Abstract
The introduction of adhesive bonding in the automotive industry is one of the key enabling technologies for the production of aluminium closures and all-aluminium car body structures. One of the main concerns limiting the use of adhesive joints is the durability of these system when exposed to service conditions. The present article primarily focuses on the different research works carried out for studying the effect of water, corrosive ions and external stresses on the performances of adhesively bonded joint structures. Water or moisture can affect the system by both modifying the adhesive properties or, more importantly, by causing failure at the substrate/adhesive interface. Ionic species can lead to the initiation and propagation of filiform corrosion and applied stresses can accelerate the detrimental effect of water or corrosion. Moreover, in this review the steps which the metal undergoes before being joined are described. It is shown how the metal preparation has an important role in the durability of the system, as it modifies the chemistry of the substrate’s top layer. In fact, from the adhesion theories discussed, it is seen how physical and chemical bonding, and in particular acid-base interactions, are fundamental in assuring a good substrate/adhesive adhesion.
1. Introduction
In the automotive industry, one of the key elements to reduce the fuel consumption, and therefore CO emission, is the switch to lighter materials than plain carbon steel. For this reason, a great interest was put on advanced high strength steels, light non-ferrous alloys (such as aluminium, magnesium and titanium alloys) and a variety of composites, including carbon fiber composites, material matrix composites and nano-composites. Thus, during the last decade, the average amount of aluminium used in passenger cars has doubled, and based on the new design concepts, this trend will be confirmed in the coming years [1,2,3]. One of the main advantages of aluminium over steel is its density which is approximately 65% lower. Both cast and wrought aluminium alloys are used in numerous applications in automobiles. The change in the automotive industry from steel to aluminum is not straightforward and needs design and process adaptations. For example, in order to have the same stiffness, the aluminium parts needs to be thicker compared to the steel ones. This is due to the fact that aluminium has an average elastic modulus of 70 GPa while for steel this is 207 GPa. As such, aluminium components need to be around 40% thicker than steel ones, but remains 50% lighter.
The aluminium alloys used cannot be easily spot-welded because of the low electrical resistance of the aluminium, the very stable and non-conductive oxide layer, and the trend of the aluminium to interact with the spot weld electrodes. On the other hand, the strength of the welded parts is reduced by the effect of heat during the welding process. Especially regarding fatigue, the negative effect of the heat during the welding process is very relevant. To avoid the heat disadvantages, cold processes such as mechanical fastening and adhesive bonding established [4]. In recent years, adhesive bonding has been used more and more in automotive joining for a variety of components including closures and structural modules. Adhesive bonding, alone or in combination with other joining techniques, shows significant advantages over more traditional joining techniques, such as lower weight, cost and improved crash performance/safety [5]. Adhesives (and sealants) can be characterized according to the way in which they cure. This can be by loss of solvent, loss of water, cooling or chemical reaction. Once hardened, the polymer in an adhesive can be linear or crosslinked. The crosslinking makes polymers insoluble and poorly fusible, greatly reducing creep [6]. A structural adhesive is one used when the load required to cause separation is substantial such that the adhesive provides for the major strength and stiffness of the structure. The structural members of the joint, which are joined together by the adhesive, are the adherends [7].
All structural adhesives are cross-linked. The best known and most widely used structural adhesives are epoxies. A schematic representation of this review outline is shown in Figure 1. In the first part, the use of adhesive joints in automotive is introduced. Then, the chemical characteristics of the epoxy adhesives will be described. However, it must be noticed that epoxies are only one, even if widely applied, of the broad class of adhesives that can used in a vehicle. A factor of pivotal importance for the long term durability of the joint is the chemistry of the substrate’s surface prior to bonding. In order to be used for bonding, in most cases the aluminium substrates is pre-treated. Different pre-treatments can be applied on the surface and the different methods will be mentioned in the successive sections. Section 5 will then briefly discuss the classical theories of adhesion. In fact, there have been different attempts in the past to develop a single theory which could explain how adhesion can occur. Finally, the focus will be on the durability of the adhesive joints and some of the extensive literature on the effect of moisture, corrosion and stress on the adhesive joints will be analyzed.
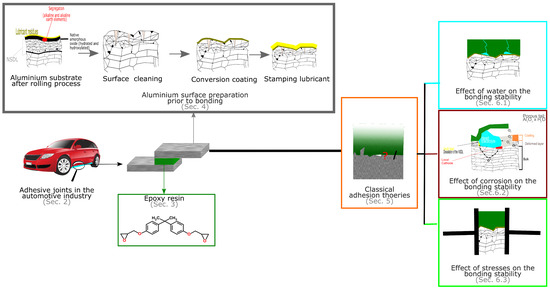
Figure 1.
Graphical outline of this chapter.
2. Adhesive Joints in Automotive
Structural adhesive bonding was initially used in aerospace industry, starting from the 1950’s, and only from the late 1960’s the application spread as well to automotive companies. Adhesive bonding is practiced more commonly with aluminium than with steel, since aluminium is more difficult to weld. Structural bonding makes it possible to increase the rigidity of the components and the crash resistance [8]. Several aluminium-intensive concept cars as well as low volume niche cars, have been produced using adhesive bonding as the primary joining methods. Also, the S-Class Coupé has more than 100 m of structural bonds in body in white (BiW) applications and the BMW 7 series has more than 10 kg of structural adhesives applied [9]. Besides of the use of structural adhesives, within the automobile body-in-white adhesive bonding is used as well for:
anti-flutter bonding to reduce or eliminate any fluttering or vibration of the outer and inner panels to each other. Anti-fluttering adhesives are commonly used on horizontal closure panels (bonnets, trunk lids or roof) and less on vertical panes.
hem flange bonding for joining the inside sheets in the hem flange areas of doors, hoods and tailgates.
sealants to seal joints and crevices before painting in order to prevent damage of joined substrates by protecting against possible aggressive environments.
2.1. Advantages
Adhesive bonding, compared to other joining techniques, does not affect the bulk of the adherends, and, therefore, does not interfere with the aluminium metallurgy or result in thermally or mechanically weakened zones. Thus, there is a uniform stress distribution over the whole bonded area which results in an increased static and dynamic stiffness of the vehicle structure. As the body structure is more rigid, the reasonance frequency modes will be higher and the structural damping faster and, therefore, the vehicle will have better noise, vibration, and harshness (NVH) characteristics. In combination with other joining techniques, also crash performance and fatigue strength are improved by the adhesive bonding, allowing an additional weight reduction of the body structure (as there is a lower application of material gauges) [10]. Furthermore, the use of adhesive bonding can enable the use of dissimilar metals. Due to the presence of the adhesive the metals are also isolated against potential galvanic corrosion [11].
The aesthetics of the final assembly represent a further advantage of the adhesive bonding technology compared to other joining techniques. In fact, there are no visible weld seams or rivet heads. Thus, adhesive bonding may minimize or eliminate secondary operations like grinding and polishing [12]. Very beneficial is also its gap filling potential. Adhesives can bridge large gaps between panels and improve the overall appearance compared to other joining methods. Therefore, in many cases, joining and sealing operations can be combined [5].
2.2. Disadvantages
The adhesive bonding entails some disadvantages as well. Among the most significant is the long-life durability of the adhesive joints when exposed to harsh conditions, such as the presence of water or corrosive environments. Moreover, especially for aluminium alloys, in order to achieve a good durability, in some cases, the substrate will need to be pre-treated. In the next sections, the focus will be on some of the pre-treatment used in automotive industry and on the main known failure mechanisms due to the presence of water or/and corrosive environments.
Besides the over-mentioned disadvantages, other limitations are present too. In fact, adhesively bonded structures (similar to welded structures) cannot be easily dismantled for in-service repairs. Moreover, in the assembly, the joint needs to be supported until the adhesive is cured which, therefore, slows down the whole production process. This is one of the resons for which most of the structural adhesive are not used alone but in combination with another joining techniques (as selfpiercing or riveting) [5].
3. Epoxy Resin
3.1. Chemical Properties
Epoxies are probably the most versatile family of adhesives because they bond well to many substrates and can be easily modified to achieve widely varying properties [13]. The term epoxy, epoxy resin, or epoxide refers to a broad group of reactive compounds which are characterized by the presence of an oxirane or epoxy ring (Figure 2).
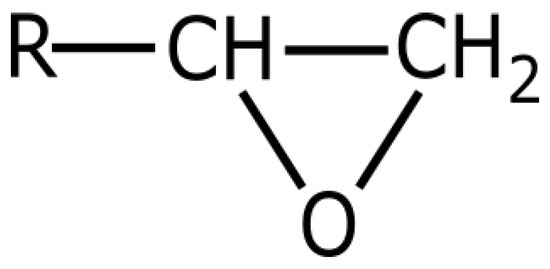
Figure 2.
The epoxy or oxirane ring structure.
An epoxy resin can be any molecule containing more than one of these epoxy groups. The number of epoxy groups per molecule is the functionality of the resin. The group can be situated internally, terminally or on cyclic structures. Epoxy groups are capable of reacting with curing agents or catalytically (homopolymerized) to form higher-molecular-weight polymers. Once cured, the epoxy polymers have a densely crosslinked, thermosetting structure with high cohesive strength and adhesion properties. However, the term epoxy can also be used to indicate an epoxy resin in the thermoplastic or uncured state [14]. A general formula for an epoxy resin can be represented by a linear polyether with terminal groups and secondary hydroxyl groups occurring at regular intervals along the length of the chain. The epoxy structure and properties are influenced by the various chemical groups. An example of an epoxy resin is illustrated in Figure 3 where the role of each chemical group is illustrated as well [15]:
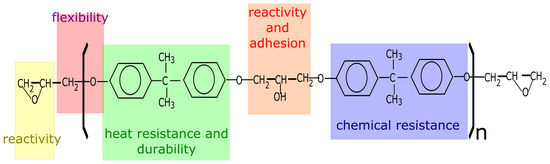
Figure 3.
The structure and properties of an epoxy resin (DGEBA).
- the epoxy groups at both terminals of the molecule and the hydroxyl groups at the midpoint of the molecule are highly reactive
- the outstanding adhesion of epoxy resin is largely due to the secondary hydroxyl groups located along the molecular chain, the epoxy groups are generally consumed during cure
- the large part of the epoxy resin backbone contains aromatic rings, which provide a high heat and chemical resistance
- the aliphatic sequence between epoxy linkages confer chemical resistance and flexibility
- the epoxy molecule can be of different molecular weight and chemistry. Resins can be low viscosity liquids or hard solids.
- a large variety of polymeric structures can be obtained depending on the polymerization reaction and the curing agents involved. This can lead to versatile resins that can cure slowly or very quickly at room or at elevated temperatures.
Epoxy resins commercially produced are not necessarily completely linear or terminated with epoxy groups. Some degree of branching occurs, with the end groups being either epoxy or hydroxyl [15].
3.2. Curing Mechanisms
The epoxy resins can react with different curing agents or with themselves (via a catalyst) to form solid, crosslinked materials with high strength and adhesion in a step usually called curing or hardening. This capability to be mould from a viscous liquid into a tough, hard thermoset is one of the most important characteristics of epoxy resins. In order for the curing to occur, a chemically active compound (as a catalyst or a curing agent) is usually added to the epoxy resin. Depending on the particular details of the epoxy formulation, curing may perform at room temperature, by applying external heat, or with the application of an external source of energy other than heat such as ultraviolet (UV) or electron beam (EB) energy [16].
The main type of epoxy curing reactions are polyaddition reactions and homopolymerization. Both reactions can result in increased molecular weight and crosslinking. Both types of reaction occur without the formation of by-products. The curing reactions are exothermic, and the rates of reaction increase with temperature. Compared to other thermosetting resins, epoxy resins present a lower degree of cure shrinkage. This is due to the fact that they cure primarily by a ring-opening mechanisms. In these reaction processes, the epoxy group may react in one of two different ways: anionically and cationically. In the anionic mechanism, the epoxy group may open in various fashion to produce an anion. The anion is an activated species capable of further reaction. In the cationic mechanism, the epoxy group may be opened by active hydrogen to produce a new chemical bond and a hydroxyl group. Depending on the curing agent and the epoxy resin, curing can take place at ambient or elevated temperatures. Room temperature curing generally cannot achieve the same performance as is obtained by curing the epoxy adhesive at elevated temperatures [13,16].
3.3. Amine Curing Agents
Amines are important curing agents for epoxy resin. They provide fast cures with a relatively high crosslink density. Usually primary amines are used for curing and these can be aromatic, cycloaliphatic or aliphatic. A primary amine has two active hydrogens which are capable of reacting with an epoxy group. Most of the primary amine curing agents that are used have more than one primary amine per molecule so that the crosslinking and, therefore, network development can occur. A secondary amine will react with only one epoxy group. The secondary amines are derived from the reaction product of primary amines and epoxies. They have rates of reactivity and crosslinking characteristics that are different from those of primary amines. The secondary amines are generally more reactive towards the epoxy group than the primary amines, because they are stronger bases. They do not always react first, however, due to steric hindrance. If they do react, they form tertiary amines. In contrast, tertiary amines which have no active hydrogens, will not react with epoxy resins but can cure epoxy resins catalytically, resulting in homopolymerization. Figure 4 shows the reaction between an epoxy and a primary or secondary aliphatic amine [14,17].
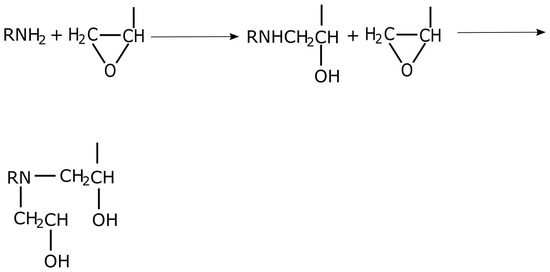
Figure 4.
Mechanism of cure of primary or secondary amines.
The amine curing agent selection depends on the desired mechanical and physical properties, enviromental resistance, pot-life, viscosity, processing etc. In many commercial epoxies more than one amine type will be used to balance the processing and properties. Table 1 shows advantages and disadvantages of different amine-based curing agents [16].

Table 1.
Advantages and disadvantages of common amine based epoxy curing agents. Data from [16].
3.4. Polyamide Resins Curing Agents
This class of curing agents (Figure 5) are made from dimerized unsaturated fatty acid and they are derived from the reaction of the dimer acid with a polyamine. Their reaction with the epoxy resins is through the amine groups and not the amide hydrogens. The presence of a polyamide backbone gives an overall good mechanical property to the polyamide amine cured adhesives. Cure times can last to several hours at room temperature to few minutes at 150 °C [18].
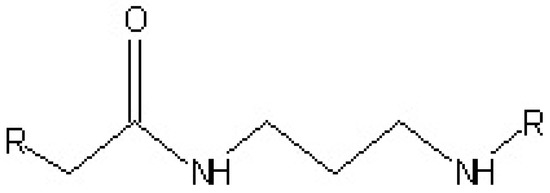
Figure 5.
Chemical structure of a polyamide.
3.5. Imidazoles Curing Agents
Imidazoles (Figure 6) are a type of anionic polymerizing curing agent for epoxy resins. They are characterized by a relatively long pot life, the ability to form resin with a high heat deformation temperature when they are treated thermally at medium temperature (between 80 and 120 °C) for a short time. The curing temperature is between 100 to 180 °C. Imidazoles can be used as a curing accelerator or co-curing agent for organic-acid anhydrides, dicyandiamide, polyhydric phenol, and aromatic amine [14].
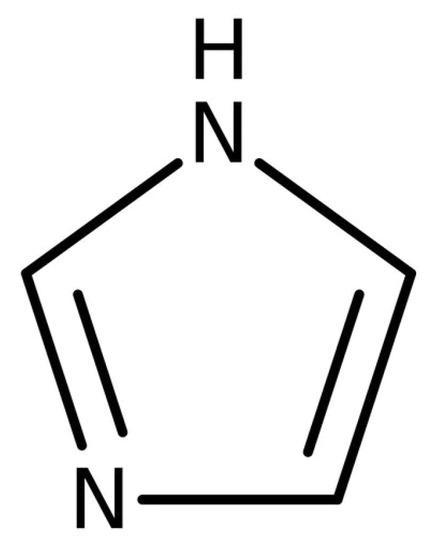
Figure 6.
Chemical structure of imidazole.
3.6. Anhydrides Curing Agents
Anhydrides curing agents are derived from the elimination of water from diacids. As the reaction is reversable, these curing agents needs to be protected from moisture.
Usually anhydrides used for curing epoxy are liquid, but solid dianhydrides have limited use as curing agents in fiber reinforced composite applications. The reactivity of most liquid anhydride curing agents with epoxy resins is very slow without the addition of a catalyst, even at high temperature. The reaction mechanism of anhydrides with epoxies is complex and it includes different competing reactions. The time, temperature, type of accelerator and concentration may have a major effect on the reaction mechanism.
Among the advantages of anhydrides curing agent there are the long pot-life, the low cost, the low viscosity (which is beneficial in composite processes) the excellent thermal stability and low curing shrinkage. The main disadvantages includes their reaction with water when uncured and the susceptibility to hydrolysis when exposed to specific temperatures and humidity, which can dissolve the matrix and reconvert back to liquid in few days [19].
3.7. Latent Curing Agents
Latent curing agents are mixtures of curing agents with epoxy resins which can be stored at room temperature and that can cure rapidly.
Lewis acids (as for example BF3, AlCl3, ZnCl2) react with resins at room temperature. Their pot-life is 30 s or less. Therefore, they are usually used in form of complexes with amines. Lewis acid presents exceptional heat deformation temperature and excellent electrical properties and therefore they have been used in electrical insulating laminates and carbon fiber reinforced plastics (CFRP). Together with Lewis acids, dicyandiamide (DCIY) are used as latent curing agents too. DICY, when dispersed in the resin as powder, can have a long pot life up to 12 months. DICY heat at high temperature (160 to 180 °C) in 20 to 60 min. As it generates a large quantity of heat during curing, it is mostly used as thin films such as paints. Finally, organic-acid hydrazide belongs to the category of latent curing agents. It is a powder which has a high melting point and it is used mostly in powder paints and one-part adhesives. It cures at around 150 °C in 1 to 6 h [20].
3.8. Epoxy Additives
In addition to the two main ingredients of an epoxy formulation, which are resin and curing agent, numerous other formulatory materials are available and have been regularly employed to modify the properties and characteristics of epoxies, both in their uncured and cured form.
Among them there are:
- Diluents They are used to reduce viscosity for both the ease of processability and allowing a greater incorporation of formulatory ingredients. Diluents are used as well to improve wettability [14]. Examples of diluents for epoxy resins include: phenylglycidyl ether, butylglycidyl ether, allylglycidyl ether, butanediol diglycidyl ether and glycerol-based epoxy resins [21].
- Fillers They are the most common ingredient used in the majority of the epoxy formulations. Hundreds of different fillers can be used to modify specific properties of the epoxy. Even if fillers are considered beneficial for most of the applications, the disadvantage is the increase of density (and therefore weight) and viscosity which can influence the way in which the formulation behaves. Table 2 shows a non exhaustive list of fillers which have been used in epoxy formulations [14].
 Table 2. Example of filler types and potential property modification, data from [14].
Table 2. Example of filler types and potential property modification, data from [14]. - Resinous modifiers Resinous materials are sometimes used together with epoxy to reduce the cost or to impart property modifications. Adding resinous materials such as nylon to epoxy has shown to increase the toughness enough to be used as structural adhesives. However, due to the presence of hygroscopic constituents, the use of these systems is limited as they lead to durability problems in presence of moisture [14].
- Flexibilising/plasticising additives Another way to overcome brittle behaviour from adhesives, besides using elastomers or fillers, is by incorporation of plasticising or flexibilising additives. The difference between them is that while plasticisers are long-chain non-reactive molecules, which are not incorporated into the epoxy network, flexibilisers react with the epoxy system during cure [22]. Examples of plasticizers are phthalates or bisphenol A diglycidyl ether, while among flexibilizers there are thermoplastic polymers such as polyvinyl ethers or polyurethanes [23].
- Miscellaneous additives In addition to the additives described above, there are other additives which can be added to epoxy systems. Here two examples are shown. It is a common practice for some epoxy manufacturers to add in the epoxy some coupling agents (such as organosilanes). By adding the coupling agent to the epoxy and not on the substrate, a step in the preparation of the substrate can be skipped. Another example is the use of “expanding monomers” which help reduce the shrinkage occuring during cure [24].
4. Aluminium Surface Preparation Prior to Bonding
As the adhesive is bonding on the surface, the chemistry of the surface prior to bonding is highly important. Therefore, before joining, aluminium substrates undergo a surface preparation which has the aim of [25]:
- Remove the weak boundary layers, including the weak oxide layers formed by heat treatment or exposure to humid atmosphere, air-borne contamination and protective oils and greases
- Enhance the molecular contact between the adhesive and the substrate to promote the formation of intrinsic adhesion
- Create a continuous film on the oxide layer which has a high stability over a wide pH range, protects against hydration, create a barrier against corrosion.Adhesive bonding is a technology applicable for various product forms such as sheets, extrusion and casting. For different aluminium products the preparation procedure or application products may somehow differ but the essential steps are still the same [5]. In the following sections the case of aluminium sheets is considered.
4.1. The Need for Surface Preparation and the Weak Boundary Layer Theory
Bikerman and his colleagues developed in 1950–1960 the early theory on the weak boundary layer. This theory is often discussed alongside the classical adhesion theories (Section 5) due to the fact that when it was formulated, 60 years ago, there was not yet a basic understanding on the adhesion mechanisms.
According to this theory, a weak layer (sometimes referred as interphase) is formed at the interfacial region of an adhesive joint causing the joint itself to fail at lower stresses than expected (Figure 7) [12]. In fact, the surface of the metal is covered by a complex layer which includes contaminants such as residual lubricant and residuals coming from manufacturing processes. This layer will mostly probably form a weak boundary layer which lowers the cohesive strength of the joint. A successful surface preparation will most likely not remove completely the contamination but it will produce a surface which will be less affected by cohesive weakness. However, not all the contaminants will form a weak boundary layer as, in some circumstances, they will be dissolved by the adhesive [26]. Even it is not completely supported today as adhesion’s theory, Bikerman’s theory did stimulate different developments in understanding adhesion. Moreover, thanks to this theory careful attention was put on the adhesive bond preparation to avoid the presence of contaminants [27] such as the residual lubricants coming from the rolling processes (described in Section 4.5).
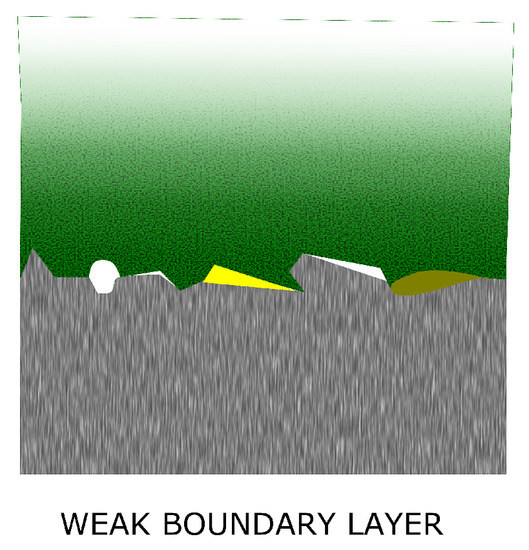
Figure 7.
The weak boundary layer.
4.2. The Aluminium Substrate after the Rolling Process
In order to produce aluminium sheet plates, Al needs to undergo a rolling process, either hot or cold. The main characteristics of the top surface of the the aluminium oxide after rolling are shown in Figure 8.
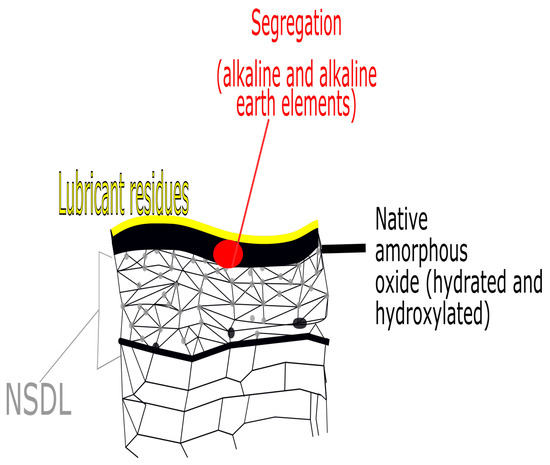
Figure 8.
Main features of the aluminium top surface after rolling. NSDL is the near surface deformed layer which is created after the rolling process.
In metal rolling processes, lubricants are applied to keep the surface of the roll and work-piece separated by a film of solid or fluid. The reason to use lubricants is to both reduce friction forces between the work-piece and the roll and to reduce the possible damage that the work-piece is applying on the surface. The lubricants used during the rolling stages are generally based on paraffin and are volatilised either during annealing or by natural evaporation. However, the rolled surface can still have a certain degree of contamination which will then be removed with a subsequent degreasing step [28].
Aluminium is a very reactive metal with a high affinity for oxygen. Therefore, when exposed to air it will instantaneously form a thin oxide. If the oxide is formed at low temperature (below about 375 °C) it is composed of a thin amorphous Al2O3 about 1–2 nm which is adjacent to the metal with hydrated surfaces oxides and hydroxides on top. The hydrated overlayer is usually composed of hydrated gel-like pseudoboehmite (AlOOH), crystalline boehmite and/or trihydroxides Al(OH)3, bayerite, or gibbsite depending on how much they were exposed to humidity and for how long. The total thickness can be between 2 and 60 nm [29]. Aluminium hydroxides are considered basic and therefore good for interaction with acid polar sites of polymers (as explained later in the acid-base theory). However, hydration (as opposed to hydroxylation) may reduce the overall adhesion performance as it creates weaker basic sites on the top surface. Oxidation and hydration can also be accelerated by the presence of alkaline and alkaline earth elements (such as Li, Na, and Mg) segregated at the surface or at the metal-metal oxide interface [30]. In particular, in case of heat treatment, Mg migrates at elevated temperatures depending on what is the initial Mg bulk concentration and the bulk Al grain size. Even if Mg oxide is basic, unlike Al2O3, it dissolves easily in neutral humid enviroments [31]. In case of heat treatment at higher temperatures (>400 °C) the amorphous oxide may crack due to thermal expansion and nuclei of crystalline -Al2O3 can be formed. If the enucleation of crystalline alumina lead to the loss of cohesion of the oxide with the metal substrate then it may have a detrimental effect on the total adhesion [32,33]. The presence of Mg also promotes the crystalline oxide growth as it acts as a nucleant for -Al2O3. Different heat treatments conditions and Mg content can form different oxides on the top surface such as MgO, Al2MgO4 or Mg doped Al2O3 [32]. Therefore, in Mg rich alloys more complex oxides can be formed. Another characteristic of the alumninium substrate after rolling is the so-called near surface deformed layer (NSDL). When the roll is biting the surface, it can initiate cracks in the oxide of the metal. During the exit of the roll, mill metal or intermetallics can stick to the roll surface and can be picked up by the roll. In successive rolling cycles, these particles are re-depositing on the surface and they can create more imperfections or they can initiate cracks in the oxide layer. Oxides are then lift up by the roll and can be redeposited on other areas of the surface [34]. These properties create a layer on the surface of the aluminium which is different from the underlying bulk [35,36,37,38,39]. This layer is known as the near-surface deformed layer. In their work Fishkis and Lin [35], studied a non-heat treatable aluminium alloy containing magnesium which has undergone different stages of hot rolling. By using a combination of wavelength-dispersive X-ray spectroscopy (WDS) and transmission electron microscopy (TEM), they could observe the different characteristics of the NSDL which are summarized in Figure 9.
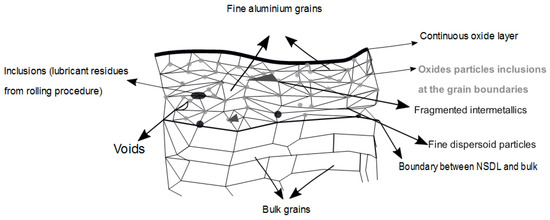
Figure 9.
Schematic representation of near surface deofrmed layer. This schematic representation was created based on the thoery of Fishkis et al. Data from [35].
The NSDL is formed by two different oxide zones: a thin continuous oxide layer and the subsurface layer containing oxide particle inclusions at the grain boundaries. Using TEM they saw that the aluminium grains in the subsurface were in the range of 0.04–0.3 m and the small oxide particles around 25–500 A. Moreover cracks, voids and inclusions were observed in the layer too. The thickness of the NSDL varies between 1.5 to 8 m depending on the rolling mills gauge’s thickness. Regarding the rolling process, the formation of the NSDL was seen for both hot [37,39,40] and cold [37,41] rolling. However, it was noticed [42] that during cold rolling, the incorporation of oxides is lower. From an electrochemical point of view, it was seen by Afseth et al. [43,44,45] that there is a strong correlation between the presence of the NSDL and the susceptibility to filiform corrosion (FFC) for 3xxx and 5xxx aluminium alloys. The influence of the NSDL on FFC will be discussed in Section 6.2.
4.3. Cleaning Step
Before pre-treatment the substrate undergoes a cleaning step. The scope of the surface cleaning is to remove residual oil, smut and surface oxides. For 5xxx alloys the main requirement for cleaning, besides removing the organic residues, is to ensure the removal of Mg rich oxides which was reported to have a detrimental effect on the wet adhesion of the coatings [46,47]. Therefore the cleaning of 5xxx aluminium alloys is less straightforward than for 6xxx [48]. The most used cleaning step for auto-sheets is a mixed acid process. The process bath is sometimes a combination of sulphuric and/or phosphoric acid with, sometimes, small additions of hydrofluoric acid, operated at 50/70 °C. This process is carried out either by spraying or by immersion [5]. For materials which are susceptible to surface-active mode of corrosion, any cleaning process which removes the NSDL leads to an inherently more corrosion resistant substrate. The thickness of the NSDL determines the amount of metal that needs to be removed [48].
4.4. Chemical Surface Pre-Treatments Options in Automotive
Pre-treatments for automotive industry need to be fast and low cost and aim to modify the chemistry of the top layer to increase adhesion and/or anti-corrosion performances.
The pre-treatments used in industry can be divided in three different groups according to the way they act:
- Metal ions and inorganic molecules which react with or precipitate on the oxidized Al to form a mixed oxide
- Coupling agents which promote adhesion
- Anodised film, which modify the aluminium oxide
The first category includes difficult to reduce transition metal oxides, such as such as Hf, Ti, Zr and Ta. These form a very stable oxide in their highest oxidation state. Soluble and mobile precoursors of these oxides are difficult to stabilize in aqueous solution while peroxo complexes and acid fluorides of these elements exist at low pH [49]. Widely used in automotive industry are conversion coatings based on titanium fluoride or a mixture of titanium and zirconium fluoride. The processing baths are based on fluorotitanate (H2TiF6) and fluorozirconate (H2ZrF6) solutions. The treatments can be carried out by immersion or spray or by no-rinse processes [25]. The advantage of using Ti-, Ti/Zr or Zr- oxides is mostly due to their fast and simple application, the possibility to “dry-in-place” and the low temperature of operation [50]. Moreover, it was seen from pull-off tests that the presence of Ti/Zr based conversion coating enhances both the corrosion performance and the adhesion of the substrate [51]. In order to obtain even a higher adhesion with the underlying substrate and to attain a homogeneous coating, organic additives can be added to the conversion baths [50]. The first additive that was used in early studies was poly(acrylic acid) PAA [52,53,54]. Other additives as phenol phosphate [55], silanes [56], and polypyrrole [57], chelating agent such as amino trimethylene phosphonic acid (ATMP) [58] and polyphenol like tannic acid (TA) [59] have been added to conversion baths as well. In all the mentioned cases the performance of the TiZr-based conversion coating was increased by the presence of organic additives.
Among coupling agents, silanes are used the most. The role of coupling agents is to improve the degree of cross-linking in the interface region to obtain an improved chemical bonding. The functionality of the silane can interact to form chemical bonds with both the substrate and the adhesive. Silanes coupling agents have the form R−SiX3, where R is an organic functional group and X is the hydrolysable group [26]. An example of how the silane is binding with the metal, with the formation of a covalent bond, is shown in Figure 10. The advantage of silanes is that they are simple and stable, mostly due to their covalent cross-linked structure [60]. Moreover, in addition to covalent bonding, other factors which have been proposed for the effectiveness of silanes include the improvement in surface wettability and the capacity of silane layers to be deformable and relieve internal stresses [61]. However, among the drawbacks of the use of silanes is their difficult to be stored which leads to a relatively short shelf-life [62]. Organophosphonic –acid based coatings have been used as well as coupling agents due to their excellent properties. It has been shown that organophosphonic acids form very stable monolayers on aluminium alloys covered with a thin oxide film [63,64,65,66,67]. It was seen that the presence of phosphonate monolayers enhances the adhesion with the aluminium oxide [68]. Moreover, having a bi-functionality enables the binding of the phosphonic acid or its anion to the oxide surface while the other group can react with the organic phase of the adhesive or coating [69]. Organophosphonic acids can be applied on the surface via dip coating or spraying [70].
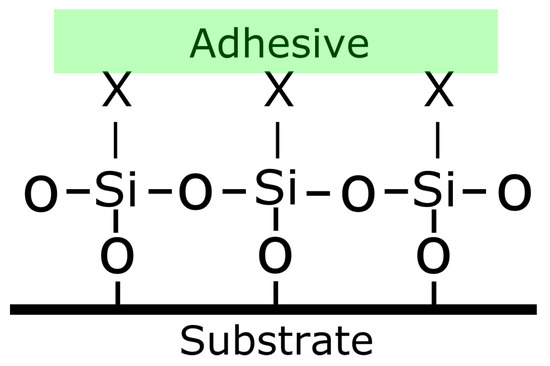
Figure 10.
Interaction between a silane coupling agent and the metal substrate.
Another form of pre-treatment consists in modifying the aluminium oxide by forming anodised films.Thin anodised films are created by AC or DC powered electrolytic processes. The oxide film is formed by an amorphous barrier layer, which gives resistance to corrosion, and a porous top which provides adhesion to adhesives or primers [5]. The anodising has been performed with both sulphuric and phosphoric acid baths or boric sulfuric acid baths [71]. The performance of the treatment used is function as well of the adhesive used. Correira et al. [72] performed an experimental study on single lap joints pre-treated with sulfuric acid anodizing and boric-sulfuric acid anodizing on aluminium-to-aluminium joints and using two similar structural adhesives for aerospace from different manufacturers. The results have shown that the optimal surface treatment is different for each type of adhesive, leading to differences in mechanical behaviour.
In automotive, it was developed the use of thinner anodized films (thin film anodising, TAF) prior to bonding for the second generation of Mercedes CLS. For the low volume Lotus Elise and Opel Speedster sports cars, a sulphuric acid anodising (SAA) on AA6060 aluminium was employed. The oxide formed in the SAA has a thickness of about 5 m which provides both adhesion and good corrosion resistance of the uncoated structure [25]. The advantage of using thin anodised films over other pre-treatments is that as they consist purely of aluminium oxide, they represent an environmental friendly alternative to transition-metal based pre-treatments. Moreover, the thickness and the morphology of the finished oxide can be easily controlled by applying a different range of potentials and voltages. However, the drawbacks include higher operation costs and low volume requirements.
4.5. The Effect of the Stamping Lubricant
After the pre-treatment process, a stamping lubricant is usually applied on the metal substrate to improve the material formability and to protect the substrate prior to bonding. The amount of oil applied is approximately 0.9 g/m. However, if the oil used is not a dry-film lubricant, there will be an inhomogeneous distribution on the surface due to run-off. Therefore, a higher amount of oil could be present in some areas [73]. Lubricants are not necessarily removed in the stamping plants and, therefore, it is important that the adhesives are compatible with the processing lubricants. Therefore, the adhesive must be able to either displace or absorb any applied lubricant [5]. Several studies have been performed to analyse how the epoxy adhesive may accommodate the oil in order to produce strong interfacial bonds with the substrate [74,75,76]. Debski et al. [74,75] proposed that, in case of an apolar oil, there is thermodynamic displacement of the oil by the epoxy followed by absorption by diffusion. Ogawa et al. [76] found that the oil layer disappears in the first steps of curing as the diffusion of the oil into the adhesive increased abruptly with temperature. Therefore, according to their study, the presence of oil does not affect the perfomance of the joint. According to Greiveldinger et al. [77] the oil diffuses over several hundred of microns when cured (therefore the typical thickness of the join), but still leaving some residues on the interface. Moreover, it was found that the crosslinking of the adhesive only starts when oil diffusion is complete and, consequently, does not affect the kinetics or mechanism of adhesive cure. Different studies have been performed to study the effect of the lubricant on the mechanical performance. Zhang et al. [78] studied that the strength of adhesive joints of AA6111/high-strength-steel (HSS) with various lubricant masses and observed that increasing the amount of lubricant slightly decreases the joint strength. Zheng et al. [79] studied the effect of a hydrophobic lubricant on the adhesive joints of different aluminium alloys. From the strength point of view, they found that below a certain value (2.21 g/m), the lubricant itself had no effect on the strength, while above this value the oil would decrease adhesion. Moreover, the lubricant itself was increasing the corrosion performance of the joint protecting the substrate from possible electrochemical reactions.
5. Adhesion Theory
It is not completely clear which is the mechanism behind adhesion. Several theories have been attempted to describe this phenomenon. However, no single theory can give a comprehensive mechanism of adhesion but each look to be more feasible for specific substrates and applications rather than for others. The most common theories are described in the following sections. The most relevant theories to explain the bonding between the metal substrate and the epoxy are the mechanical and the adsorption theory. A schematic representation for the different adhesion theories is illustrated in Figure 11.
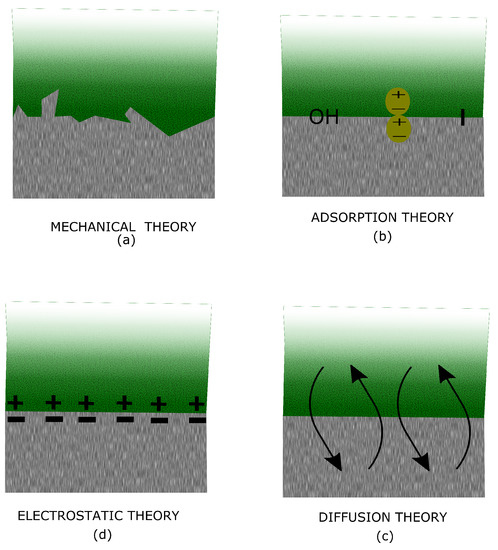
Figure 11.
Schematic view of the different adhesion theories: mechanical (a), adsorption (b), electrostatic (d) and diffusion (c) theory.
5.1. Mechanical Theory
According to this theory, the degree of adhesion for a bonded joint is directly linked with the porosity and roughness of the substrate. The adhesive must fill the cavities of the adherends’ surface in order to achieve an intimate contact referred to as mechanical interlocking. Experimental results show an increase in joint strengths after mechanical roughening of the surface, supporting this theory [80]. It was seen that in case of anodized aluminium substrates, when a porous open structure is formed, the adhesion strength measured was even higher [81,82,83] than in the case of a non-anodized substrate. Nonetheless, when the roughness is excessive there could be an incomplete wetting of the surface and voids may be created which can act as concentration points initiating failure [26]. Criticism against this theory are based on the fact that good adhesion can anyway be obtained when using a smooth surface [84]. Boutar et al. [85] studied the effect of surface roughness on the strength of single lap joints. It was seen that changes in the roughness are not enough to explain a change in strength and other factors such as physical and chemical properties of the interface need to be taken into account as well.
5.2. Adsorption Theory
According to the adsorption theory, macro-molecules of the mobile phase are adsorbed onto the substrate by forces which range from weak dispersion forces to chemical bonds. As a consequence, an “interface” is formed. The typical strengths from different kind of interactions are shown in Table 3 [6].

Table 3.
Typical strength of attraction forces, data from [6].
5.2.1. Van der Waals Interactions
Van der Waals forces are the one responsible for the physical adsorption. These involve attractions between permanent dispoles and induced dipoles and are of three types: London, Keesom and Debye interaction. Even if these interactions are weak (in comparison with chemical bonds), as they occur between any two molecules in contact, they contribute to all adhesive bonds. By measuring the contact angle it is possible to asses the Van der Waals forces and to predict the stability of the joint by calculating the thermodynamic work of adhesion [12].
5.2.2. Chemical Bonds
Chemical bonds are considered as primary bonds when compared to the physical interactions which are called secondary force interactions. The choice of this nomenclature comes from the strength of the interaction as it can be seen from Table 3. The formation of a chemical bond depends on the reactivity of both the adhesive and the substrate. Different types of chemical bonds can be formed according to their strength. The covalent bond is usually the strongest and the most durable. The presence of covalent bonds was found from some studies on the action of organosilanes promoters used to bond epoxies to metal and glass surfaces [86]. It has been long suggested that the effectiveness of silanes was partially associated with their capability to form a covalent bond between the silane and the metal surface [87,88,89]. Adhesive can bond to the substrates in a different manner as well. It was shown by different studies [90,91,92,93,94,95,96], that when a carboxylic acid is in contact with a metal oxide substrate, a carboxylate bond is formed (COO−). This bonds forming an ion with the substrate. Brand et al. [97] studied the interaction of different carboxylic acid based model compounds with differently prepared aluminium substrates via infrared reflection absorption spectroscopy (FTIR-RAS). Their study shows that all the carboxylic acid groups are deprotonated to form a carboxylate bonded with the alumina substrate.
5.2.3. Acid-Base Theory
Fowkes [98] was the first that considered that the interaction between two materials has two main contributions. A contribution due to dispersion interactions in the form of a geometric mean relationship and a contribution of an acid-base interaction. The acid-base interactions are considered the most important interaction which exist across the metal-polymer interface. This theory is based on the interaction between an acceptor and a donor of electron pair. In acid-base interactions both covalent and non-covalent factors are involved [99]. An acceptor is a molecular system which has unoccupied levels and an affinity to electrons. A donor, on the other side, is a system which has a lone electron pair. According to Brønsted, an acid is a proton donor and a base is a proton acceptor. These reagents may be both molecules or ions. The reaction of transfer of a proton is referred to as protolytic. A protolytic reaction is a reversible reaction. Therefore a proton transfer can occur also in the reverse process, the reaction products can act as an acid and a base relative to each other. The acid-base properties of substances are dependent on the thermodynamics of protolytic reactions. An example of this is HCl with H+ the acid and Cl− the conjugated base [84]. Lewis definition of acid-base interactions (1923) states that a basic substance is one which has a lone pair of electrons which may be used to complete the valence shell of another atom (the acid) and that an acid substance is one which can employ a lone pair from another molecule (the base) in completing the valence shell of its own atoms [100]. The hydrogen bond is a sub-class of the Lewis base interactions. A hydrogen bond can exist between two electronegative atoms or groups of which one has a hydrogen atom covalently attached to it. A larger electronegativity of the attached atom results in more electronic charge withdrawn from the proton. Therefore, the H is charged more positively and can form a stronger hydrogen-bond. A molecule which contains hydroxyl (OH) group can act as a base through the O or as a acid through the H [101].
If the oxide surface of a metal is exposed to the environment or immersed in aqueous solutions it forms hydroxyl groups due to the interaction with water molecules. When exposed to an aqueous solution the surface hydroxyl groups remain undissociated if the pH of the the aqueous solution is the same as the isoelectric point (IEP) of the oxide. If the pH is less than the IEP the surface will have a positive charge
If the pH is greater than the IEP, the surface will acquire a negative charge
or
In the first case, the surface species is a Brønsted acid because it is a proton donor. is a Brønsted base because it is a proton acceptor. Therefore, is amphoteric as it is both an acid and a base [102].
Using the definition of Lewis for acid and bases the types of acid sites on metal oxide surfaces are (with being the partial charge):
- Unhydroxylated metal ions, M
- Protonated surface hydroxyls,
- Surface hydroxyls, −
The basic sites are
- Unhydroxylated oxygen anions,
- Dissociated surface hydroxyls,
- Surface hydroxyls,
Therefore a metal surface can be seen as a sequence of Lewis acid-base and Brønsted acid-base sites [101].
What is present on the surface depends on the metal oxide and on the preparation of the oxide itself [101,103,104,105]. By using photoelectrochemistry, XANES and XPS, Lopez et al. [106,107] studied the basicity of differently pre-treated aluminium surfaces. Formic acid and pyridine were used to probe respectively surface Brønsted basic and acid sites and it was observed that a Mg-containing oxide has the highest basicity while the alkaline detergent degreased (sodium silicates and phosphate) has the lowest.
The acid/base theory has been used to identify the type of interactions which occurs between an epoxy and a substrate. Nakemae et al. [108] studied an epoxy resin/4,4-diaminodiphenylmethane (DDM) curing agent system on oxidized aluminium via XPS, FTIR and contact angle measurements. It was seen that the curing agent (DDM) is preferentially adsorbed onto the Al2O3 substrate while the epoxy resin was adsorbed on Al2O3 particles whose surface was covered with the epoxy. From their study they concluded that the interaction between the cured epoxy resin and the oxidized aluminium interface was an acid-base interaction between amino groups of the curing agent and acid sites of the oxidized aluminium. Similar results were found by Hong et al. [109] which studied the behaviour of an epoxy/amidoamine system on iron, aluminium and zinc oxides and they saw that the amidoamine curing agent is preferentially adsorbed on the three metal oxide surfaces due to the surface acid-base interaction between the metal surface and the curing agent. Therefore, curing agents are often used as model epoxy systems to study the interactions between the epoxy and the metal oxide. Abel et al. [110] studied the interaction between diethanolamine (DEA) and two substrates: grit-blasted aluminium and aluminium coated with an organosilane adhesion promoter, -glycidoxy propyl trimethoxy silane (GPS) by X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS). It was found that on oxidized aluminium DEA interacts with the surface via the formation of a hydrogen bond or via donor-acceptor interaction with an aluminium atom. When deposited onto GPS-coated aluminium, DEA undergoes two types of interactions: formation of covalent bond with nucleophilic addition or Brønsted type of interaction between nitrogen of DEA and silanol functionality of hydrolyzed GPS.
5.3. Diffusion Theory
The diffusion theory suggests that the adhesion is due to an interdiffusion of molecules in and between the adhesive and the adherend. This theory is applicable when both adherend and adhesive are polymers with relatively long chain molecules capable of moving. An interphase layer will be formed typical in the thickness range of 10–1000 A. As no physical discontinuity exists in the interphase area, there will be no stress concentration. To interpret the diffusion bonding, cohesive energy density (CED) can be used as defined in Equation (1) where: is the amount of energy required to separate molecules to an infinite distance, V is the molar volume and is the solubility parameter. Bond strength is maximized when solubility parameters between adhesive and adherend are similar.
There is a small number of polymer pairs which make this interaction possible. Schreiber and Ouhlal [111] annealed a number of polymer pairs in contact for up to 72 h at 60–160 °C and found an increase in adhesive strength for polypropylene/linear low density and polyethlene and polystyrene/PVC but not for polystyrene/PMMA and PVC/polyvinylidene chloride. Their data shows that there are significant contributions to bond strength coming from diffusion when dispersion forces and acid-base interactions are favourable at the interface [112].
5.4. Electrostatic Theory
This theory proposes that there is an electrostatic effect which joins adherend and adhesive. An electron transfer takes place between the adhesive and the adherend as results of unlike electronic band structures and a electric double layer is formed between adhesive and adherend. According to this theory adhesive and adherends which contain polar molecules or permanent dipoles are most likely to form electrostatic bondings. As polymers are insulators it seems difficult to apply this theory to adhesives [113]. However, this theory was supported by the study of Randow et al. [114] which investigated the adhesion of some commercial films used in food packaging to glass, steel and polyolefin substrates. The films were made of PVC. It was seen that after separation there was a residual electrical charge on both film and substrate and that all the films showed sparking when repeatedly applied to glass [112].
6. Environmental Degradation of Adhesive Joints
One of the main drawbacks of adhesive joints is their long term durability when exposed to environmental conditions. In the following section the effect of water, corrosive environment and external stress of the durability of the adhesives joints will be discussed.
6.1. The Effect of Water
Water can enter the bonded system by bulk diffusion through the adhesive, interfacial diffusion along the interface between the adhesive and substrate, and by capillary action through cracks or defects in the adhesive or conversion layer. Moreover, it can affect the system by either modifying the adhesion properties or by displacing the adhesive at the interface causing failure [12].
6.1.1. The Effect of Water on The Adhesive
All adhesives, even if in different extent, absorb water. The diffusion of water in polymers follows Fick’s law.
where F is the flux or rate of transfer per unit area, c is the concentration of the diffusing substance, x is the space coordinate measured normal to the section, and D is the diffusion coefficient. Furthermore, the diffusion of water in structural adhesives follows the Arrhenius equation, and therefore the rate of diffusion increases strongly with temperature [12].
Once it reaches the joint, there are several ways in which the water can affect the adhesive. These, reviewed by Comyn (1983), include the following:
- causing plasticization by altering the properties of the adhesive in a reversible way
- causing the adhesive to crack, craze or hydrolize, in this case the properties of the adhesive are altered in an irreversible manner
- attacking the adhesive-adherent interface
- causing stress due to swelling
Weitsman [115] studied the stresses introduced into an adhesive joint by water swelling. In the case of an epoxide adhesive, the joints were evaluated to swell 3% in water and the stresses were localized at the edges of the joint. However, due to drying the stress concentration was decreasing with time suggesting that swelling does not give a contribution on the long term. Morsch et al. [116] studied the effect of water uptake on epoxy-phenolic coatings. It was seen that the water uptake is not completely a reversible process. In fact, in case of pre-soaked and dried coatings, an exposure to humid environment led to a greater amount of water absorbed. This was interpreted as a result of water plasticized macromolecular relaxation mechanism which resulted in the formation of nanoscale hydrophilic regions in the epoxy during immersion. Xu and Dillard [117] exposed electrically conductive adhesives to saturated air at 85 °C for up to 50 days and then dried the samples at 150 °C. They measured water absorption and mechanical properties. Part of the joint strength was recovered, indicating that some reversible processes are taking place and that interfacial irreversible phenomena are of greater impact in the failure of the joint than bulk properties of the adhesive.
6.1.2. The Effect of Water at the Adhesive Metal Oxide Interface
While water uptake in the bulk follows Fick’s law, the speed of water diffusion at the interface is higher, and it is attributed to the capillary diffusion occurring between the adhesive and the adherends [118].
The water penetration process to the adhesive/metal oxide interface was analyzed by Gledhill and Kinloch [119] based on thermodynamics. They defined the work of adhesion as the energy required to separate the unit area of two phases which are forming an interface. In an inert medium the work of adhesion is expressed as:
where and are the surface free energies of the adhesive and substrate, respectively and is the interfacial free energy. In the presence of a liquid, the work of adhesion is:
where and are the interfacial free energies between the adhesive-liquid and substrate-liquid interfaces, respectively. For an adhesive-substrate interface, in an inert environment, the work of adhesion always has a positive value, indicating thermodynamic stability of the interface. However, in the presence of a liquid, the value of may have a negative value, indicating that the interface is now unstable.
Some values of work of adhesion for different adhesive/substrates are shown in Table 4 [26]. The thermodynamic approach shows that substrates with high energy, such as metal oxides, are very susceptible to water to displace the adhesive from the substrate. However, it is not straightforward to give a complete description of the metal/polymer adhesive interface. This is due to the tendency of the metal oxides to hydrate when exposed to the atmosphere.

Table 4.
Values of work of adhesion for various interfaces in dry air and water, data from [26].
When water interacts with an aluminium substrate it will form hydrated oxides according to the reactions:
If the hydration occurs under an adhesive, the resulting increase in volume may induce high stresses and crack growth [25]. Therefore, the adhesive, to tightly bond with the substrate, must reach through layers of water molecules to reach the hydroxyl base connected to the metal surface. Water can act both as acid or base. Thus, when there is a weak acid or basic group present at the interface, water can easily penetrate and lead to eventual destruction of the adhesive/substrate interface. The approach mentioned above is only valid when the forces are a result of van der Waals forces and not due to stronger primary forces. Therefore, pre-treating the surface and creating primary bonds with structural adhesive leads to an improvement on the adhesive performances [120].
The stability in water between organic coatings of PAA and PMMA and aluminium oxide was studied by Pletincx et al. [96,121] by mean of in-situ techniques such as ambient pressure X-ray photoelectron spectroscopy (APXPS) and attenuated total reflectance Fourier transform infrared spectroscopy in the Kretschmann geometry (ATR-FTIR Kretschmann). For both cases it was seen that when water reaches the interface, the water molecules deprotonate the carboxylic acid forming carboxylate ions. The carboxylate ions are then reacting with the surface hydroxyl groups of the aluminium surface to form ionic bonds and hydroxide ions. However, after a certain exposure time it was seen taht the interfacial interactions are destroyed by water, which eventually leads to macroscopic delamination [122].
Abrahami et al. [123] studied the adhesion of epoxy resin as a function of the surface chemistry of barrier-type anodic oxides prepared in sulfuric acid (SAA), phosphoric acid (PAA), and mixtures of phosphoric-sulfuric acids (PSA). It was seen that, bonding stability under wet conditions is highly influenced by the surface chemistry. The wet adhesion strength increases with the hydroxyl concentration at the aluminum (oxide) surface, indicating that interfacial bonding is established through these surface hydroxyls. In this study the presence of phosphates and sulfates anions were not found to contribute to bonding with this type of adhesive.
6.1.3. Adhesive Joint Strength in Humid Environment
Brewis, Comny et al. [124,125,126] exposed aluminium joints with different structural adhesives at 50 °C and 100 % RH. It was observed that the strength decreases significantly. However, when the same samples were exposed successively for the same amount of time at 50% RH and 50 °C, the strength would partially recover. Therefore, it seems that the mechanism responsible for the decrease in joint strength in humidity is at least partially reversible. Similar results were observed by Mubashar et al. [127] on AC anodized single lap joints of AA7075. The weakening of the joints is function of the humidity at which they are exposed as well. Although joints decrease in strength at high humidity (80–100 % RH), it has been frequently observed that joints can withstand exposure at lower humidity (lower than 50%) for long periods without weakening. Brewis et al. [124] found that exposing aluminium adhesive joints for 10,000 h at 45% RH and 20 °C did not significantly decrease the joint strength. Gledhill et al. [128] proposed that there is a critical concentration of water in the adhesive above which the joints will be negatively affected (1.35 g/100 g). Wang et al. [129] studied the lap-shear strength of an adhesive-bonded 5052 aluminium alloy exposed to neutral salt spray for up to 1200 h. It was seen that the lap-shear strength of the joints decreased sharply in the first 240 h. The main reason for this was associated with the strong polar water molecules which dissolve the hydrogen bonds responsible of the adhesion. Few studies have been done on the effect of the Ti/Zr conversion coating on the strength of the adhesive joint exposed to humid environments. Critchlow and Brewis [130] studied the effect of zirconium-based pre-treatments on AA5251 on single lap shear joints immersed in water at 60 °C and they observed an increase in strength compared to degreased-only or grit-blasted substrates. Lunder et al. [131] studied the effect of Ti/Zr pre-treatment on the durability of epoxy-bonded AA6060 aluminium joints. According to their findings, the Ti/Zr based pre-treatment improved the adhesion of the epoxy bonded aluminium, but it was still performing worse than a chromate pre-treatment.
6.2. The Effect of Corrosion on the Durability of the Adhesive Joints
H2O can diffuse directly through the organic coating but most of the adhesive are a good barrier for ion transport due to their low dielectric constant and small free volumes due to the high cross-linking network. However both defects or cut-edges can allow the ions to reach the polymer/substrate interface.
The corrosion protection performance depends also on the adhesion of the coating layer to the substrate. When the bonding between the coating layer and the substrate is strong, so that the penetration of water into the interface is difficult, the corrosion does not develop fast. However, when the bond is weak, the corrosion due to the presence of water/ions can easily propagate at the interface [120,132,133].
Wu et al. [134] studied the effect of long-term salt spray (50 g/L of salt solution) on the strength of Zr-Ti coated and bare lap-shear aluminium joints bonded with hem flange. It was seen that, for the first 130 h the Zr-Ti protected the aluminium substrate from electrochemical reaction, therefore reducing the joint degradation. However, after 1400 h, the Zr-Ti coating provided a worse protection than the bare aluminium.
For adhesive joints, the most relevant form of corrosion, which will be discussed in the following section, is filiform corrosion. However, even if not explicitly reported in literature, it is not excluded that other forms of corrosion with a slower kinetics may affect the durability of the system.
6.2.1. Filiform Corrosion
Filiform corrosion (FFC) is a form of atmospheric corrosion which occurs under organic coatings in the form of narrow interconnected thread-like filaments [135].
FFC was observed for the first time in 1944 on steel, while on aluminium it was observed for the first time in the late 1960’ s where it occurred around rivet heads and from the edges of the aluminium skins on certain aircrafts exposed to aggressive tropical environments [136,137].
In case of polymer layers on non-conductive oxide surfaces, like on Al-alloys, filiform corrosion is due to anodic delamination. Anodic undermining corresponds to a situation in which the loss of adhesion is caused by the anodic dissolution of the substrate. The metal at the edge of the filament is therefore anodic. While on steel the anodic delamination usually occurs under an applied potential, aluminium substrates are particularity prone to anodic undermining. The main environmental factors that are crucial for the initiation and proliferation of this form of corrosion were found to be relative humidity (above 80%), the presence of aggressive ions such as Cl− and defected sites in the coatings. The filaments are made of an “active head”, which is filled with liquid, and a “tail”, with dry corrosion products and it may reach the length of several centimetres [138].
The primary driving force for filament advancement is thought to be an oxygen concentration cell which forces the anodic metal dissolution reaction (Equation (2)) in the case of aluminium to occur at the leading edge and cathodic oxygen reduction (Equation (3)) to occur at the trailing edge of the active head.
Reaction (1) and (2) will occur simultaneously. The metal therefore adopts a mixture potential relative to the electrolyte, known as the free corrosion potential, which lies between the equilibrium potentials of the couples present and is determined by their relative reversibility.
William et al. [138] described in their work the kinetics and the mechanism of FFC using a scanning kelvin probe (SKP). A defect was formed on epoxy coated aluminium alloys which were successively exposed for some time to HCl vapour. The exposed samples were than placed in the SKP which was maintained at 50 °C and 95 % RH. The proposed initiation and propagation mechanism is explained here.
Initiation. When the adhesive joint is exposed to high humidity, several layers of water molecules are formed on the bare aluminium which is exposed due to the scratch. As the transport of O2 is much easier through the thin electrolyte than through the polymer film, the reaction in Equation (3) will occur on the bare metal. On the other side, the reaction in Equation (2) will occur where there is a deficiency of O2, thus on the metal/electrolyte/coating interface. Therefore a local anode in which Al is dissolved will be formed. As the Al dissolution proceeds the Cl− ions will migrate beneath the delaminated coating to preserve electroneutrality and water will be drawn in by osmosis to produce an electrolyte droplet (Figure 12a).
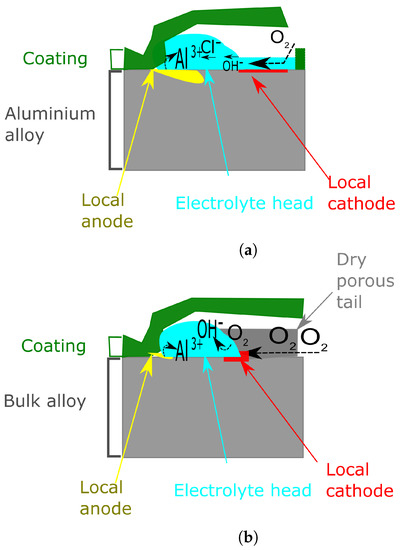
Figure 12.
Schematic diagram showing the process of initiation (a) and propagation (b) of FFC on aluminium. This schematic was based on the theory illustrated byWilliams et al. Data from [138].
Propagation Once the corrosion process starts, it will try to propagate. The reaction described in Equation (2) occurs mostly at the leading edge. The cations created at the leading edge will than migrate towards the trailing edge and will combine with OH anions generated by the reaction in Equation (3). Water insoluble products will eventually precipitate. Cl− anions will keep moving towards the anodic leading edge and all water soluble ions and all liquid water molecules will be in the FFC active head. The Al(OH)3 corrosion products left behind will slowly lose water and convert into porous hydrated aluminium. However, O2 will easily pass through Al2O3 and hence filament propagation will be maintained over important distances (Figure 12b).
Alloying elements and especially the presence of electrochemically noble second phase particles play an important role for FFC to occur. The intermetallic particles in aluminium alloys may either act as local cathodes or anodes. Afseth et al. [44] studied the corrosion mechanisms of 2xxx series Al-alloys showing that the enrichment in Cu creates areas for cathodic reactions to occur. Therefore, when a filament propagates, it will use the intermetallic particles which are present at or near the surface, to jump from particle to particle. The detrimental effect of Cu enrichment at the surface was seen as well by Colemal et al. [139] on a high levels copper AA6111. Regarding the role of surface pre-treatments to protect from filament corrosion, Lunder et al. [131] studied the effect of Ti/Zr pre-treatment on AA6060. In their study it was found that this did not provide significant protection against FFC as the Ti–Zr oxide deposit did not inhibit the cathodic activity on the electrochemically active particles. Moreover, Coleman et al. [140], dissolved phenylphosphonic acid (H2PP) in a polyvinyl butryal coating deposited on AA6111-T4 and followed the FFC, after application of HCl in a penetrative coating defect, with use of SKP. They saw that the presence of the H2PP inhibits the propagation of the filament by reducing up to 0.35 V (compared to a coating without H2PP) the difference in free corrosion potential between the intact and the scratched part of the sample.
6.2.2. The Effect of the Near-Surface Deformed Layer on Filiform Corrosion
It was demonstrated that there is a strong correlation between the presence of a NSDL and the susceptibility to corrosion, in particular FFC [139,141]. This is due to the fact that the NSDL is more reactive than the bulk, promoting therefore rapid delamination. In their work, McMurray et al. [142] investigated, by use of SKP, the effect of NSDL on AA6111. Moreover, they proposed a delamination mechanism which is presented here. According to their work the delamination process in presence of a NSDL proceeds in two different phases. The first part consists of the intergranular anodic attack. However, as the NSDL presents a high density of grain boundaries and small grain size, this will result in the consumption of the NSDL. Therefore, the propagation of the filament can be distinguished in 4 different areas as shown in Figure 13a.
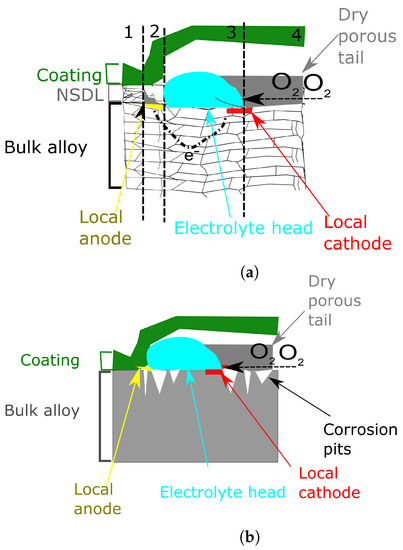
Figure 13.
Schematic diagram showing the mechanism of FFC in the presence of NSDL. This schematic was based on the theory illustrated by McMurray et al. Filament propagation (a) and successive pitting (b). Data from [142].
- Area 1, not corroded with NSDL still present
- Area 2, anodic site with dissolution of the NSDL
- Area 3, in which the NSDL is com ely dissolved and the bulk aluminium acts as cathodic O2 reduction site
- Area 4, comprising a dry porous tail as described in the previous section.
It was shown that, in presence of a NSDL, together with differential aeriation (which is still the most important driving force for the FFC to occur), an additional force is represented by the difference in potential of the NSDL and bulk material which will lead to anodic activation.
In the second phase, there is a proceeding of the FFC. However, as the NSDL has been already consumed, the bulk metal will not be cathodically protected by an anodically active NSDL. Therefore, a successive-pitting FFC will be present (Figure 13b). Again, in this case, the driving force is the differential aeriation arising from the ease at which atmospheric O2 diffuses through the porous corrosion products.
6.3. The Effect of Static and Dynamic Stresses on the Durability of the Adhesive Joints
One of the main advantages of using adhesive joints over mechanical fastening, is the more uniform stress distribution over a large area. However, this does not imply that loads are uniform or well understood in adhesive joints.
Applying a stress will cause an adhesive bond to degrade at a faster rate than an unstressed bond especially if the bonds are subject to high loads for prolonged periods.
Adhesively bonded joints can be exposed to both static and dynamic stresses. The stresses are not only due to external loads. They can in fact originate as well from adhesive shrinkage (after curing), adhesive swelling (water adsorption) or from a thermal mismatch between adhesive and adherend. Moreover, stress can also accelerate other processes such as the rate of diffusion of moisture in the joint [143]. The effect of the stress on the durability can be determined by the time that the joint need to fail when imposing a given load or by imposing a load for a certain time.
Stress-durability testing of adhesively bonded joints is a common method to classify the performance of the joint. Marceau et al. [144] compared the performance of AA2024-T3 bonded joints under static and cyclic loads in different controlled environments. It was seen that an increase in temperature was leading to a shortened life of the lap shear subjected to fatigue. Ashcroft et al. tested different CFRP (carbon firber reinforced polymer)- epoxy lap-straps under fatigue in different environments. They showed that samples in hot-wet conditions experience a significant reduction in the fatigue threshold [145]. Samples were even conditioned at high humidity conditions until saturation. For wet tested samples at 22 °C there was no change in the fatigue threshold while for samples tested at 90 °C in both wet and dry conditions, they experienced a large reduction in fatigue threshold.
The effect of environmental exposure on the fatigue of mild steel joints bonded with different adhesives has been investigated as well [143]. Double lap shear joints were kept under different loading and environmental conditions for 8 years. It was seen that the adhesive used had a big impact as some were showing excellent durability while others were adversely affected by the environment. The adhesives which showed a better performance were the ones cured with polyamide hardeners and with an initial high strength and Young’s modulus.
So et al. [146] studied the fatigue response of epoxy bonded poly(methyl methacrylate) (PMMA) and PMMA to aluminium joints. It was seen that the PMMA-to aluminium joints were more sensitive to temperature than PMMA-to-PMMA joints and for both systems the stress applied had a higher influence than the frequency.
Small and Fay [147] studied the creep of steel lap joints in high humidity. A single-part, heat curing epoxy was used as adhesive and the joints were subjected to a creep test for three and a half years at 70 °C and 45 °C at 95% RH. The time to fail was ten times faster in humid conditions than at dry conditions at high temperature. For dry conditions the failure was associated with a combination of creep of the adhesive and peeling of the adhesive from the adherends. Moreover it was seen that the way the joints are assembled has an influence on the joint durability [148,149].
A number of techniques have been used to model fatigue and creep in bonded joints. The best is to combine load-life approach together with monitoring techniques, like embedded sensors, and modelling analysis such as finite element analysis (FEA) [150].
7. Conclusions
Adhesive joints are an important joining technique in the automotive industry as their introduction into car manufacturing can be viewed as one of the main enabling technologies for the production of all-aluminium car body structures. One of the main issues related with a wider use of adhesive joints is the durability of these systems when exposed to service conditions where water, corrosive environments and external stresses are present. This work presents the main parameters influencing the durability of adhesive joints. The different preparation phases which lead to the final adhesive system were analysed. It was seen how the top surface’s chemistry, which has an important role in the final adhesion, changes in each step. In particular, a wide variety of surface pre-treatments can be applied on the substrate to obtain a beneficial effect on the adhesion/corrosion protection. The way in which the substrate interacts with the epoxy, is explained by the different classical adhesion theories. In particular, the adsorption theory is the most relevant for the polymer/metal oxide bonding and it was used to describe a different range of interactions between the adhesive and the substrate. It was seen how those interactions can deteriorat by the presence of water/moisture or corrosive ions. Water can in fact reach the epoxy/substrate interface displacing the adhesive at the interface and causing interfacial failure. Moreover, water can be absorbed by the adhesive and weaken it. The presence of ionic species, together with high moisture content, can lead to the corrosion of the substrate. In particular, adhesive joints are prone to filiform corrosion. The detrimental effect of water or corrosive ions seems to be accelerated by the presence of dynamic or static stresses.
Author Contributions
Writing—original draft: F.C. Supervision: T.H., H.T., M.B. Review: T.H., H.T., M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Njuguna, J. Lightweight Composite Structures in Transport: Design, Manufacturing, Analysis and Performance; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferr. Met. Soc. China 2014. [Google Scholar] [CrossRef]
- Crolla, D.; Ribbens, W.; Heisler, H.; Blundell, M.; Harty, D.; Brown, J.; Serpento, S.; Robertson, A.; Garrett, T.; Fenton, J.; et al. Automotive Engineering e-Mega Reference; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Barnes, T.; Pashby, I. Joining techniques for aluminium spaceframes used in automobiles. J. Mater. Process. Technol. 2002, 99, 72–79. [Google Scholar] [CrossRef]
- European Aluminium Association. EAA Aluminium Automotive Manual—Joining. In The Aluminium Automotive Manual; European Aluminium Association: Ljubljana, Slovenia, 2015; pp. 1–5. [Google Scholar]
- Adams, R. Adhesive Bonding: Science, Technology and Applications; Woodhead Publishing Series in Welding and Other Joining Technologies; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Schwartz, M. Adhesive Bonding; CRC Press: Boca Raton, FL, USA, 2010; pp. 11–52. [Google Scholar] [CrossRef]
- Brockmann, W.; Geiß, P.; Klingen, J.; Mikhail, B.; Schröder, K. Adhesive Bonding: Materials, Applications and Technology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Omar, M. The Automotive Body Manufacturing Systems and Processes; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dunn, D. Update on Engineering and Structural Adhesives; Smithers Rapra Technology: Akron, OH, USA, 2010; Volume 15. [Google Scholar]
- Ozer, H. Applied Adhesive Bonding in Science and Technology; IntechOpen: London, UK, 2018. [Google Scholar]
- Adams, R.; Adams, R.; Comyn, J.; Wake, W.; Wake, W. Structural Adhesive Joints in Engineering; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- May, C. Epoxy Resins: Chemistry and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Dodiuk, H.; Goodman, S.H. Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–768. [Google Scholar] [CrossRef]
- Petrie, E. Epoxy Adhesive Formulations; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Hara, O. Curing agents for epoxy resins. In Chemistry and Technology of Epoxy Resins; Springer: Berlin/Heidelberg, Germany, 1993; pp. 37–71. [Google Scholar] [CrossRef]
- Kent, J. Riegel’s Handbook of Industrial Chemistry; Springer: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kulshreshtha, A.; Vasile, C. Handbook of Polymer Blends and Composites; Number vol. 1 Handbook of Polymer Blends and Composites; Rapra Technology: Akron, OH, USA, 2002. [Google Scholar]
- Fink, J. Reactive Polymers: Fundamentals and Applications: A Concise Guide to Industrial Polymers; Plastics Design Library; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Licari, J.; Swanson, D. Adhesives Technology for Electronic Applications: Materials, Processing, Reliability; Materials and Processes for Electronic Applications; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hamerton, I. Recent Developments in Epoxy Resins; Smithers Rapra Technology: Akron, OH, USA, 1996. [Google Scholar]
- Minges, M.; Committee, A. Electronic Materials Handbook: Packaging; Electronic Materials Handbook; Taylor & Francis: Abingdon, UK, 1989. [Google Scholar]
- Meister, J. Polymer Modification: Principles, Techniques, and Applications; Plastics Engineering; Taylor & Francis: Abingdon, UK, 2000. [Google Scholar]
- Lunder, O. Chromate-Free Pre-Treatment of Aluminium for Adhesive Bonding. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2003. [Google Scholar]
- Da Silva, L.; Öchsner, A.; Adams, R. Handbook of Adhesion Technology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Messler, R. Joining of Materials and Structures: From Pragmatic Process to Enabling Technology; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- King, R. Surface Treatment & Finishing of Aluminium; Pergamon Materials Engineering Practice Series; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Totten, G.; MacKenzie, D. Handbook of Aluminum: Volume 2: Alloy Production and Materials Manufacturing; Number vol. 2 in Handbook of Aluminum; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- Esposto, F.J.; Zhang, C.S.; Norton, P.R.; Timsit, R.S. Segregation of Mg to the surface of an AlMg single crystal alloy and its influence on the initial oxidation at room temperature. Surf. Sci. 1994, 302, 109–120. [Google Scholar] [CrossRef]
- Textor, M.; Amstutz, M. Surface analysis of thin films and interfaces in commercial aluminium products. Anal. Chim. Acta 1994, 297, 15–26. [Google Scholar] [CrossRef]
- Dunlop, H.; Benmalek, M. Role and Characterization of Surfaces in the Aluminium Industry. J. Phys. IV 1997, 7, C6–C174. [Google Scholar] [CrossRef]
- Kumar, A.K.; Prasanna, S.; Subramanian, B.; Jayakumar, S.; Rao, G.M. A transmission electron microscopy and X-ray photoelectron spectroscopy study of annealing induced γ-phase nucleation, clustering, and interfacial dynamics in reactively sputtered amorphous alumina thin films. J. Appl. Phys. 2015, 117. [Google Scholar] [CrossRef]
- Frolish, M.F.; Walker, J.C.; Jiao, C.; Rainforth, W.M.; Beynon, J.H. Formation and structure of a subsurface layer in hot rolled aluminium alloy AA3104 transfer bar. Tribol. Int. 2005, 38, 1050–1058. [Google Scholar] [CrossRef]
- Fishkis, M.; Lin, J.C. Formation and evolution of a subsurfacenext term layer in a metalworking process. Wear 1997, 206, 156–170. [Google Scholar] [CrossRef]
- Keuong, Y.W.; Nordlien, J.H.; Nisancioglu, K. Characterization of Electrochemically Active Surface Layers on Rolled Commercial Alloys AA8006 and AA5182 Aluminum. J. Electrochem. Soc. 2002, 148, B497. [Google Scholar] [CrossRef]
- Buytaert, G.; Terryn, H.; Van Gils, S.; Kernig, B.; Grzemba, B.; Mertens, M. Study of the near-surface of hot- and cold-rolled AlMg0.5 aluminium alloy. Surf. Interface Anal. 2005, 37, 534–543. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Thompson, G.E.; Hashimoto, T.; Scamans, G.M.; Afseth, A. Precipitation and corrosion behaviour of nano-structured near-surface layers on an AA6111 aluminium alloy. J. Phys. Conf. Ser. 2006, 26, 103–106. [Google Scholar] [CrossRef]
- Liu, Y.; Hashimoto, T.; Zhou, X.; Thompson, G.E.; Scamans, G.M.; Rainforth, W.M.; Hunter, J.A. Influence of near-surface deformed layers on filiform corrosion of AA3104 aluminium alloy. Surf. Interface Anal. 2013, 45, 1553–1557. [Google Scholar] [CrossRef]
- Li, K.; Zhou, X.R.; Thompson, G.E.; Hunter, J.A.; Yuan, Y.D. Evolution of Near-Surface Deformed Layers on AA3104 Aluminium Alloy. Mater. Sci. Forum 2013, 765, 358–362. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Thompson, G.E.; Hunter, J.A.; Yuan, Y. Delamination of near-surface layer on cold rolled AlFeSi alloy during sheet forming. Mater. Charact. 2015, 99, 109–117. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Thompson, G.E.; Scamans, G.M.; Skeldon, P.; Hunter, J.A. Near-surface deformed layers on rolled aluminum alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 1373–1385. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Influence of heat treatment and surface conditioning on filiform corrosion of aluminium alloys AA3005 and AA5754. Corros. Sci. 2001, 43, 2359–2377. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Effect of thermo-mechanical processing on filiform corrosion of aluminium alloy AA3005. Corros. Sci. 2002, 44, 2491–2506. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Effect of heat treatment on filiform corrosion of aluminium alloy AA3005. Corros. Sci. 2001, 43, 2093–2109. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Bishop, H.E.; Smart, N.R. Surface Analysis and Bonding of Aluminium-Magnesium Alloys. J. Adhes. 1982, 14, 105–118. [Google Scholar] [CrossRef]
- Kozma, L.; Olefjord, I. Basic processes of surface preparation and bond formation of adhesively joined aluminium. Mater. Sci. Technol. 1987, 3, 860–874. [Google Scholar] [CrossRef]
- Scamans, G.M.; Afseth, A.; Thompson, G.E.; Liu, Y.; Zhou, X.R. Corrosion of Painted Aluminium Sheet. Mater. Sci. Forum 2009, 519–521, 647–654. [Google Scholar] [CrossRef]
- Buchheit, R. Critical Factors for the Transition from Chromate to Chromate-Free Corrosion Protection; Technical Report; The Ohio State University: Columbus, OH, USA, 2005. [Google Scholar]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Andreatta, F.; Turco, A.; de Graeve, I.; Terryn, H.; de Wit, J.H.; Fedrizzi, L. SKPFM and SEM study of the deposition mechanism of Zr/Ti based pre-treatment on AA6016 aluminum alloy. Surf. Coat. Technol. 2007, 201, 7668–7685. [Google Scholar] [CrossRef]
- Karmaschek, U.; Roland, A.; Vennshott, H.; Wennemann, E. Chromium-Free Conversion Coating Treatment of Aluminum. U.S. Patent 55,849,46A, 17 May 1994. [Google Scholar]
- Deck, P.D.; Moon, M.; Sujdak, R.J. Investigation of fluoacid based conversion coatings on aluminum. Prog. Org. Coat. 1997, 34, 39–48. [Google Scholar] [CrossRef]
- Hubert, H.; Puderbach, H.; Pulm, H.; Roland, W.A. Investigations on zirconium-containing conversion layers on aluminium. Fresenius’ Z. Anal. Chem. 1989, 333, 304–307. [Google Scholar] [CrossRef]
- Posner, R.; Fink, N.; Wolpers, M.; Grundmeier, G. Electrochemical electrolyte spreading studies of the protective properties of ultra-thin films on zinc galvanized steel. Surf. Coat. Technol. 2013, 228, 286–295. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Paussa, L.; Fedrizzi, L. Addition of phosphates or copper nitrate in a fluotitanate conversion coating containing a silane coupling agent for aluminium alloy AA6014. Prog. Org. Coat. 2014, 77, 2107–2115. [Google Scholar] [CrossRef]
- Paloumpa, I.; Yfantis, A.; Hoffmann, P.; Burkov, Y.; Yfantis, D.; Schmeißer, D. Mechanisms to inhibit corrosion of Al alloys by polymeric conversion coatings. Surf. Coat. Technol. 2004, 180–181, 308–312. [Google Scholar] [CrossRef]
- Wang, L.; Peng, B.; Guo, X.; Ding, W.; Chen, Y. Ferric molybdate nanotubes synthesized based on the Kirkendall effect and their catalytic property for propene epoxidation by air. Chem. Commun. 2009, 1565–1567. [Google Scholar] [CrossRef]
- Smit, M.; Hunter, J.; Sharman, J.; Scamans, G.; Sykes, J. Effect of organic additives on the performance of titanium-based conversion coatings. Corros. Sci. 2003, 45, 1903–1920. [Google Scholar] [CrossRef]
- Zakir, M.; Ashraf, U.; Tian, T.; Han, A.; Qiao, W.; Jin, X.; Zhang, M.; Tsoi, J.K.H.; Matinlinna, J.P. The Role of Silane Coupling Agents and Universal Primers in Durable Adhesion to Dental Restorative Materials—A Review. Curr. Oral Health Rep. 2016, 3, 244–253. [Google Scholar] [CrossRef]
- Comyn, J. Adhesion Science; RSC Paperbacks; Royal Society of Chemistry: London, UK, 1997. [Google Scholar]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Hauffman, T.; Hubin, A.; Terryn, H. Study of the self-assembling of n-octylphosphonic acid layers on aluminum oxide from ethanolic solutions. Surf. Interface Anal. 2013, 45, 1435–1440. [Google Scholar] [CrossRef]
- Bulusu, A.; Paniagua, S.A.; Macleod, B.A.; Sigdel, A.K.; Berry, J.J.; Olson, D.C.; Marder, S.R.; Graham, S. Efficient modification of metal oxide surfaces with phosphonic acids by spray coating. Langmuir 2013, 29, 3935–3942. [Google Scholar] [CrossRef] [PubMed]
- Luschtinetz, R.; Oliveira, A.F.; Frenzel, J.; Joswig, J.O.; Seifert, G.; Duarte, H.A. Adsorption of phosphonic and ethylphosphonic acid on aluminum oxide surfaces. Surf. Sci. 2008, 602, 1347–1359. [Google Scholar] [CrossRef]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of self-assembled monolayers of alkylphosphonic acid on the native oxide surface of SS316L. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef]
- Textor, M.; Ruiz, L.; Hofer, R.; Rossi, A.; Feldman, K.; Hähner, G.; Spencer, N.D. Structural chemistry of self-assembled monolayers of octadecylphosphoric acid on tantalum oxide surfaces. Langmuir 2000, 16, 3257–3271. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Thissen, P.; Valtiner, M.; Grundmeier, G. Stability of phosphonic acid self-assembled monolayers on amorphous and Single-crystalline aluminum oxide surfaces in aqueous solution. Langmuir 2010, 26, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Rupper, P.; Gaan, S. Recent Development in Phosphonic Acid-Based Organic Coatings on Aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef]
- Rowe, J. Advanced Materials in Automotive Engineering; Woodhead Publishing in Materials; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Correia, S.; Anes, V.; Reis, L. Effect of surface treatment on adhesively bonded aluminium-aluminium joints regarding aeronautical structures. Eng. Fail. Anal. 2018, 84, 34–45. [Google Scholar] [CrossRef]
- Meiler, M.; Jaschke, H. Lubrication of aluminium sheet metal within the automotive industry. Adv. Mater. Res. 2005, 6–8, 551–558. [Google Scholar] [CrossRef]
- Debski, M.; Shanahan, M.; Schultz, J. Mechanisms of contaminant elimination by oil-accommodating adhesives Part 1: Displacement and absorption. Int. J. Adhes. Adhes. 1986, 6, 145–149. [Google Scholar] [CrossRef]
- Debski, M.; Shanahan, M.E.R.; Schultz, J. Mechanisms of contaminant elimination by oil-accommodating adhesives Part 2: A model of the processes involved. Int. J. Adhes. Adhes. 1986, 6, 150–152. [Google Scholar] [CrossRef]
- Ogawa, T.; Ochiai, K.; Masuichi, M. Simulation Analyses on the Diffusion of a Rust-Preventing Oil Into an Oil-Accommodating Adhesive. J. Adhes. 1995, 49, 83–96. [Google Scholar] [CrossRef]
- Greiveldinger, M.; Shanahan, M.E.; Jacquet, D.; Verchère, D. Oil-covered substrates: A model study of the evolution in the interphase during cure of an epoxy adhesive. J. Adhes. 2000, 73, 179–195. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.P.; Hicks, C.; Yang, X.; Carlson, B.E.; Zhou, Q. Experimental study of initial strengths and hygrothermal degradation of adhesive joints between thin aluminum and steel substrates. Int. J. Adhes. Adhes. 2013, 43, 14–25. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, J.; Wang, P.C.; Wu, Q.; Wu, Y. Effects of a sheet metal stamping lubricant on static strength of adhesive-bonded aluminum alloys. J. Adhes. Sci. Technol. 2015, 29, 1382–1402. [Google Scholar] [CrossRef]
- Packham, D.E.; Van Ooij, W.J.; Anderson, H.R.; Mittel, K.L. The mechanical theory of adhesion—A seventy year perspective and its current status. In First International Congress on Adhesion Science and Technology; CRC Press: Boca Raton, FL, USA, 1998; pp. 81–108. [Google Scholar]
- Brockmann, W.; Hennemann, O.D.; Kollek, H.; Matz, C. Adhesion in bonded aluminium joints for aircraft construction. Int. J. Adhes. Adhes. 1986, 6, 115–143. [Google Scholar] [CrossRef]
- Venables, J.; McNamara, D.; Chen, J.; Sun, T.; Hopping, R. Oxide morphologies on aluminum prepared for adhesive bonding. Appl. Surf. Sci. 1979, 3, 88–98. [Google Scholar] [CrossRef]
- Yendall, K.A.; Critchlow, G.W. Novel methods, incorporating pre- and post-anodising steps, for the replacement of the Bengough–Stuart chromic acid anodising process in structural bonding applications. Int. J. Adhes. Adhes. 2009, 29, 503–508. [Google Scholar] [CrossRef][Green Version]
- Pizzi, A.; Mittal, K. Handbook of Adhesive Technology, Revised and Expanded; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- Boutar, Y.; Naïmi, S.; Mezlini, S.; Ali, M.B.S. Effect of surface treatment on the shear strength of aluminium adhesive single-lap joints for automotive applications. Int. J. Adhes. Adhes. 2016, 67, 38–43. [Google Scholar] [CrossRef]
- Ishida, H.; Koenig, J.L. Molecular Organization of the Coupling Agent Interphase of Fiber-Glass Reinforced Plastics. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 1807–1813. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Adhesion Through Silane Coupling Agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Mohseni, M.; Mirabedini, M.; Hashemi, M.; Thompson, G. Adhesion performance of an epoxy clear coat on aluminum alloy in the presence of vinyl and amino-silane primers. Prog. Org. Coat. 2006, 57, 307–313. [Google Scholar] [CrossRef]
- Jang, J.; Kim, E.K. Corrosion Protection of Epoxy-Coated Steel Using Different Silane Coupling Agents. J. Appl. Polym. Sci. 1999, 71, 585–593. [Google Scholar] [CrossRef]
- Romero, M.A.; Chabert, B.; Claude, U.; Lyon, B. IR Spectroscopy Approach for the Study of Interactions Between an Oxidized Aluminium Surface and a Poly(Propylene-g-Acrylic Acid) Film. J. Appl. Polym. Sci. 1993, 47, 543–554. [Google Scholar] [CrossRef]
- Konstadinidis, K.; Thakkar, B.; Chakraborty, A.; Potts, L.W.; Tannenbaum, R.; Tirrell, M.; Evans, J.F. Segment Level Chemistry and Chain Conformation in the Reactive Adsorption of Poly ( methy1 methacrylate ) on Aluminum Oxide Surfaces. Langmuir 1992, 8, 1307–1317. [Google Scholar] [CrossRef]
- Ulren, L.; Hjertberg, T.; Ishida, H. An FT-IR Study on Interfacial Interactions in Ethylene Copolymers/Aluminium Laminates in Relation to Adhesion Properties. J. Adhes. 1990, 31, 117–136. [Google Scholar] [CrossRef]
- Vermohlen, K.; Lewandowski, H.; Narres, H.; Koglin, E. Adsorption of polyacrylic acid on aluminium oxide: DRIFT spectroscopy and ab initio calculations. Colloids Surf. A 2000, 170, 181–189. [Google Scholar] [CrossRef]
- Tannenbaum, R.; King, S.; Lecy, J.; Tirrell, M.; Potts, L. Infrared Study of the Kinetics and Mechanism of Adsorption of Acrylic Polymers on Alumina Surfaces. Langmuir 2004, 20, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Payan, S.; Duc, T.M. Interfacial Interactions of Plasma-polymerized Acrylic Acid and an Oxidized Aluminium Surface Investigated using XPS, FTIR and Poly(acrylic acid) as a Model Compound. Surf. Interface Anal. 1998, 26, 961–973. [Google Scholar] [CrossRef]
- Pletincx, S.; Trotochaud, L.; Fockaert, L.L.; Mol, J.M.; Head, A.R.; Karslloǧlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. In situ characterization of the initial effect of water on molecular interactions at the interface of organic/inorganic hybrid systems. Sci. Rep. 2017, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.V.D.; Blajiev, O.; Beentjes, P.C.J.; Terryn, H.; Wit, J.H.W.D. Interaction of Anhydride and Carboxylic Acid Compounds with Aluminum Oxide Surfaces Studied Using Infrared Reflection Absorption Spectroscopy. Langmuir 2004, 20, 6308–6317. [Google Scholar] [CrossRef]
- Fowkes, F.M. Acid-Base Interactions in Polymer Adhesion. Tribol. Ser. 1981, 7, 119–137. [Google Scholar] [CrossRef]
- Lee, L. Fundamentals of Adhesion; Language of Science; Springer: New York, NY, USA, 2013. [Google Scholar]
- Lewis, G.N. Valence and the Structure of Atoms and Molecules; The Chemical Catalog Company: New York, NY, USA, 1923. [Google Scholar]
- Van Den Brand, J. On the Adhesion Between Aluminium and Polymers. Ph.D. Thesis, Technische Universiteit Delft, Delft, The Netherlands, 6 October 2004. [Google Scholar]
- McCafferty, E. Lewis acid/Lewis base effects in corrosion and polymer adhesion at aluminum surfaces. J. Electrochem. Soc. 2003, 150, 342–347. [Google Scholar] [CrossRef]
- Prolongo, S.; Ureña, A. Effect of surface pre-treatment on the adhesive strength of epoxy–aluminium joints. Int. J. Adhes. Adhes. 2009, 29, 23–31. [Google Scholar] [CrossRef]
- Abrahami, S.; Hauffman, T.; Kok, J.; Terryn, H.; Mol, J. The role of acid-base properties in the interactions across the oxide-primer interface in aerospace applications. Surf. Interface Anal. 2015. [Google Scholar] [CrossRef]
- Okanat, O.; Wit, F.; Dewit, J.; Terryn, H.; Mol, J.M.C. Influence of pretreatments and aging on the adhesion performance of epoxy-coated aluminum. Surf. Coat. Technol. 2013, 215, 260–265. [Google Scholar] [CrossRef]
- Lopez, S.; Petit, J.P.; Dunlop, H.M.; Butruille, J.R.; Tourillon, G. Acid-base properties of passive films on aluminum: I. A photoelectrochemical study. J. Electrochem. Soc. 1998, 145, 823–829. [Google Scholar] [CrossRef]
- Lopez, S.; Petit, J.P.; Dunlop, H.M.; Butruille, J.R.; Tourillon, G. Acid-base properties of passive films on aluminum: II. An X-ray Photoelectron Spectroscopy and X-ray Absorption Near Edge Structure Study. J. Electrochem. Soc. 1998, 145, 829–834. [Google Scholar] [CrossRef]
- Nakamae, K.; Nishino, T.; Airu, X.; Asaoka, S. Localization of the curing agent at an epoxy resin/oxidized aluminium interface. Int. J. Adhes. Adhes. 1995, 15, 15–20. [Google Scholar] [CrossRef]
- Hong, S.G.; Tsai, J.S. Adsorption and curing behaviors of the epoxy/aminoamine system in the presence of metal oxides. J. Therm. Anal. Calorim. 2001, 63, 31–46. [Google Scholar] [CrossRef]
- Abel, M.L.; Rattana, A.; Watts, J.F. Interaction of epoxy analogue molecules with organosilane-treated aluminum: A study by XPS and ToF-SIMS. Langmuir 2000, 16, 6510–6518. [Google Scholar] [CrossRef]
- Schreiber, H.P.; Ouhlal, A. Polymer diffusion and the evolution of adhesive bond strength. J. Adhes. 2003, 79, 141–153. [Google Scholar] [CrossRef]
- Landrock, A.; Ebnesajjad, S. Adhesives Technology Handbook; Plastics Design Library; Elsevier Science: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lee, L. Adhesive Bonding; Springer: New York, NY, USA, 2013. [Google Scholar]
- Randow, C.L.; Williams, C.A.; Ward, T.C.; Dillard, D.A.; Dillard, J.G.; Wightman, J.P. An Investigation of the Cling of Thin Polymeric Films. J. Adhes. 1997, 63, 285–307. [Google Scholar] [CrossRef]
- Weitsman, Y. Stress assisted diffusion in elastic and viscoelastic materials. J. Mech. Phys. Solids 1987, 35, 73–93. [Google Scholar] [CrossRef]
- Morsch, S.; Lyon, S.; Greensmith, P.; Smith, S.D.; Gibbon, S.R. Mapping water uptake in organic coatings using AFM-IR. Faraday Discuss. 2015, 180, 527–542. [Google Scholar] [CrossRef]
- Xu, S.; Dillard, D.A.; Dillard, J. Environmental aging effects on the durability of electrically conductive adhesive joints. Int. J. Adhes. Adhes. 2003, 23, 235–250. [Google Scholar] [CrossRef]
- Viana, G.; Costa, M.; Banea, M.D.; da Silva, L.F. Water diffusion in double cantilever beam adhesive joints. Lat. Am. J. Solids Struct. 2017, 14, 188–201. [Google Scholar] [CrossRef]
- Gledhill, R.A.; Kinloch, A.J. Environmental Failure of Structural Adhesive Joints. J. Adhes. 1974, 6, 315–330. [Google Scholar] [CrossRef]
- Da Silva, L.; Sato, C. Design of Adhesive Joints Under Humid Conditions; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Pletincx, S.; Marcoen, K.; Trotochaud, L.; Fockaert, L.L.; Mol, J.M.; Head, A.R.; Karslioǧlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. Unravelling the chemical influence of water on the PMMA/Aluminum oxide hybrid interface in situ. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pletincx, S.; Mol, J.M.; Terryn, H.; Hubin, A.; Hauffman, T. An in situ spectro-electrochemical monitoring of aqueous effects on polymer/metal oxide interfaces. J. Electroanal. Chem. 2019, 848, 113311. [Google Scholar] [CrossRef]
- Abrahami, S.T.; Hauffman, T.; De Kok, J.M.; Mol, J.M.; Terryn, H. Effect of Anodic Aluminum Oxide Chemistry on Adhesive Bonding of Epoxy. J. Phys. Chem. C 2016, 120, 19670–19677. [Google Scholar] [CrossRef]
- Brewis, D.M.; Comyn, J.; Tegg, J.L. The uptake of water vapour by an epoxide adhesive formed from the diglycidyl ether of bisphenol-A and di-(1-aminopropyl-3-ethoxy) ether. Polymer 1980, 21, 134–138. [Google Scholar] [CrossRef]
- Brewis, D.M.; Comyn, J.; Tegg, J.L. The durability of some epoxide adhesive-bonded joints on exposure to moist warm air. Int. J. Adhes. Adhes. 1980, 1, 35–39. [Google Scholar] [CrossRef]
- Brewis, D.M.; Comyn, J.; Cope, B.C.; Moloney, A.C. Effect of carriers on the performance of aluminum alloy joints bonded with a structural film adhesive. Polym. Eng. Sci. 1981, 21, 797–803. [Google Scholar] [CrossRef]
- Mubashar, A.; Ashcroft, I.A.; Critchlow, G.W.; Crocombe, A.D. Moisture absorption-desorption effects in adhesive joints. Int. J. Adhes. Adhes. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Gledhill, R.A.; Kinloch, A.J.; Shaw, S.J. A Model for Predicting Joint Durability. J. Adhes. 1980, 11, 3–15. [Google Scholar] [CrossRef]
- Wang, S.; Min, J.; Lin, J.; Wu, Y. Effect of neutral salt spray (NSS) exposure on the lap-shear strength of adhesive-bonded 5052 aluminum alloy (AA5052) joints. J. Adhes. Sci. Technol. 2019, 33, 549–560. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Brewis, D.M. A comparison of chromate-phosphate and chromate-free conversion coatings for adhesive bonding. J. Adhes. 1997, 61, 213–230. [Google Scholar] [CrossRef]
- Lunder, O.; Lapique, F.; Johnsen, B.; Nisancioglu, K. Effect of pre-treatment on the durability of epoxy-bonded AA6060 aluminium joints. Int. J. Adhes. Adhes. 2004, 24, 107–117. [Google Scholar] [CrossRef]
- Hua, D.; Lin, J.; Zhang, B. Effects of salt spray on the mechanical properties of aluminum-epoxy adhesive joints. J. Adhes. Sci. Technol. 2013, 27, 1580–1589. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.D.; Xv, H.Y.; Liu, W.B. The durability of adhesive/carbon-carbon composites joints in salt water. Int. J. Adhes. Adhes. 2004, 24, 471–477. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Wang, P.C.; Zheng, R.; Wu, Q. Effect of long-term neutral salt spray exposure on durability of adhesive-bonded Zr-Ti coated aluminum joint. Int. J. Adhes. Adhes. 2016, 64, 97–108. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Shaw, B.; Buchheit, R.; Moran, J.; Division, E.S.C.; Meeting, E.S. Corrosion and Corrosion Prevention of Low Density Metals and Alloys: Proceedings of the International Symposium; Proceedings (Electrochemical Society); Electrochemical Society: Pennington, NJ, USA, 2001. [Google Scholar]
- Vargel, C. Corrosion of Aluminium; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Williams, G.; McMurray, H.N.; Hayman, D.; Morgan, P.C. Time-lapse potentiometric imaging of active filiform corrosion using a scanning Kelvin probe technique. PhysChemComm 2001, 4, 1–6. [Google Scholar] [CrossRef]
- Coleman, A.; McMurray, H.; Williams, G.; Afseth, A.; Scamans, G.M. Filiform Corrosion on 6000 Series Aluminium: Kinetics and Inhibition Strategies. Mater. Sci. Forum 2006, 519–521, 629–634. [Google Scholar] [CrossRef]
- Coleman, A.J.; McMurray, H.N.; Williams, G.; Afseth, A.; Scamans, G.M. Inhibition of Filiform Corrosion on AA6111-T4 Using In-Coating Phenylphosphonic Acid. Electrochem. Solid-State Lett. 2007, 10, C35. [Google Scholar] [CrossRef]
- Liu, Y.; Laurino, A.; Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E.; Scamans, G.M.; Blanc, C.; Rainforth, W.M.; Frolish, M.F. Corrosion behaviour of mechanically polished AA7075-T6 aluminium alloy. Surf. Interface Anal. 2010, 42, 185–188. [Google Scholar] [CrossRef]
- McMurray, H.N.; Holder, A.; Williams, G.; Scamans, G.M.; Coleman, A.J. The kinetics and mechanisms of filiform corrosion on aluminium alloy AA6111. Electrochim. Acta 2010, 55, 7843–7852. [Google Scholar] [CrossRef]
- Institute, W.; Cho, G. The Automotive Industry: Core Research from TWI; Core Research from TWI; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Marceau, J.A.; McMillan, J. Exploratory Development on Durability of Adhesive Bonded Joints; Technical Report; Boeing Commercial Airplane Company: Seattle, WA, USA, 1976. [Google Scholar]
- Wahab, M.; Ashcroft, I.; Crocombe, A.; Hughes, D.; Shaw, S. The effect of environment on the fatigue of bonded composite joints. Part 2: Fatigue threshold prediction. Compos. Part A Appl. Sci. Manuf. 2001, 32, 59–69. [Google Scholar] [CrossRef]
- So, H.W.; Chen, N.N.; Niem, P.I. Fatigue performance of adhesive joints immersed in different solutions. J. Adhes. 1994, 44, 245–256. [Google Scholar] [CrossRef]
- Small, G.; Fay, P.A. Creep of adhesive lap-joints in dry and high humidity environments. In Proceedings of the Adhesion ’90, Plastic and Rubber Inst. Meetings, Cambridge, UK, 10–12 September 1990. [Google Scholar]
- Croccolo, D.; De Agostinis, M.; Mauri, P. Influence of the assembly process on the shear strength of shaft-hub hybrid joints. Int. J. Adhes. Adhes. 2013, 44, 174–179. [Google Scholar] [CrossRef]
- Croccolo, D.; De Agostinis, M.; Fini, S.; Olmi, G. Influence of the engagement ratio on the shear strength of an epoxy adhesive by push-out tests on pin-and-collar joints: Part II: Campaign at different temperature levels. Int. J. Adhes. Adhes. 2016, 67, 76–85. [Google Scholar] [CrossRef]
- Da Silva, L. Modeling of Adhesively Bonded Joints; Modeling of Adhesively Bonded Joints; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).