Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
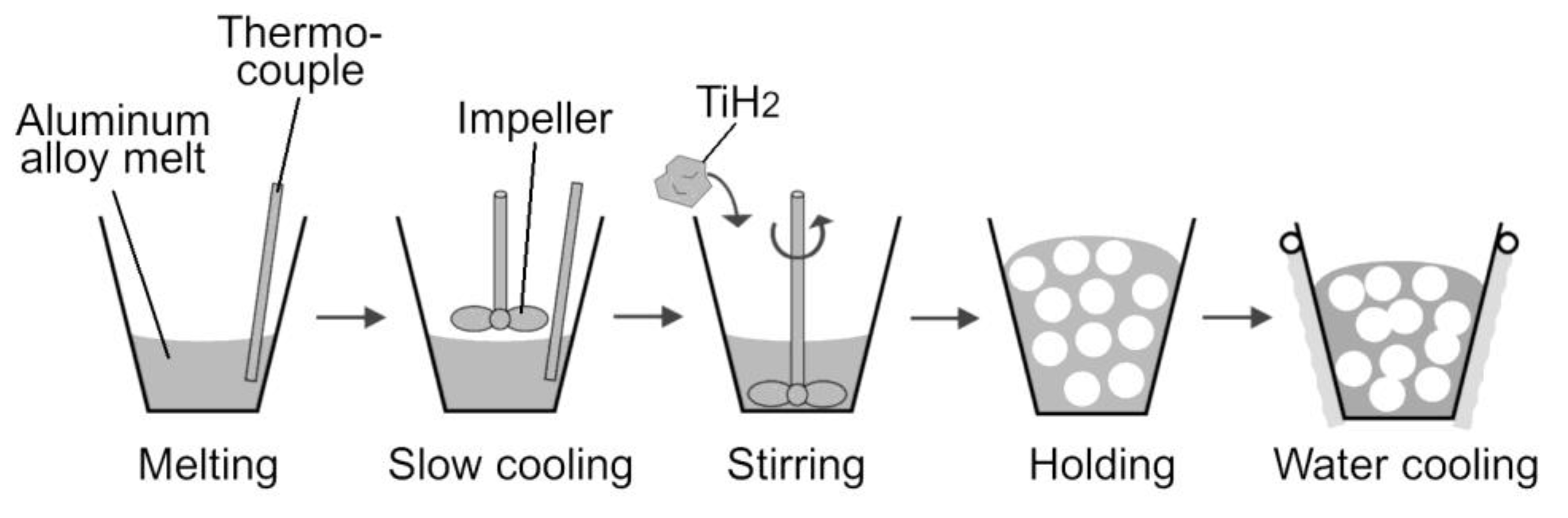
2.2. Foaming Process
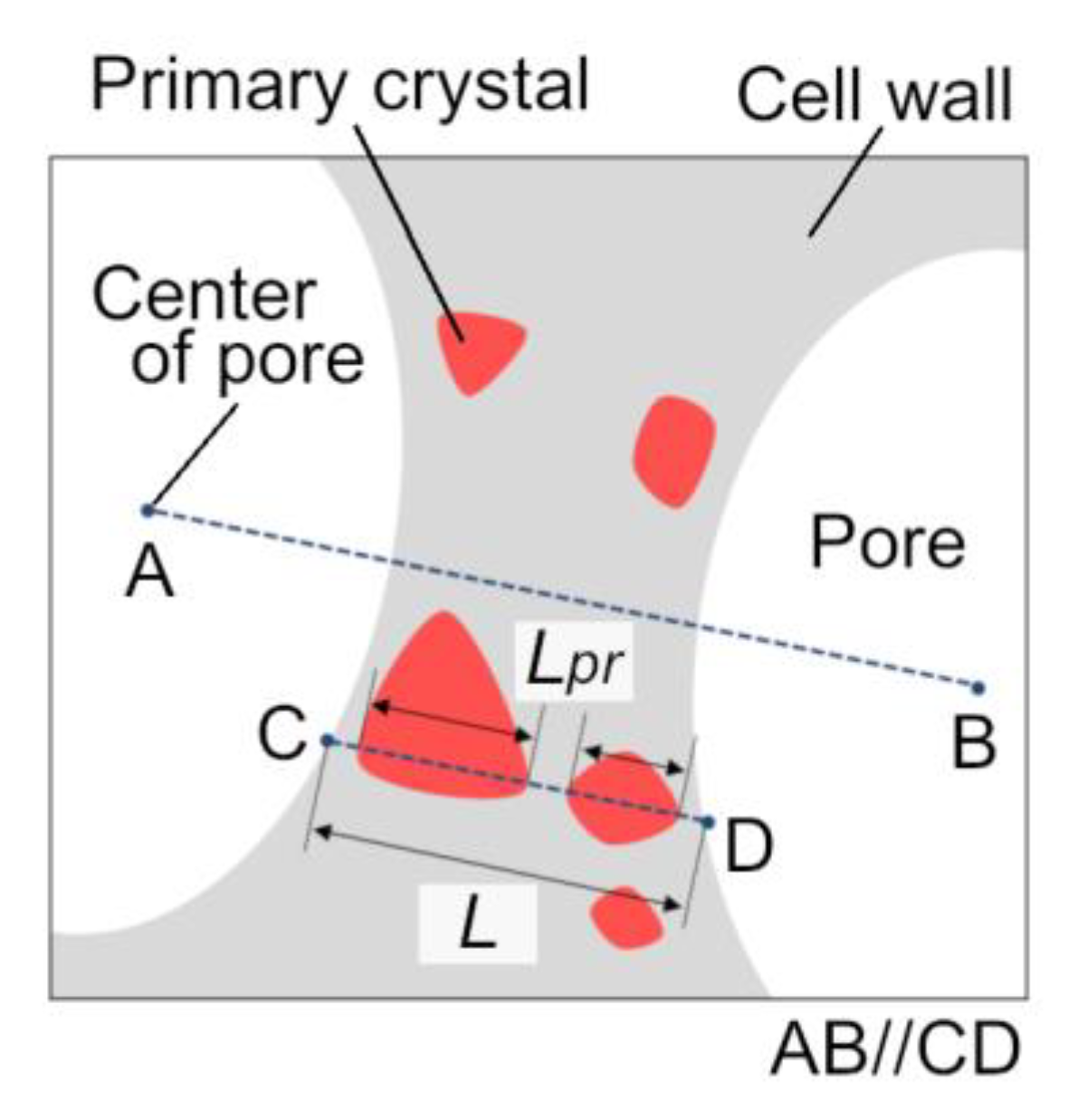
2.3. Evaluation of Pore Structure
2.4. Microstructural Observation
3. Results
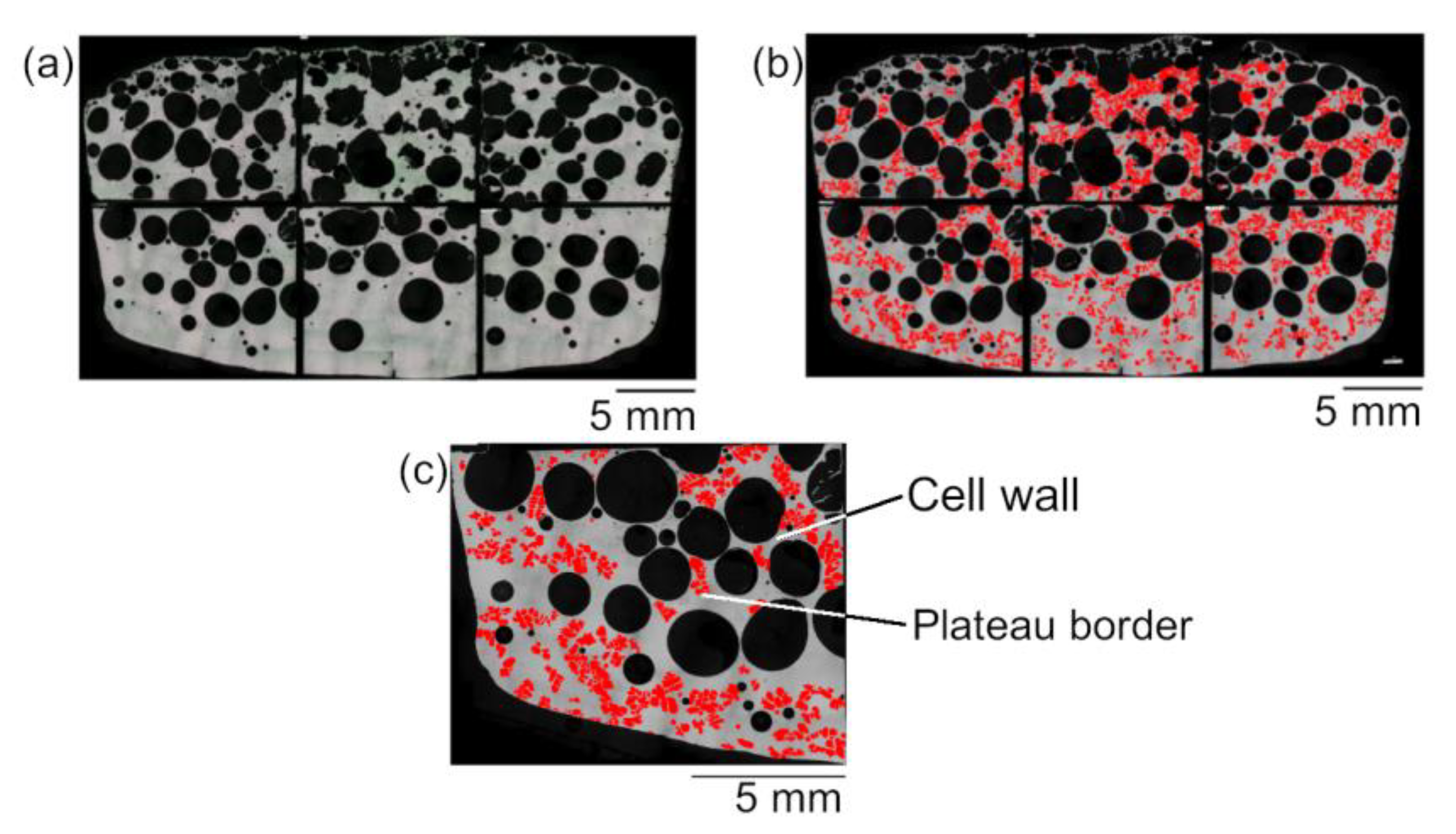
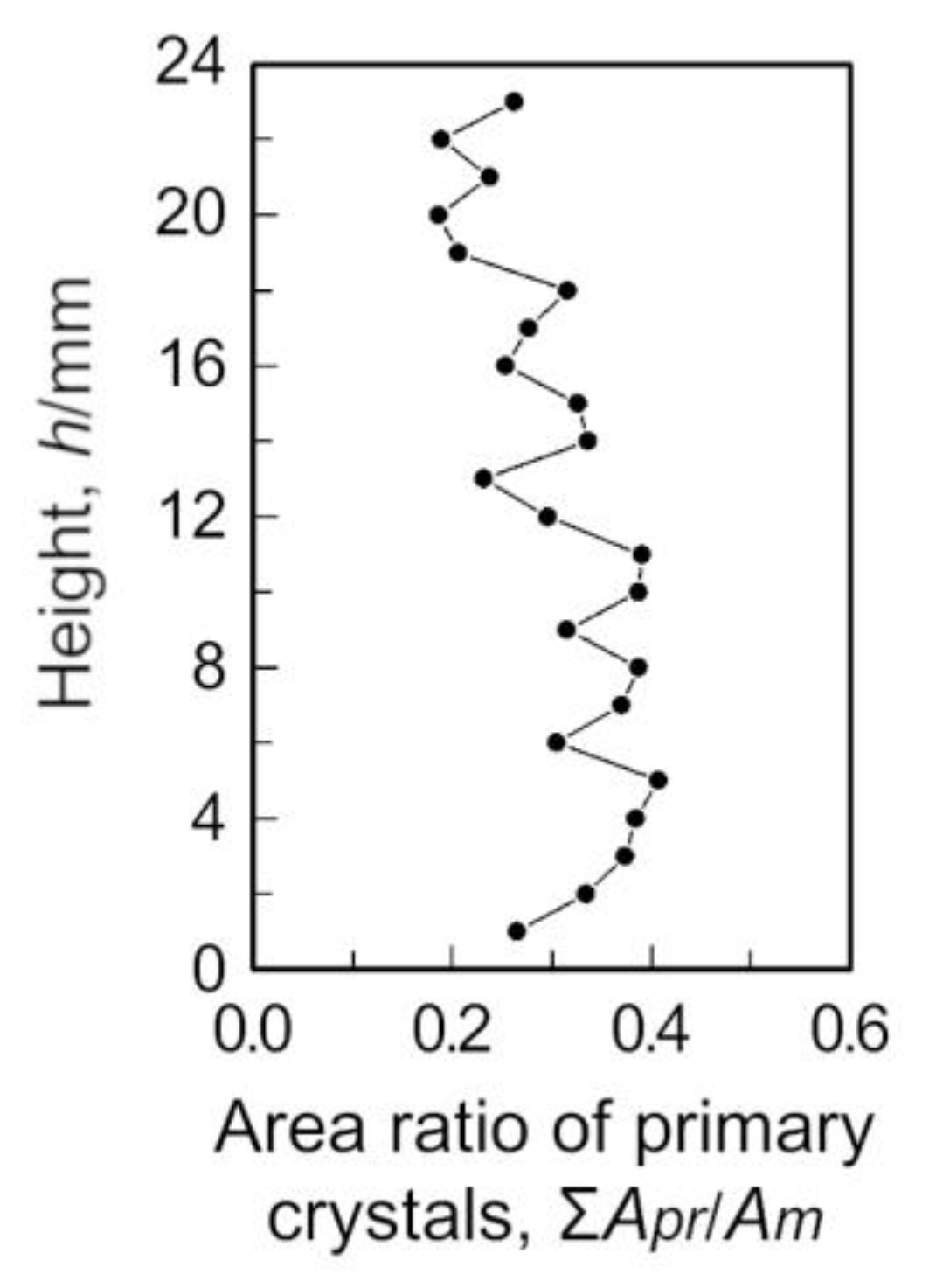
3.1. Pore Distributions
3.2. Microscopic Observation for the Primary Crystals
4. Discussion
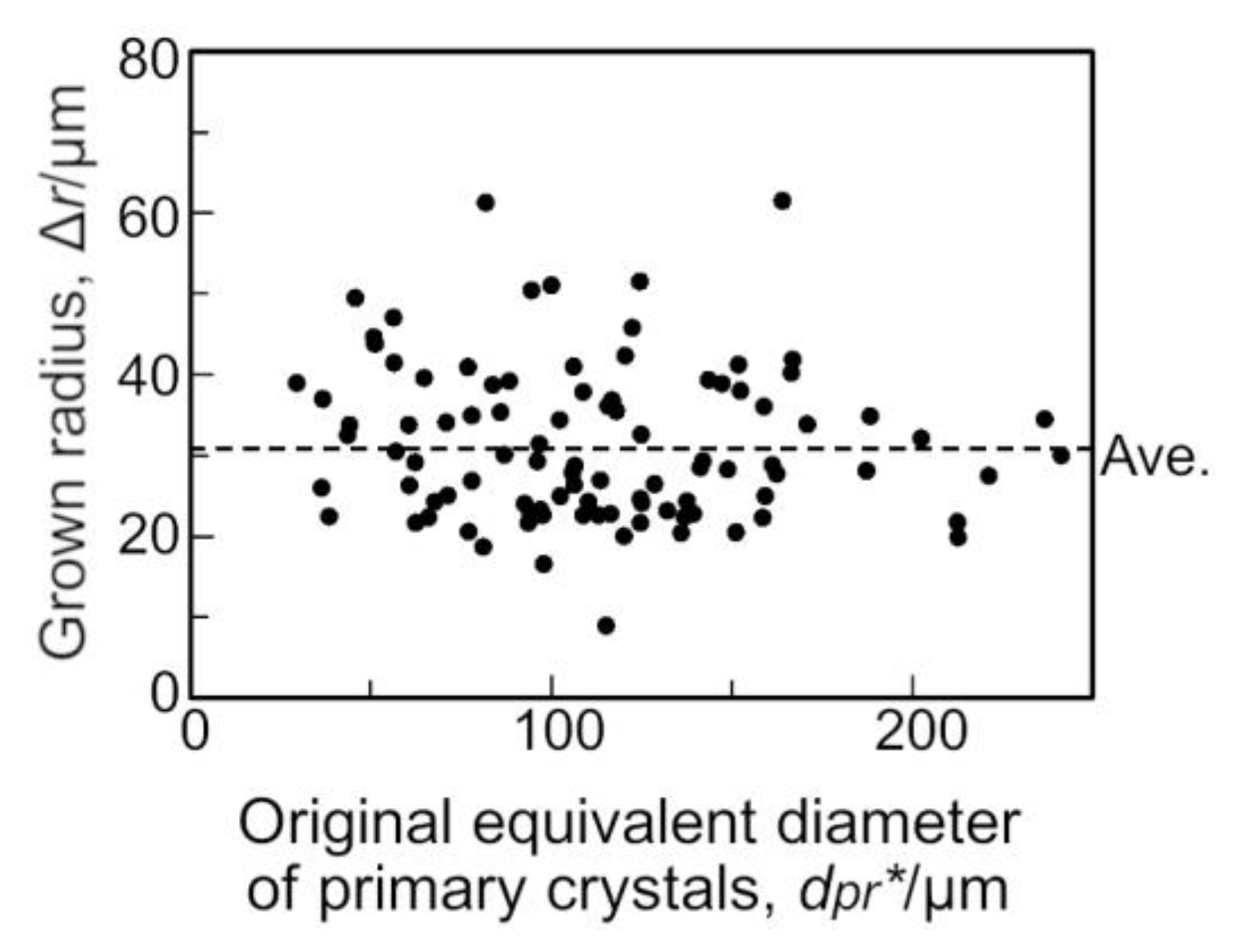
4.1. The Original Size of the Primary Crystals in Semi-Solid State
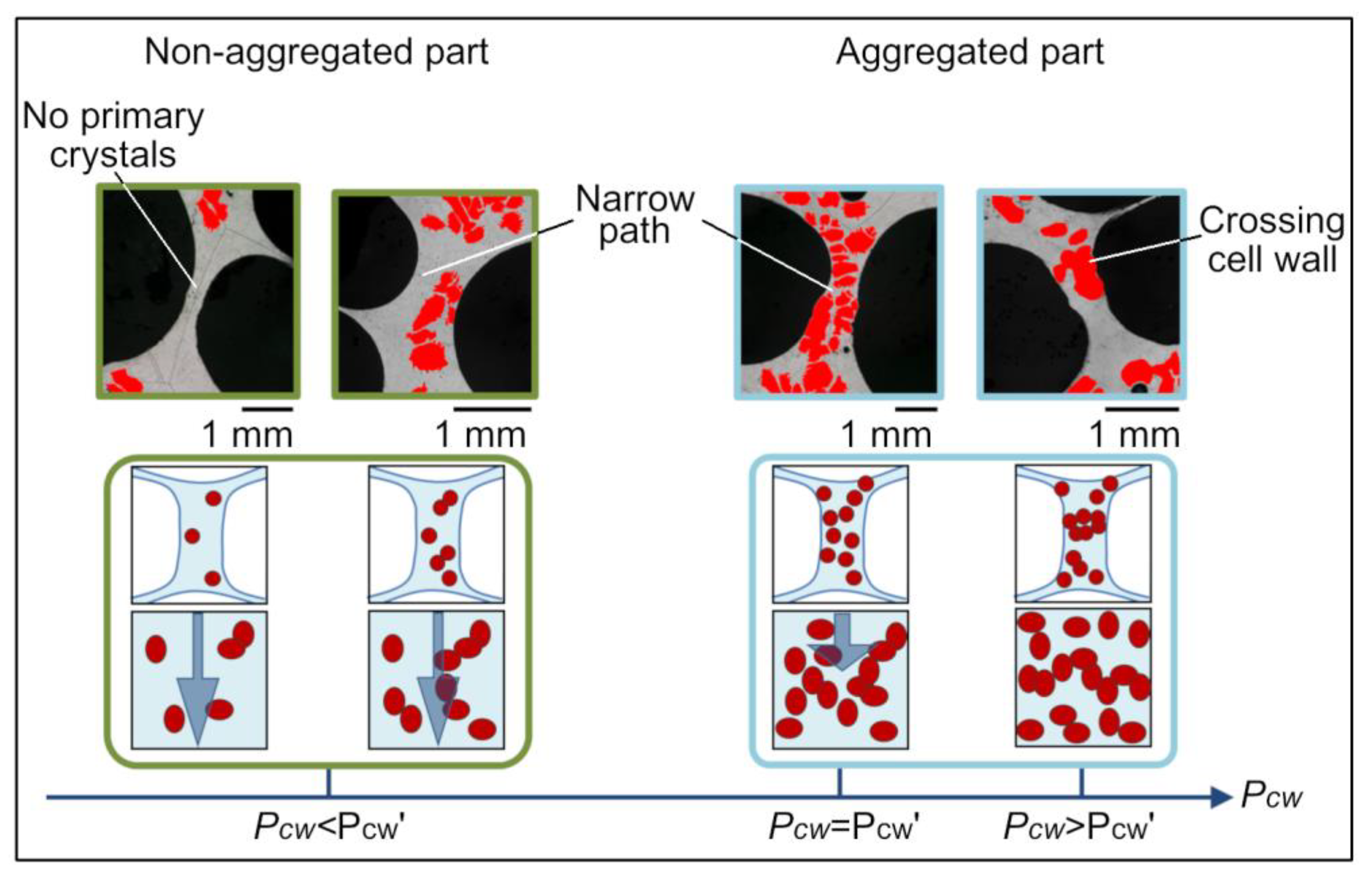
4.2. Percolation Theory on Stabilization Mechanism
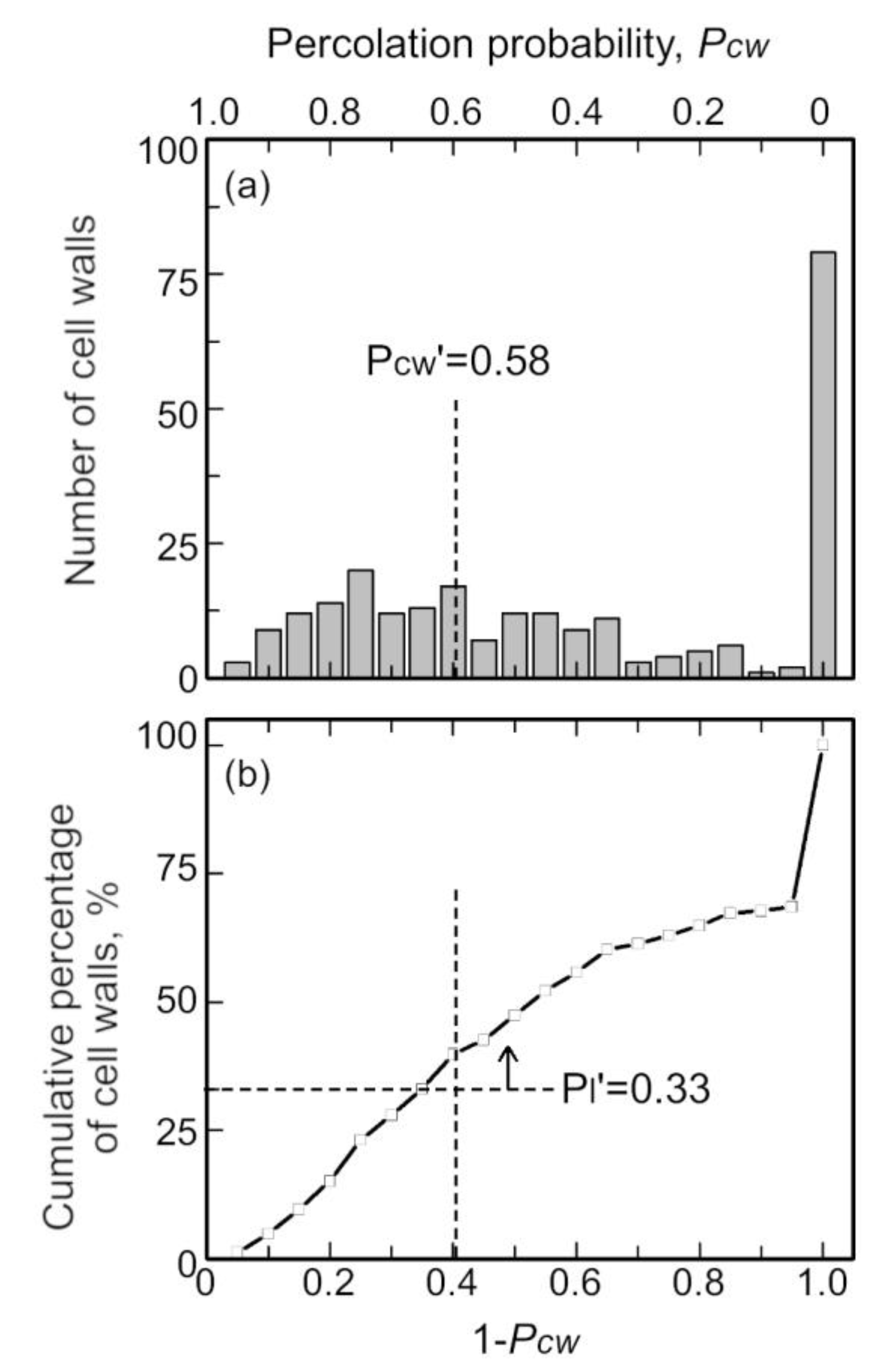
4.2.1. Percolation Theory for Cell Walls
4.2.2. Percolation Theory for the Structure of the Foam
4.2.3. Discussion of Percolation Theory in Three Dimension
5. Conclusions
- Some primary crystals were aggregated in the stable cell walls. Additionally, its percolation probability exceeded the reference percolation threshold (0.581) in some stable cell walls (aggregated cell walls). It is considered that the aggregated cell wall can retain its shape for a long time due to the presence of the primary crystals.
- Cell walls with insufficient primary crystals also remained stable. Therefore, the fact that the aggregated cell walls inhibit the drainage of non-aggregated cell walls is considered to be one of the factors that maintain the stability of the foam.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.; Vinod-Kumar, G.S.; García-Moreno, F.; Banhart, J. Stability of various particle-stabilised aluminum alloys foams made by gas injection. J. Mater. Sci. 2017, 52, 6401–6414. [Google Scholar] [CrossRef]
- Dang, F.; Liu, Y.; Lu, X.; Fan, J. Improved Stability of Aluminum Foam Through Heat Treatment of Foamable Precursor. Met. Mater. Int. 2019. only online available. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M.; Risal, A.R. Influence of Silicon Oxide and Aluminum Oxide Nanoparticles on Air and CO2 Foams Stability in Presence and Absence of Oil. Chem. Eng. Trans. 2017, 56, 1243–1248. [Google Scholar]
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide, 1st ed.; Butterworth-Heinemann: Woburn, MA, USA, 2000; pp. 8–11. [Google Scholar]
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS aluminum foam: Production process, properties, and applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Suzuki, S.; Murakami, H.; Kadoi, K.; Saiwai, T.; Nakae, H.; Babcán, N. Aluminum foam fabrication through the melt route by adding Mg and Bi. In Proceedings of the 7th International Conference on Porous Metals and Metallic Foams, Busan, Korea, 18–21 September 2012. [Google Scholar]
- Jin, I.; Kenny, J.D.; Sang, H. Method of Producting Lightweight Foamed Metal. U.S. Patent 4,973,358, 27 November 1990. [Google Scholar]
- Hanafusa, T.; Ohishi, K. Making of Porous Metallic Material by the Semi-Solid Aluminum Alloy. Available online: https://www.pref.hiroshima.lg.jp/uploaded/life/206737_384148_misc.pdf (accessed on 3 June 2020).
- Kuwahara, T.; Osaka, T.; Saito, M.; Suzuki, S. Compressive properties of A2024 alloy foam fabricated through a melt route and a semi-solid route. Metals 2019, 9, 153. [Google Scholar] [CrossRef]
- Saito, M.; Suzuki, S.; Kamm, P.H.; García-Moreno, F.; Banhart, J. In-situ observation of foam evolution of liquid and semi-solid A2024 alloy through X-ray radioscopy. In Proceedings of the 9th International Conference on Porous Metals and Metallic Foams, Barcelona, Spain, 31 August–2 September 2015. (submitted). [Google Scholar]
- Heim, K.; García-Moreno, F.; Banhart, J. Particle size and fraction required to stabilise aluminium alloy foams created by gas injection. Scr. Mater. 2018, 153, 54–58. [Google Scholar] [CrossRef]
- Kuwahara, T.; Kaya, A.; Osaka, T.; Takamatsu, S.; Suzuki, S. Stabilization mechanism of semi-solid film simulating the cell walls during fabrication of aluminum foam. Metals 2020, 10, 333. [Google Scholar] [CrossRef]
- Murray, J.L.; McAlister, A.J. The Al-Si (Aluminum-Silicon) system. Bull. Alloy Phase Diagrams 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Gao, L.; Harada, Y.; Kumai, S. Analysis of microstructure evolution and precise solid fraction evaluation of A356 aluminum alloy during partial re-melting by a color etching method. J. Mater. Sci. 2012, 47, 6553–6564. [Google Scholar] [CrossRef]
- Smith, L.N.; Lobb, C.J. Percolation in two-dimensional conductor-insulator networks with controllable anisotropy. Phys. Rev. B 1979, 20, 3653–3658. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Cluster Numbers. Introduction to percolation theory, 2nd. Ed.; Taylor & Francis: London, UK, 1994; pp. 26–34. [Google Scholar]
- Hensler, J.H. The Relation between Grain Section and Grain Size. J. Inst. Metals 1968, 96, 190–192. [Google Scholar]
- Van der Marck, S.C. Percolation thresholds and universal formulas. Phys. Rev. E 1997, 55, 1514–1517. [Google Scholar] [CrossRef]






| Element | Si | Fe | Cu | Al |
|---|---|---|---|---|
| High-purity aluminum | 0.004 | 0.003 | 0.0001 | Bal. |
| Al-Si | 25.3 | 0.15 | 0.00 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamatsu, S.; Kuwahara, T.; Kochi, R.; Suzuki, S. Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route. Metals 2020, 10, 847. https://doi.org/10.3390/met10070847
Takamatsu S, Kuwahara T, Kochi R, Suzuki S. Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route. Metals. 2020; 10(7):847. https://doi.org/10.3390/met10070847
Chicago/Turabian StyleTakamatsu, Satomi, Takashi Kuwahara, Ryunosuke Kochi, and Shinsuke Suzuki. 2020. "Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route" Metals 10, no. 7: 847. https://doi.org/10.3390/met10070847
APA StyleTakamatsu, S., Kuwahara, T., Kochi, R., & Suzuki, S. (2020). Percolation of Primary Crystals in Cell Walls of Aluminum Alloy Foam via Semi-Solid Route. Metals, 10(7), 847. https://doi.org/10.3390/met10070847





