In-Process Fabrication of Carbon-Dispersed Aluminum Matrix Composite Using Selective Laser Melting
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussion
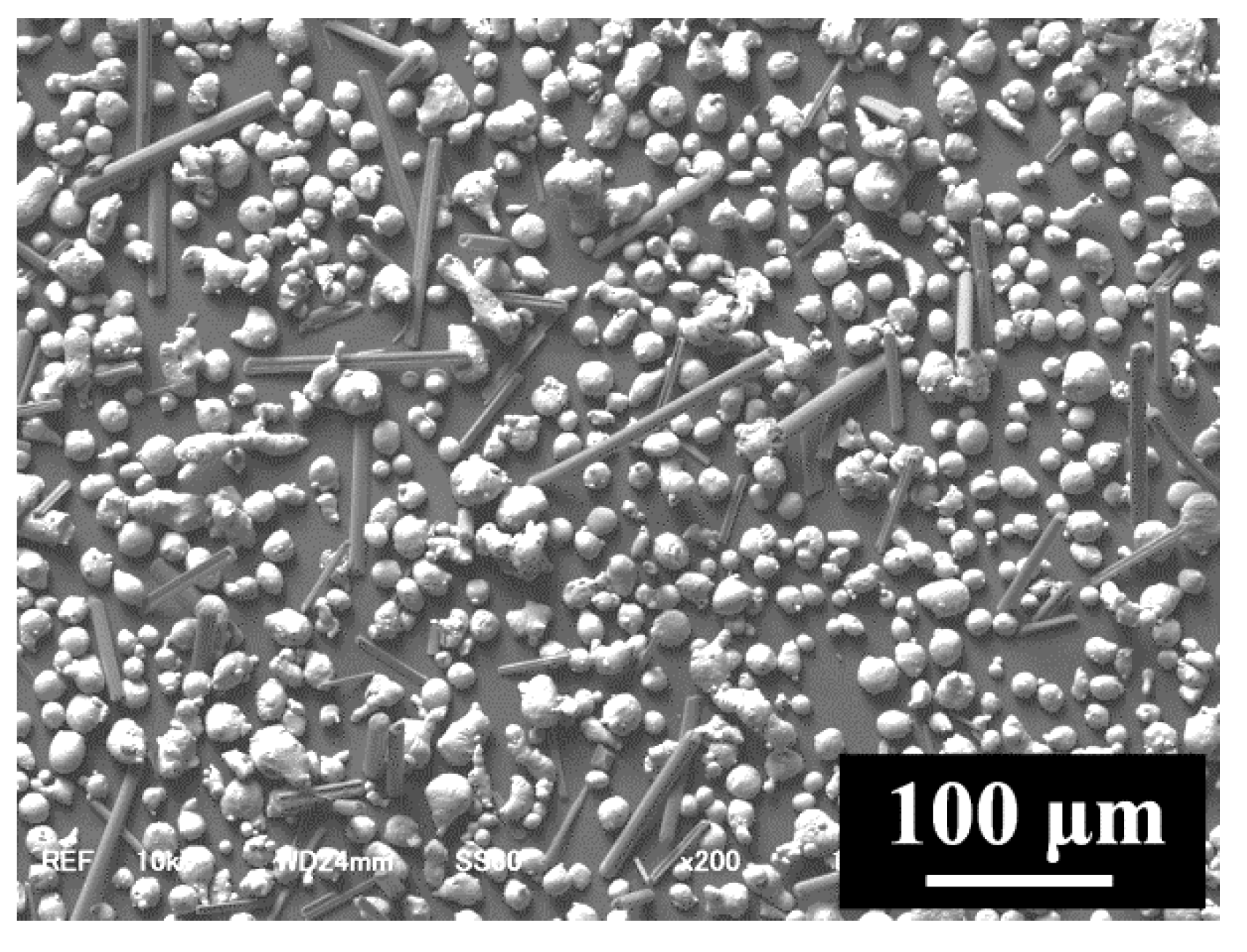
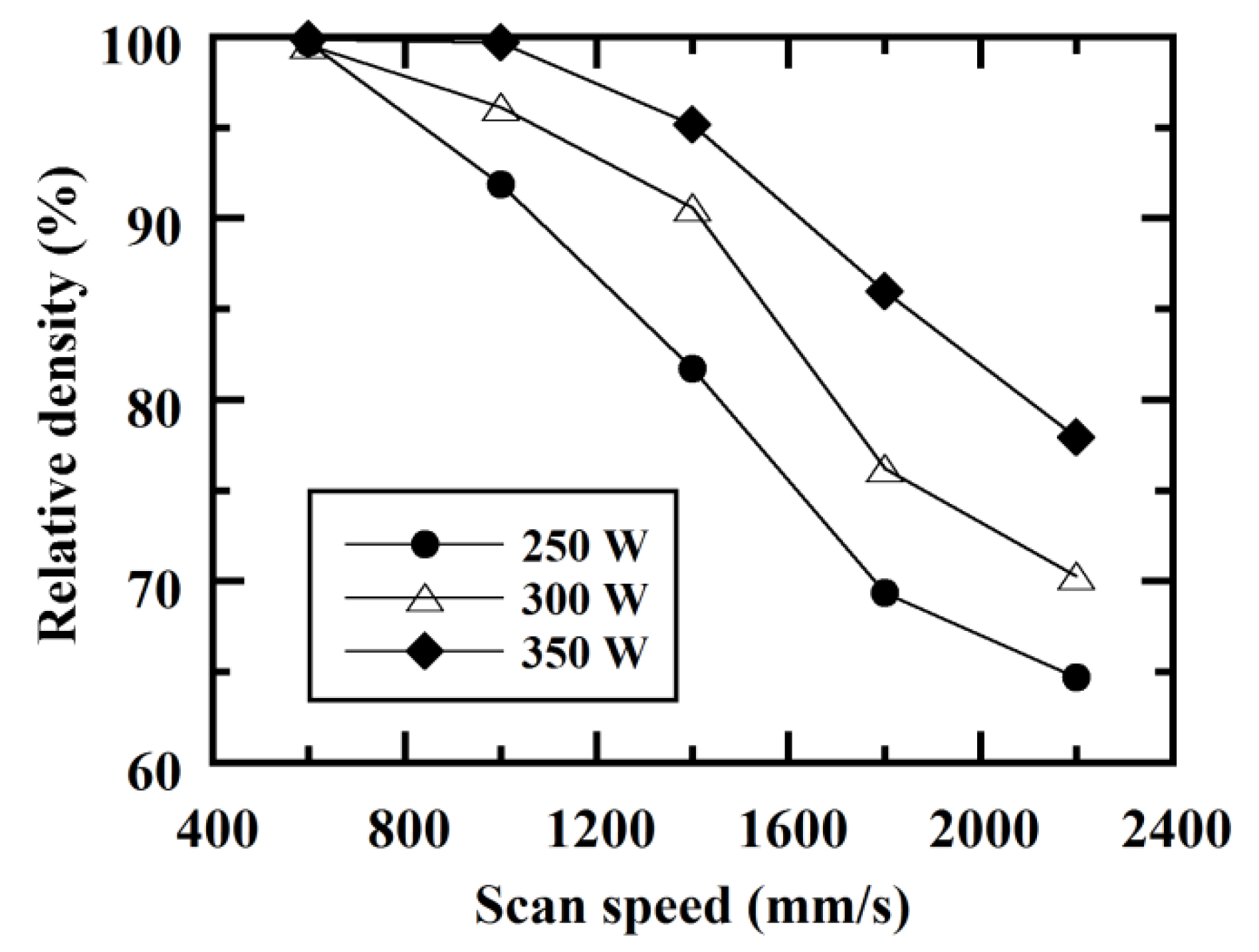
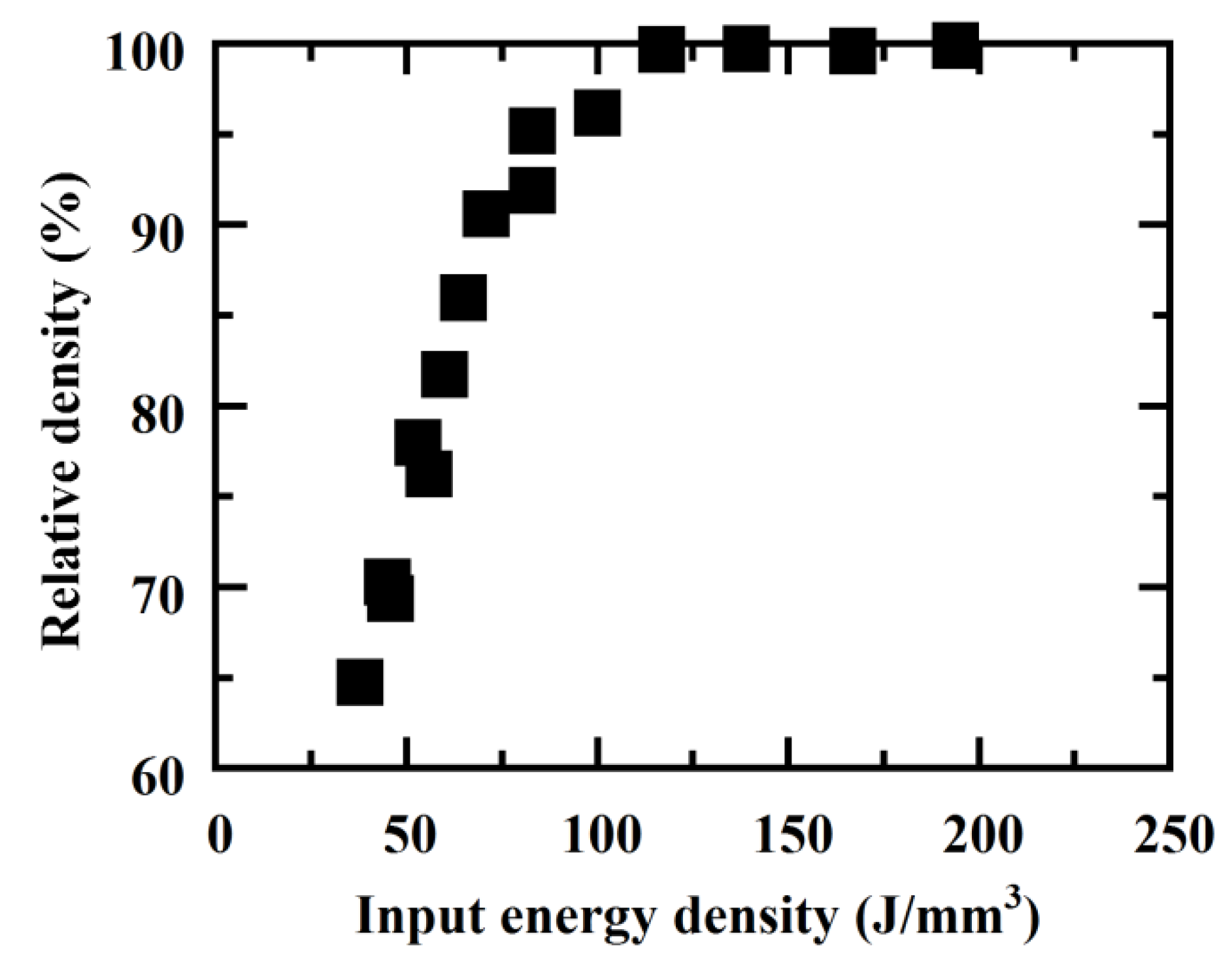
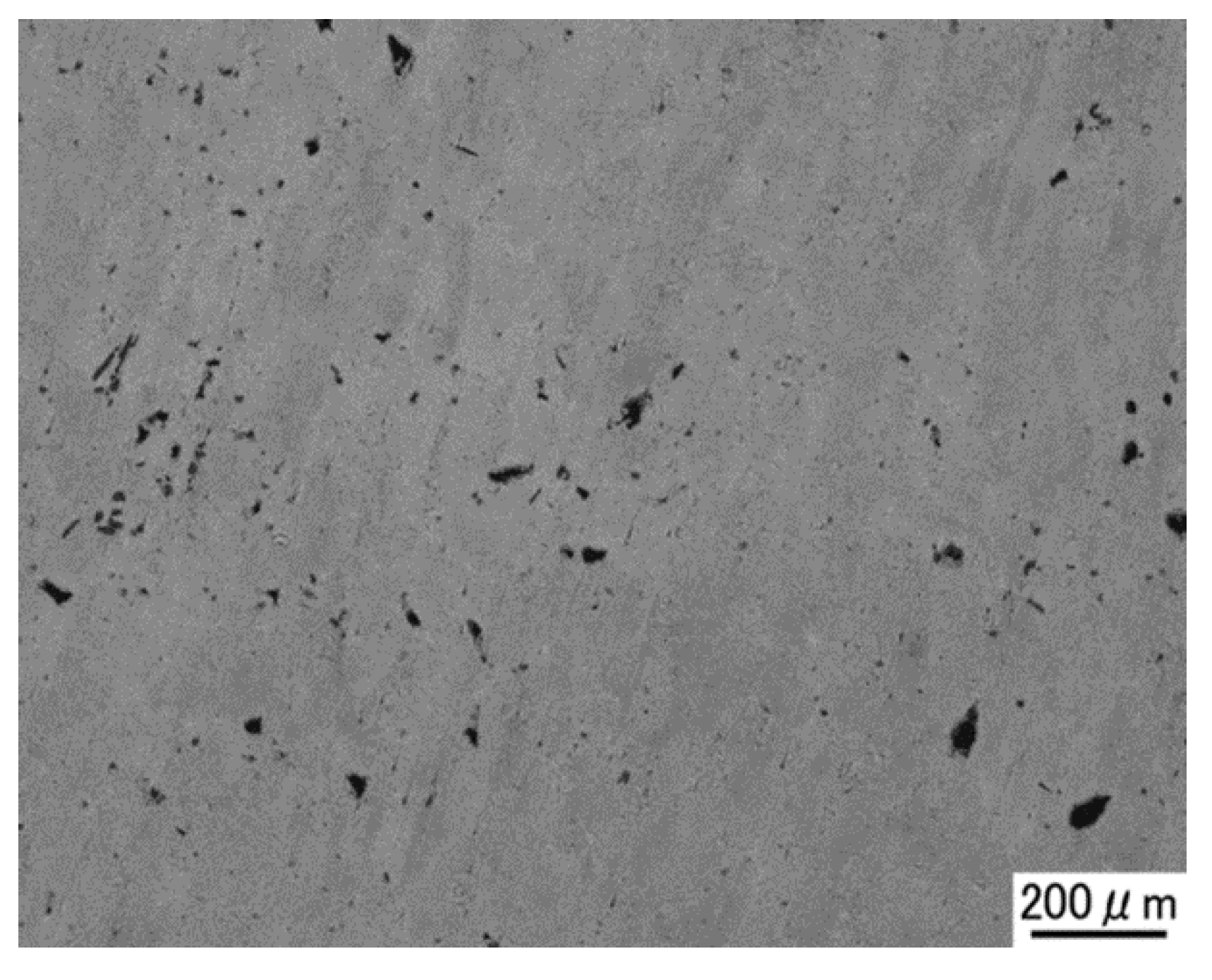
3.1. Densification of C/Al SLM Composite
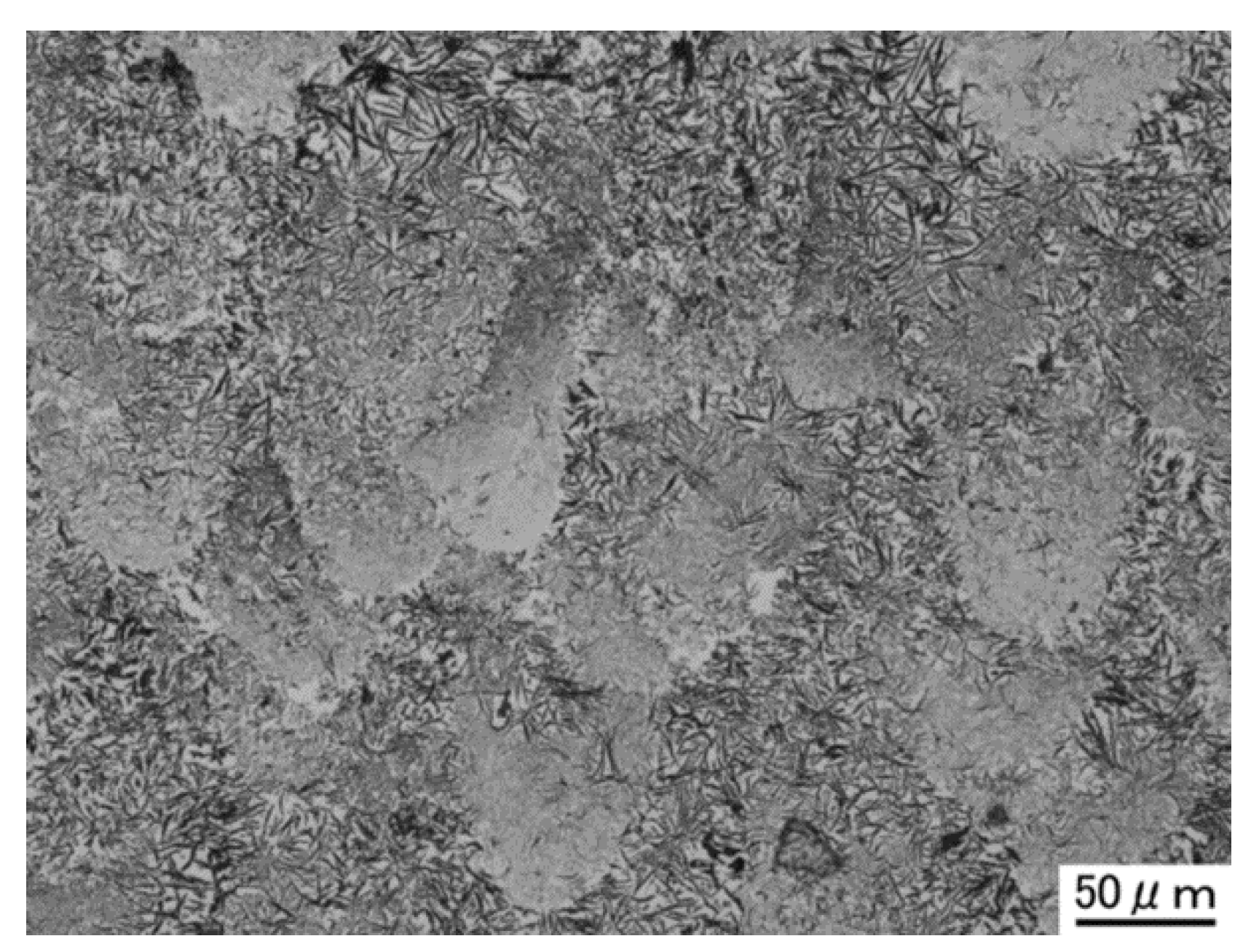
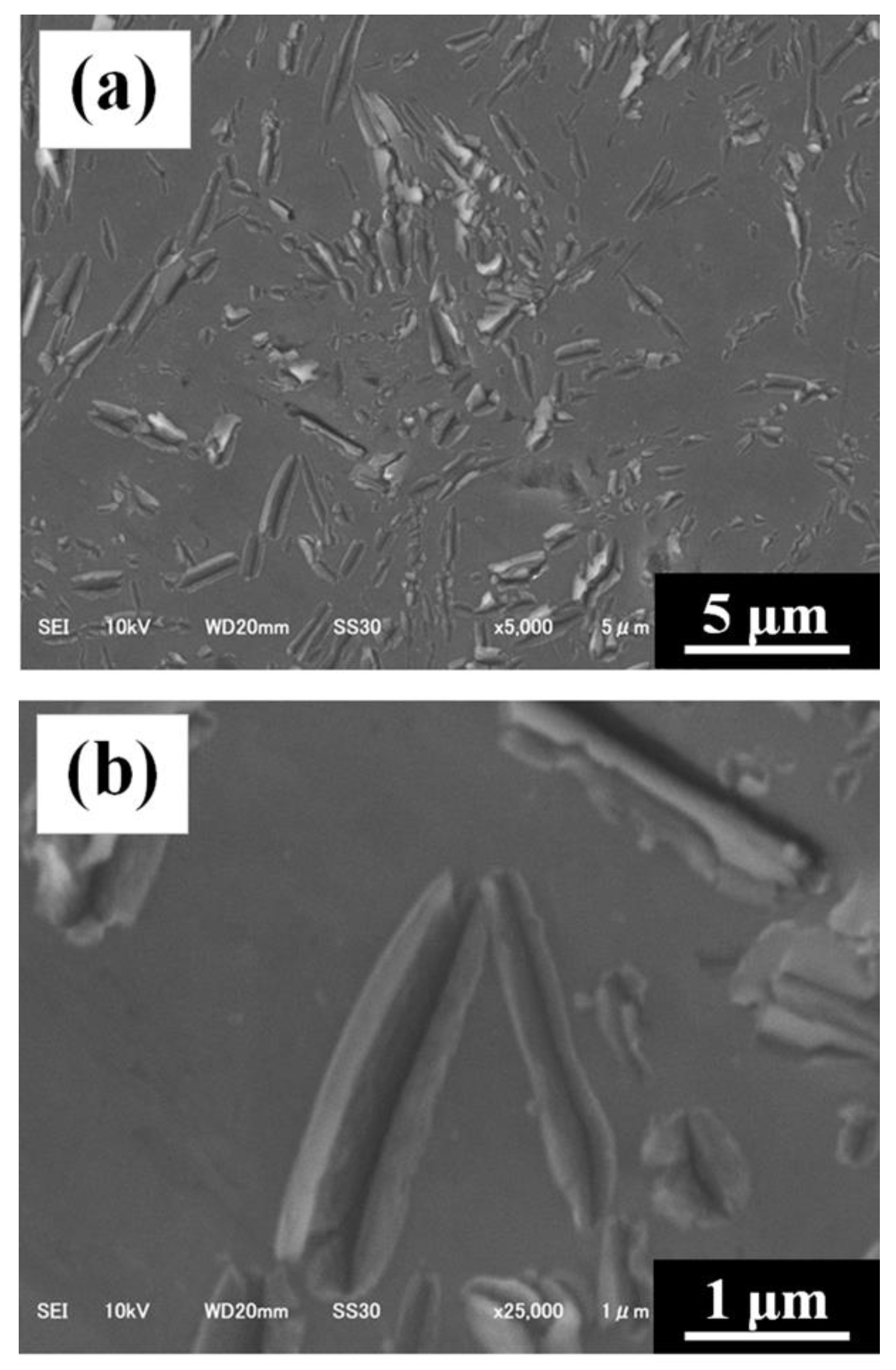
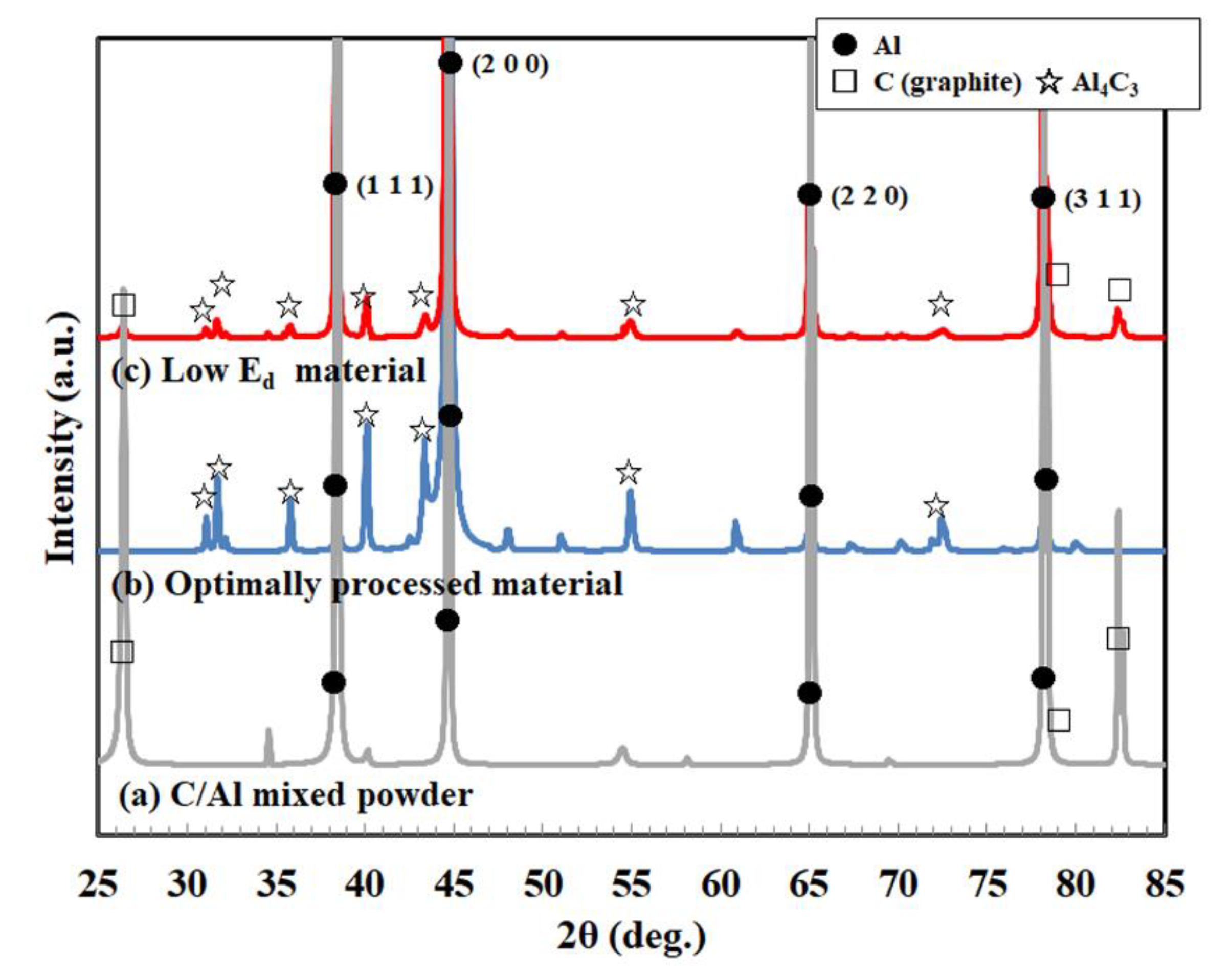
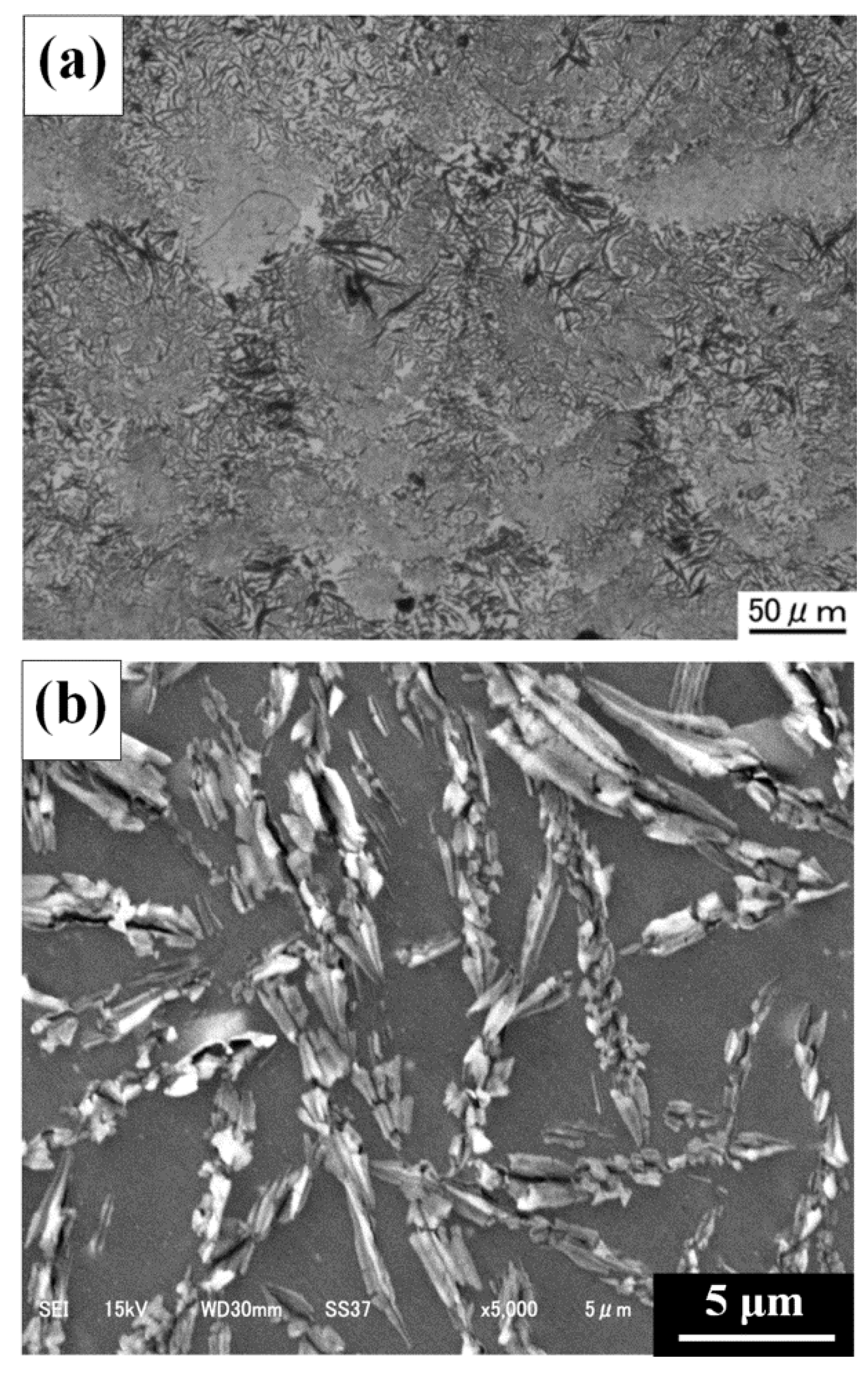
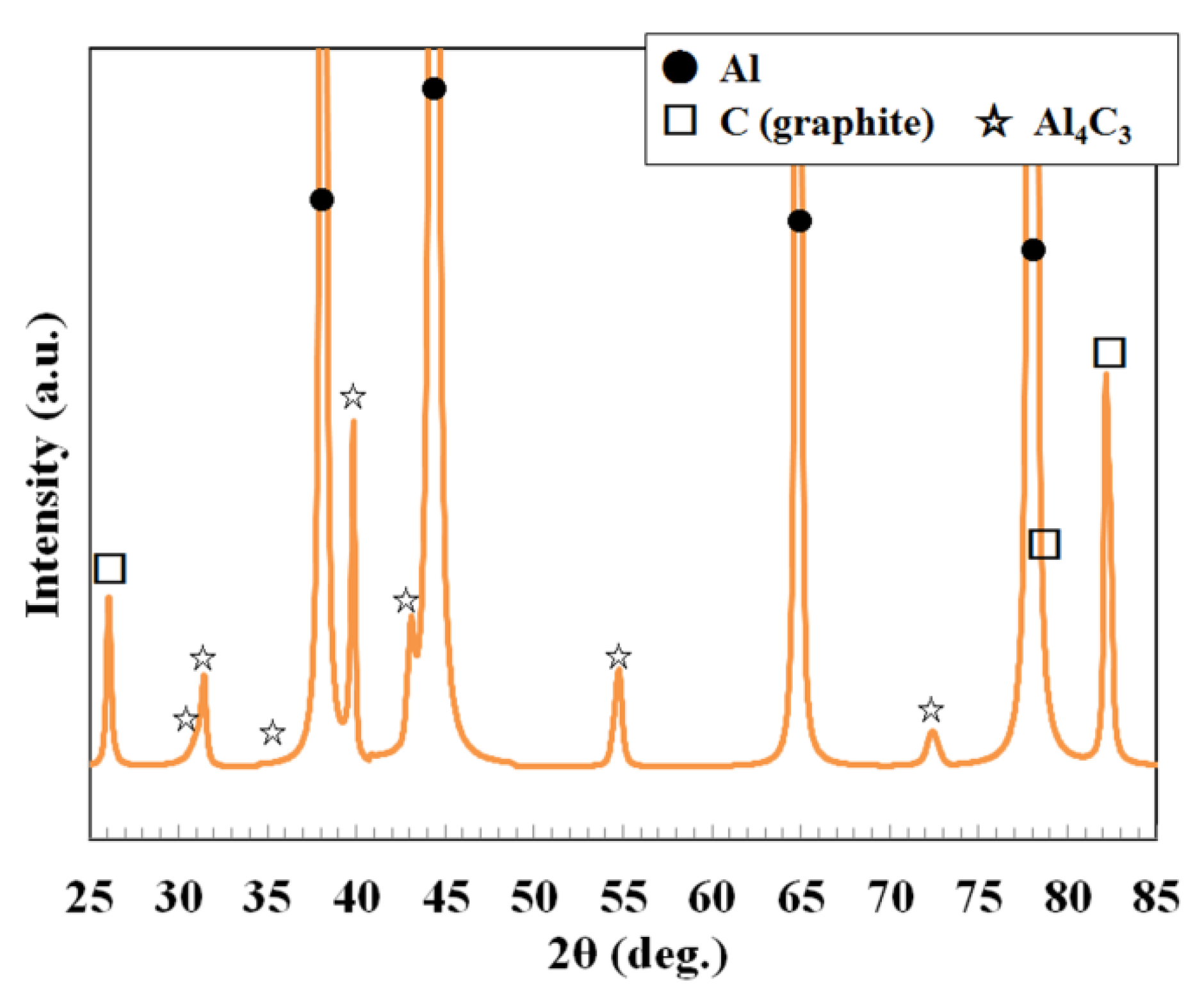
3.2. Characterization of C/Al SLM Composite
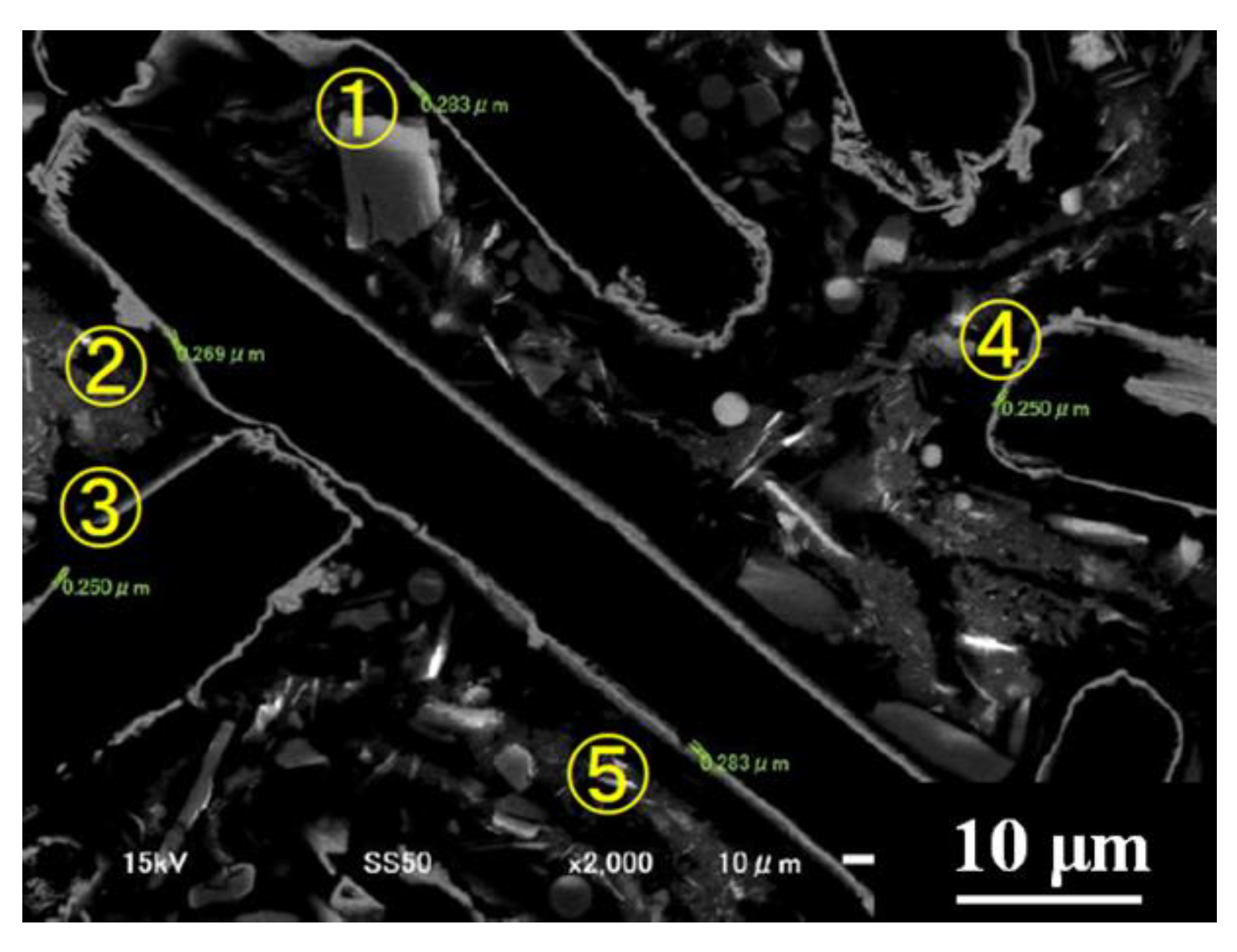
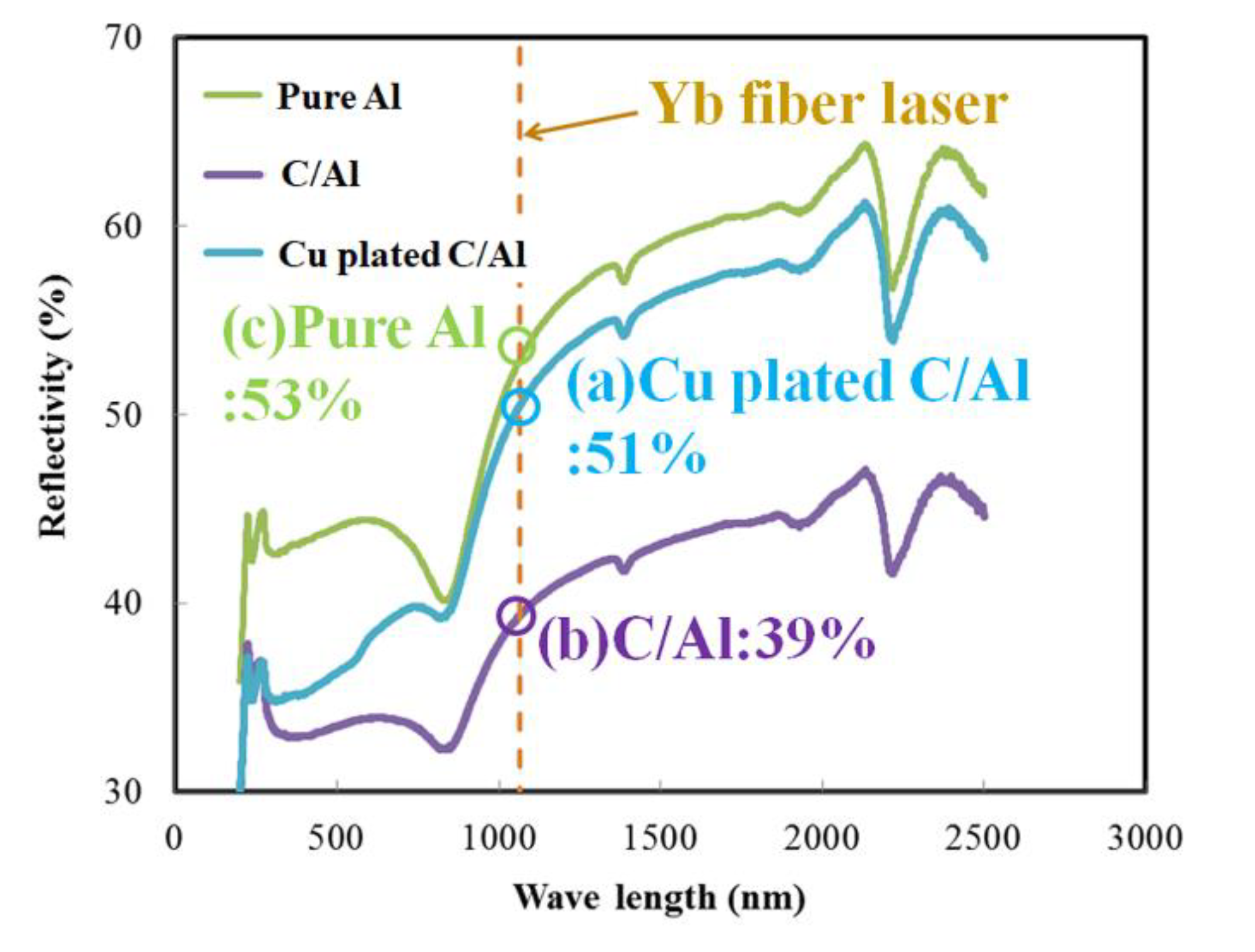
3.3. Control of Laser Reflectivity by Copper Plating on Carbon Fiber
- Reaction control between aluminum and carbon: the generation of Al4C3 phase and supersaturated solid-solution carbon in the α-Al matrix via the Al-C reaction led to difficulties in controlling the properties, specifically the thermal conductivity.
- Control of the laser absorptivity of mixed powders for densification: since the carbon dominantly absorbed the laser, the aluminum powder could not gain the thorough input energy to melt, leading to difficult densification.
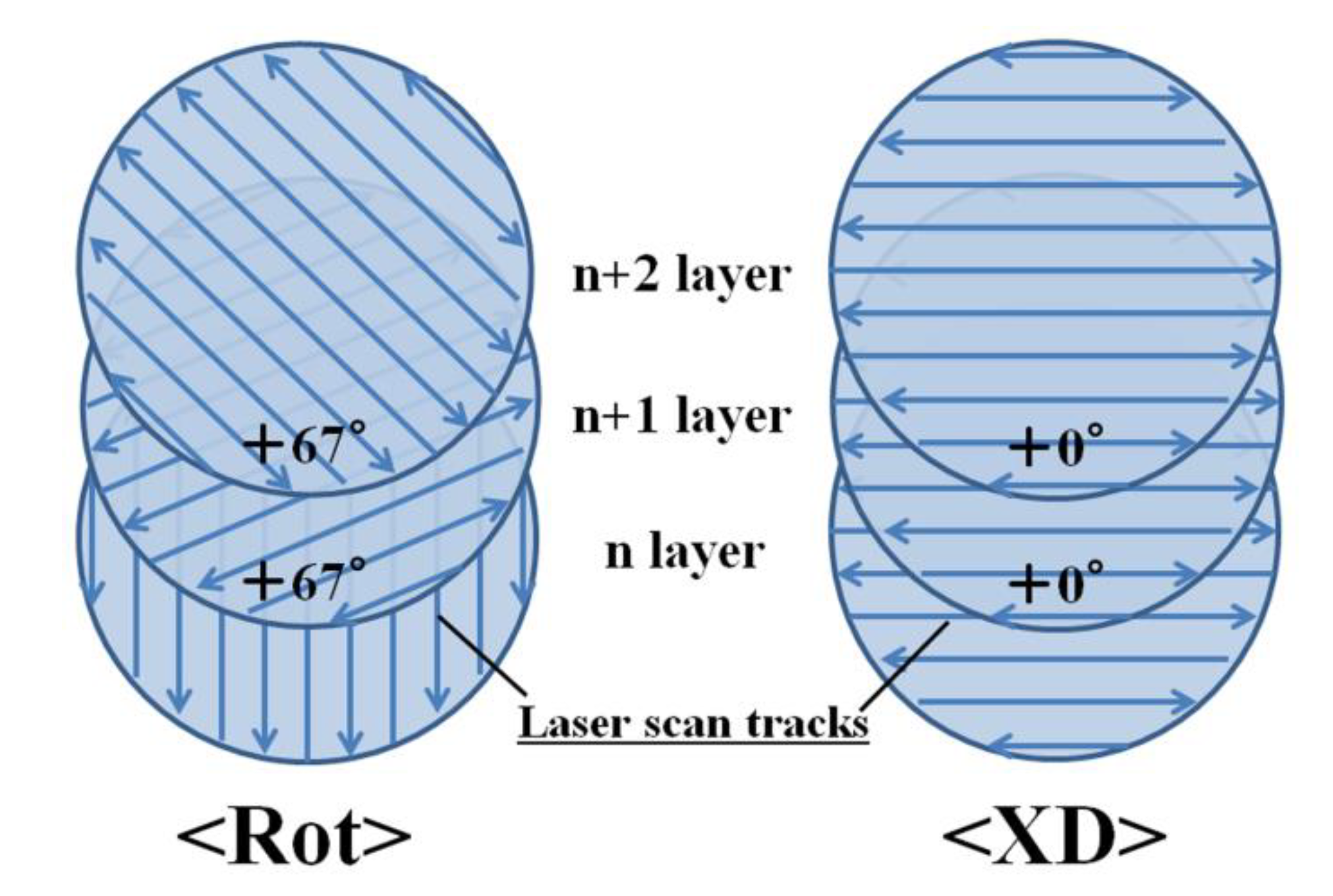
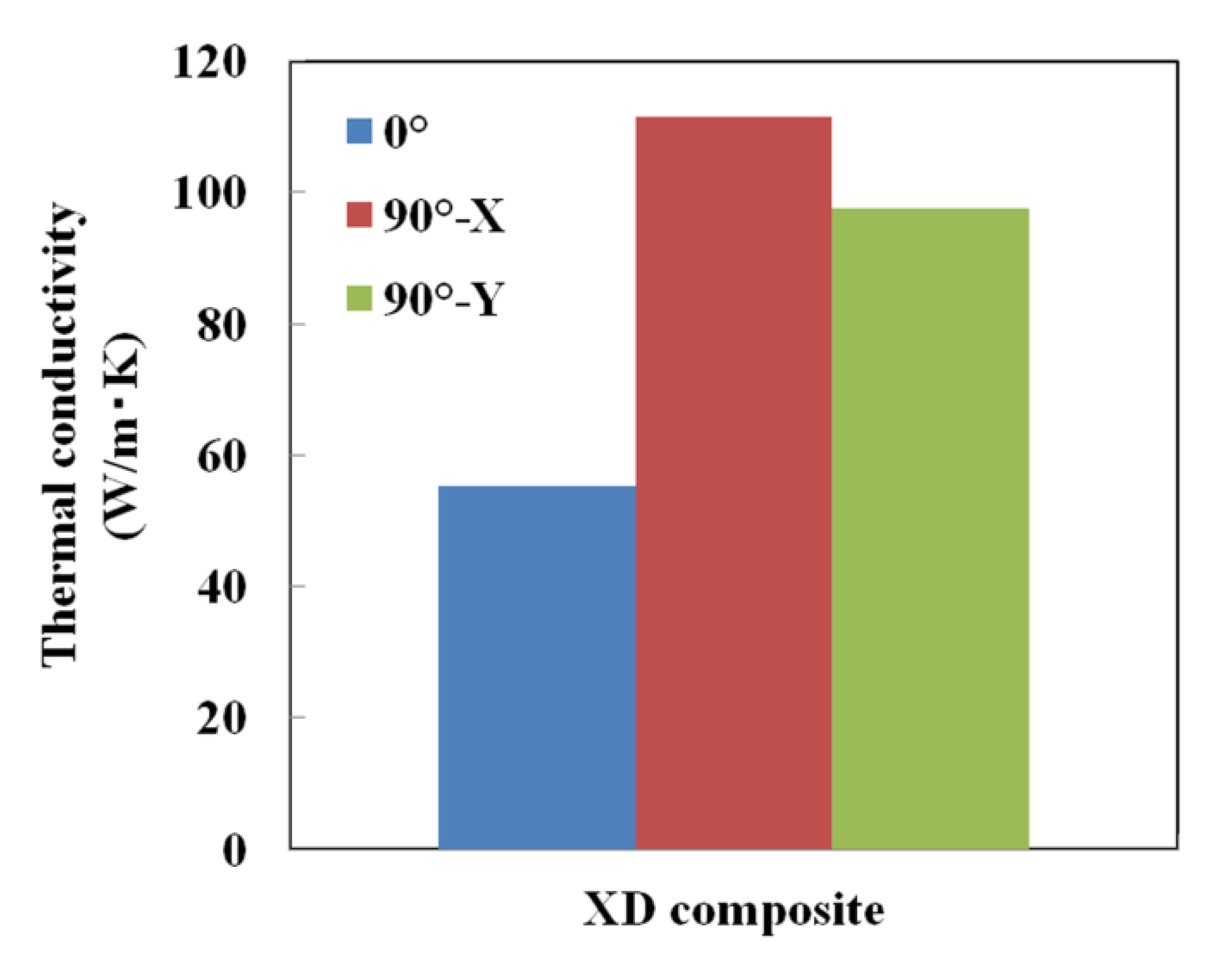
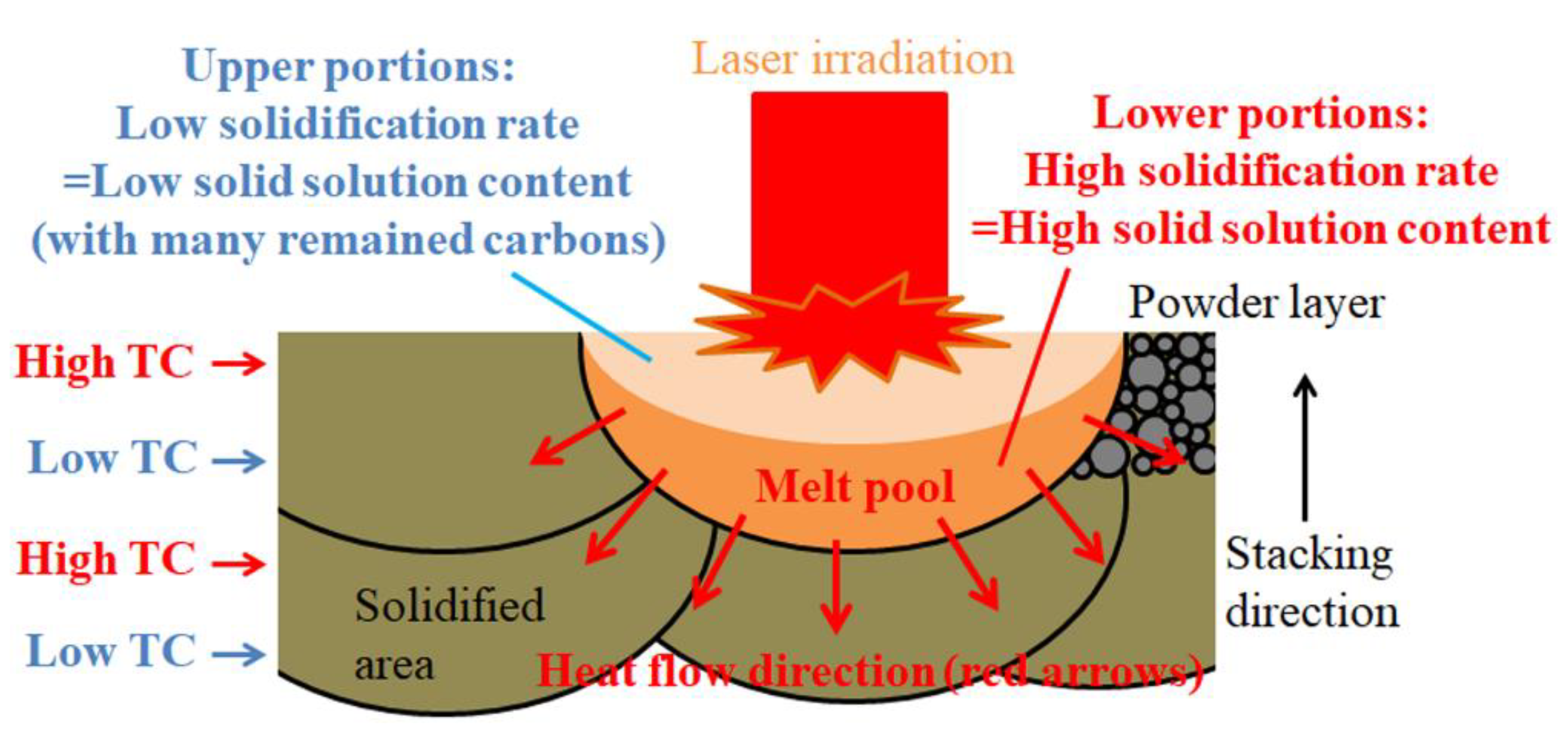
3.4. Generation of Anisotropy in Thermal Conductivity by Laser Scanning Pattern
4. Conclusions
- (1)
- C/Al SLM composites with a high relative density of 99.7% could be achieved by optimizing laser scan conditions with an energy density of greater than 100 J/mm3.
- (2)
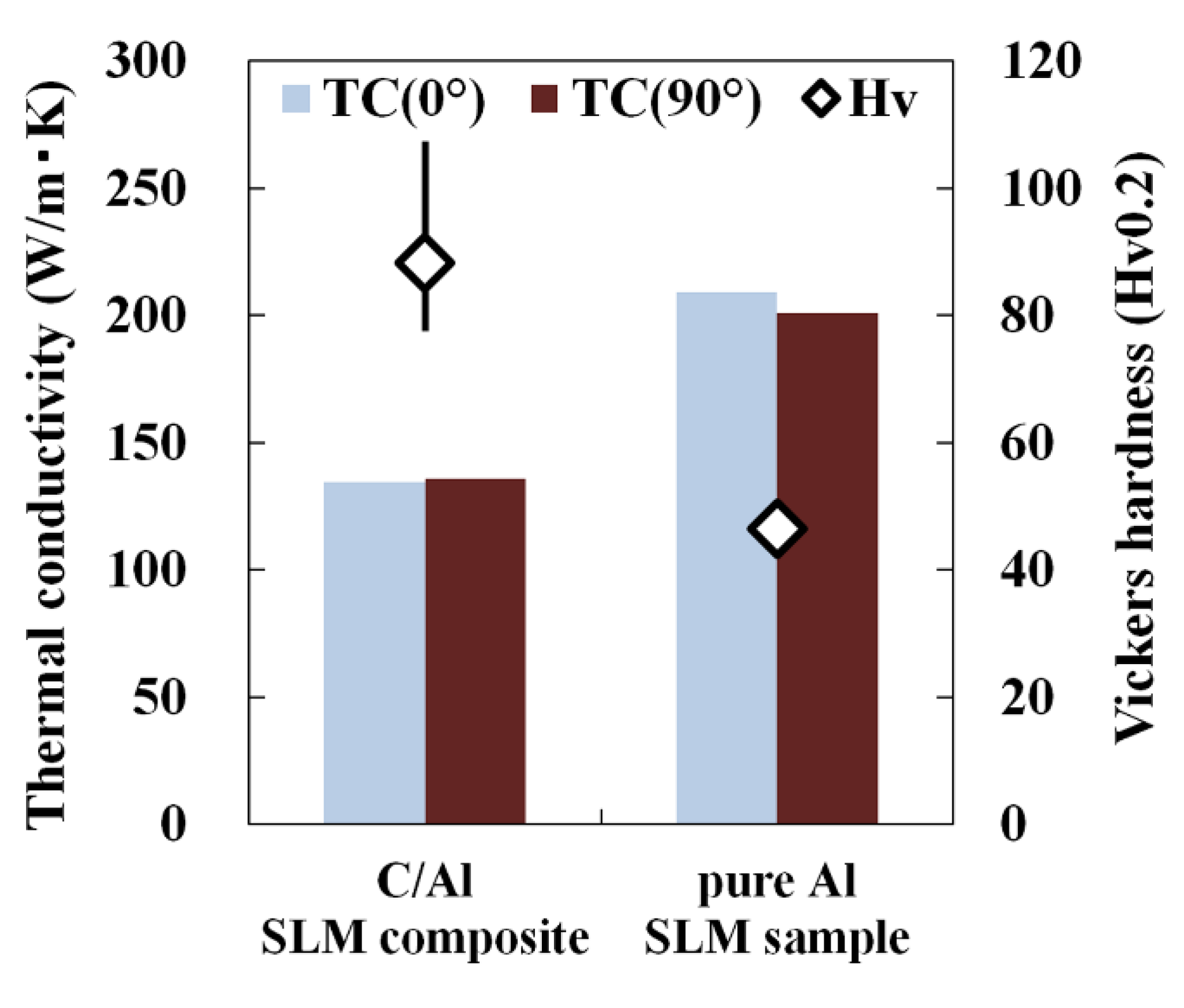
- Vickers hardness of C/Al SLM composites was significantly higher than that of pure Al SLM material. The strengthening was attributed to the dispersion hardening by the generated Al4C3 phase and solid-solution hardening by solid-solution carbon in the α-Al matrix caused via the Al-C reaction. Meanwhile, the thermal conductivity of C/Al SLM composite decreased due to the increased content of solid-solution carbon.
- (3)
- Plating of copper on carbon fiber could control laser reflectivity in Cu plated C/Al mixed powder and prevent the Al-C reaction during SLM processing. As a result, the Vickers hardness of copper-plated C/Al SLM composites increased compared to that of the C/Al SLM composite, although the thermal conductivity remained unchanged.
- (4)
- A thermal management material possessing anisotropic thermal conductivity was obtained by applying a unidirectional laser scanning pattern in the SLM process for Cu plated C/Al mixed powder.
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, I.; Rosen, D.W.; Stucker, B. Powder Bed Fusion Processes. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2010; pp. 103–142. [Google Scholar]
- Steen, W.M.; Mazumder, J. Rapid Prototyping and Low-volume Manufacture. In Laser Material Processing, 4th ed.; Springer: New York, NY, USA, 2010; pp. 349–369. [Google Scholar]
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Frontiers Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Milewski, J.O. Additive Manufacturing Metal, the Art of the Possible. In Additive Manufacturing of Metals; Springer: Cham, Switzerland, 2017; pp. 7–33. [Google Scholar]
- Yang, L.; Hsu, K.; Baughman, B.; Godfrey, D.; Medina, F.; Menon, M.; Wiener, S. Introduction to Additive Manufacturing. In Additive Manufacturing of Metals: The Technology, Materials, Design and Production; Springer: Cham, Switzerland, 2017; pp. 1–31. [Google Scholar]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Matthew, W.; Tsopanos, S.; Chris, J.; Leuan, O. Selective Laser Melting of Heat Transfer Devices. Rapid Prototyp. J. 2007, 13, 291–297. [Google Scholar]
- Vilaro, T.; Abed, S.; Knapp, W. Direct Manufacturing of Technical Parts Using Selective Laser Melting: Example of Automotive Application. In Proceedings of the 12th European Forum Rapid Prototype, Paris, France, 5–6 March 2008. [Google Scholar]
- Kempen, K.; Thijs, L.; Humbeeck, J.V.; Kruth, J.P. Mechanical Properties of AlSi10Mg Produced by Selective Laser Melting. Phys. Procedia. 2012, 39, 439–446. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Ambrosio, E.P.; Atzeni, E. From Powders to Dense Metal Parts. Characterization of a Commercial AlSi10Mg Alloy Processed through Direct Metal Laser Sintering. Materials 2013, 6, 856–869. [Google Scholar] [CrossRef]
- Buchbinder, D.; Meiners, W.; Wissenbach, K.; Lohmeier, K.M.; Brandl, E. Rapid Manufacturing von Aluminium-bauteilen fur die Serienproduktion durch Selective Laser Melting (SLM). In Proceedings of the Euro-uRapid, Frankfurt, Germany, 3–4 December 2007. [Google Scholar]
- Kimura, T.; Nakamoto, T. Microstructures and Mechanical Properties of Al-10%Si-0.4%Mg Fabricated by Selective Laser Melting. J. Jpn. Soc. Powder Powder Metall. 2014, 61, 531–537. [Google Scholar] [CrossRef][Green Version]
- Aboulkhair, N.T.; Everitt, N.M.; Ashcroft, I.; Tuck, C. Reducing porosity in AlSi10Mg parts processed by selective laser melting. Addit. Manufact. 2014, 1–4, 77–86. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T. Microstructures and Mechanical Properties of A356 (AlSi7Mg0.3) aluminum alloy fabricated by selective laser melting. Mater. Des. 2016, 89, 1294–1301. [Google Scholar] [CrossRef]
- Rao, H.; Giet, S.; Yang, K.; Wu, X.; Davies, C.H.J. The influence of processing conditions on aluminium alloy A357 manufactured by Selective Laser Melting. Mater. Des. 2016, 109, 334–346. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Klauss, H.J.; Surreddi, K.B.; Lober, L.; Wang, Z.; Chaubey, A.K.; Kuhn, U.; Eckert, J. Microstructure and mechanical properties of Al-12Si produced by selective laser melting: Effect of heat treatment. Mater. Sci. Eng. A 2014, 590, 153–160. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Parametric analysis of thermal behavior during selective laser melting additive manufacturing of aluminum alloy powder. Mater. Des. 2014, 63, 856–867. [Google Scholar] [CrossRef]
- Tang, M.; Pistorius, P.C.; Narra, S.; Beuth, J.L. Rapid Solidification: Selective Laser Melting of AlSi10Mg. JOM 2016, 68, 960–966. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T.; Ozaki, T.; Sugita, K.; Mizuno, M.; Araki, H. Microstructural formation and characterization mechanisms of selective laser melted Al–Si–Mg alloys with increasing magnesium content. Mater. Sci. Eng. A 2019, 754, 786–798. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, O.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Esawi, A.M.K.; Morsi, K.; Sayed, A.; Gawad, A.A.; Borah, P. Fabrication and properties of dispersed carbon nanotube–aluminum composites. Mater. Sci. Eng. A 2009, 508, 167–173. [Google Scholar] [CrossRef]
- Poirier, D.; Gauvin, R.; Drew, R.A.L. Structural characterization of a mechanically milled carbon nanotube/aluminum mixture. Compos. Part A 2009, 40, 1482–1489. [Google Scholar] [CrossRef]
- Luo, Z.P.; Song, Y.G.; Zhang, S.Q. A TEM study of the microstructure of SiCp/Al composite prepared by pressureless infiltration method. Scr. Mater. 2001, 45, 1183–1189. [Google Scholar] [CrossRef]
- Liu, H.N.; Ogi, K. Compressive failure of alumina continuous fibre reinforced Al–4.5 wt-%Cu alloy at elevated temperatures. Mater. Sci. Technol. 1999, 15, 821–825. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Ogawa, F.; Masuda, C. Fabrication of VGCF reinforced aluminum matrix composites by powder can extrusion and investigation of their reinforcing mechanism. J. Jpn. Inst. Light Met. 2013, 63, 350–357. [Google Scholar] [CrossRef][Green Version]
- Han, Q.; Setchi, R.; Lacan, F.; Gu, D.; Evans, S.L. Selective laser melting of advanced Al-Al2O3 nanocomposites: Simulation, microstructure and mechanical properties. Mater. Sci. Eng. A 2017, 698, 162–173. [Google Scholar] [CrossRef]
- Li, X.P.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.W.; Vleugels, J.; Humbeeck, J.V.; Kruth, J.P. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta. Mater. 2017, 129, 183–193. [Google Scholar] [CrossRef]
- Wang, P.; Gammer, C.; Brenne, F.; Niendorf, T.; Eckert, J.; Scudino, S. A heat treatable TiB2/Al-3.5Cu-1.5Mg-1Si composite fabricated by selective laser melting: Microstructure, heat treatment and mechanical properties. Compos. Part B 2018, 147, 162–168. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, L.; Wen, S.; Wei, Q.; Shi, Y. Enhanced micro-hardness and wear resistance of Al-15Si/TiC fabricated by selective laser melting. Comp. Comm. 2018, 10, 64–67. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, H.; Xue, G.; Zeng, X. Alumina loss mechanism of Al2O3-AlSi10Mg composites during selective laser melting. J. Alloys Compd. 2019, 785, 286–295. [Google Scholar] [CrossRef]
- Astfalck, L.C.; Kelly, G.K.; Li, X.; Sercombe, T.B. On the Breakdown of SiC during the Selective Laser Melting of Aluminum Matrix Composites. Adv. Eng. Mater. 2017, 19, 1600835. [Google Scholar] [CrossRef]
- Aversa, A.; Marchese, G.; Lorusso, M.; Calignano, F.; Biamino, S.; Ambrosio, E.P.; Manfredi, D.; Fino, P.; Lombardi, M.; Pavese, M. Microstructural and Mechanical Characterization of Aluminum Matrix Composites Produced by Laser Powder Bed Fusion. Adv. Eng. Mater. 2017, 19, 1700180. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T. Thermal and Mechanical Properties of Commercial-Purity Aluminum Fabricated Using Selective Laser Melting. Mater. Trans. 2017, 58, 799–805. [Google Scholar] [CrossRef]
- Nakamoto, T.; Shirakawa, N.; Miyata, Y.; Sone, T.; Inui, H. Selective laser sintering of high carbon steel powders studied as a function of carbon content. J. Mater. Process. Technol. 2009, 209, 5653–5660. [Google Scholar] [CrossRef]
- Gu, H.; Gong, H.; Pal, D.; Rafi, K.; Starr, T.; Stucker, B. Influences of Energy Density on Porosity and Microstructure of Selective Laser Melted 17-4PH stainless Steel. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 12–14 August 2013; pp. 474–489. [Google Scholar]
- Mondolfo, L.F. PART 2—BINARY ALLOYS Aluminum-Carbon system. In Aluminum Alloys, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1976; pp. 236–238. [Google Scholar]
- Kobashi, M.; Takata, N.; Suzuki, A. Reactive processing of aluminum matrix composites. J. Jpn. Inst. Light Met. 2017, 67, 571–575. [Google Scholar] [CrossRef]
- Nakae, H. Wettability of liquid aluminum to non-metallic materials. J. Jpn. Inst. Light Met. 1989, 39, 136–146. [Google Scholar] [CrossRef]
- Steen, W.M.; Mazumder, J. Basic Laser Optics—Reflection or Absorption. In Laser Material Processing, 4th ed.; Springer: New York, NY, USA, 2010; pp. 89–91. [Google Scholar]
- Mondolfo, L.F. PART 2—BINARY ALLOYS Al-Cu Aluminum-Copper system. In Aluminum Alloys, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1976; pp. 253–278. [Google Scholar]

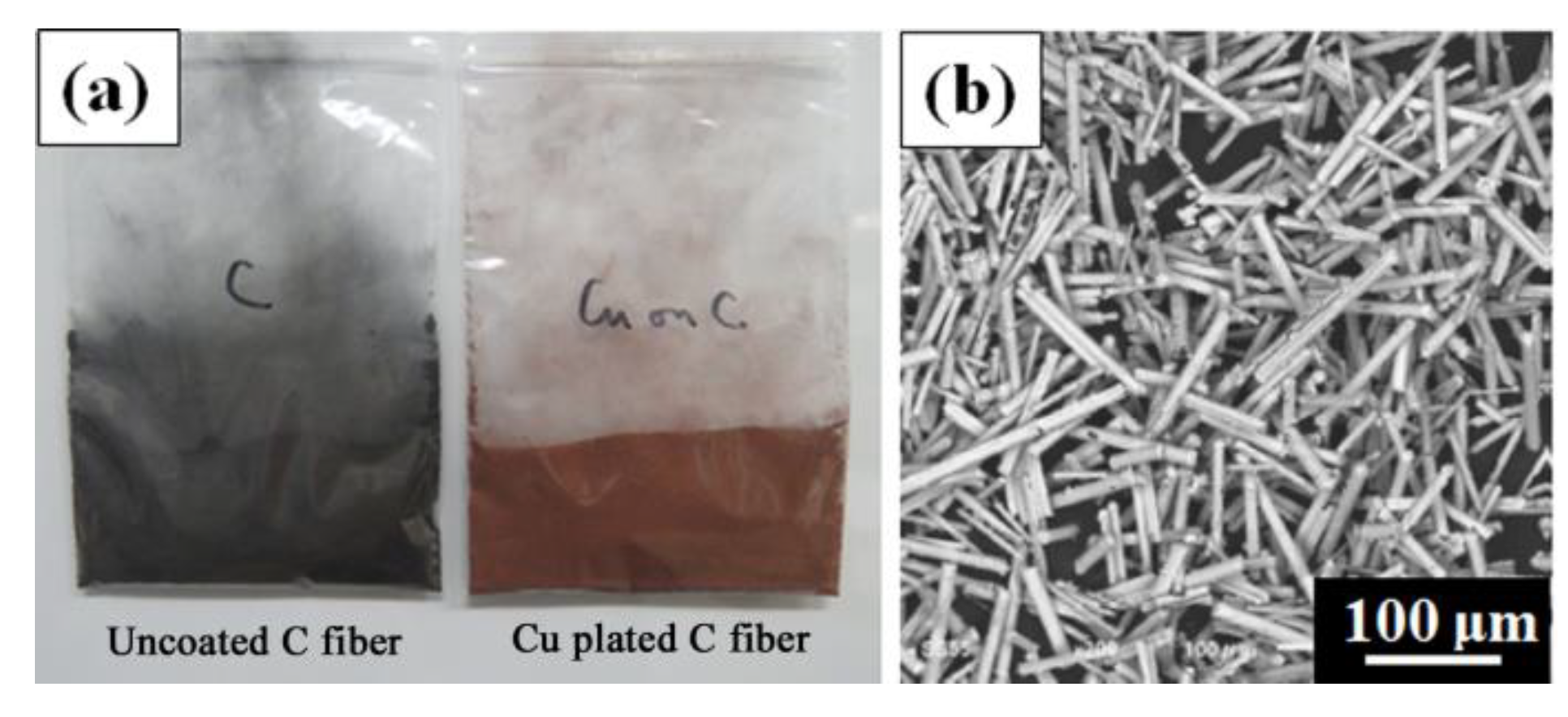


| Laser Scan Conditions | Setting Range |
|---|---|
| Layer thickness (mm) | 0.03 |
| Laser power (W) | 250–350 |
| Scan speed (mm/s) | 600–2200 |
| Scan interval (mm) | 0.1 |
| Copper Plate Thickness (μm) |
|---|
| ① 0.238 |
| ② 0.269 |
| ③ 0.250 |
| ④ 0.250 |
| ⑤ 0.283 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, T.; Nakamoto, T.; Suyama, T.; Miki, T. In-Process Fabrication of Carbon-Dispersed Aluminum Matrix Composite Using Selective Laser Melting. Metals 2020, 10, 619. https://doi.org/10.3390/met10050619
Kimura T, Nakamoto T, Suyama T, Miki T. In-Process Fabrication of Carbon-Dispersed Aluminum Matrix Composite Using Selective Laser Melting. Metals. 2020; 10(5):619. https://doi.org/10.3390/met10050619
Chicago/Turabian StyleKimura, Takahiro, Takayuki Nakamoto, Takeshi Suyama, and Takao Miki. 2020. "In-Process Fabrication of Carbon-Dispersed Aluminum Matrix Composite Using Selective Laser Melting" Metals 10, no. 5: 619. https://doi.org/10.3390/met10050619
APA StyleKimura, T., Nakamoto, T., Suyama, T., & Miki, T. (2020). In-Process Fabrication of Carbon-Dispersed Aluminum Matrix Composite Using Selective Laser Melting. Metals, 10(5), 619. https://doi.org/10.3390/met10050619




