Analysis of Different Solution Treatments in the Transformation of β-AlFeSi Particles into α-(FeMn)Si and Their Influence on Different Ageing Treatments in Al–Mg–Si Alloys
Abstract
:1. Introduction
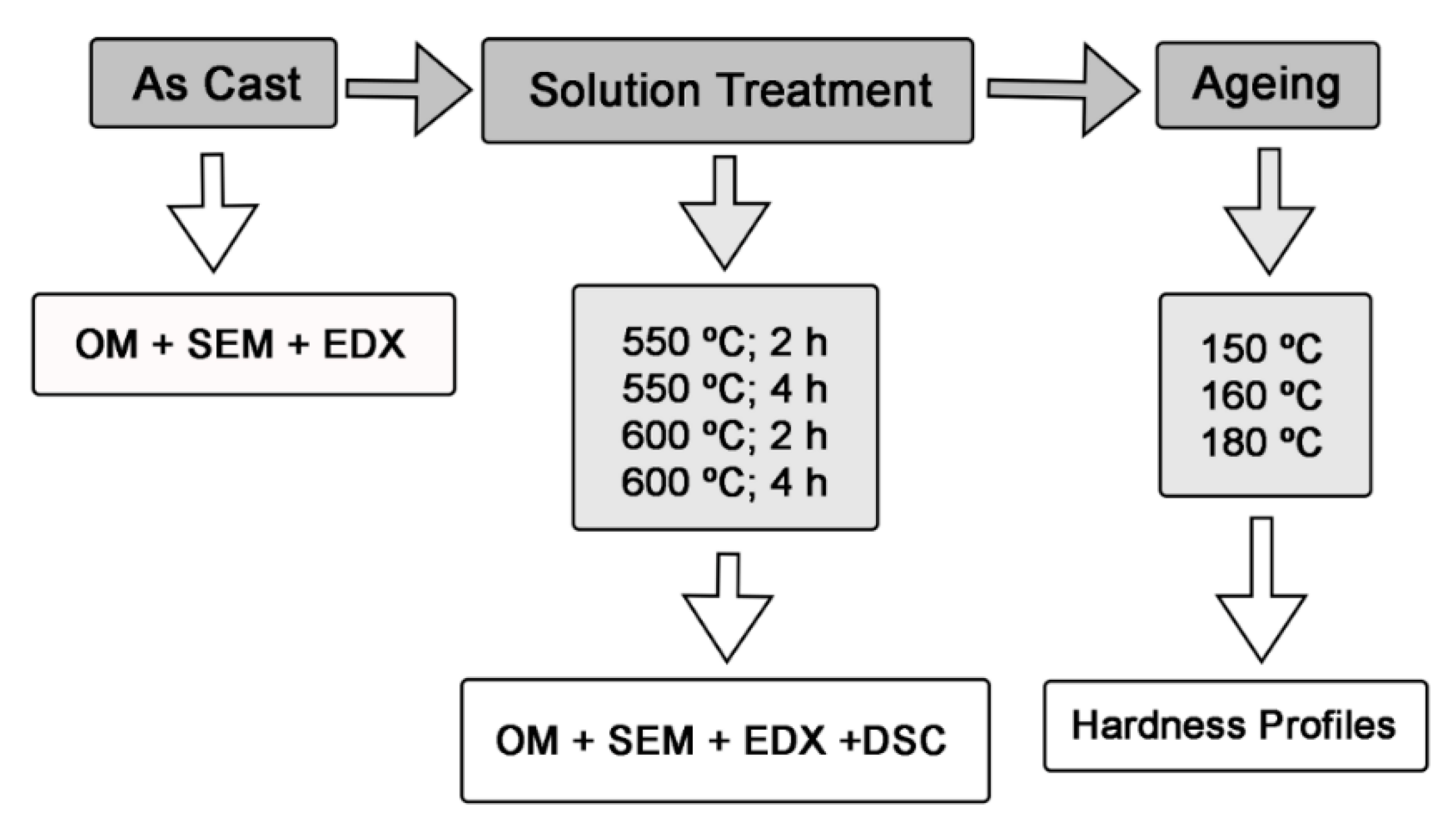
2. Materials and Methods
- (1)
- 108 specimens were used to obtain the hardness profiles: 4 different solution treatments were carried out, employing 3 ageing treatments per solution treatment, with a total of 9 specimens in each ageing treatment.
- (2)
- 8 specimens had no ageing. There were 2 specimens after each solution treatment, one was used for metallographic inspection and calculation of the material’s hardness, while the other was used for differential scanning calorimetry (DSC).
- (3)
- The remaining 4 specimens were analysed in the as-cast state.
- (1)
- 2 mL HF, 3 mL HCl, 5 mL HNO3 and 190 mL distilled water.
- (2)
- A solution made of 4 g KMnO4, 1 g NaOH and 100 mL distilled water. This reagent reveals the chemical heterogeneities derived from dendritic microsegregation.
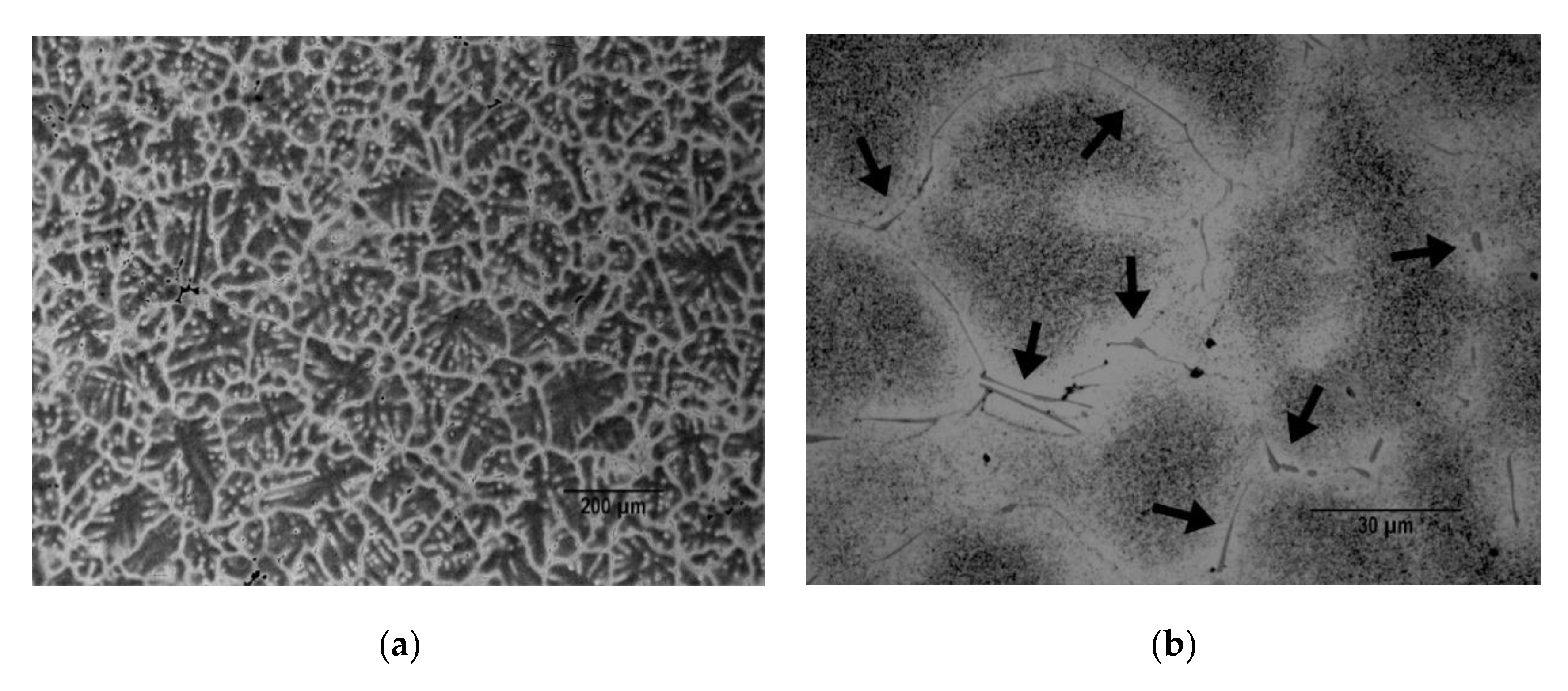
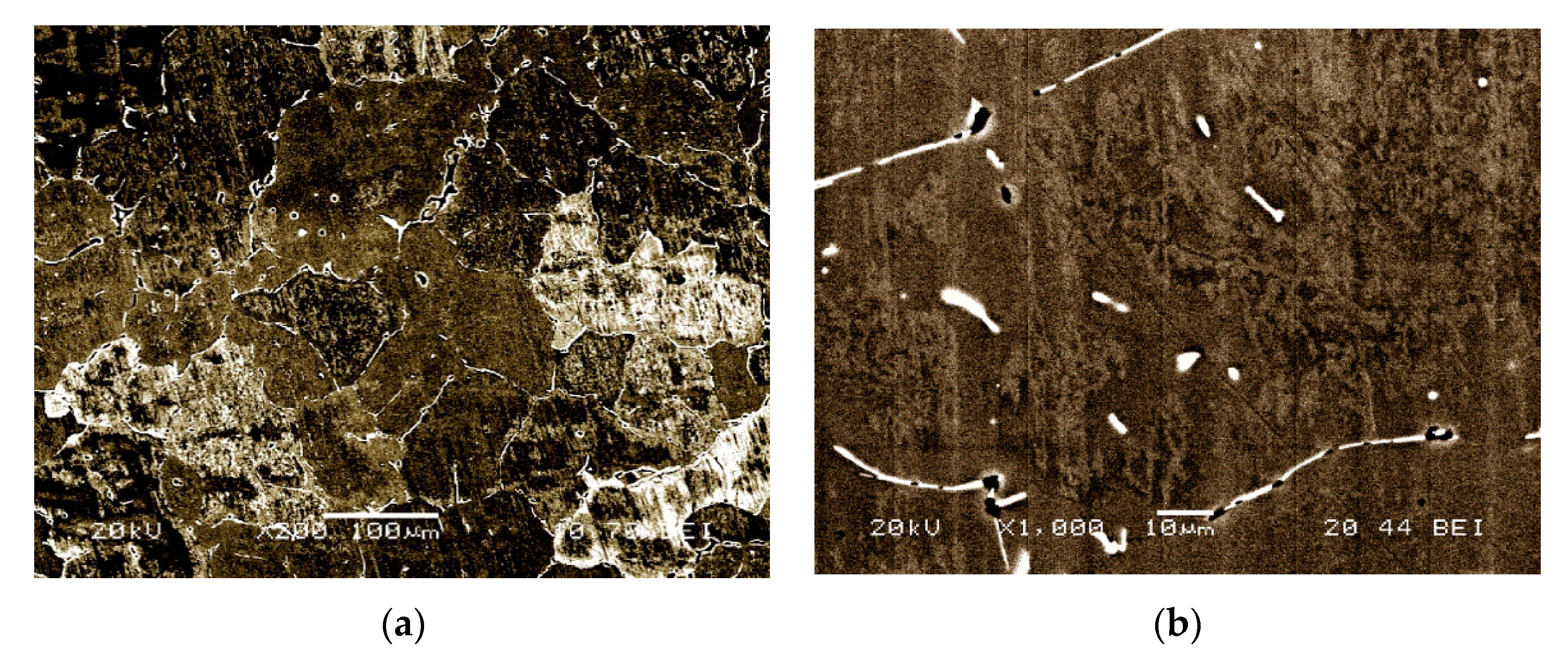
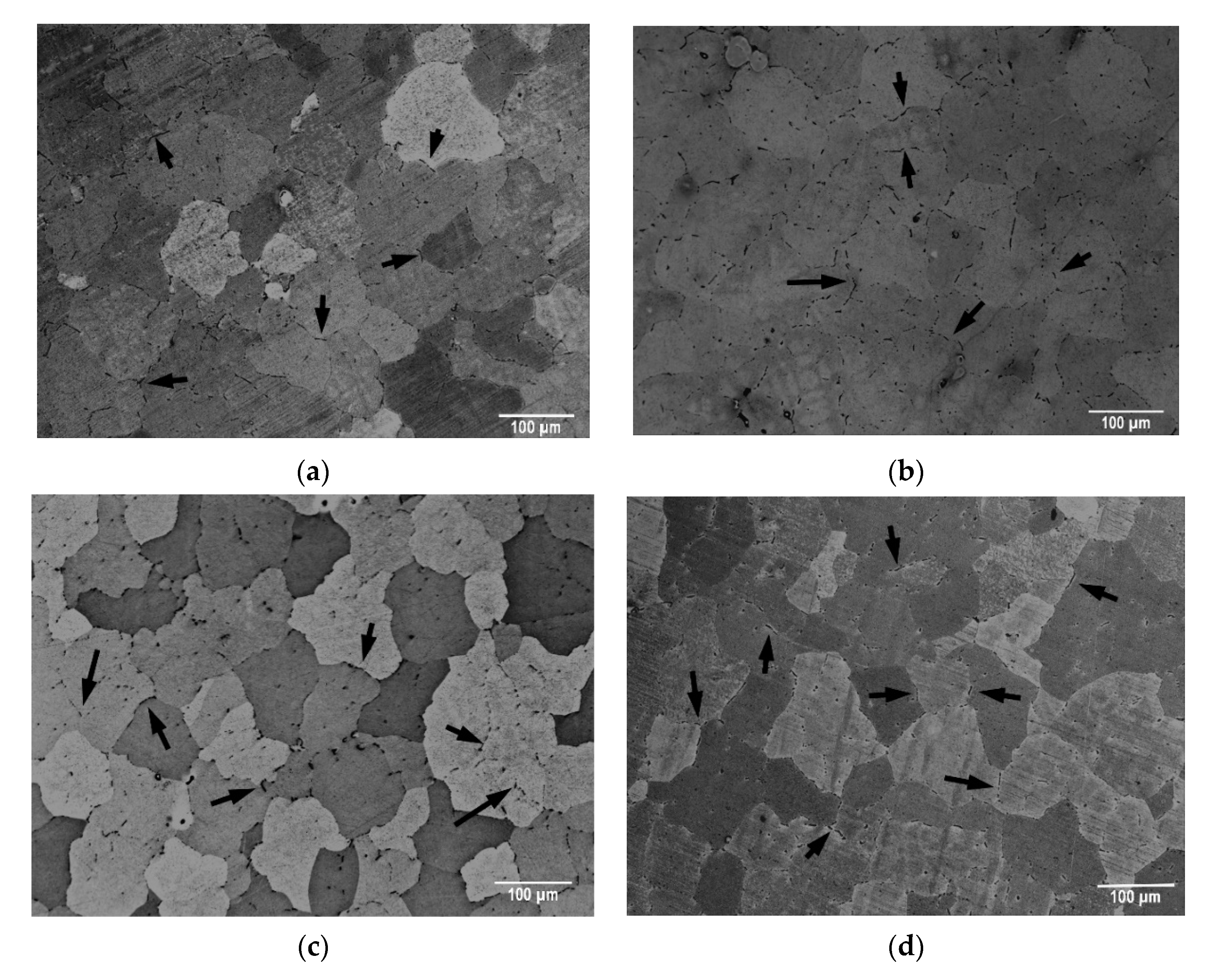
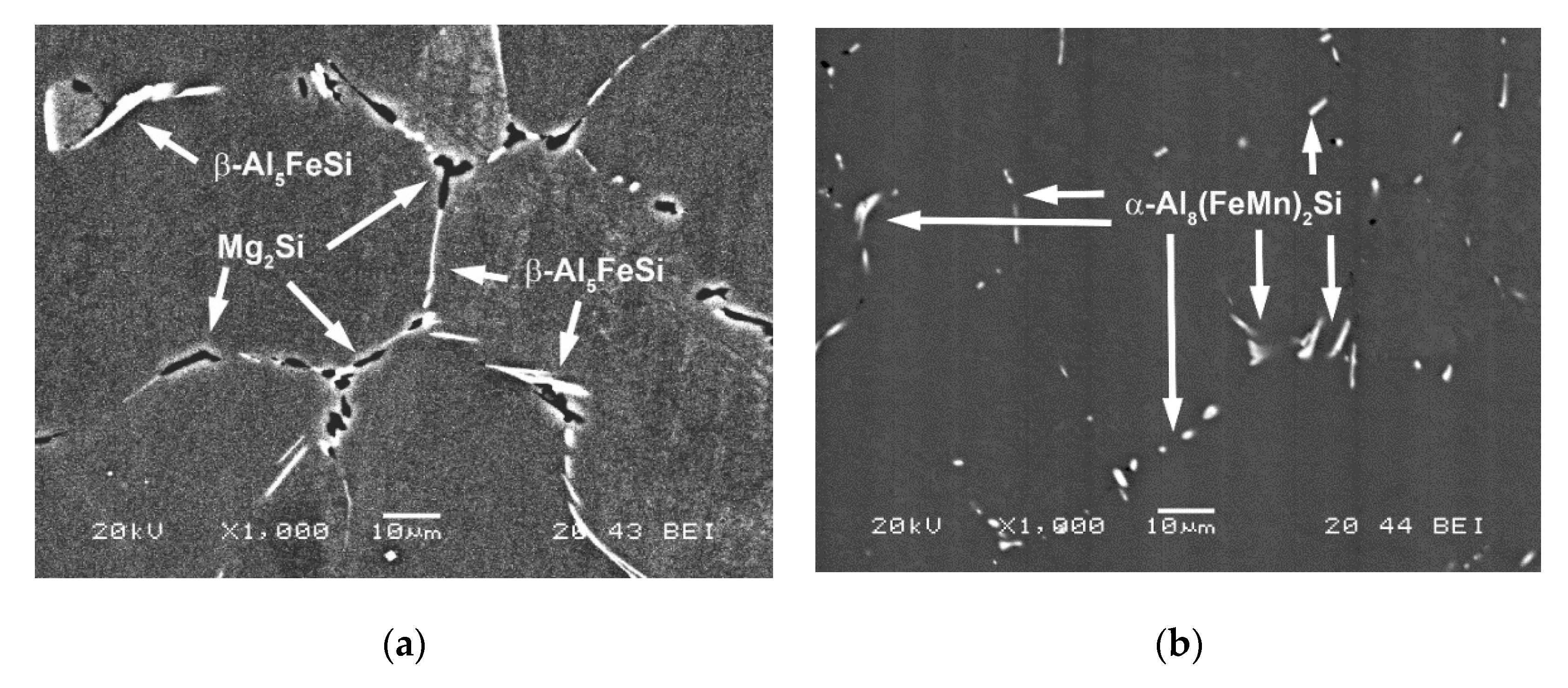
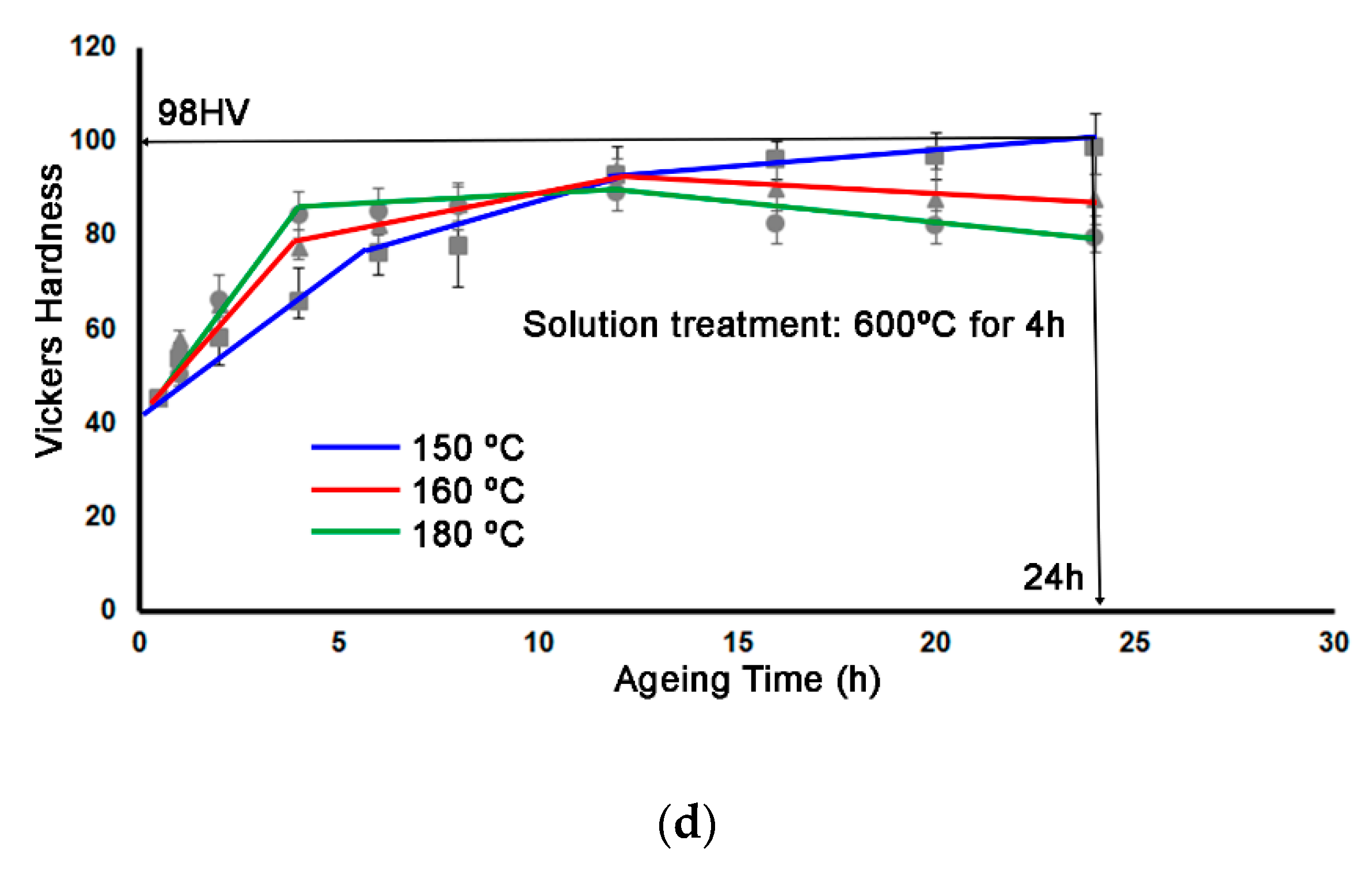
3. Results
4. Conclusions
- (1)
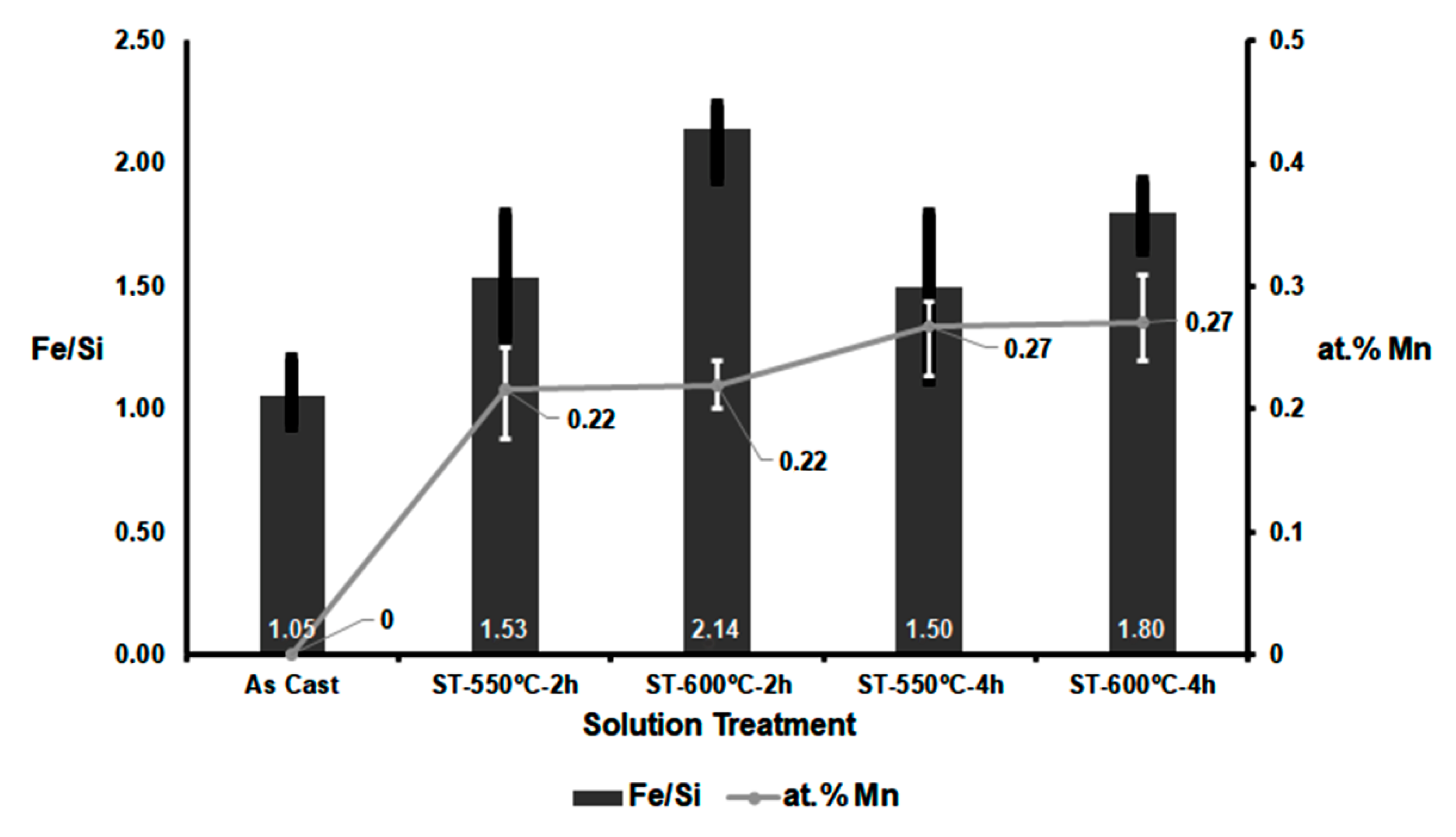
- The Fe/Si atomic ratio increased with increasing solution treatment temperature from 550 to 600 °C. This reflects a greater degree of transformation of β-Al5FeSi particles into α-Al8(FeMn)2Si particles, and a greater potential for structural hardening.
- (2)
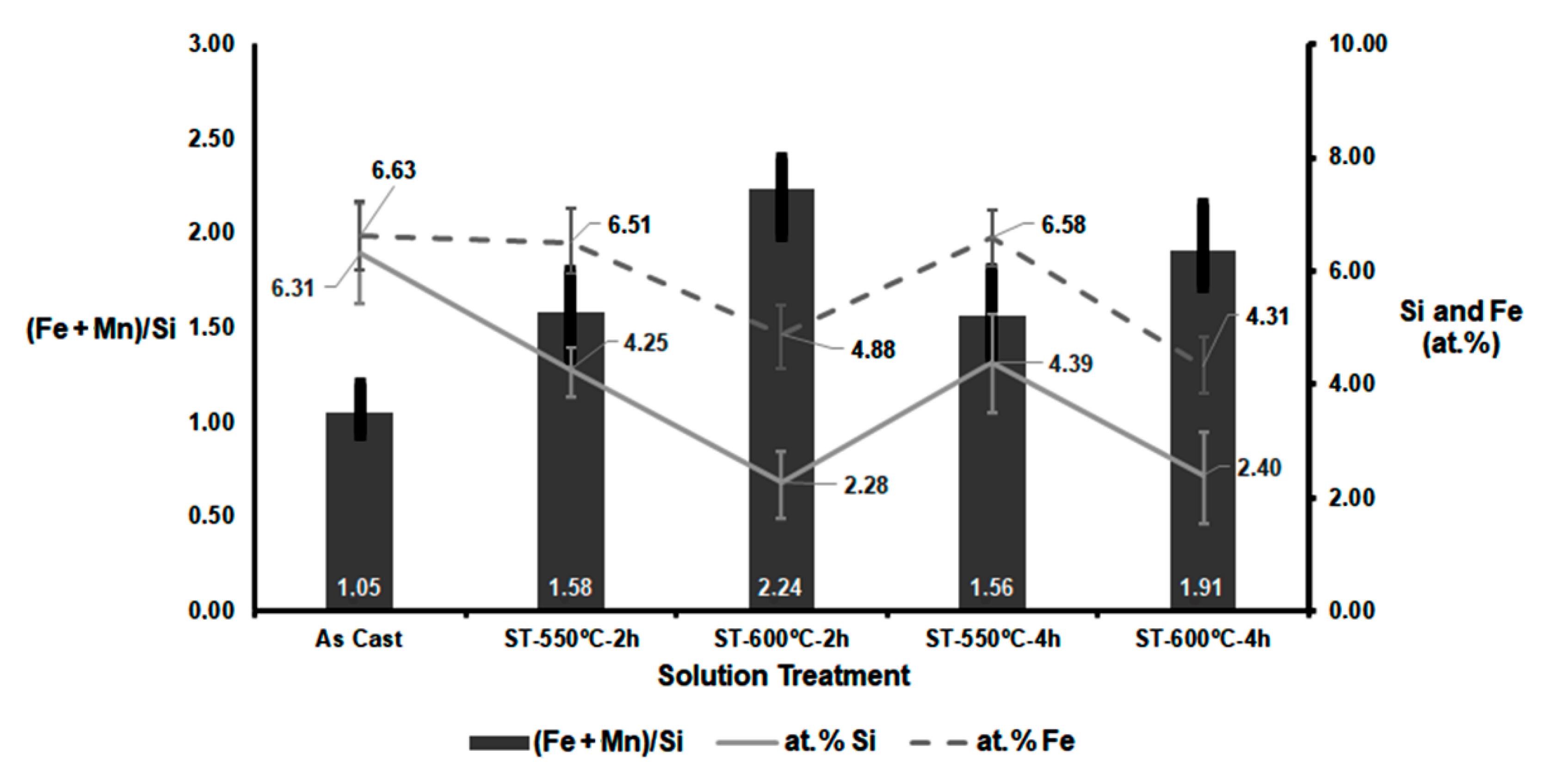
- In the transformation of β-Al5FeSi particles into α-Al8(FeMn)2Si, a greater dissolution of both Si and Fe atoms was observed in the matrix when the solution treatment was carried out at 600 °C. The dissolution of Fe was somewhat more pronounced when the dwell times were increased from 2 to 4 h.
- (3)
- At a solution temperature of 550 °C, the (Fe + Mn)/Si atomic ratio remained practically constant. However, at 600 °C, this ratio decreased when the dwell time was increased from 2 to 4 h. This suggests that the rate of dissolution of Fe atoms exceeded the rate of incorporation of Mn atoms. This could lead to a delay in reaching peak hardness values during ageing at temperatures between 150 and 180 °C.
- (4)
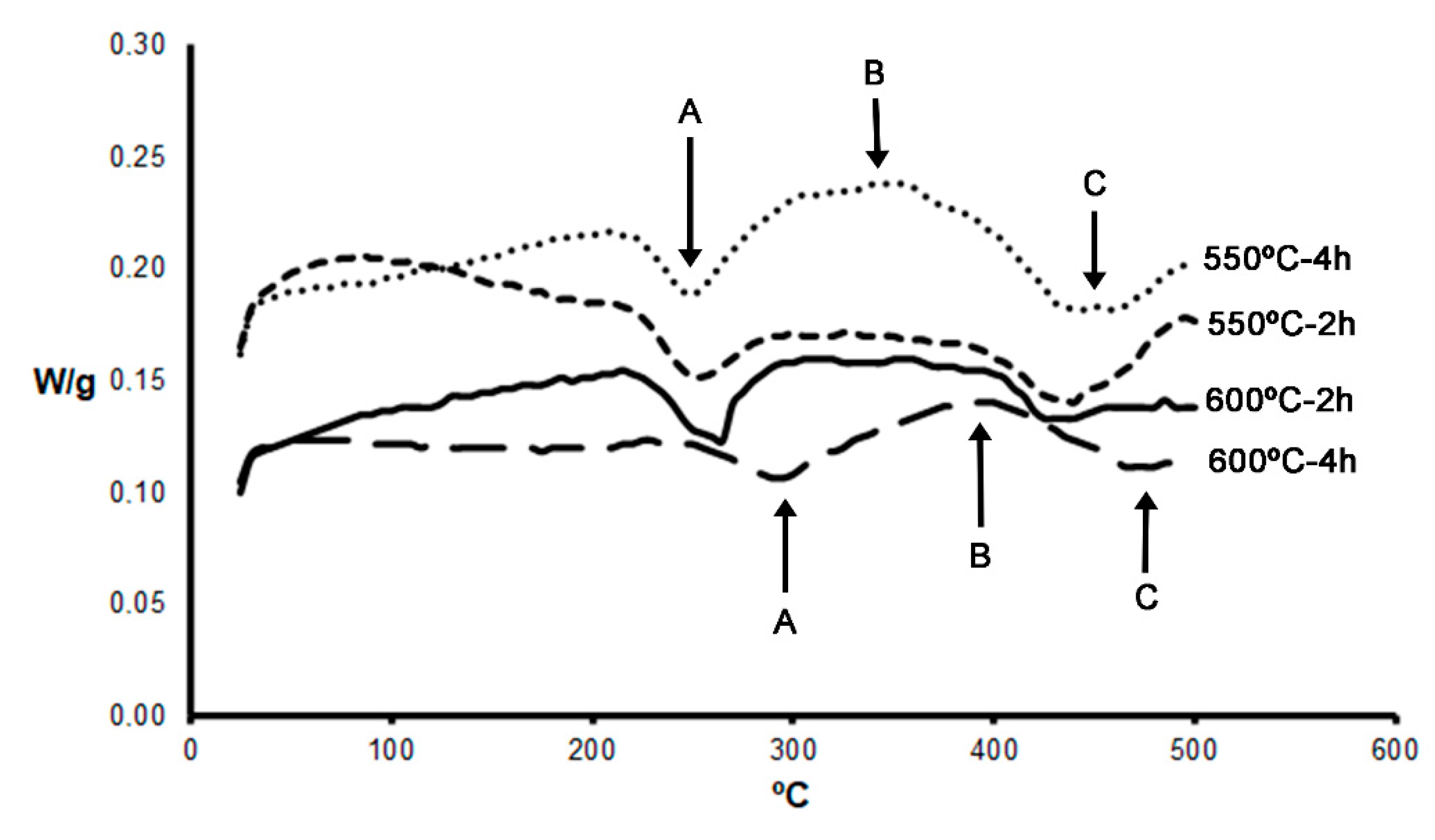
- The peak hardness value obtained was 104 HV, following a solution treatment at 600 °C for 2 h and ageing at 160 °C for 12 h.
Author Contributions
Funding
Conflicts of Interest
References
- Chanyathunyaroj, K.; Patakham, U.; Kou, S.; Limmaneevichitr, C. Microstructural evolution of iron-rich intermetallic compounds in scandium modified Al-7Si-0.3Mg alloys. J. Alloy. Compd. 2017, 692, 865–875. [Google Scholar] [CrossRef]
- Hsu, C.; O’Reilly, K.A.Q.; Cantor, B.; Hamerton, R. Non-equilibrium reactions in 6xxx series Al alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2001, 304, 119–124. [Google Scholar] [CrossRef]
- Sha, G.; O’Reilly, K.; Cantor, B.; Worth, J.; Hamerton, R. Growth related metastable phase selection in a 6xxx series wrought Al alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2001, 304, 612–616. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.Y.; Jiang, H.T.; Lo, Z.F.; Zhu, Z.F. Characterizations of precipitation behavior of Al-Mg-Si alloys under different heat treatments. China Foundry 2018, 15, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, N.C.W.; Vermolen, F.J.; Vuik, K.; van der Zwaag, S. A model of the beta-AlFeSi to alpha-Al(FeMn)Si transformation in Al-Mg-Si alloys. Mater. Trans. 2003, 44, 1448–1456. [Google Scholar] [CrossRef] [Green Version]
- Gorny, A.; Manickaraj, J.; Cai, Z.H.; Shankar, S. Evolution of Fe based intermetallic phases in Al-Si hypoeutectic casting alloys: Influence of the Si and Fe concentrations, and solidification rate. J. Alloy. Compd. 2013, 577, 103–124. [Google Scholar] [CrossRef]
- Fan, C.; Long, S.Y.; Yang, H.D.; Wang, X.J.; Zhang, J.C. Influence of Ce and Mn addition on alpha-Fe morphology in recycled Al-Si alloy ingots. Int. J. Miner. Metall. Mater. 2013, 20, 890–895. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhang, H.R.; Zhang, F.X.; Ma, Z.; Jia, L.N.; Yang, B.; Tao, T.X.; Zhang, H. Effect of cooling rate and Co content on the formation of Fe-rich intermetallics in hypoeutectic A17Si0.3Mg alloy with 0.5%Fe. Mater. Charact. 2018, 139, 116–124. [Google Scholar] [CrossRef]
- Zajac, S.; Gullman, L.O.; Johansson, A.; Bengtsson, B. Hot ductility of some Al-Mg-Si alloys. Mater. Sci. Forum 1996, 217, 1193–1198. [Google Scholar] [CrossRef]
- Bayat, N.; Carlberg, T.; Cieslar, M. In-situ study of phase transformations during homogenization of 6060 and 6063 Al alloys. J. Phys. Chem. Solids 2019, 130, 165–171. [Google Scholar] [CrossRef]
- Sirichaivetkul, R.; Wongpinij, T.; Euaruksakul, C.; Limmaneevichitr, C.; Kajornchaiyakul, J. In-situ study of microstructural evolution during thermal treatment of 6063 aluminum alloy. Mater. Lett. 2019, 250, 42–45. [Google Scholar] [CrossRef]
- Couto, K.B.S.; Claves, S.R.; Van Geertruyden, W.H.; Misiolek, W.Z.; Goncalves, M. Effects of homogenisation treatment on microstructure and hot ductility of aluminium alloy 6063. Mater. Sci. Technol. 2005, 21, 263–268. [Google Scholar] [CrossRef]
- Kumar, S.; Grant, P.S.; O’Reilly, K.A.Q. Evolution of Fe Bearing Intermetallics During DC Casting and Homogenization of an Al-Mg-Si Al Alloy. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2016, 47A, 3000–3014. [Google Scholar] [CrossRef] [Green Version]
- Zajac, S.; Hutchinson, B.; Johansson, A.; Gullman, L.O. Microstructure control and extrudability of al-mg-si alloys microalloyed with manganese. Mater. Sci. Technol. 1994, 10, 323–333. [Google Scholar] [CrossRef]
- Asensio-Lozano, J.; Suarez-Pena, B. Quantitative analysis and morphological characterization of 6063 alloy. Microestructural and mechanical comparison between periphery and center of semi-continuous casting round billets. Rev. De Metal. 2012, 48, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, N.C.W.; Vermolen, F.J.; Vuik, C.; Koenis, P.T.G.; Nilsen, K.E.; van der Zwaag, S. The dependence of the beta-AlFeSi to alpha-Al(FeMn)Si transformation kinetics in Al-Mg-Si alloys on the alloying elements. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2005, 394, 9–19. [Google Scholar] [CrossRef]
- Osada, Y. EPMA mapping of small particles of alpha-AlFeSi and beta-AlFeSi in AA6063 alloy billets. J. Mater. Sci. 2003, 38, 1457–1464. [Google Scholar] [CrossRef]
- Liu, C.L.; Azizi-Alizamini, H.; Parson, N.C.; Poole, W.J.; Du, Q. Microstructure evolution during homogenization of Al-Mg-Si-Mn-Fe alloys: Modelling experimental results. Trans. Nonferrous Met. Soc. China 2017, 27, 747–753. [Google Scholar] [CrossRef]
- Gavgali, M.; Totik, Y.; Sadeler, R. The effects of artificial aging on wear properties of AA 6063 alloy. Mater. Lett. 2003, 57, 3713–3721. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Y.J.; Zhu, T.; Li, X.Y. The effects of Solid-solution on properties and microstructure of 6063 aluminum alloy. Adv. Mater. Res. 2014, 881–883, 1346–1350. [Google Scholar] [CrossRef]
- Lang, P.; Povoden-Karadeniz, E.; Falahati, A.; Kozeschnik, E. Simulation of the effect of composition on the precipitation in 6xxx Al alloys during continuous-heating DSC. J. Alloy. Compd. 2014, 612, 443–449. [Google Scholar] [CrossRef]
- Sjolander, E.; Seifeddine, S. The heat treatment of Al-Si-Cu-Mg casting alloys. J. Mater. Process. Technol. 2010, 210, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, R.A.; Abdullah, H.A.; Al-Belushi, K.R. Influence of aging parameters on the mechanical properties of 6063 aluminium alloy. J. Mater. Process. Technol. 2000, 102, 234–240. [Google Scholar] [CrossRef]
- Milkereit, B.; Wanderka, N.; Schick, C.; Kessler, O. Continuous cooling precipitation diagrams of Al-Mg-Si alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2012, 550, 87–96. [Google Scholar] [CrossRef]
- Yuksel, B. Effect of artificial aging on hardness and intergranular corrosion of 6063 Al alloy. Pamukkale Univ. J. Eng. Sci. -Pamukkale Univ. Muhendis. Bilimleri Derg. 2017, 23, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Wang, X.D.; Pan, Q.L.; Li, M.J.; Ye, J.; Li, K.; Peng, Z.W.; Sun, Y.Q. Investigation of microstructure evolution and quench sensitivity of Al-Mg-Si-Mn-Cr alloy during isothermal treatment. J. Alloy. Compd. 2020, 826, 11. [Google Scholar] [CrossRef]
- Saito, T.; Marioara, C.D.; Royset, J.; Marthinsen, K.; Holmestad, R. The effects of quench rate and pre-deformation on precipitation hardening in Al-Mg-Si alloys with different Cu amounts. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2014, 609, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Meyveci, A.; Karacan, I.; Caligulu, U.; Durmus, H. Pin-on-disc characterization of 2xxx and 6xxx aluminium alloys aged by precipitation age hardening. J. Alloy. Compd. 2010, 491, 278–283. [Google Scholar] [CrossRef]
- Nandy, S.; Abu Bakkar, M.; Das, D. Influence of Ageing on Mechanical Properties of 6063 Al Alloy. Mater. Today-Proc. 2015, 2, 1234–1242. [Google Scholar] [CrossRef]
- Zohrabyan, D.; Milkereit, B.; Kessler, O.; Schick, C. Precipitation enthalpy during cooling of aluminum alloys obtained from calorimetric reheating experiments. Thermochim. Acta 2012, 529, 51–58. [Google Scholar] [CrossRef]
- Li, H.Y.; Zeng, C.T.; Han, M.S.; Liu, J.J.; Lu, X.C. Time-temperature-property curves for quench sensitivity of 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 38–45. [Google Scholar] [CrossRef]
- Cavazos, J.L.; Colas, R. Quench sensitivity of a heat treatable aluminum alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2003, 363, 171–178. [Google Scholar] [CrossRef]
- Giersberg, L.; Milkereit, B.; Schick, C.; Kessler, O. In-situ isothermal calorimetric measurement of precipitation behaviour in Al-Mg-Si alloys. Mater. Sci. Forum 2014, 794–796, 939. [Google Scholar] [CrossRef]
- Luoh, T.; Bow, J.S.; Peng, A.; Tsai, S.Y.; Tseng, M.R. Observation of recording marks in phase-change media using scanning electron microscopy channelling contrast image. Jpn. J. Appl. Phys. Part 1-Regul. Pap. Short Notes Rev. Pap. 1999, 38, 1698–1700. [Google Scholar] [CrossRef]
- Frock, H.; Kappis, L.V.; Reich, M.; Kessler, O. A Phenomenological Mechanical Material Model for Precipitation Hardening Aluminium Alloys. Metals 2019, 9, 1165. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, I.; Boumerzoug, Z.; Chabane, F. Study of precipitation kinetics of an Al-Mg-Si alloy using differential scanning calorimetry. Acta Metall. Slovaca 2017, 23, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Vedani, M.; Angella, G.; Bassani, P.; Ripamonti, D.; Tuissi, A. DSC analysis of strengthening precipitates in ultrafine Al-Mg-Si alloys. J. Therm. Anal. Calorim. 2007, 87, 277–284. [Google Scholar] [CrossRef]
- van de Langkruis, J.; Vossenberg, M.S.; Kool, W.H.; van der Zwaag, S. A study on the beta ’ and beta ’’ formation kinetics in AA6063 using differential scanning calorimetry. J. Mater. Eng. Perform. 2003, 12, 408–413. [Google Scholar] [CrossRef]
- Ohmori, Y.; Doan, L.C.; Matsuura, Y.; Kobayashi, S.; Nakai, K. Morphology and crystallography of beta-Mg2Si precipitation in Al-Mg-Si alloys. Mater. Trans. 2001, 42, 2576–2583. [Google Scholar] [CrossRef] [Green Version]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Tsao, C.S.; Chen, C.Y.; Jeng, U.S.; Kuo, T.Y. Precipitation kinetics and transformation of metastable phases in Al-Mg-Si alloys. Acta Mater. 2006, 54, 4621–4631. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]

| %Si | %Fe | %Cu | %Mg | %Mn | %Ti |
|---|---|---|---|---|---|
| 0.42 | 0.22 | 0.018 | 0.47 | 0.031 | 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Antolin, F.; Asensio-Lozano, J.; Cofiño-Villar, A.; Gonzalez-Pociño, A. Analysis of Different Solution Treatments in the Transformation of β-AlFeSi Particles into α-(FeMn)Si and Their Influence on Different Ageing Treatments in Al–Mg–Si Alloys. Metals 2020, 10, 620. https://doi.org/10.3390/met10050620
Alvarez-Antolin F, Asensio-Lozano J, Cofiño-Villar A, Gonzalez-Pociño A. Analysis of Different Solution Treatments in the Transformation of β-AlFeSi Particles into α-(FeMn)Si and Their Influence on Different Ageing Treatments in Al–Mg–Si Alloys. Metals. 2020; 10(5):620. https://doi.org/10.3390/met10050620
Chicago/Turabian StyleAlvarez-Antolin, Florentino, Juan Asensio-Lozano, Alberto Cofiño-Villar, and Alejandro Gonzalez-Pociño. 2020. "Analysis of Different Solution Treatments in the Transformation of β-AlFeSi Particles into α-(FeMn)Si and Their Influence on Different Ageing Treatments in Al–Mg–Si Alloys" Metals 10, no. 5: 620. https://doi.org/10.3390/met10050620





