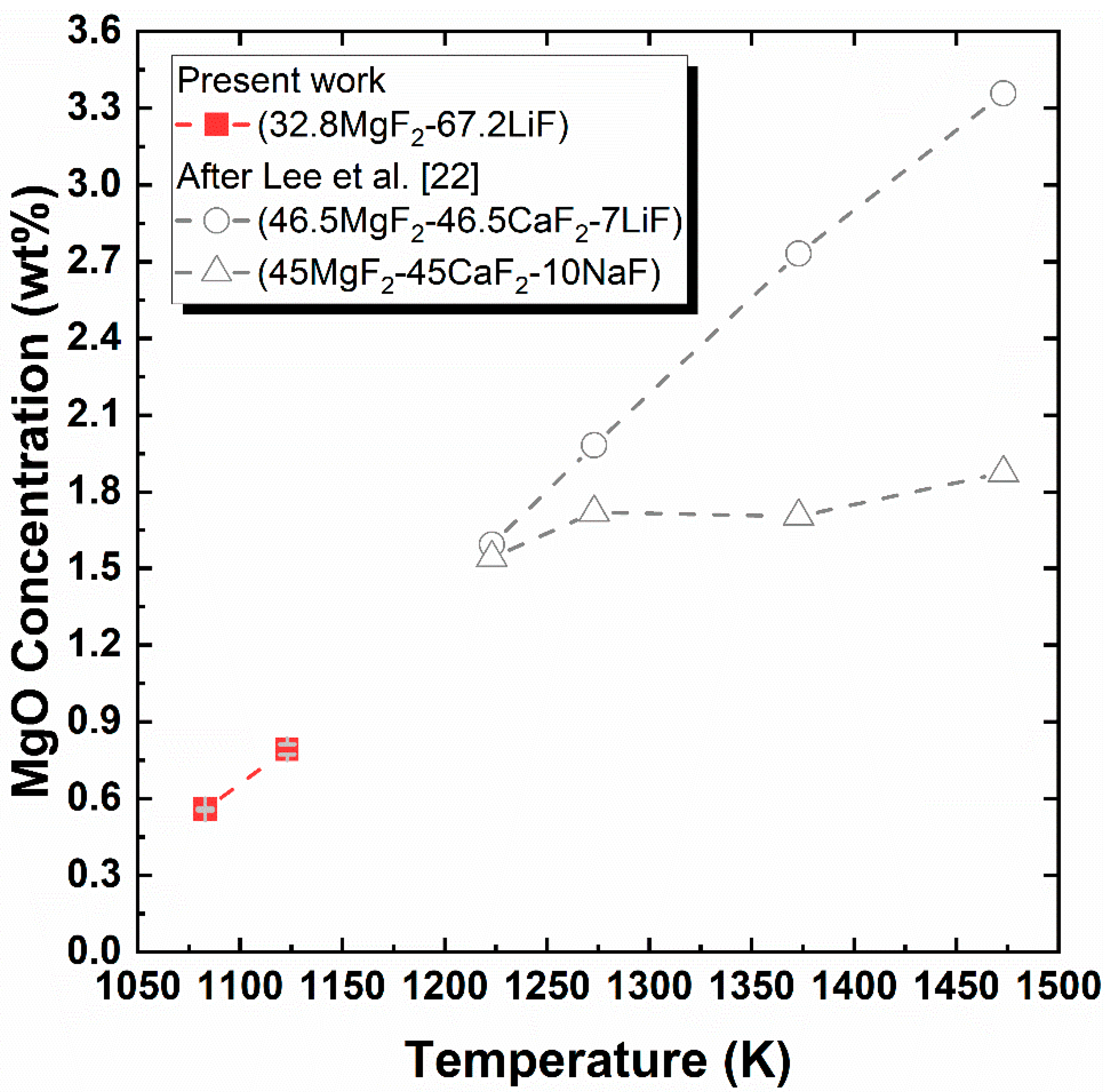
3.1. Determination of the Saturated MgO Concentration in Molten Fluoride
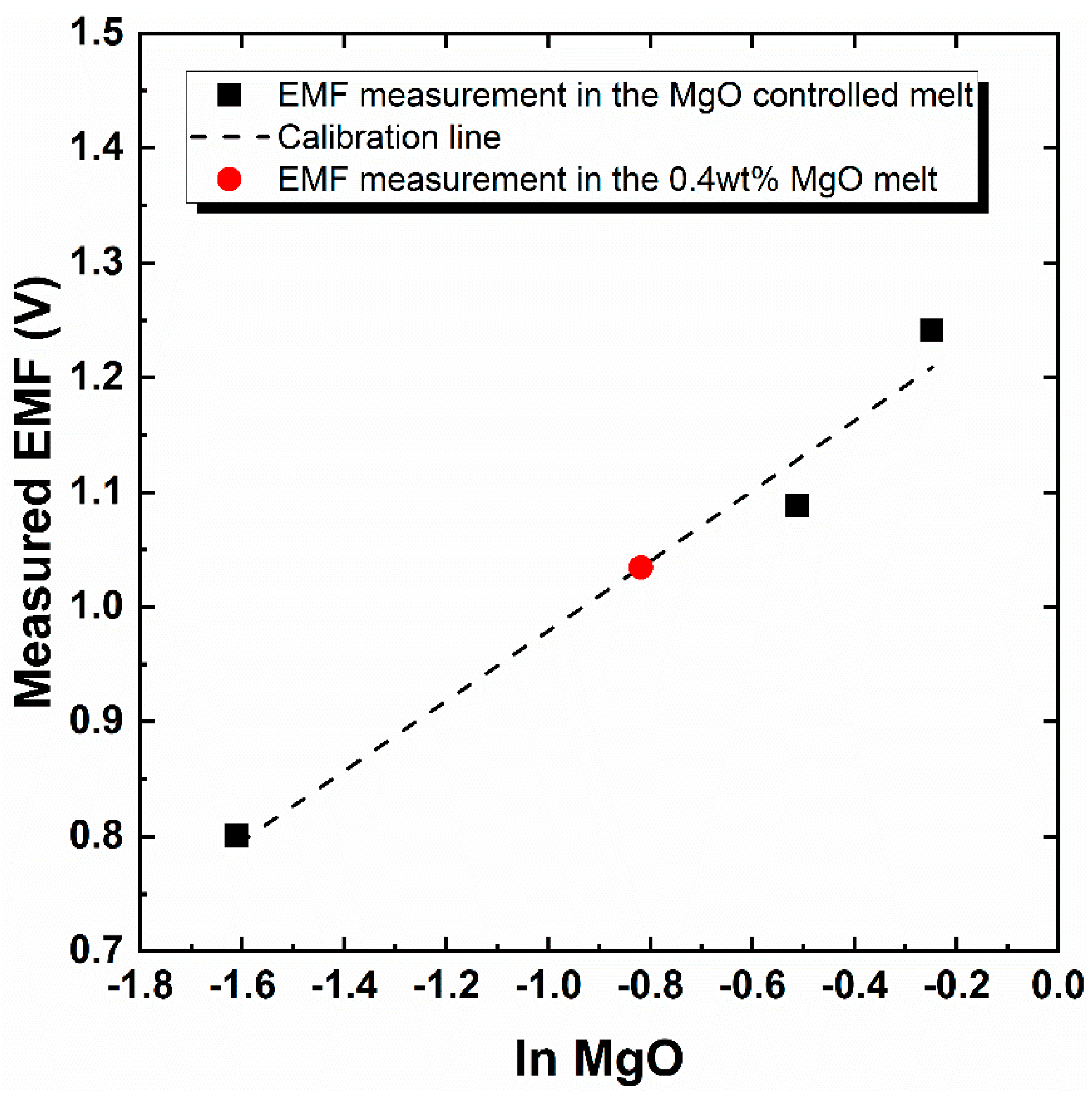
3.2. Application of the Electrochemical Oxygen Sensor for the Determination of MgO Solubility in the Molten Fluoride System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Cardarelli, F. Materials Handbook—A Concise Desktop Reference, 3rd ed.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-38923-3. [Google Scholar]
- Dieringa, H.; Kainer, K.U. Magnesium and Magnesium Alloys. In Springer Handbook of Materials Data; Warlimont, H., Martienssen, W., Eds.; Springer: Cham, Switzerland, 2018; pp. 151–159. [Google Scholar]
- Hsu, C.-H. Process Performance Analysis and Improvement for the Manufacture of 6063 Aluminum Alloy. Metals Basel 2020, 10, 605. [Google Scholar] [CrossRef]
- Santos, J.; Dahle, A.K.; Jarfors, A.E.W. Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg. Metals Basel 2020, 10, 614. [Google Scholar] [CrossRef]
- Ren, J.; Wang, X.; Niu, L. A New Kind of Crude Magnesium Extraction Process and Device. In Proceedings of the 2017 International Conference on Manufacturing Engineering and Intelligent Materials (ICMEIM 2017), Guangzhou, China, 25–26 February 2017; Atlantis Press: Guangzhou, China. [Google Scholar]
- Baek, U.H.; Lee, B.D.; Lee, K.W.; Han, G.S.; Han, J.W. Study of the thermal reduction behavior of dolomite by the pidgeon process. J. Korean Inst. Met. Mater. 2016, 54, 104–112. [Google Scholar] [CrossRef]
- Ad-Hoc Working Group on Defining Critical Raw Materials. Report on Critical Raw Materials for the EU; European Comission: Brussels, Belgium, 2014. [Google Scholar]
- Deloitte Sustainability; British Geological Survey; Bureau de Recherches Géologiques et Minières; Netherlands Organisation for Applied Scientific Research. Study on the Review of the List of Critical Raw Materials—Criticality Assessments; European Comission: Luxembourg, 2017. [Google Scholar]
- Zang, J.C.; Ding, W. The Pidgeon Process in China and Its Future. In Essential Readings in Magnesium Technology; Springer: Cham, Switzerland, 2016; pp. 113–116. [Google Scholar]
- Ramakrishnan, S.; Koltun, P. Global warming impact of the magnesium produced in China using the Pidgeon process. Resour. Conserv. Recycl. 2004, 42, 49–64. [Google Scholar] [CrossRef]
- Pal, U.B.; Powell, A.C. The use of solid-oxide-membrane technology for electrometallurgy. JOM 2007, 59, 44–49. [Google Scholar] [CrossRef]
- Guan, X.; Pal, U.B.; Powell, A.C. Energy-Efficient and Environmentally Friendly Solid Oxide Membrane Electrolysis Process for Magnesium Oxide Reduction: Experiment and Modeling. Metall. Mater. Trans. E 2014, 1, 132–144. [Google Scholar] [CrossRef]
- Suput, M.; Delucas, R.; Pati, S.; Ye, G.; Pal, U.; Powell, A.C., IV. Solid oxide membrane technology for environmentally sound production of titanium. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2008, 117, 118–122. [Google Scholar] [CrossRef]
- Krishnan, A.; Lu, X.G.; Pal, U.B. Solid Oxide Membrane (SOM) technology for environmentally sound production of tantalum metal and alloys from their oxide sources. Scand. J. Metall. 2005, 34, 293–301. [Google Scholar] [CrossRef]
- Su, S.; Pal, U.; Guan, X. Solid Oxide Membrane Electrolysis Process for Aluminum Production: Experiment and Modeling. J. Electrochem. Soc. 2017, 164, F248–F255. [Google Scholar] [CrossRef]
- Xu, J.P.; Lo, B.; Jiang, Y.; Pal, U.; Basu, S. Stability of yttria stabilized zirconia in molten oxy-fluorite flux for the production of silicon with the solid oxide membrane process. J. Eur. Ceram. Soc. 2014, 34, 3887–3896. [Google Scholar] [CrossRef]
- Guan, X.; Pal, U.B.; Powell, A.C. An environmentally friendly process involving refining and membrane-based electrolysis for magnesium recovery from partially oxidized scrap alloy. JOM 2013, 65, 1285–1292. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.; Lee, T. Metal refining method by using liquid metal cathode. Korea patent 10-2004920, 23 July 2019. [Google Scholar]
- Korenko, M.; Šimko, F.; Mlynáriková, J.; Larson, C.; Mikšíková, E.; Priščák, J.; Ambrová, M.; Palumbo, R. Physico—Chemical properties of (MgF2—CaF2—(LiF))eut—MgO system as a molten electrolyte for Mg electrowinning. J. Mol. Liq. 2019, 275, 535–543. [Google Scholar] [CrossRef]
- Lee, Y.; Yang, J.K.; Min, D.J.; Park, J.H. Mechanism of MgO dissolution in MgF2–CaF2–MF (M=Li or Na) melts: Kinetic analysis via in-situ high temperature confocal scanning laser microscopy (HT-CSLM). Ceram. Int. 2019, 45, 20251–20257. [Google Scholar] [CrossRef]
- Lee, Y.; Yang, J.K.; Park, J.H. Thermodynamics of fluoride-based molten fluxes for extraction of magnesium through the low temperature solid oxide membrane (LT-SOM) process. Calphad Comput. Coupling Phase Diagr Thermochem. 2018, 62, 232–237. [Google Scholar] [CrossRef]
- Liu, S.-H.; Fruehan, R.J.; Morales, A.; Ozturk, B. Measurement of FeO activity and solubility of MgO in smelting slags. Metall. Mater. Trans. B 2001, 32, 31–36. [Google Scholar] [CrossRef]
- Baucke, F.G.K. Electrochemistry of Glass-Forming Melts. In Electrochemistry of Glasses and Glass Melts, Including Glass Electrodes; Bach, H., Baucke, F.G.K., Krause, D., Eds.; Schott Series on Glass and Glass Ceramics; Springer: Berlin/Heidelberg, Germany, 2001; pp. 269–436. ISBN 978-3-642-08206-1. [Google Scholar]
- Seidl, A.; Marten, R.; Müller, G. Oxygen distribution in Czochralski silicon melts measured by an electrochemical oxygen sensor. Mater. Sci. Eng. B 1996, 36, 46–49. [Google Scholar] [CrossRef]
- Thoma, R.E. Phase diagrams of binary and ternary fluoride systems. In Advances in Molten Salt Chemistry; Braunstein, J., Mamantov, G., Smith, G.P., Eds.; Springer: Boston, MA, USA, 1975; Volume 3, p. 289. ISBN 978-1-4615-8272-4. [Google Scholar]
- Ito, M.; Morita, K. The Solubility of MgO in Molten MgCl2-CaCl2 Salt. Mater. Trans. 2004, 45, 2712–2718. [Google Scholar] [CrossRef]
- Kim, Y.; Min, D.J. Effect of FeO and Al2O3 on the MgO Solubility in CaO-SiO2-FeO-Al2O3-MgO Slag System at 1823 K. Steel Res. Int. 2012, 83, 852–860. [Google Scholar] [CrossRef]
- Cherginets, V.L. On studies of oxide solubilities in melts based on alkaline halides. Electrochim. Acta 1997, 42, 3619–3627. [Google Scholar] [CrossRef]
- Ito, Y.; Hayashi, H.; Itoh, Y.; Yoshizawa, S. The thermodynamic behavior of nickel in a molten LiF-KF-O2- system. Bull. Chem. Soc. Jpn. 1985, 58, 3172–3175. [Google Scholar] [CrossRef]
- Kwon, S.; Cho, S.H.; Nersisyan, H.H.; Lee, J.; Kang, J.; Lee, J.H. High-temperature stability of YSZ and MSZ ceramic materials in CaF2–MgF2–MgO molten salt system. J. Am. Ceram. Soc. 2018, 101, 2074–2083. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Yoo, J.; Kang, J. Applicability of the Electrochemical Oxygen Sensor for In-Situ Evaluation of MgO Solubility in the MgF2–LiF Molten Salt Electrolysis System. Metals 2020, 10, 906. https://doi.org/10.3390/met10070906
Kim Y, Yoo J, Kang J. Applicability of the Electrochemical Oxygen Sensor for In-Situ Evaluation of MgO Solubility in the MgF2–LiF Molten Salt Electrolysis System. Metals. 2020; 10(7):906. https://doi.org/10.3390/met10070906
Chicago/Turabian StyleKim, Youngjae, Junsoo Yoo, and Jungshin Kang. 2020. "Applicability of the Electrochemical Oxygen Sensor for In-Situ Evaluation of MgO Solubility in the MgF2–LiF Molten Salt Electrolysis System" Metals 10, no. 7: 906. https://doi.org/10.3390/met10070906
APA StyleKim, Y., Yoo, J., & Kang, J. (2020). Applicability of the Electrochemical Oxygen Sensor for In-Situ Evaluation of MgO Solubility in the MgF2–LiF Molten Salt Electrolysis System. Metals, 10(7), 906. https://doi.org/10.3390/met10070906




