Production of Oxide Dispersion Strengthened Mg-Zn-Y Alloy by Equal Channel Angular Pressing of Mechanically Alloyed Powder
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructural Characterization
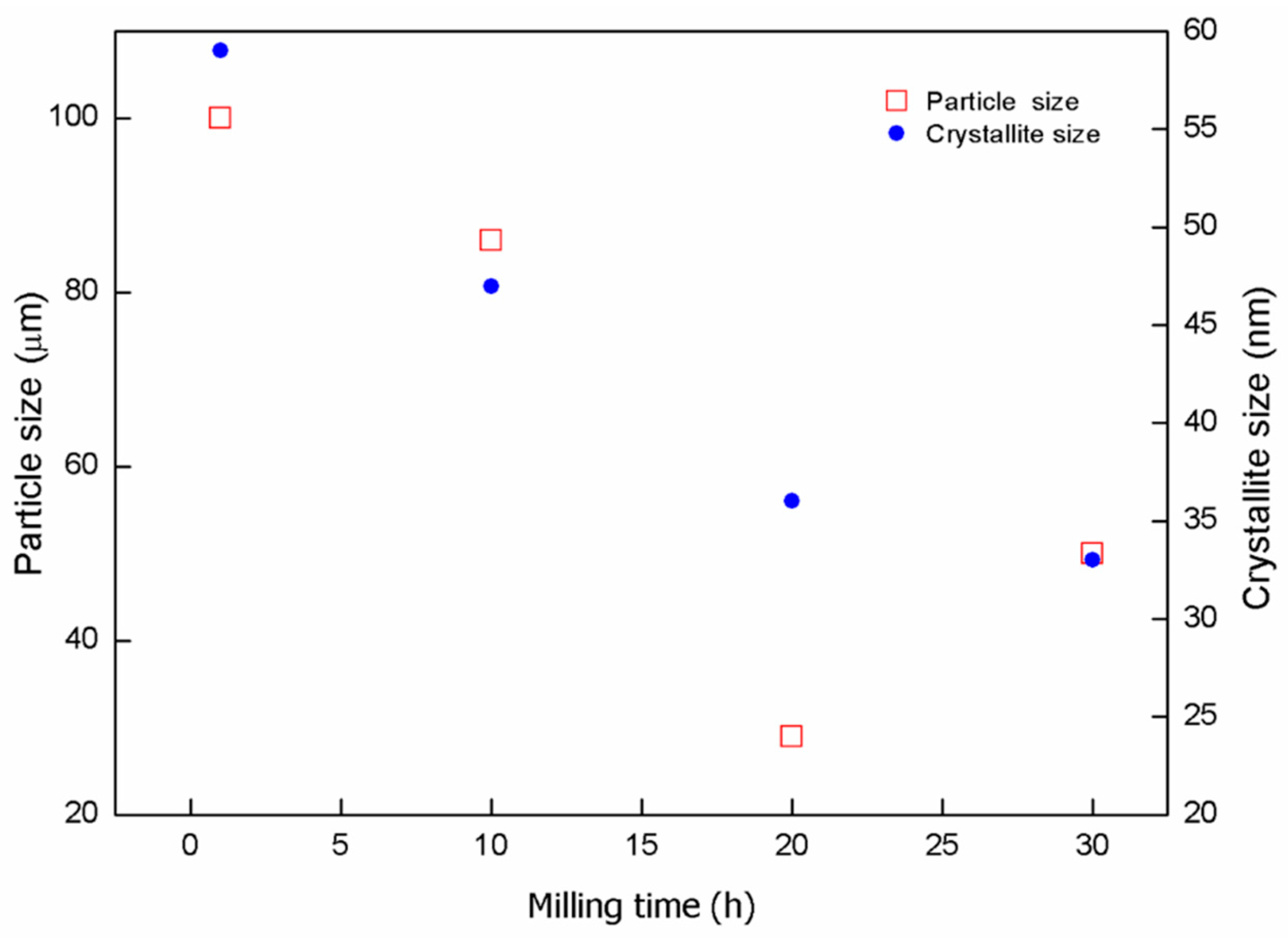
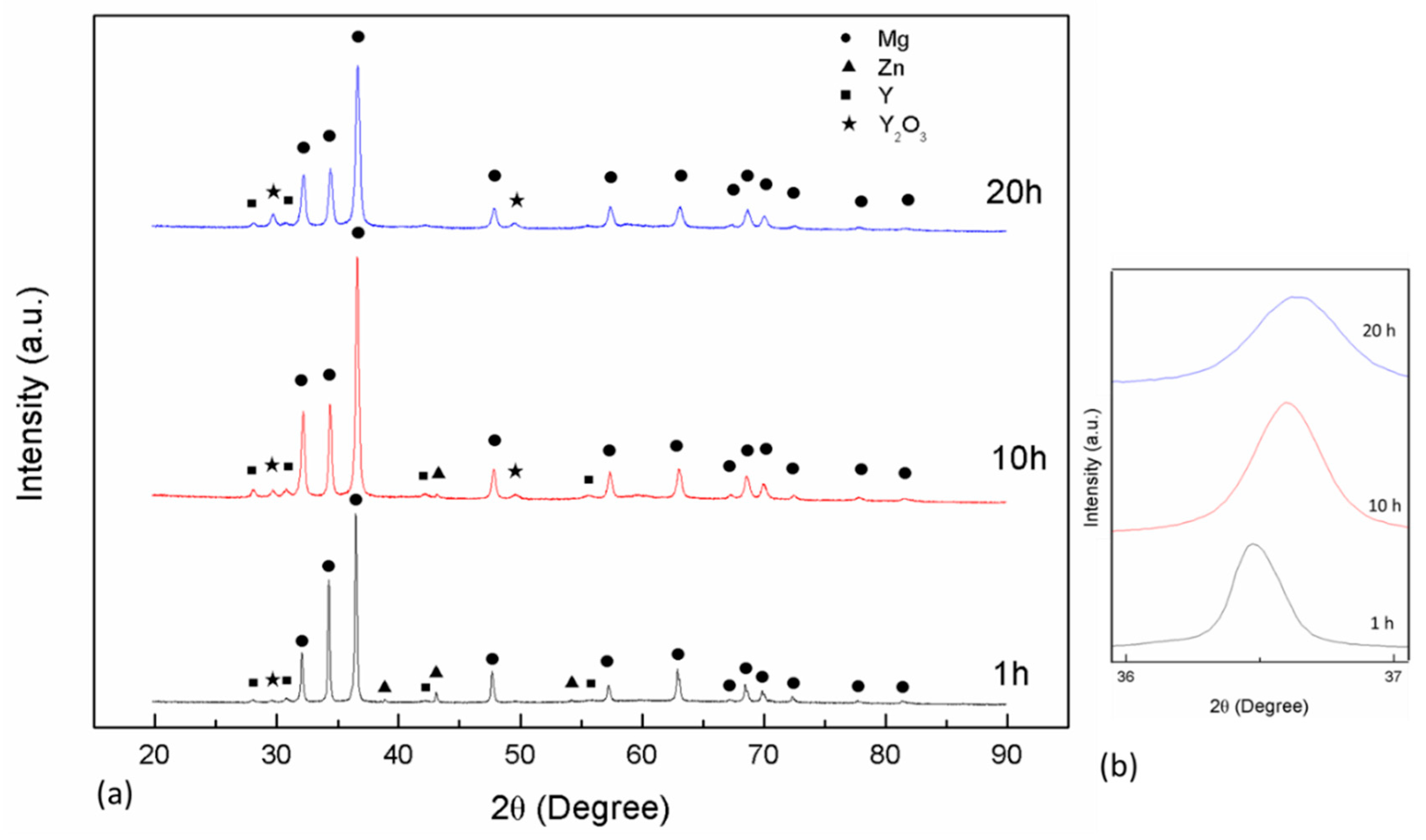
3.1.1. Mechanically-Alloyed Mg-Zn-Y Powders
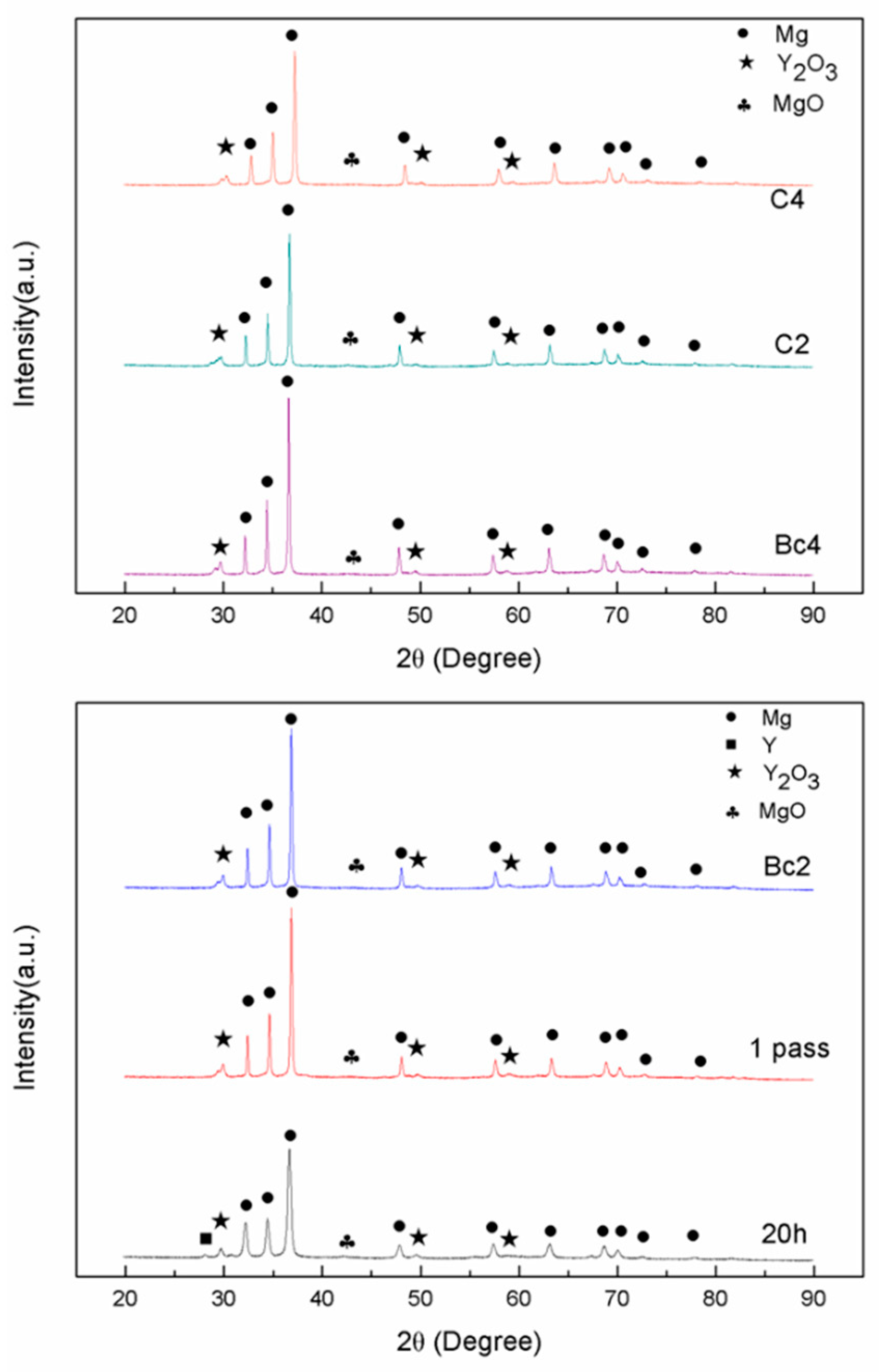
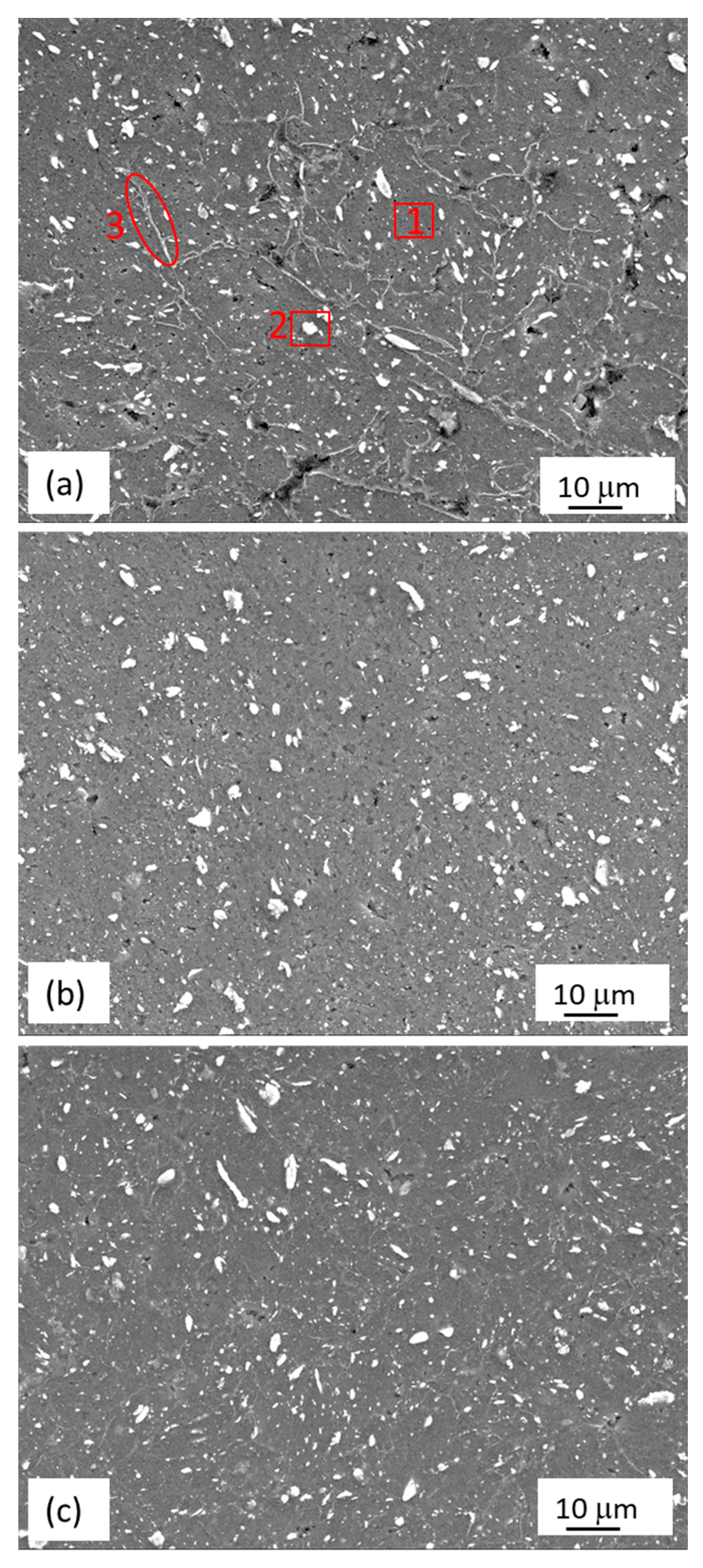
3.1.2. Mg-Zn-Y Powders Consolidated by ECAP
3.2. Mechanical Properties
4. Conclusions
- The Mg97Zn1Y2 alloy powder after mechanical alloying for 20 h consisted of α-Mg, Y and Y2O3 phases. The ECAP-compacted bulk alloy contained the α-Mg matrix, uniformly dispersed Y2O3 and MgO phase.
- The powder compacted with ECAP route Bc for four passes exhibited a hardness of 110 HV and an ultimate compressive strength of 185 MPa. The hardness was higher than those of as-cast Mg97Zn1Y2 alloy and compacted Mg; however, the UCS was lower.
- The improved hardness observed in the ECAP-compacted alloy was mainly attributed to the dispersion hardening of Y2O3 particles. The decrease in ultimate compressive strength was due to the higher amount of porosity in the ECAP-compacted alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Schumann, S. The paths and strategies for increased magnesium applications in vehicles. Mater. Sci. Forum 2005, 488, 1–8. [Google Scholar] [CrossRef]
- Cabibbo, M.; Spigarelli, S. A TEM quantitative evolution of strengthening in an Mg-RE alloy reinforced with SiC. Mater. Charact. 2011, 62, 959–969. [Google Scholar] [CrossRef]
- Pan, F.S.; Yang, M.B.; Chen, X.H. A review on casting magnesium alloys: Modification of commercial alloys and development of new alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Kainer, K.U.; Hort, N. Recent research and developments on wrought magnesium alloys. J. Magnesium Alloys 2017, 5, 239–253. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Langdon, T.G. Processing Magnesium and Its Alloys by High-Pressure Torsion: An Overview. Adv. Eng. Mater. 2019, 21, 1801039. [Google Scholar] [CrossRef]
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly solidified powder metallurgy Mg97Zn1Y2 alloy with excellent tensile yield strength above 600 MPa. Mater. Trans. 2001, 42, 1172–1176. [Google Scholar] [CrossRef]
- Inoue, A.; Matsushita, M.; Kawamura, Y.; Amiya, K.; Hayashi, K.; Koike, J. Novel hexagonal structure of ultra-high strength magnesium-based alloys. Mater. Trans. 2002, 43, 580–584. [Google Scholar] [CrossRef]
- Lee, H.C.; Chao, C.G.; Liu, T.F.; Lin, C.Y.; Wang, H.C. Effect of temperature and extrusion pass on the consolidation of magnesium powders using equal channel angular extrusion. Mater. Trans. 2013, 54, 765–768. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Guo, Q.; Wang, R. Fabrication of the nanometer Al2O3/Cu composite by internal oxidation. J. Mater. Process. Tech. 2005, 170, 336–340. [Google Scholar] [CrossRef]
- Broyles, S.E.; Anderson, K.R.; Groza, J.R.; Gibeling, J.C. Creep deformation of dispersion-strengthened copper. Metall. Mater. Trans A 1996, 27, 1217–1227. [Google Scholar] [CrossRef]
- Rapp, R.A. Kinetics, Microstructures and mechanism of internal oxidation—Its effect and prevention in high temperature alloy oxidation. Corrosion 1965, 21, 382–401. [Google Scholar] [CrossRef]
- Chino, Y.; Furuta, T.; Hakamada, M.; Mabuchi, M. Influence of distribution of oxide contaminants on fatigue behavior in AZ31 Mg alloy recycled by solid-state processing. Mater. Sci. Eng. A 2006, 424, 355–360. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, L.; Sum, Y.; Zhang, H.; Duan, C.; Yu, H. Synthesis of nanocrystalline AZ31 magnesium alloy with titanium addition by mechanical milling. Mater. Charact. 2016, 113, 108–116. [Google Scholar] [CrossRef]
- Koch, C.C.; Scattergood, R.O.; Youssef, K.M.; Chan, E.; Zhu, Y.T. Nanostructured materials by mechanical alloying: New results on property enhancement. J. Mater. Sci. 2010, 45, 4725–4732. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Scott, J.M.; Senkova, S.V. Compaction of amorphous aluminum alloy powder by direct extrusion and equal angular extrusion. Mater. Sci. Eng. A 2005, 393, 12–21. [Google Scholar] [CrossRef]
- Kim, H.S.; Seo, M.H.; Oh, C.S.; Kim, S.J. Equal channel pressing of metallic powders. Mater. Sci. Forum 2003, 437, 89–92. [Google Scholar] [CrossRef]
- Nagasekhar, A.V.; Yip, T.H.; Ramakanth, K.S. Mechanics of single pass equal channel angular extrusion pf powder in tubes. Appl. Phys. A 2006, 85, 185–194. [Google Scholar] [CrossRef]
- Baker, I.; Iliescu, D.; Liao, Y. Containerless consolidation of Mg powders using ECAE. Mater. Manuf. Processes 2010, 25, 1381–1384. [Google Scholar] [CrossRef]
- Nagasekhar, A.V.; Yip, T.H.; Guduru, R.K.; Ramakanth, K.S. Multipass equal channel angular pressing of MgB2 powder in tubes. Physica C 2007, 466, 174–180. [Google Scholar] [CrossRef]
- Karaman, I.; Haouaoui, M.; Maier, H.J. Nanoparticle consolidation using equal channel angular extrusion at room temperature. J. Mater. Sci. 2007, 42, 1561–1576. [Google Scholar] [CrossRef]
- Chiu, C.; Lu, C.; Chen, S.; Ou, K. Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy. Materials 2017, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Huang, H. Microstructure and properties of Mg-Zn-Y alloy powder compacted by equal channel angular pressing. Materials 2018, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Subramanian, S.; Raj, B.; Ravi, K.R. Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg–Zn–Ca bulk metallic glass for biomedical applications. Mater. Des. 2015, 86, 829–835. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1974; pp. 618–708. [Google Scholar]
- Figueiredo, R.B.; Langdon, T.G. Grain refinement and mechanical behavior of a magnesium alloy processed by ECAP. J. Mater. Sci. 2010, 45, 4827–4836. [Google Scholar] [CrossRef]
- Furukawa, M.; Iwahashi, Y.; Horita, Z.; Nemoto, M.; Langdon, T.G. The shearing characteristics associated with equal-channel angular pressing. Mater. Sci. Eng. A 1998, 257, 328–332. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Horita, Z.; Nemoto, M.; Langdon, T.G. An investigation of microstructural evolution during equal-channel angular pressing. Acta Mater. 1997, 45, 4733–4741. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substance, 3rd ed.; VCH Publishers: New York, NY, USA, 1995; p. 993. [Google Scholar]
- Mallick, A.; Tun, K.S.; Gupta, M. Deformation behavior of Mg/Y2O3 nanocomposite at elevated temperatures. Mater. Sci. Eng. A 2012, 551, 222–230. [Google Scholar] [CrossRef]
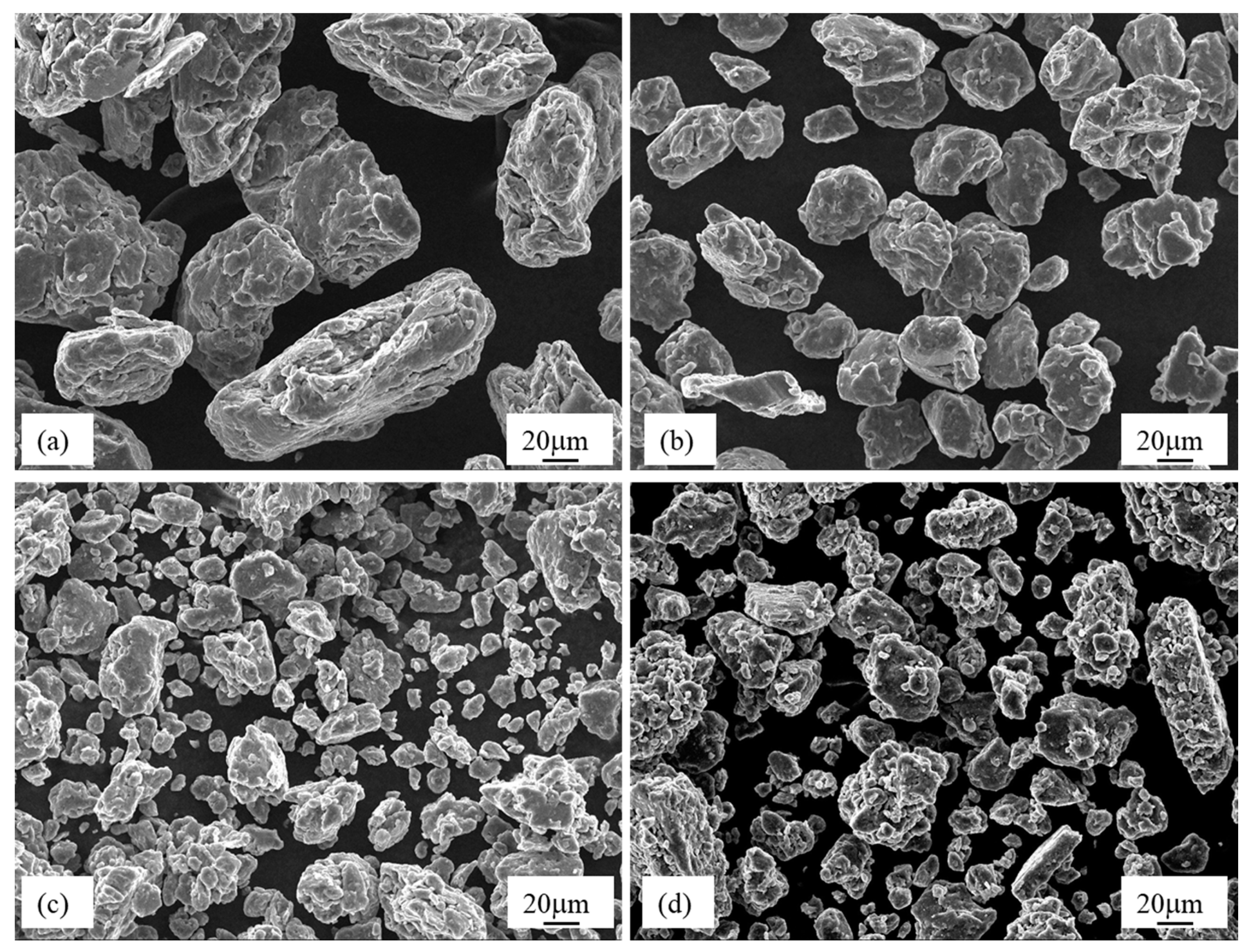

| Route | Process |
|---|---|
| A | The sample is not rotated. |
| BA | Rotating the sample around its longitudinal axis by 90° clockwise and counterclockwise alternatively |
| Bc | Rotating the sample around its longitudinal axis after each pass by 90° clockwise |
| C | Rotating the sample around its longitudinal axis after each pass by 180° clockwise |
| Milling Time (h) | Particle Size (μm) | Crystallite Size (nm) | Lattice Parameter, a (nm) | Lattice Parameter, c (nm) | Unit Cell Volume (10−2·nm3) |
|---|---|---|---|---|---|
| 1 | 100 | 59 | 0.3217 | 0.5223 | 4.6811 |
| 10 | 86 | 47 | 0.3211 | 0.5212 | 4.6528 |
| 20 | 29 | 36 | 0.3208 | 0.5208 | 4.6430 |
| 30 | 50 | 32 | 0.3208 | 0.5207 | 4.6417 |
| Route and Pass | Crystallite Size (nm) | Porosity (%) | Fraction of Oxide (%) |
|---|---|---|---|
| 1 Pass | 77 | 9.7 | 12.4 |
| Bc 2 | 67 | 7.6 | 13.2 |
| Bc 4 | 64 | 4.3 | 12.0 |
| C2 | 73 | 9.2 | 12.1 |
| C4 | 73 | 8.1 | 13.4 |
| Route and Pass | Hardness (HV) | Compressive Strength (MPa) | Failure Strain (%) |
|---|---|---|---|
| 1 Pass | 101 | 67 | 8 |
| Bc 2 | 101 | 90 | 3 |
| Bc 4 | 110 | 185 | 4 |
| C2 | 103 | 60 | 6 |
| C4 | 100 | 44 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, K.-L.; Chen, C.-C.; Chiu, C. Production of Oxide Dispersion Strengthened Mg-Zn-Y Alloy by Equal Channel Angular Pressing of Mechanically Alloyed Powder. Metals 2020, 10, 679. https://doi.org/10.3390/met10050679
Ou K-L, Chen C-C, Chiu C. Production of Oxide Dispersion Strengthened Mg-Zn-Y Alloy by Equal Channel Angular Pressing of Mechanically Alloyed Powder. Metals. 2020; 10(5):679. https://doi.org/10.3390/met10050679
Chicago/Turabian StyleOu, Keng-Liang, Chia-Chun Chen, and Chun Chiu. 2020. "Production of Oxide Dispersion Strengthened Mg-Zn-Y Alloy by Equal Channel Angular Pressing of Mechanically Alloyed Powder" Metals 10, no. 5: 679. https://doi.org/10.3390/met10050679
APA StyleOu, K.-L., Chen, C.-C., & Chiu, C. (2020). Production of Oxide Dispersion Strengthened Mg-Zn-Y Alloy by Equal Channel Angular Pressing of Mechanically Alloyed Powder. Metals, 10(5), 679. https://doi.org/10.3390/met10050679





