Numerical Simulation of the Melting Behavior of Steel Scrap in Hot Metal
Abstract
1. Introduction
2. Model Description
2.1. Assumptions
2.2. Continuity Equations
2.3. Energy Equation
2.4. Momentum Equations
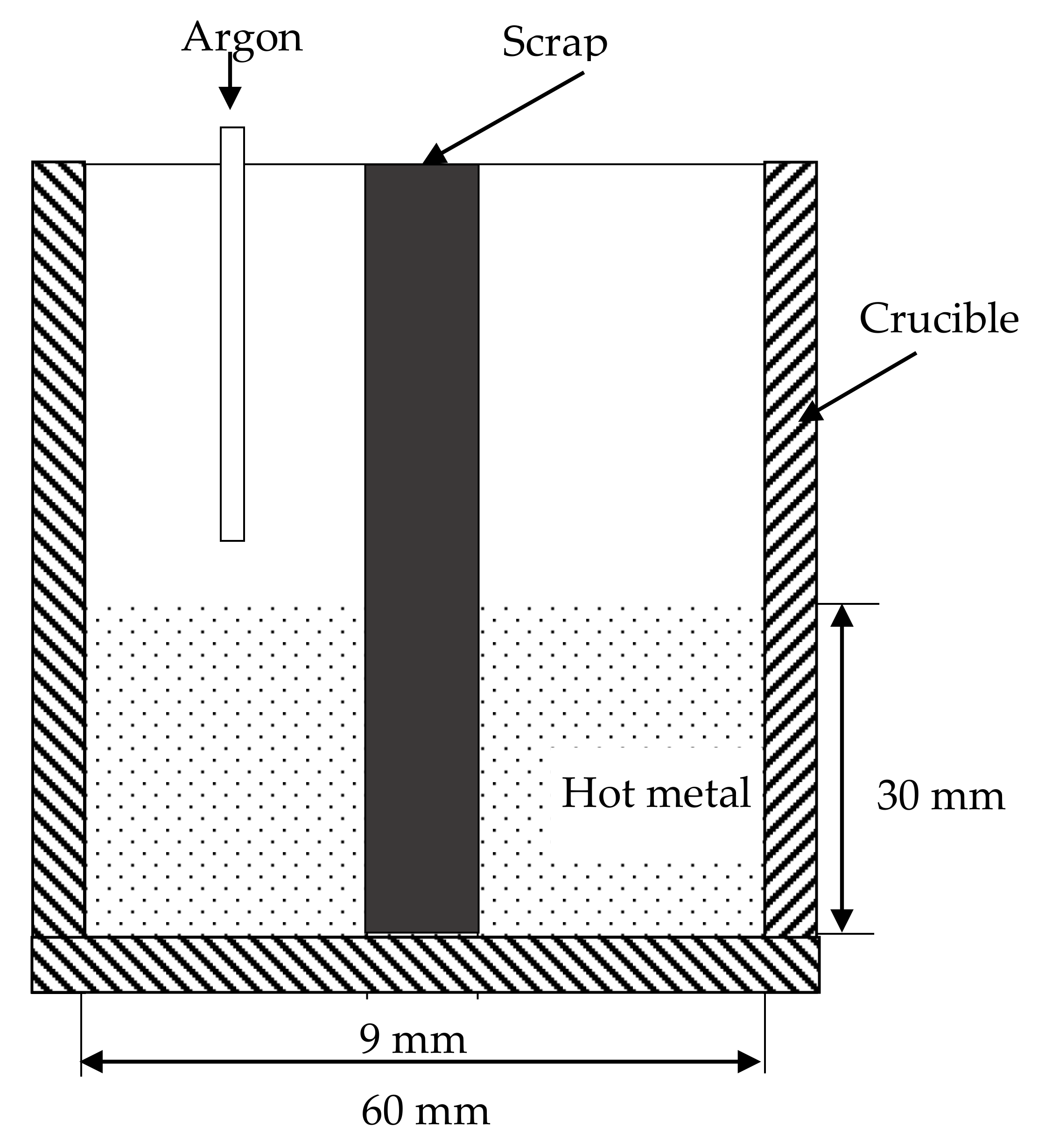
3. Experiment and Mathematical Modeling
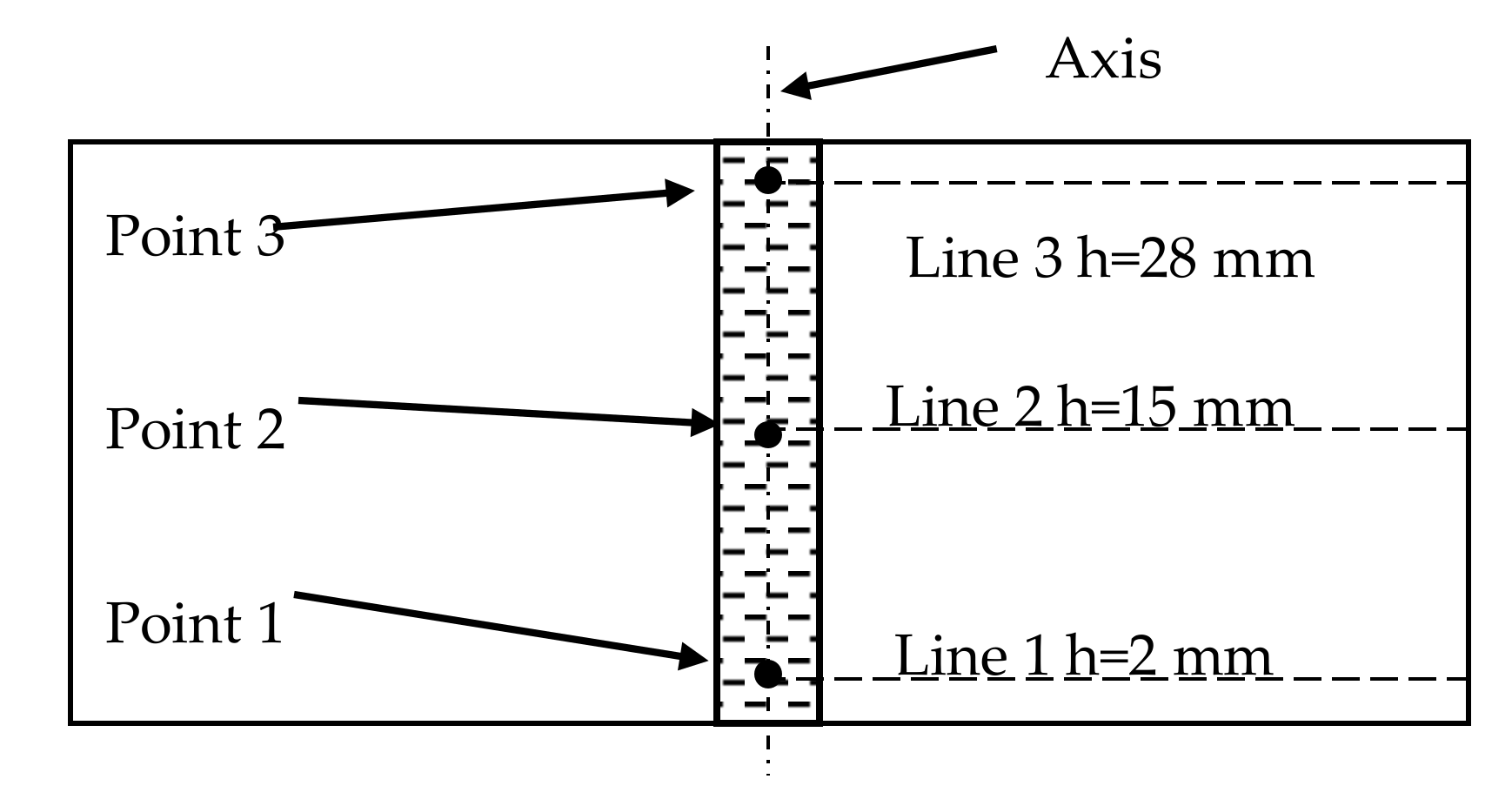
3.1. Experiment
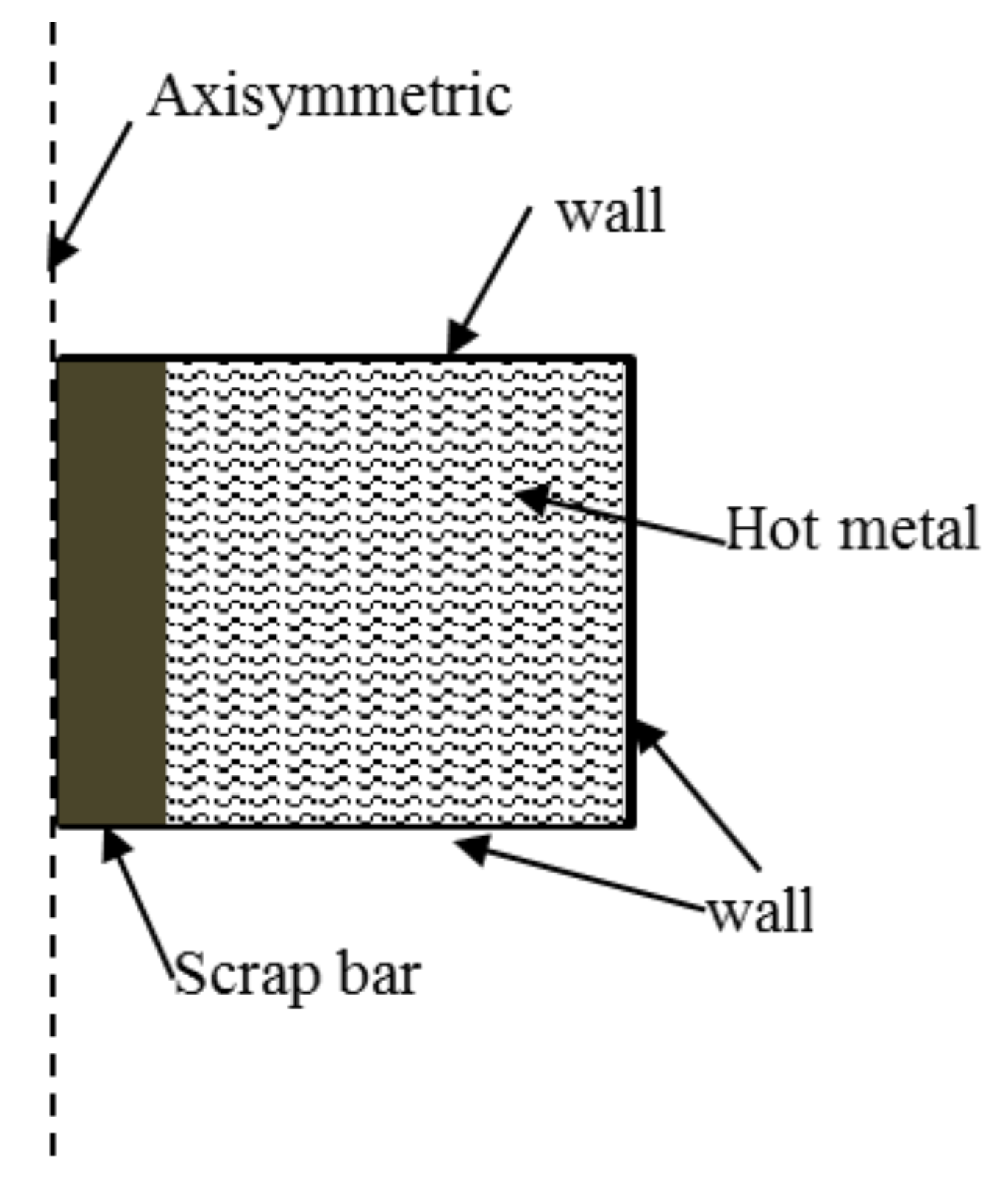
3.2. Mathematical Modeling
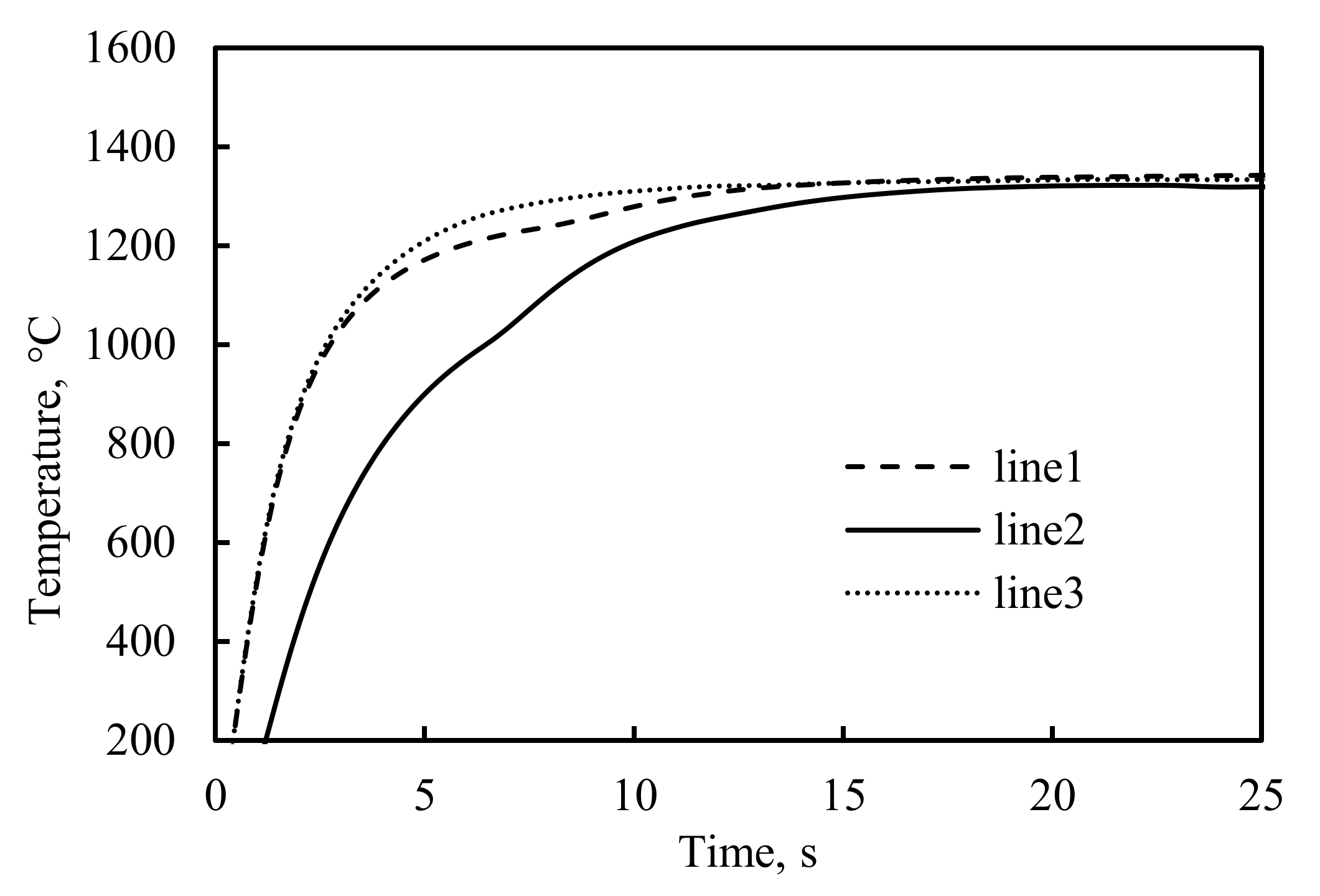
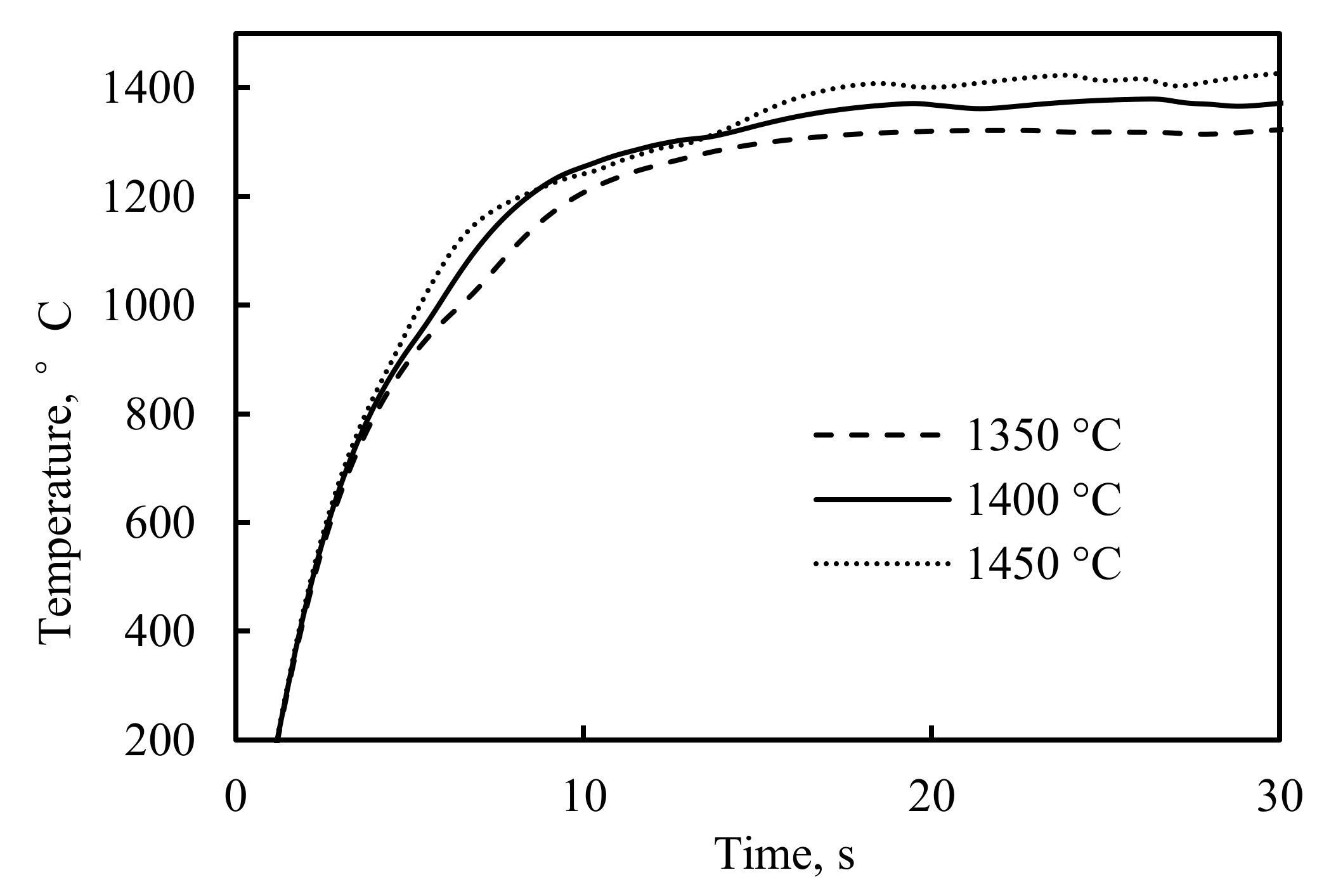
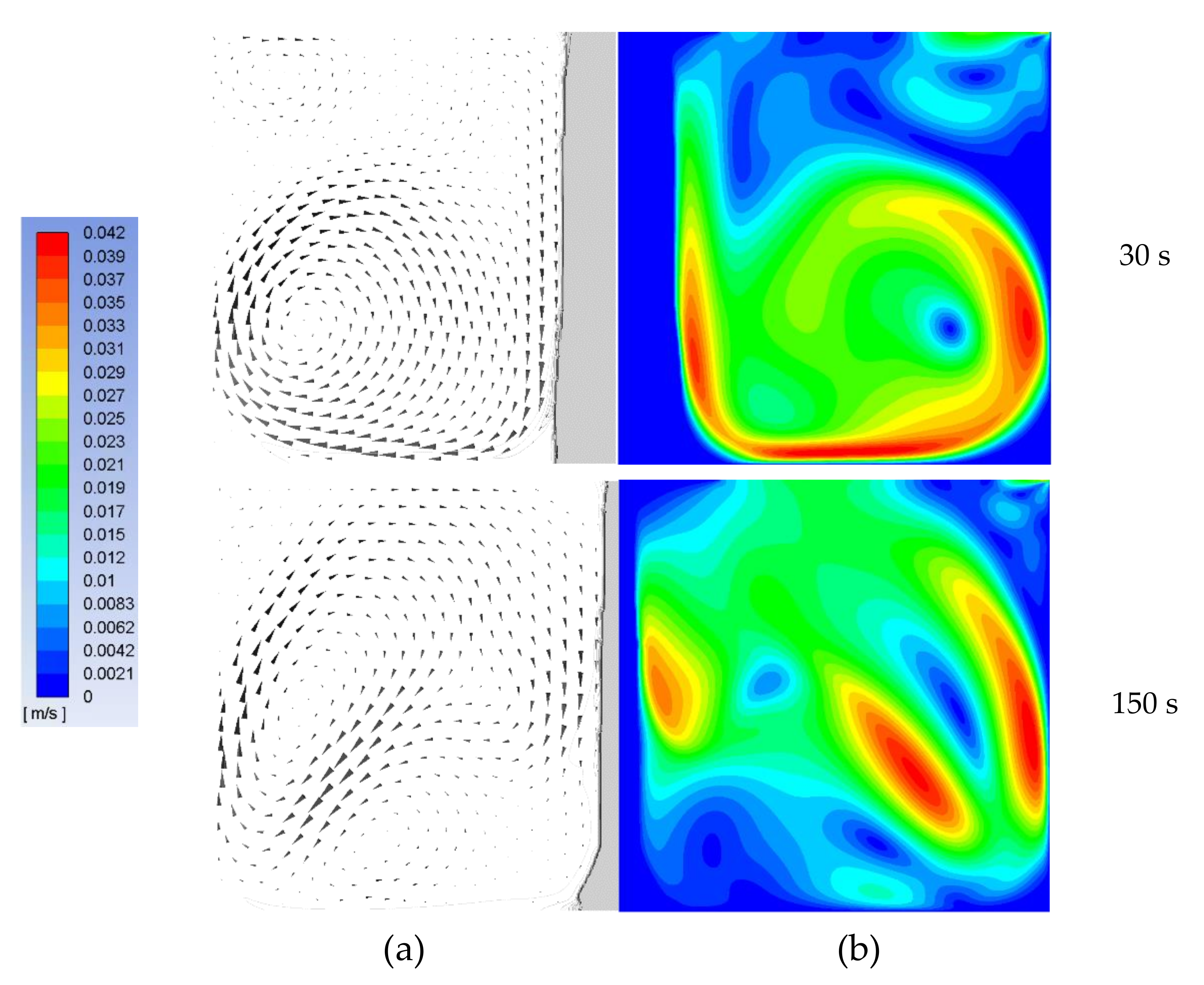
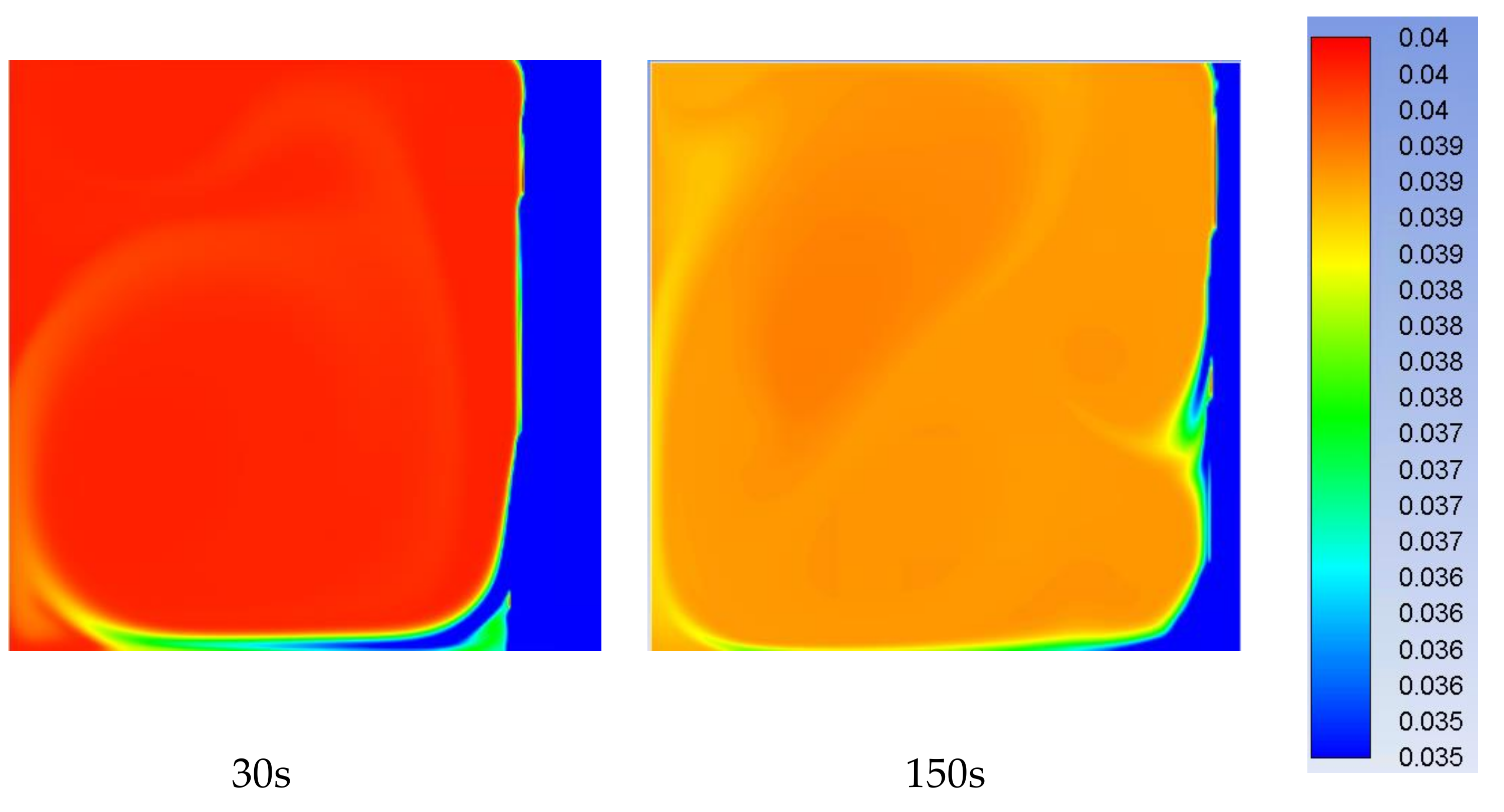
4. Results and Discussion
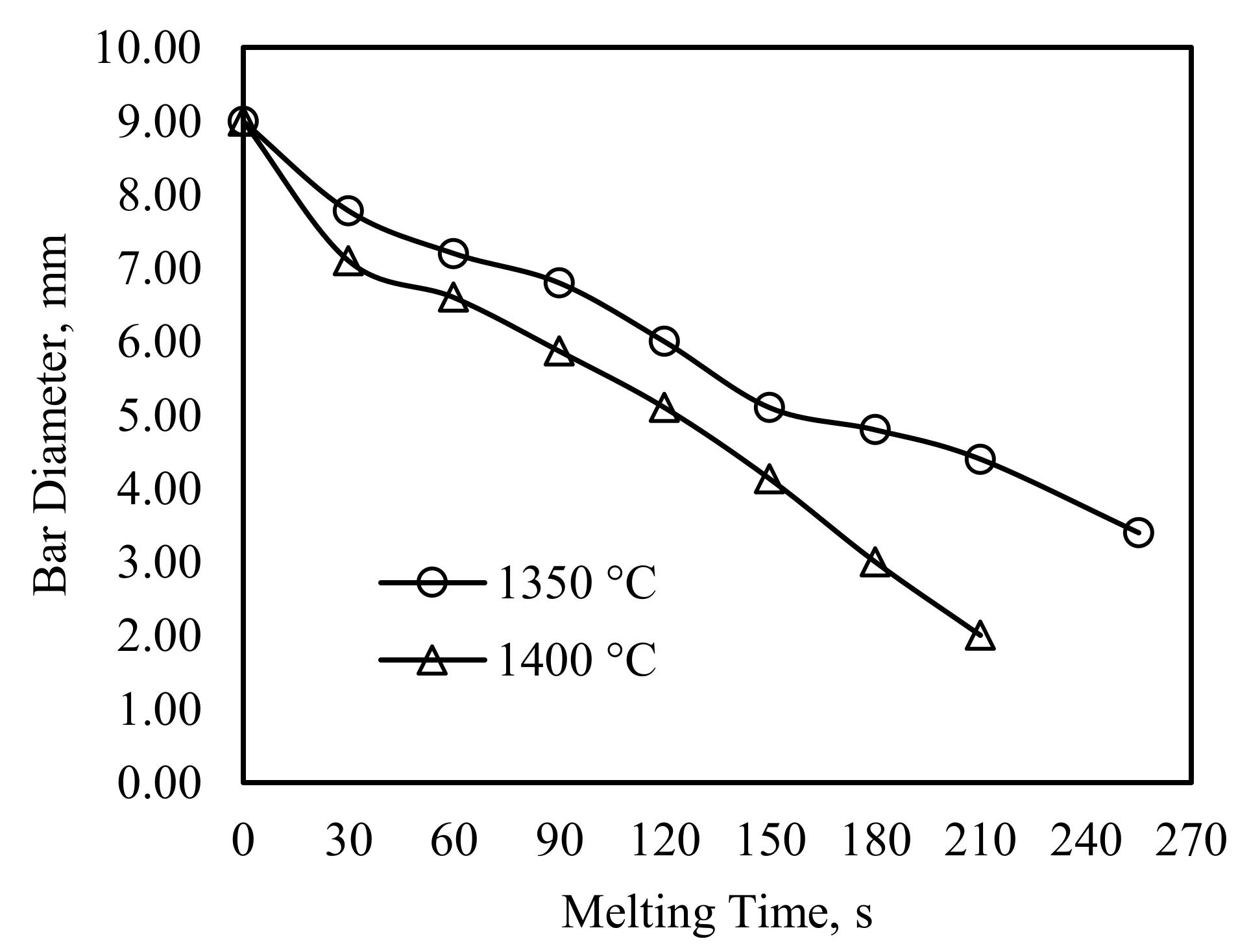
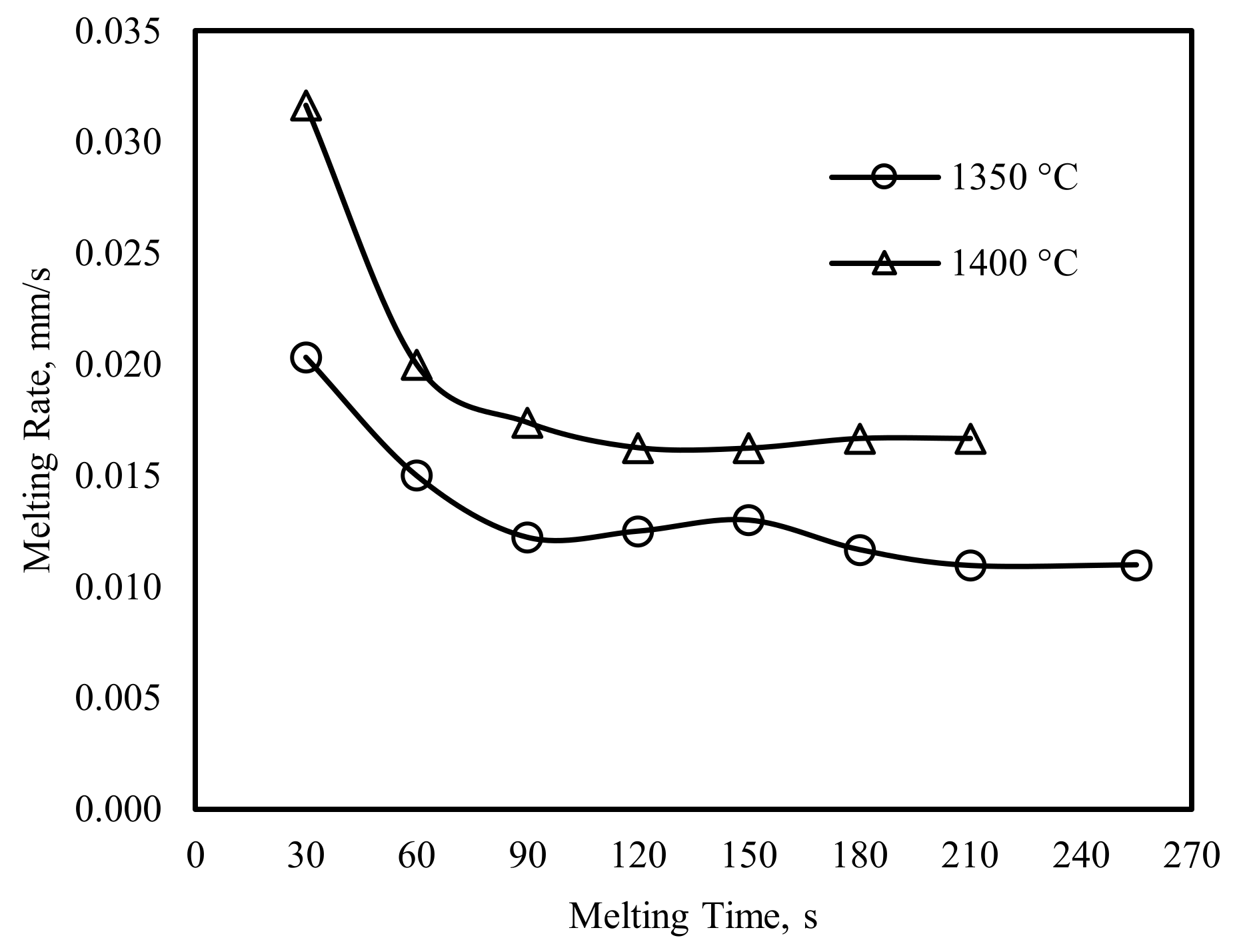
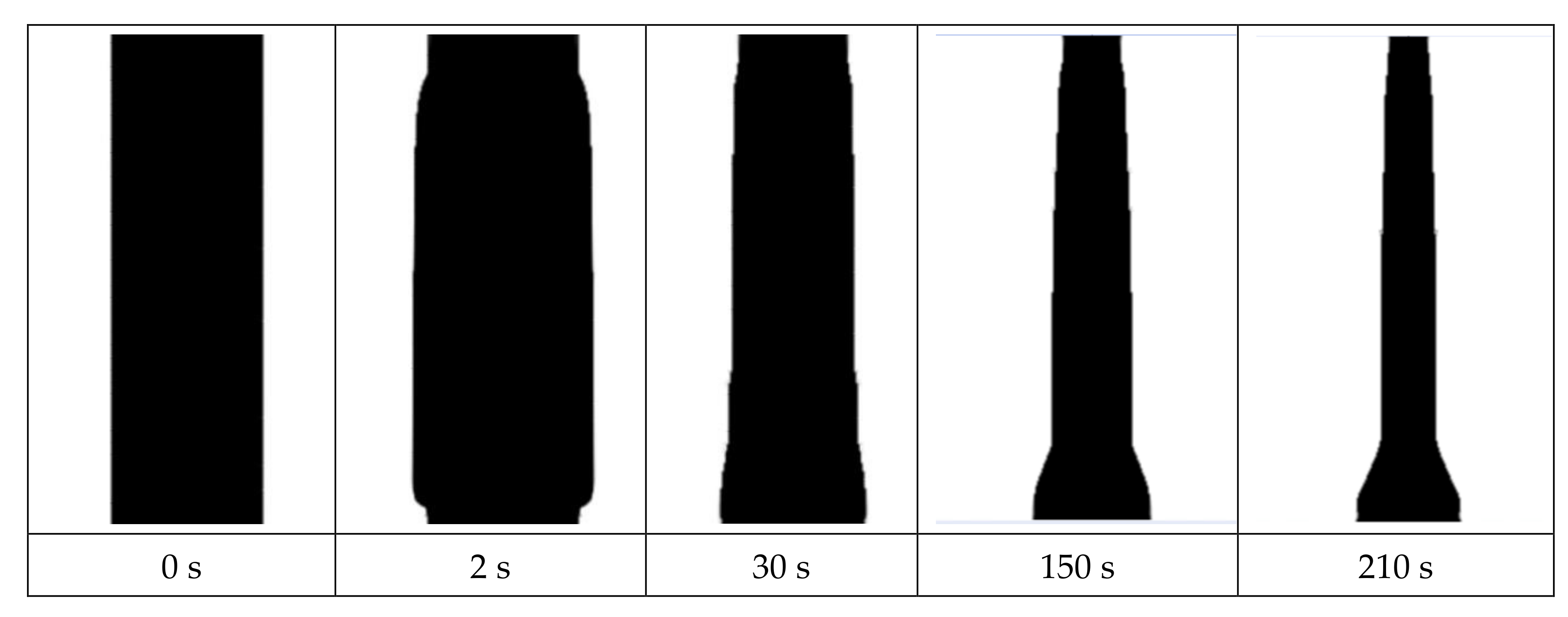
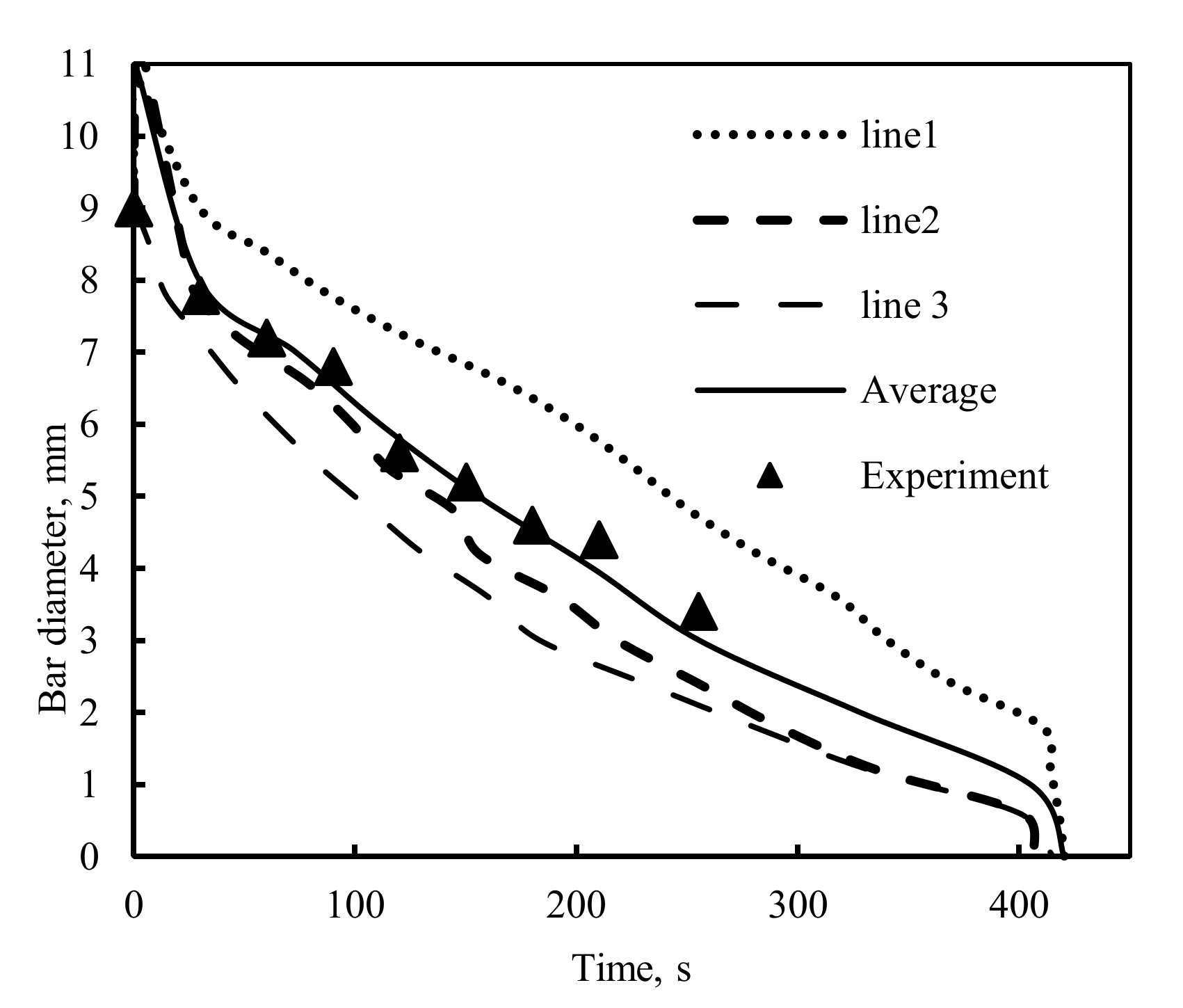
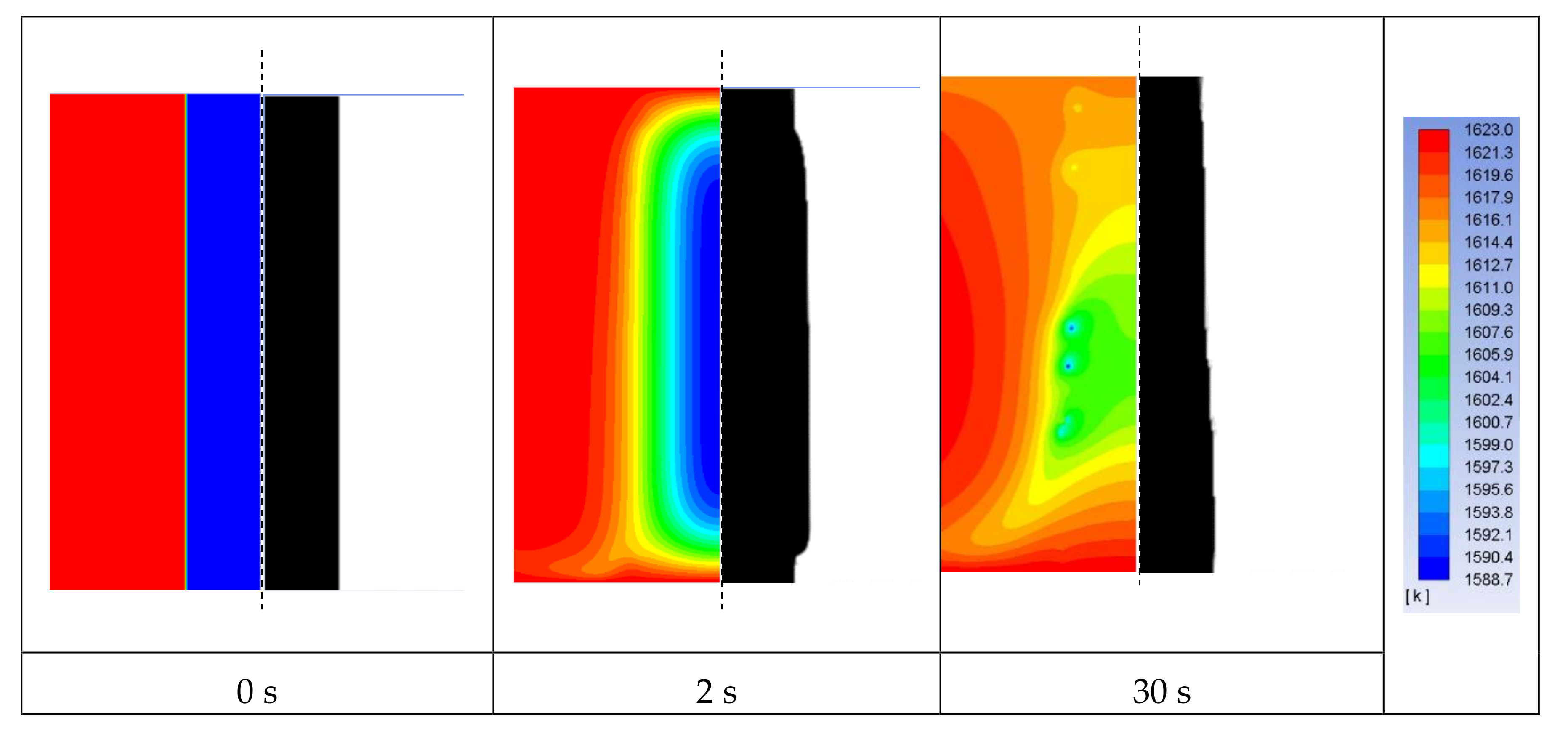
4.1. The Experimental Results and Discussions
4.2. The Mathematical Results and Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Szekely, J.; Chuang, Y.K.; Hlinka, J.W. The melting and dissolution of low carbon steels in iron carbon melts.pdf. Metall. Trans. 1972, 3, 2825–2833. [Google Scholar] [CrossRef]
- Kim, Y.U.; Pehlke, R.D. Transient heat transfer during initial stages of steel scrap melting. Metall. Trans. B 1975, 6B, 585–591. [Google Scholar] [CrossRef]
- Kawakami, M.; Takatani, K.; Brabie, L.C. Heat and Mass Transfer Analysis of Scrap Melting in Steel Bath.pdf. Tetsu Hagane 1999, 85, 20–27. [Google Scholar]
- Yamamoto, T.; Ujisawa, Y.; Ishida, H.; Takatan, K. operation and design of scrap melting process of the packed bed type. ISIJ Int. 1999, 39, 705–714. [Google Scholar] [CrossRef]
- Li, J.; Brooks, G.; Provatas, N. Kinetics of Scrap Melting in Liquid Steel. Metall. Mater. Trans. B 2005, 36, 293–302. [Google Scholar] [CrossRef]
- Li, J.; Provatas, N. Kinetics of Scrap Melting in Liquid Steel: Multipiece Scrap Melting. Metall. Mater. Trans. B 2008, 39, 268–279. [Google Scholar] [CrossRef]
- Wei, G.; Zhu, R.; Tang, T.; Dong, K. Study on the melting characteristics of steel scrap in molten steel. Ironmak. Steelmak. 2019, 46, 609–617. [Google Scholar] [CrossRef]
- Sethia, G.; Shuklaa, A.K.; Dasb, P.C.; Chandrab, P.; Deo, B. Theoretical aspects of scrap dissolution in oxygen steelmaking converters.pdf. AISTech Proc. 2004, 2, 915–926. [Google Scholar]
- Shukla, A.K.; Deo, B.; Millman, S.; Snoeijer, B.; Overbosch, A.; Kapilashrami, A. An Insight into the Mechanism and Kinetics of Reactions In BOF Steelmaking: Theory vs. Practice. Steel Res. Int. 2010, 81, 940–948. [Google Scholar] [CrossRef]
- Arzpeyma, N.; Widlund, O.; Ersson, M.; Jönsson, P. Mathematical Modeling of Scrap Melting in an EAF Using Electromagnetic Stirring. ISIJ Int. 2013, 53, 48–55. [Google Scholar] [CrossRef]
- Kruskopf, A. A Model for Scrap Melting in Steel Converter. Metall. Mater. Trans. B 2015, 46, 1195–1206. [Google Scholar] [CrossRef]
- Shukla, A.K.; Deo, B.; Robertson, D.G.C. Scrap Dissolution in Molten Iron Containing Carbon for the Case of Coupled Heat and Mass Transfer Control. Metall. Mater. Trans. B 2013, 44, 1407–1427. [Google Scholar] [CrossRef]
- Guo, D.; Swickard, D.; Slavanja, M.; Bradley, J. numerical simulation of heavy scrap melting in BOF steelmaking. In Proceedings of the Iron & Steel Technology Conference and Exposition, Atlanta, GA, USA, 7–10 May 2012; pp. 125–132. [Google Scholar]
- Ramirez-Argaez, M.A.; Conejo, A.N.; López-Cornejo, M.S. Mathematical Modeling of the Melting Rate of Metallic Particles in the EAF under Multiphase Flow. ISIJ Int. 2015, 55, 117–125. [Google Scholar] [CrossRef]
- Pineda-Martínez, E.; Hernández-Bocanegra, C.A.; Conejo, A.N.; Ramirez-Argaez, M.A. Mathematical Modeling of the Melting of Sponge Iron in a Bath of Non-reactive Molten Slag. ISIJ Int. 2015, 55, 1906–1915. [Google Scholar] [CrossRef]
- Berjeza, N.A.; Misuchenko, N.I. Computational Modelling of Heat/Mass Transfer Near the Liquid-Solid Interface During Rapid Solidification of Binary Metal Alloys Under Laser Treatment. Can. Metall. Q. 2013, 37, 313–321. [Google Scholar] [CrossRef]
- Ludwig, A.; Wu, M. Modeling of globular equiaxed solidification with a two-phase approach. Metall. Mater. Trans. A 2002, 33, 3673–3683. [Google Scholar] [CrossRef]
- Wu, M.H.; Ludwig, A.; Luo, J.L. Numerical Study of the Thermal-Solutal Convection and Grain Sedimentation during Globular Equiaxed Solidification. Mater. Sci. Forum 2005, 475–479, 2725–2730. [Google Scholar]
- Wu, M.; Ludwig, A. Using a Three-Phase Deterministic Model for the Columnar-to-Equiaxed Transition. Metall. Mater. Trans. A 2007, 38, 1465–1475. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Ludwig, A.; Kharicha, A. Simulation of macrosegregation in a 2.45-ton steel ingot using a three-phase mixed columnar-equiaxed model. Int. J. Heat Mass Transf. 2014, 72, 668–679. [Google Scholar] [CrossRef]
- Wu, M.; Li, J.; Ludwig, A.; Kharicha, A. Modeling diffusion-governed solidification of ternary alloys—Part 1: Coupling solidification kinetics with thermodynamics. Comput. Mater. Sci. 2013, 79, 830–840. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Hao, J.; Ludwig, A. Simulation of channel segregation using a two-phase columnar solidification model—Part I: Model description and verification. Comput. Mater. Sci. 2012, 55, 407–418. [Google Scholar] [CrossRef]
- Moelans, N.; Blanpain, B.; Wollants, P. An introduction to phase-field modeling of microstructure evolution. Calphad 2008, 32, 268–294. [Google Scholar] [CrossRef]
- Fluent User’s Manual; ANSYS: Canonsburg, PA, USA, 2018; pp. 646–648.
- Voller, V.R.; Brent, A.D.; Reid, K.J. A Computational Modeling Framework for the Analysis of Metallurgical Solidification Process and Phenomena. Technical report. In Proceedings of the Conference for Solidification Processing Ranmoor House, Sheffield, UK, 21–24 September 1987. [Google Scholar]
- Voller, V.R.; Prakash, C. A Fixed-Grid Numerical Modeling Methodology for Convection-Diffusion Mushy Region Phase-Change Problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1720. [Google Scholar] [CrossRef]


| Material | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| Hot metal | 4.01 | 4.3 | 0.3 | 0.014 | 0.018 |
| Scrap bar | 0.1 | 0.2 | 0.02 | 0.016 | 0.02 |
| Property | Quantity | Units |
|---|---|---|
| Temperature the hot metal | 1350, 1400 | °C |
| Temperature the scrap | 25 | °C |
| Melting time | 30, 60, 90, 120, 150, 180, 210 | s |
| Diameter of the scrap | 9 | mm |
| Property | Quantity | Units |
|---|---|---|
| Melting point of pure iron | 1805.1 | K |
| Viscosity | 0.0062 | Pa·s |
| Equilibrium partition coefficient | 0.36 | - |
| Specific heat | 500 | J/(kg·K) |
| Thermal conductivity | 34 | W/(m·K) |
| Latent heat | 2.71 × 105 | J/kg |
| Thermal expansion coefficient | 1.07 × 10−4 | K−1 |
| Solutal expansion coefficient | 1.4 × 10−2 | Wt.%−1 |
| Diffusion coefficient in liquid | 2.0 × 10−8 | m2/s |
| Diffusion coefficient in solid | 1.0 × 10−9 | m2/s |
| Property | Quantity | Units |
|---|---|---|
| Initial temperature the hot metal | 1350, 1400, 1450 | °C |
| Initial temperature the scrap | 25 | °C |
| Initial carbon content of the hot metal | 4.0 | wt.% |
| Initial carbon content of the scrap | 0.1 | wt.% |
| Initial diameter of the scrap | 9 | mm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, N.; Zhou, X.; Zhou, M.; Peng, S. Numerical Simulation of the Melting Behavior of Steel Scrap in Hot Metal. Metals 2020, 10, 678. https://doi.org/10.3390/met10050678
Deng N, Zhou X, Zhou M, Peng S. Numerical Simulation of the Melting Behavior of Steel Scrap in Hot Metal. Metals. 2020; 10(5):678. https://doi.org/10.3390/met10050678
Chicago/Turabian StyleDeng, Nanyang, Xiaobin Zhou, Moer Zhou, and Shiheng Peng. 2020. "Numerical Simulation of the Melting Behavior of Steel Scrap in Hot Metal" Metals 10, no. 5: 678. https://doi.org/10.3390/met10050678
APA StyleDeng, N., Zhou, X., Zhou, M., & Peng, S. (2020). Numerical Simulation of the Melting Behavior of Steel Scrap in Hot Metal. Metals, 10(5), 678. https://doi.org/10.3390/met10050678





