Effect of Ti Content on the Behavior of Primary Carbides in H13 Ingots
Abstract
1. Introduction
2. Materials and Methods
3. Results
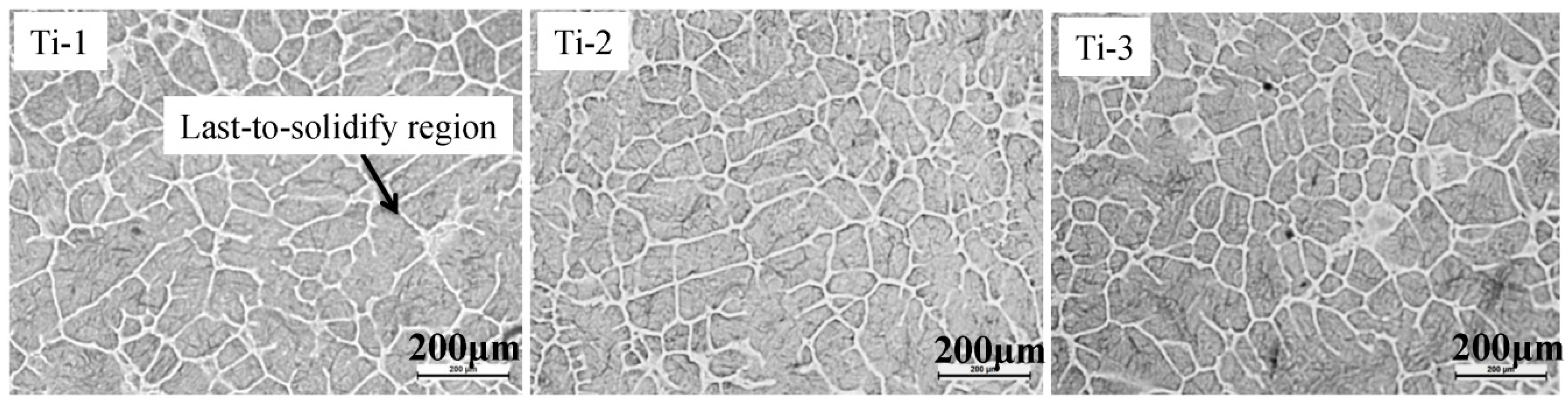
3.1. Matrix Structure
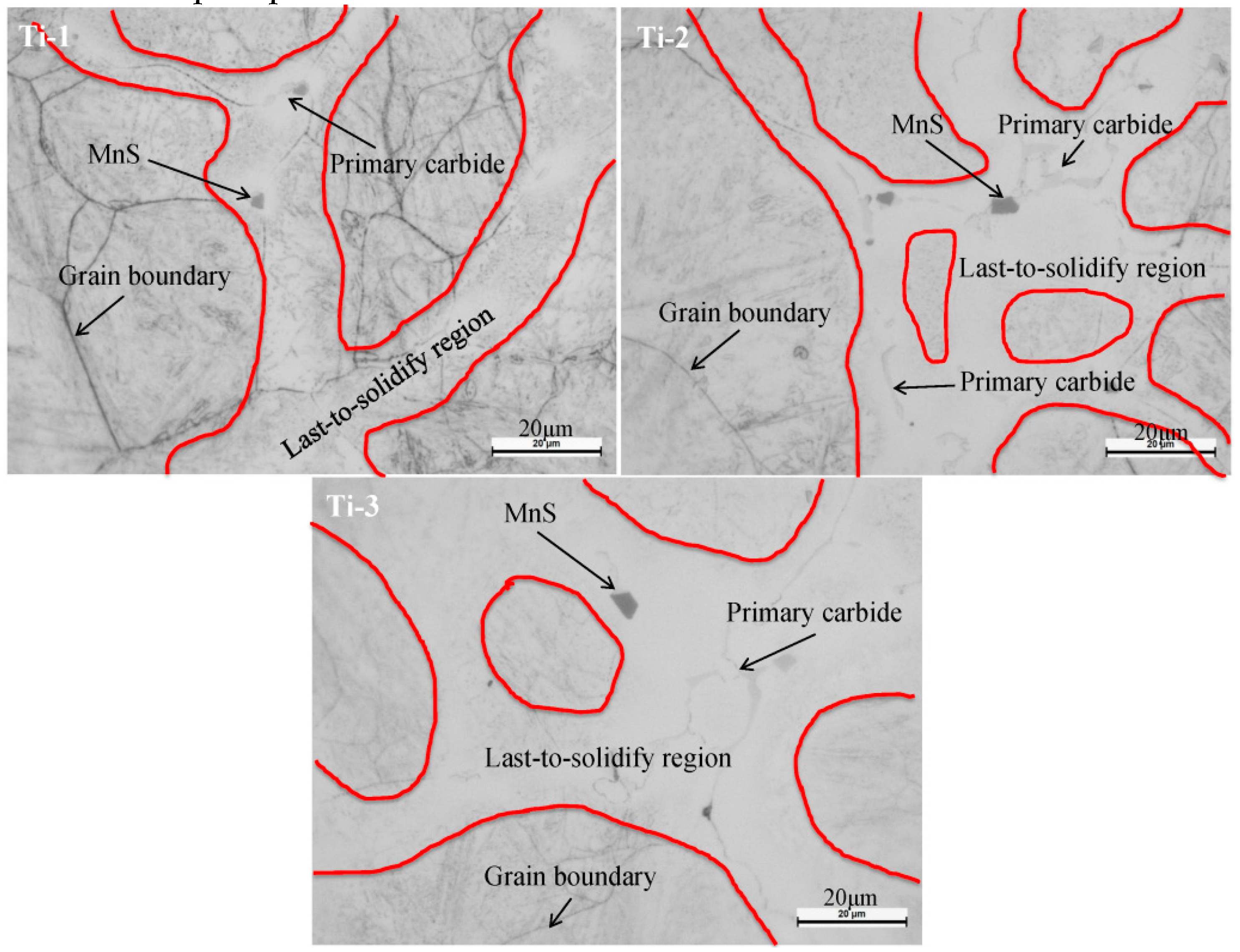
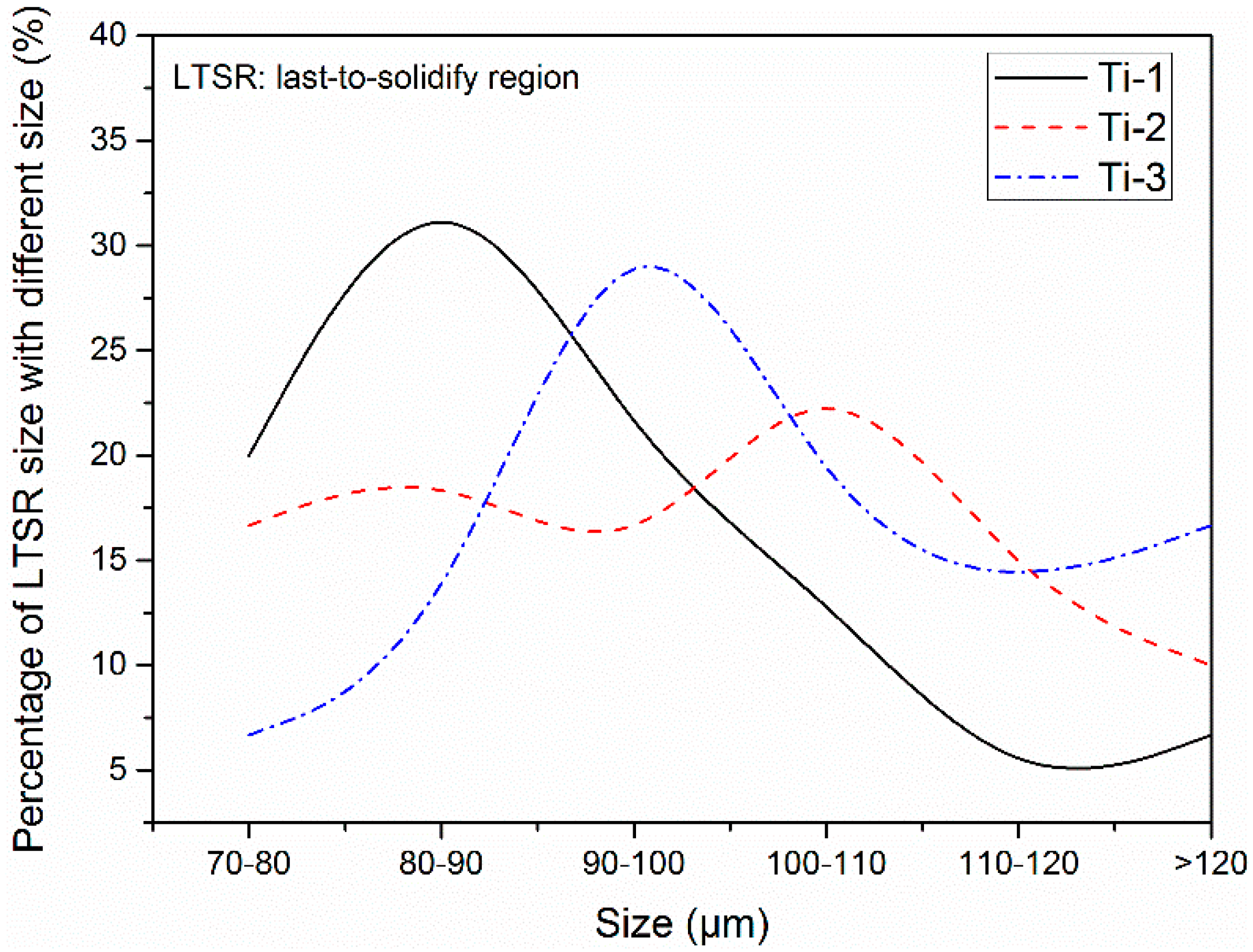
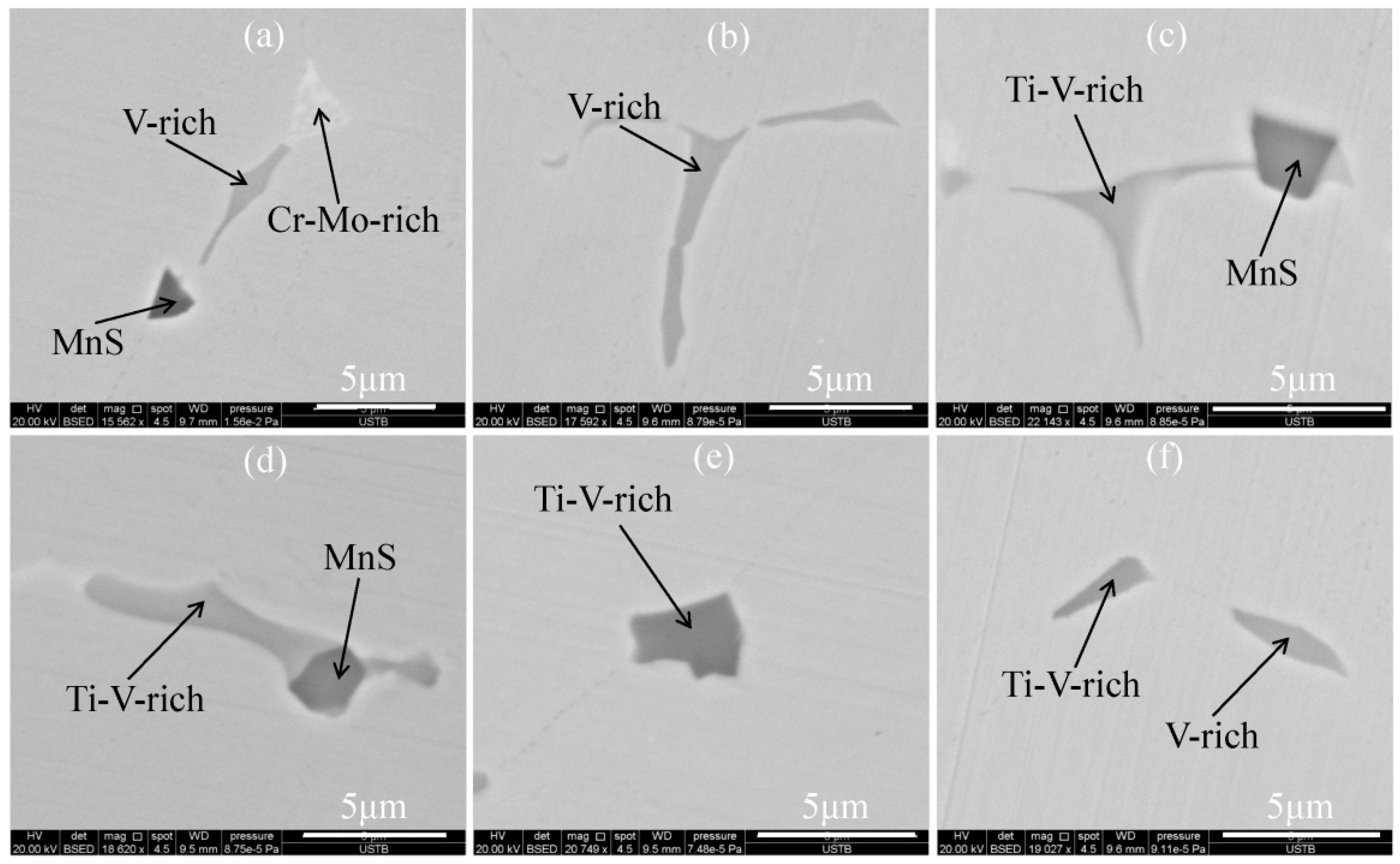
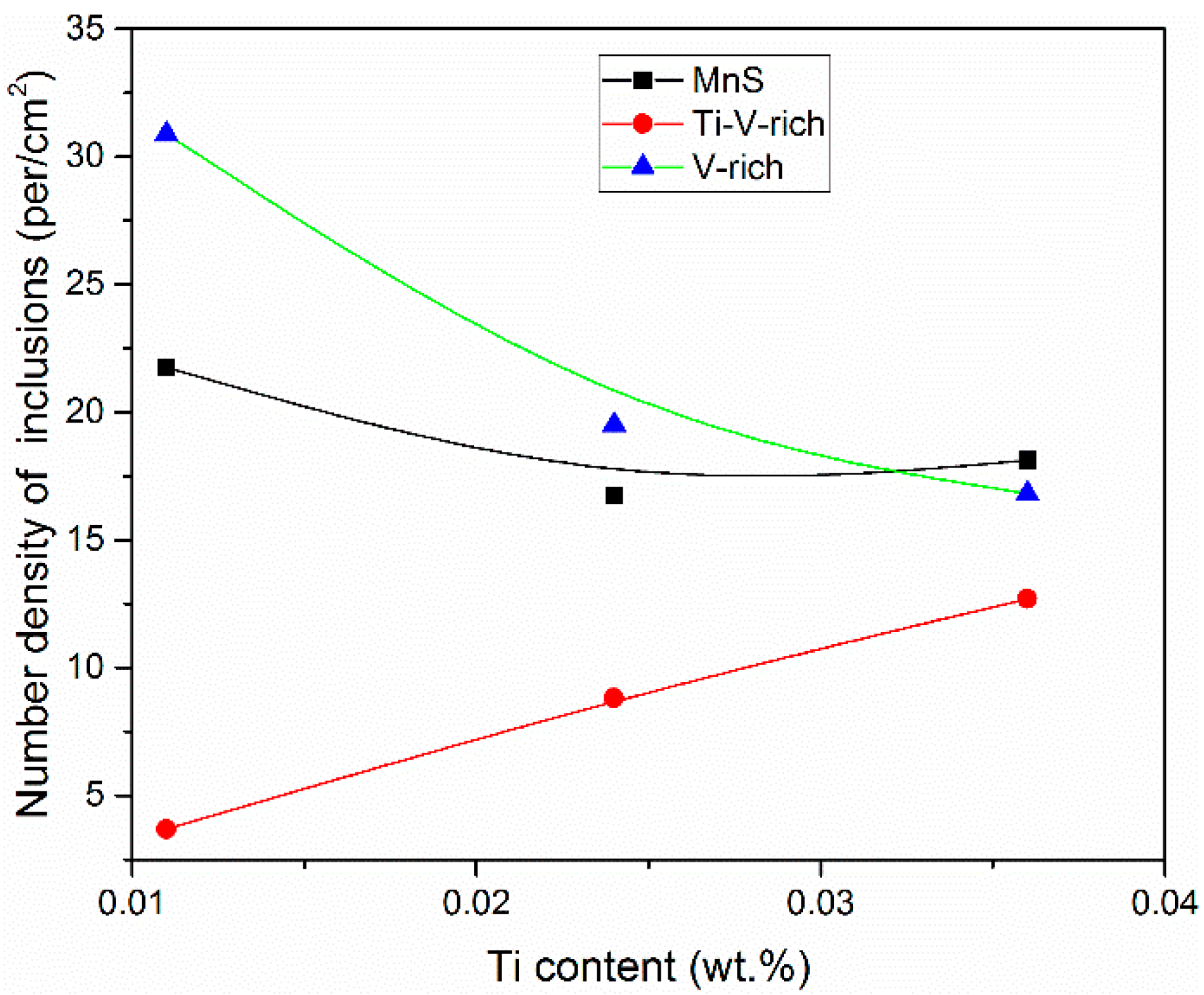
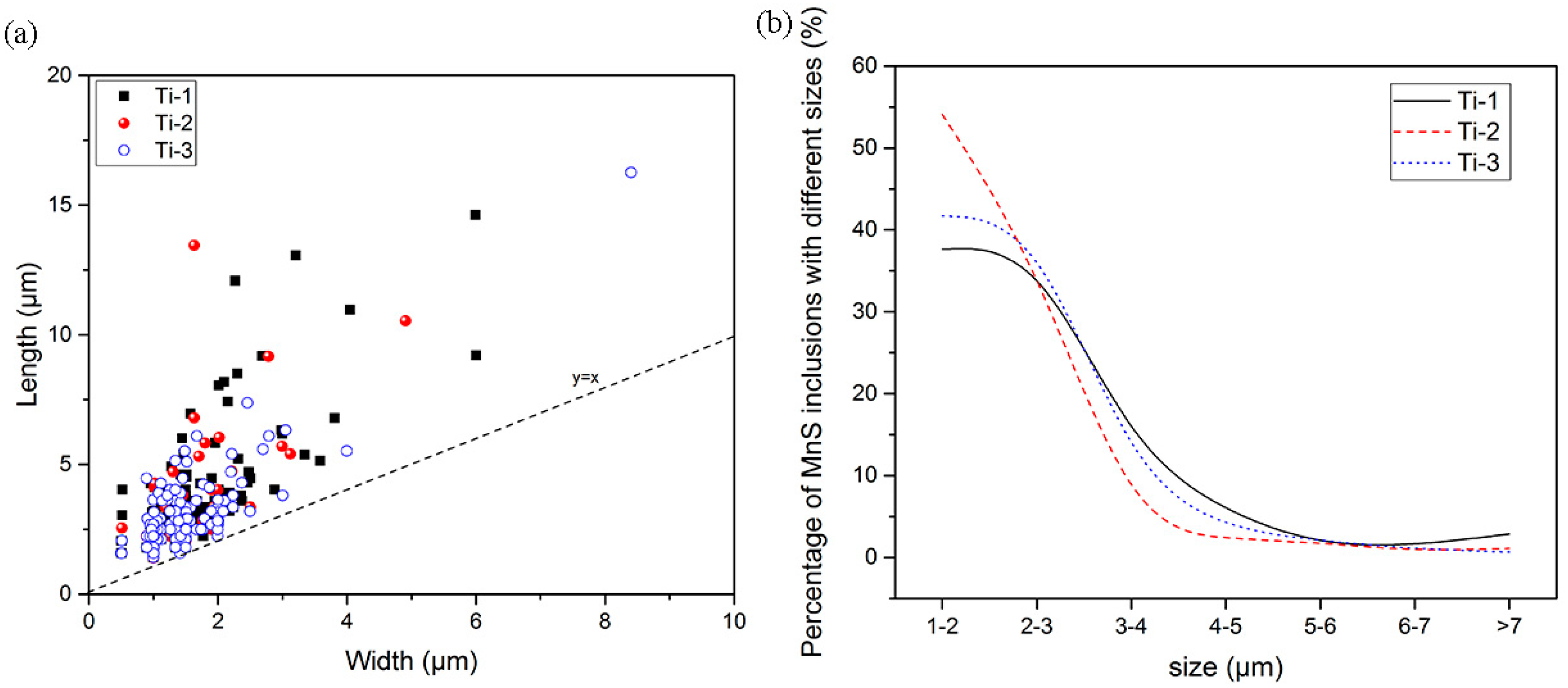
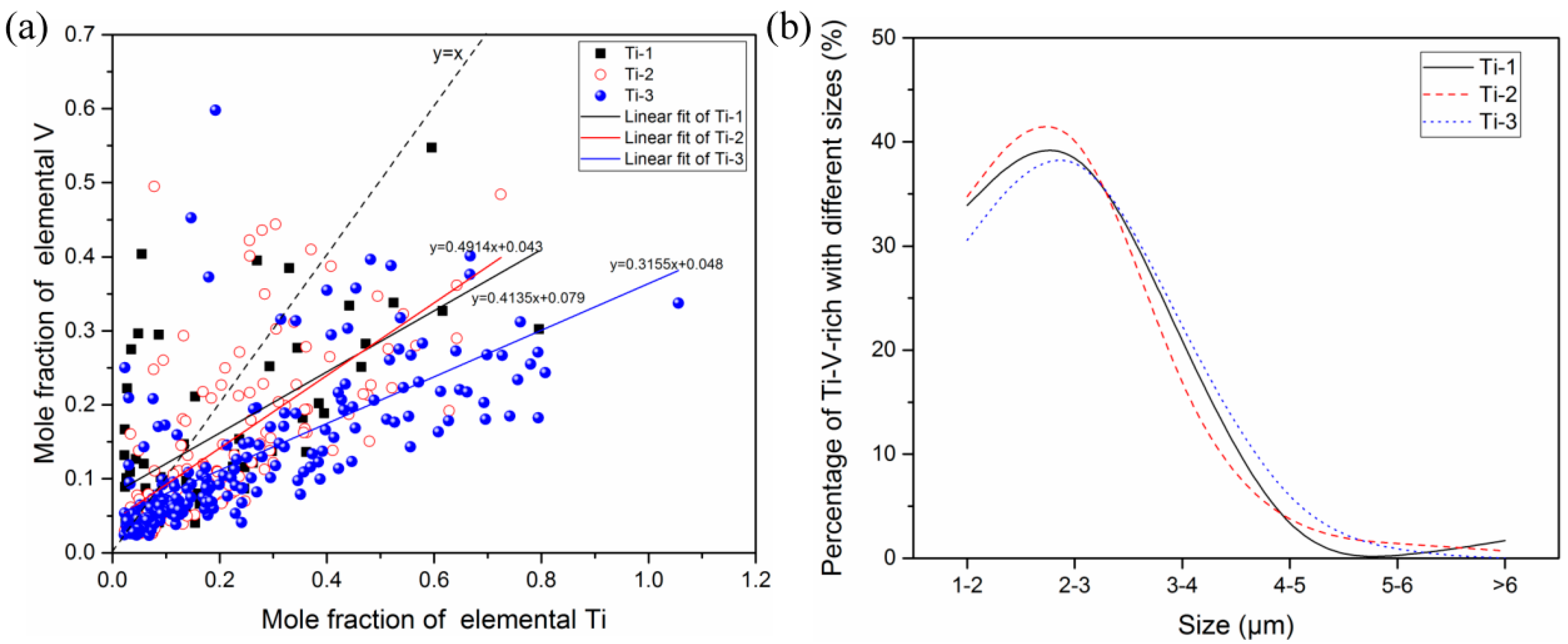
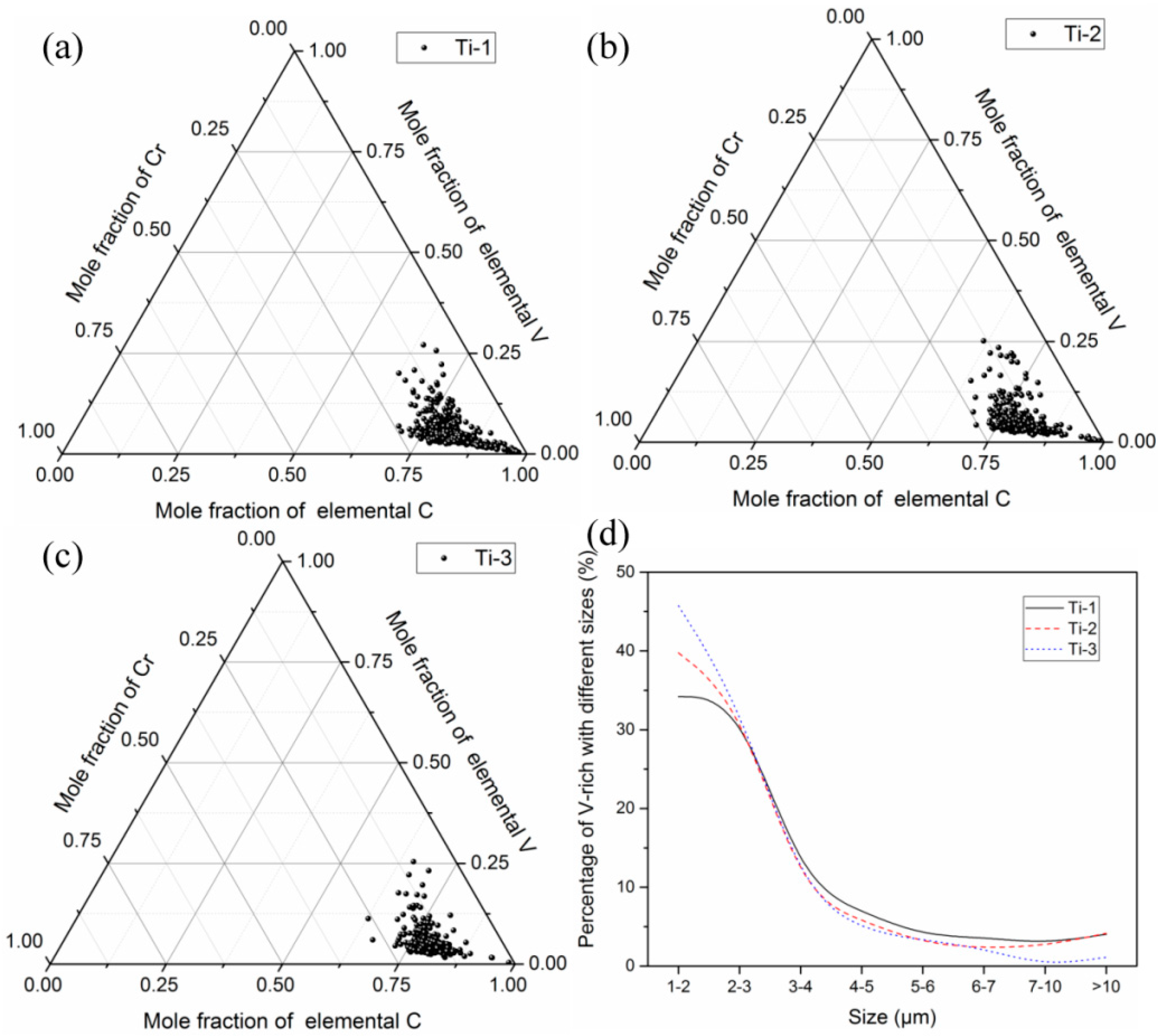
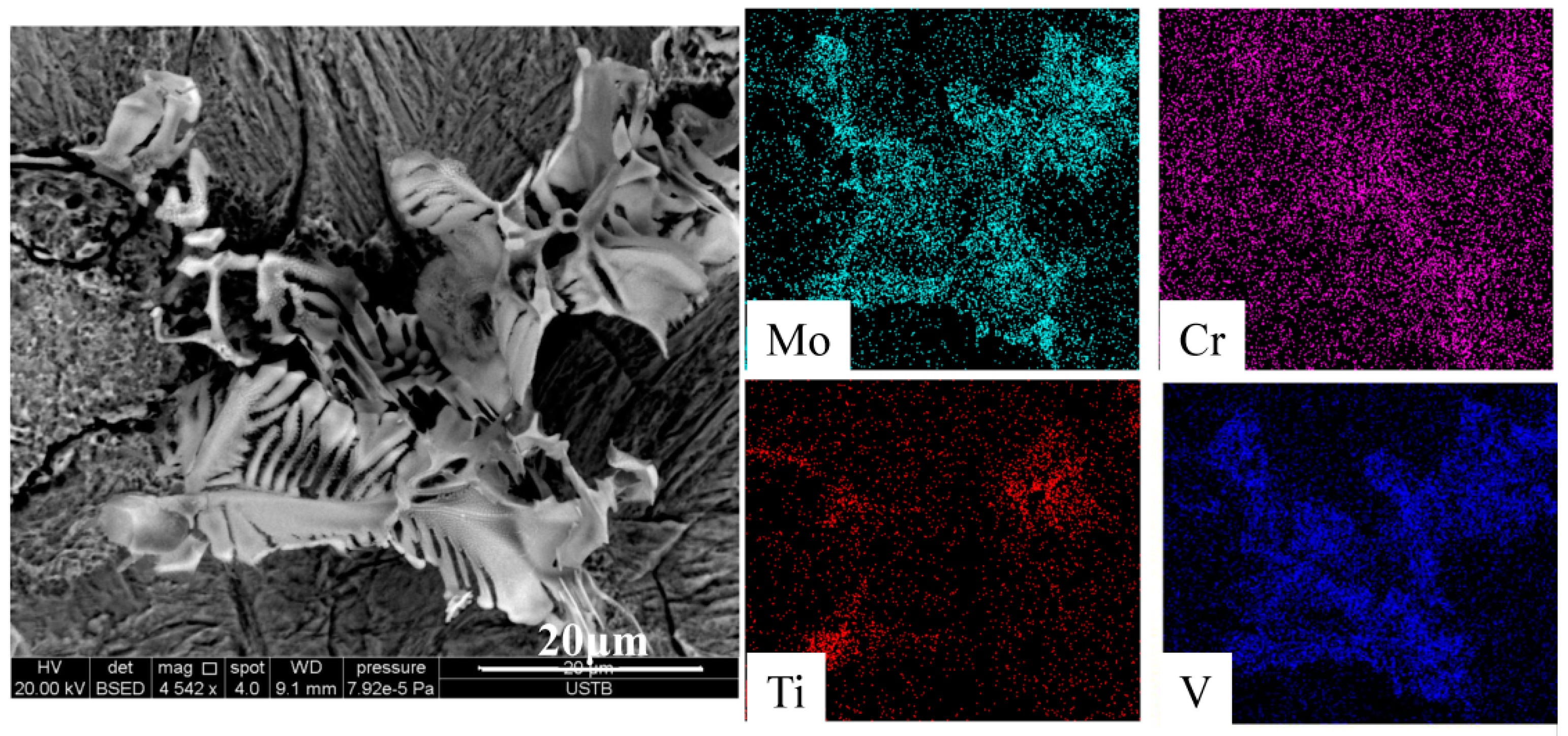
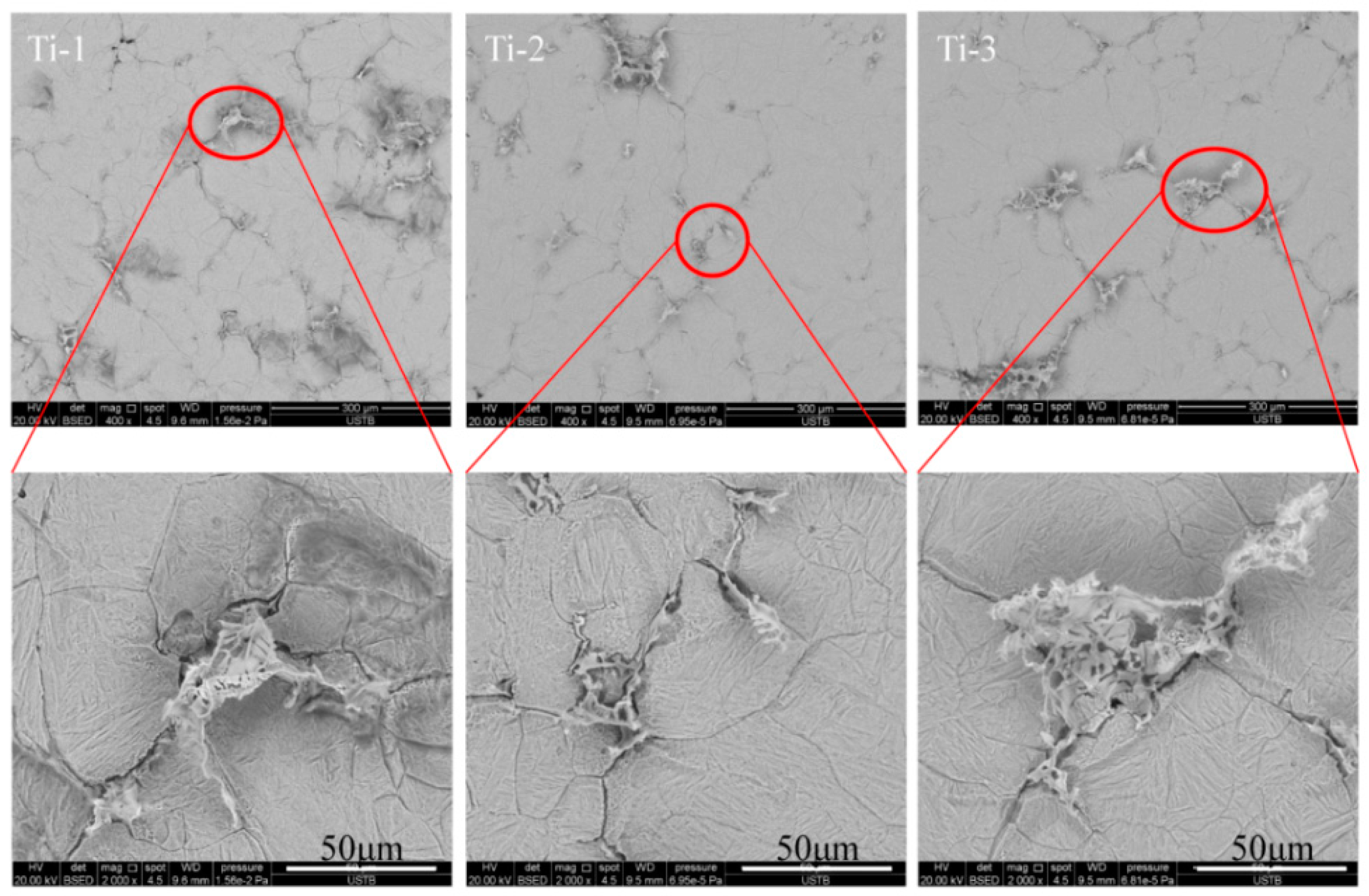
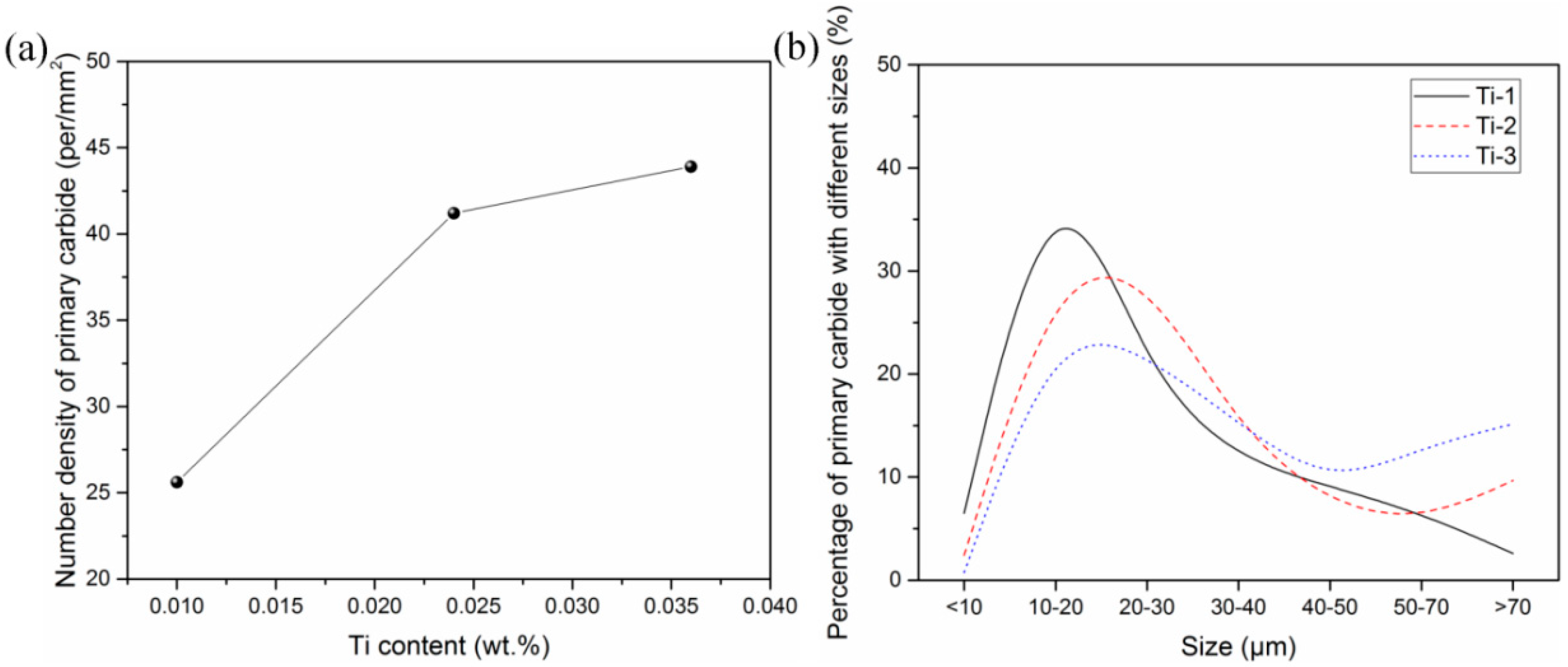
3.2. Two-Dimensional Characteristics of the Inclusions in the Samples
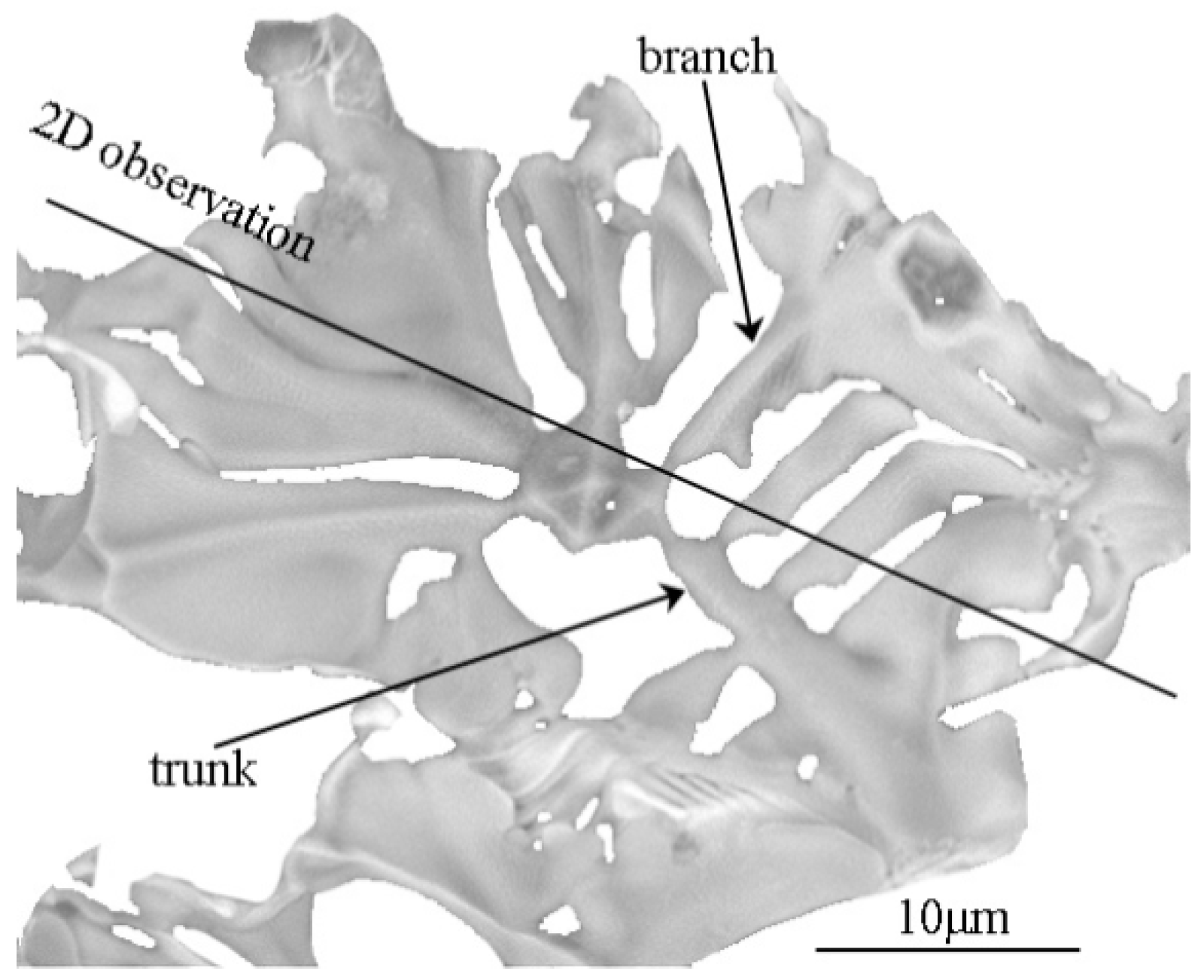
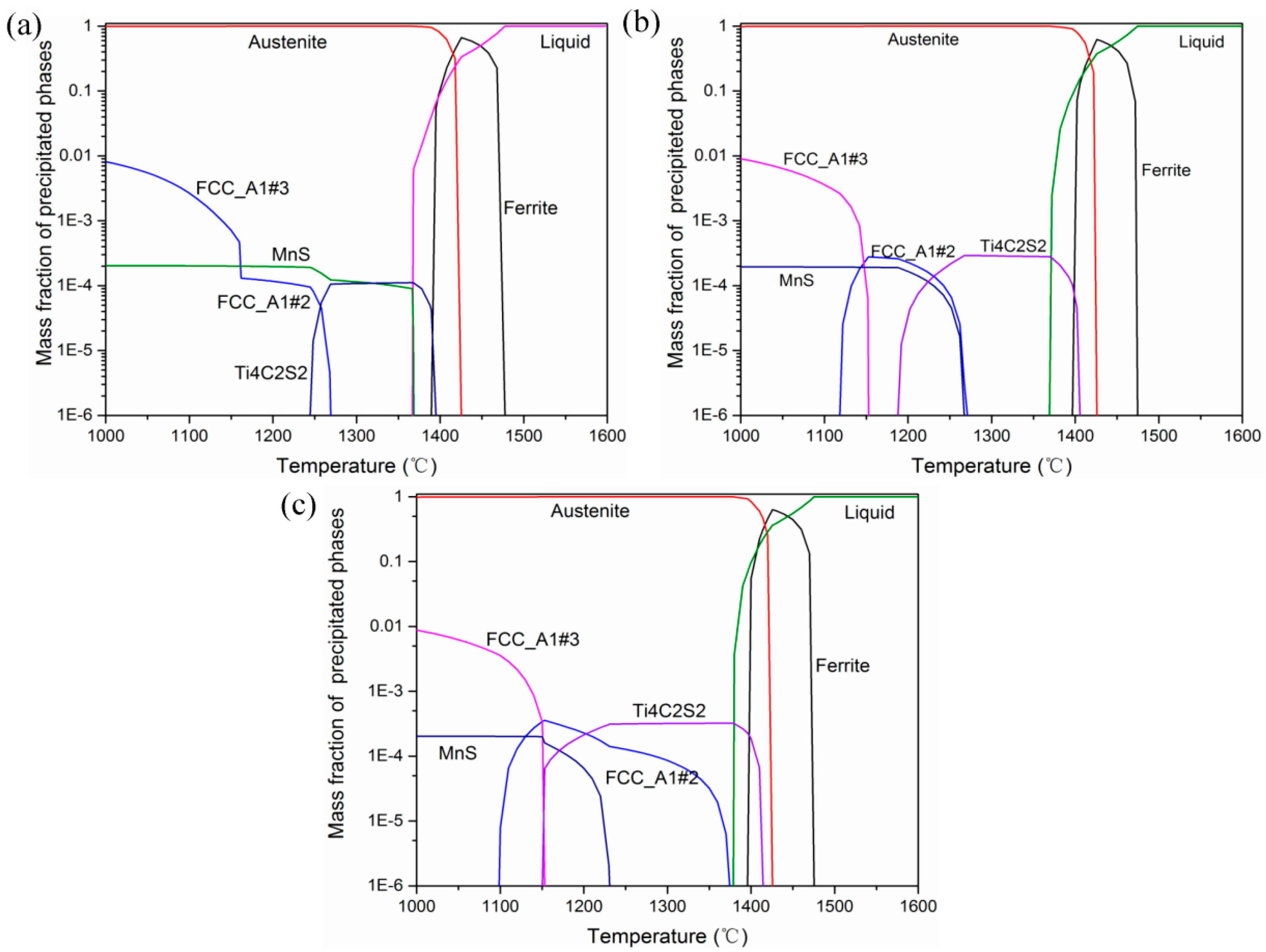
3.3. Three-Dimensional Characteristics of the Primary Carbides in the Samples
4. Discussion
4.1. Influence of the 3D Characteristics of Primary Carbides on the Mechanical Properties of Steel
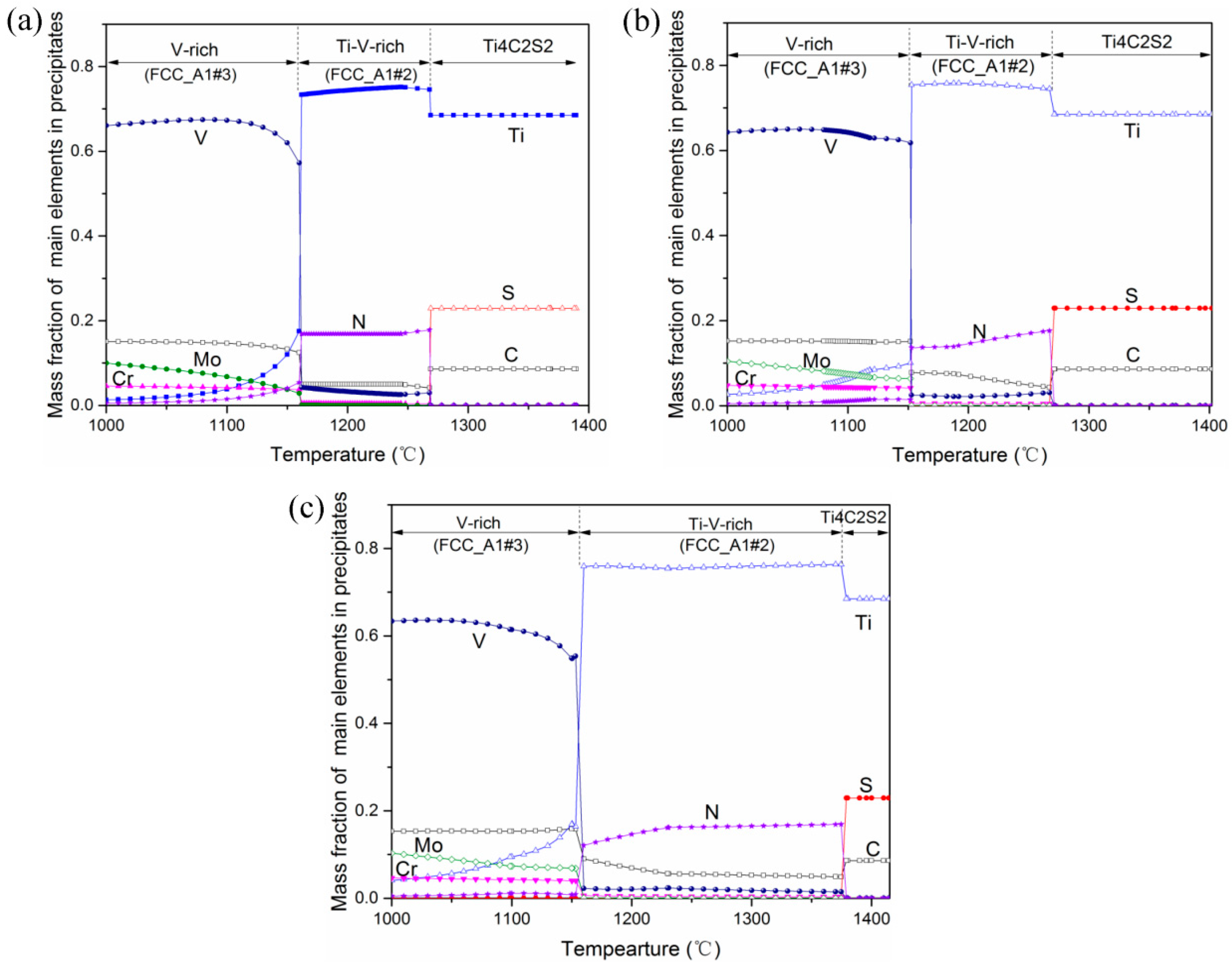
4.2. Mechanism by Which the Ti Content Influences the 3D Characteristics of Primary Carbides
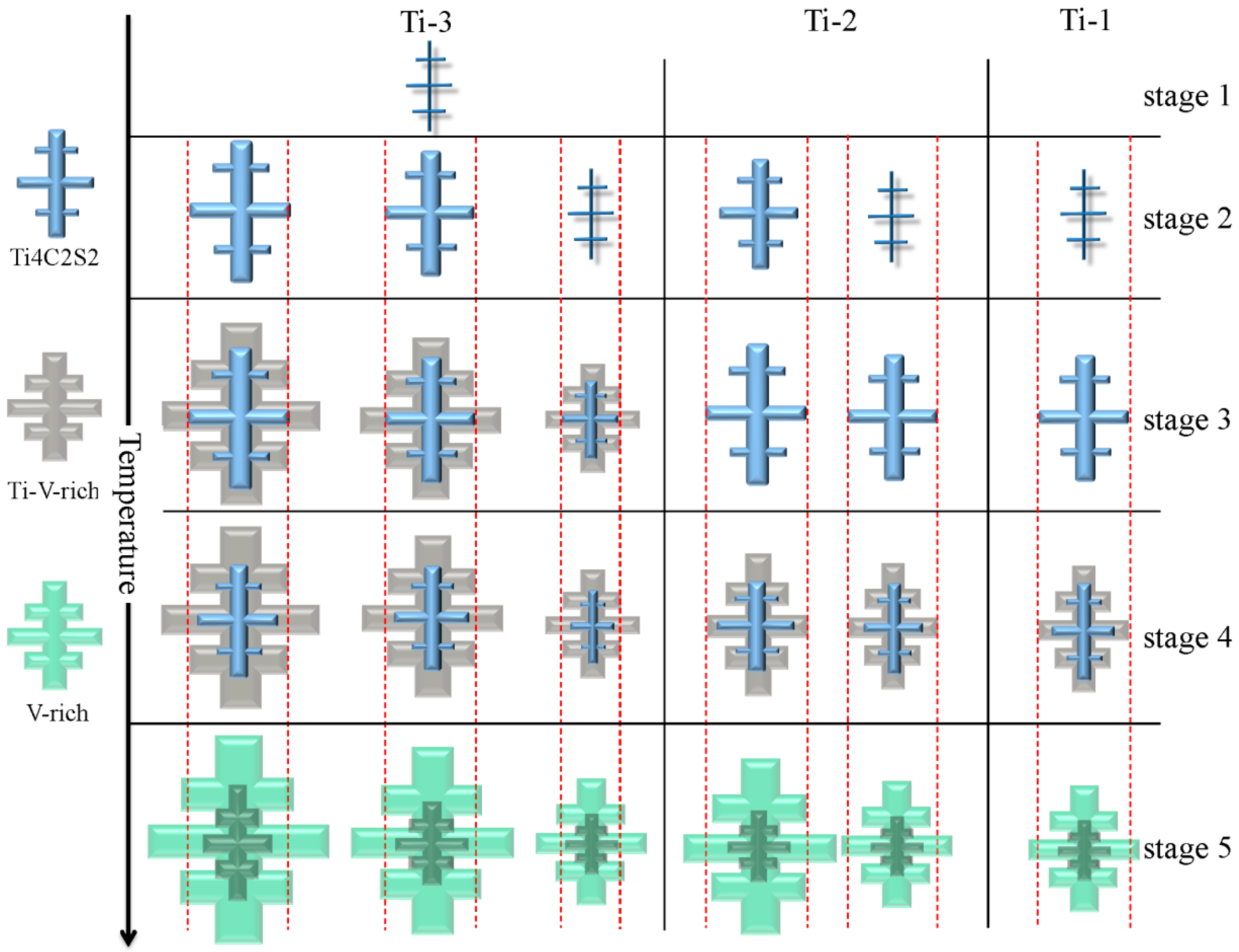
4.3. Precipitation Process of Primary Carbides in H13 Ingots with Different Ti Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.; Reddy, K.; Song, S.; Wang, J.; Wang, X. Microstructure and mechanical properties of Nb and Ti microalloyed lightweight δ-trip steel. Mater. Charact. 2020, 164, 110324. [Google Scholar] [CrossRef]
- Cao, Y.; Wan, X.; Hou, Y.; Niu, C.; Liu, Y.; Li, G. In situ observation of grain refinement in the simulated heat-affected zone of al–ti-0.05% ce-deoxidized steel. Steel Res. Int. 2019, 90, 1900084. [Google Scholar] [CrossRef]
- Zhan, D.; Qiu, G.; Li, C.; Qi, M.; Jiang, Z.; Zhang, H. Effects of Ti addition on the microstructure and tensile properties of china low activation martensitic steel for nuclear fusion reactors. Steel Res. Int. 2019, 90, 1900109. [Google Scholar] [CrossRef]
- Sasaki, M.; Matsuura, K.; Ohsasa, K.; Ohno, M. Refinement of as-cast austenite grain in carbon steel by addition of titanium. ISIJ Int. 2009, 49, 1362–1366. [Google Scholar] [CrossRef][Green Version]
- Maity, S.; Ballal, N.; Kawalla, R. Development of ultrahigh strength steel by electroslag refining: Effect of inoculation of titanium on the microstructures and mechanical properties. ISIJ Int. 2006, 46, 1361–1370. [Google Scholar] [CrossRef]
- Ohta, H.; Inoue, R.; Suito, H. Effect of TiN precipitation on austenite grain size in Fe-1.5%Mn-0.12%Ti-Si(<1.1%)-C(0.05 and 0.15%) alloy. ISIJ Int. 2008, 48, 294–300. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Liu, X.; Wang, G. Inclusion characteristics and acicular ferrite nucleation in Ti-containing weld metals of X80 pipeline steel. Metall. Mater. Trans. A 2018, 49, 2124–2138. [Google Scholar] [CrossRef]
- Cui, X.; Song, B.; Yang, Z.; Liu, Z.; Li, L.; Wang, L. Effect of Mg on the evolution of inclusions and formation of acicular ferrite in La–Ti-treated steels. Steel Res Int. 2020, 91, 1900563. [Google Scholar] [CrossRef]
- Ito, A.; Suito, H.; Inoue, R. Size distribution of multi-phase deoxidation particles for heterogeneous crystallization of TiN and solidification structure in Ti-added ferritic stainless steel. ISIJ Int. 2012, 52, 1196–1205. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, G.; Hou, Y. Effect of titanium addition on As-cast structure and high-temperature tensile property of 20Cr-8Ni stainless steel for heavy castings. Metals 2020, 10, 529. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Pan, J.; Chen, X. Characteristics of nozzle clogging and evolution of oxide inclusion for Al-killed Ti-stabilized 18Cr stainless steel. Metall. Mater. Trans. B 2019, 50, 2769–2779. [Google Scholar] [CrossRef]
- Basu, S.; Choudhary, S.; Girase, N. Nozzle clogging behavior of Ti-bearing Al-killed ultra low carbon steel. ISIJ Int. 2004, 44, 1653–1660. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W.; Xie, Y. Effect of Ti(C, N) particle on the impact toughness of B-microalloyed steel. Metals 2018, 8, 868. [Google Scholar] [CrossRef]
- Zhang, L.; Davis, C.; Strangwood, M. Dependency of fracture toughness on the inhomogeneity of coarse TiN particle distribution in a low alloy steel. Metall. Mater. Trans. A 2001, 32, 1147–1156. [Google Scholar] [CrossRef]
- Yan, W.; Shan, Y.; Yang, K. Effect of TiN inclusions on the impact toughness of low-carbon microalloyed steels. Metall. Mater. Trans. A 2006, 37, 2147–2159. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Liu, Y.; Cao, Y.; Wand, F.; Wand, Q. Investigation of primary carbides in a commercial-sized electroslag remelting ingot of H13 steel. Metals 2019, 9, 1247. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, G.; Chen, L.; Zhang, Y.; Yan, Q. Characteristics and generating mechanism of large precipitates in Nb–Ti-microalloyed H13 tool steel. ISIJ Int. 2016, 56, 995–1002. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wang, L.; Zhu, Q. Study of the effect of trace Mg additions on carbides in die steel H13. Met. Sci. Heat Treat. 2016, 58, 330–334. [Google Scholar] [CrossRef]
- Ning, A.; Mao, W.; Chen, X.; Guo, H.J. Precipitation behavior of carbides in H13 hot work die steel and its strengthening during tempering. Metals 2017, 7, 70. [Google Scholar] [CrossRef]
- Mao, M.; Guo, H.; Wang, F.; Sun, X. Effect of cooling rate on the solidification microstructure and characteristics of primary carbides in H13 steel. ISIJ Int. 2019, 59, 848–857. [Google Scholar] [CrossRef]
- Yoshida, J.; Katsumata, M.; Yamazaki, Y. Effect of primary carbide on fatigue life in die steel for cold working. Tetsu-To-Hagane 1998, 84, 79–84. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, G.; Meng, X.; Chen, L.; Zhang, Y. Precipitation behavior of primary carbide precipitates in Ti-microalloyed H13 tool steel. ISIJ Int. 2016, 56, 1996–2005. [Google Scholar] [CrossRef]
- Voller, V.; Beckermann, C. A unified model of microsegregation and coarsening. Metall. Mater. Trans. A 1999, 30, 2183–2189. [Google Scholar] [CrossRef]
- Won, Y.; Thomas, B. Simple model of microsegregation during solidification of steels. Metall. Mater. Trans. A 2001, 32, 1755–1767. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W. Precipitation Behavior of Large Primary Carbides in Cast H13 Steel. Steel Res. Int. 2019, 90, 1900035. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W. Distribution characteristics and thermal stability of primary carbide in cast Ce-H13 steel. ISIJ Int. 2020, 60, 267–275. [Google Scholar] [CrossRef]
- Ozaki, K. Effect of the Distribution of Primary Carbide on Fatigue Strength of Cold Work Die Steels. Denki Seiko 2005, 76, 249–257. [Google Scholar] [CrossRef][Green Version]
- Du, J.; Strangwood, M.; Davis, C. Effect of TiN particles and grain size on the charpy impact transition temperature in steels. J. Mater. Sci. Technol. 2012, 28, 878–888. [Google Scholar] [CrossRef]
- He, B.; Li, J.; Shi, C.; Wang, H. Effect of Mg addition on carbides in H13 steel during electroslag remelting process. Metall. Res. Technol. 2018, 115, 501–508. [Google Scholar] [CrossRef]
- Mao, M.; Wand, F.; Sun, X.; Guo, H. In situ research of partial melt in as-cast H13 steel at elevated temperature. Ironmak Steelmak. 2020, 47, 159–167. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, G.; Meng, X.; Huang, Y. Thermal stability of primary elongated V-rich carbonitrides in H13 tool steel. Metall. Res. Technol. 2017, 114, 206–213. [Google Scholar] [CrossRef]
- Hufenbach, J.; Helth, A.; Lee, M.; Wendrock, H.; Giebeler, L.; Choe, C.; Kim, K.; Kuhn, U.; Kim, T.; Eckert, J. Effect of cerium addition on microstructure and mechanical properties of high-strength Fe85Cr4Mo8V2C1 cast steel. Mater. Sci. Eng. A 2016, 674, 366–374. [Google Scholar] [CrossRef]
- Liu, H.; Fu, P.; Liu, H.; Sun, C.; Gao, J.; Li, D. Carbides evolution and tensile property of 4Cr5MoSiV1 die steel with rare earth addition. Metals 2017, 7, 436. [Google Scholar] [CrossRef]
| Samples | C | Si | Mn | P | S | Cr | Mo | V | Ti | N |
|---|---|---|---|---|---|---|---|---|---|---|
| Ti-1 | 0.36 | 1.00 | 0.60 | 0.015 | 0.0075 | 5.50 | 1.40 | 0.97 | 0.011 | 0.0045 |
| Ti-2 | 0.39 | 1.00 | 0.60 | 0.014 | 0.0072 | 5.50 | 1.40 | 0.98 | 0.024 | 0.0045 |
| Ti-3 | 0.38 | 1.00 | 0.60 | 0.015 | 0.0075 | 5.50 | 1.40 | 0.96 | 0.036 | 0.0044 |
| Samples | Solid | Ti4C2S2 | Ti-V-Rich | V-Rich | MnS |
|---|---|---|---|---|---|
| Ti-1 | 1366.7 | 1395.1 | 1269.1 | 1160 | 1368.4 |
| Ti-2 | 1369.3 | 1405.6 | 1271.2 | 1152.8 | 1267.2 |
| Ti-3 | 1378.9 | 1414.4 | 1374.6 | 1150.3 | 1230.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Cheng, G.; Zhu, M. Effect of Ti Content on the Behavior of Primary Carbides in H13 Ingots. Metals 2020, 10, 837. https://doi.org/10.3390/met10060837
Huang Y, Cheng G, Zhu M. Effect of Ti Content on the Behavior of Primary Carbides in H13 Ingots. Metals. 2020; 10(6):837. https://doi.org/10.3390/met10060837
Chicago/Turabian StyleHuang, Yu, Guoguang Cheng, and Meiting Zhu. 2020. "Effect of Ti Content on the Behavior of Primary Carbides in H13 Ingots" Metals 10, no. 6: 837. https://doi.org/10.3390/met10060837
APA StyleHuang, Y., Cheng, G., & Zhu, M. (2020). Effect of Ti Content on the Behavior of Primary Carbides in H13 Ingots. Metals, 10(6), 837. https://doi.org/10.3390/met10060837





