3.1. As-Cast Microstructure of the Composite
In the experiment, the alloy components were all hypereutectic, so in the Mg
2Si–Al solidification process, Mg
2Si particles first appeared, and then Al and Mg
2Si solidified in a eutectic form. The composition of the composite in this experiment was Al–20Mg
2Si–10Si. According to previous research [
18], the solidification process of Mg
2Si–Al composites is as follows:
where the subscript P represents the primary phase and e represents the eutectic phase. Because of non-equilibrium in the solidification process, other phases, such as the eutectic Si phase, appeared.
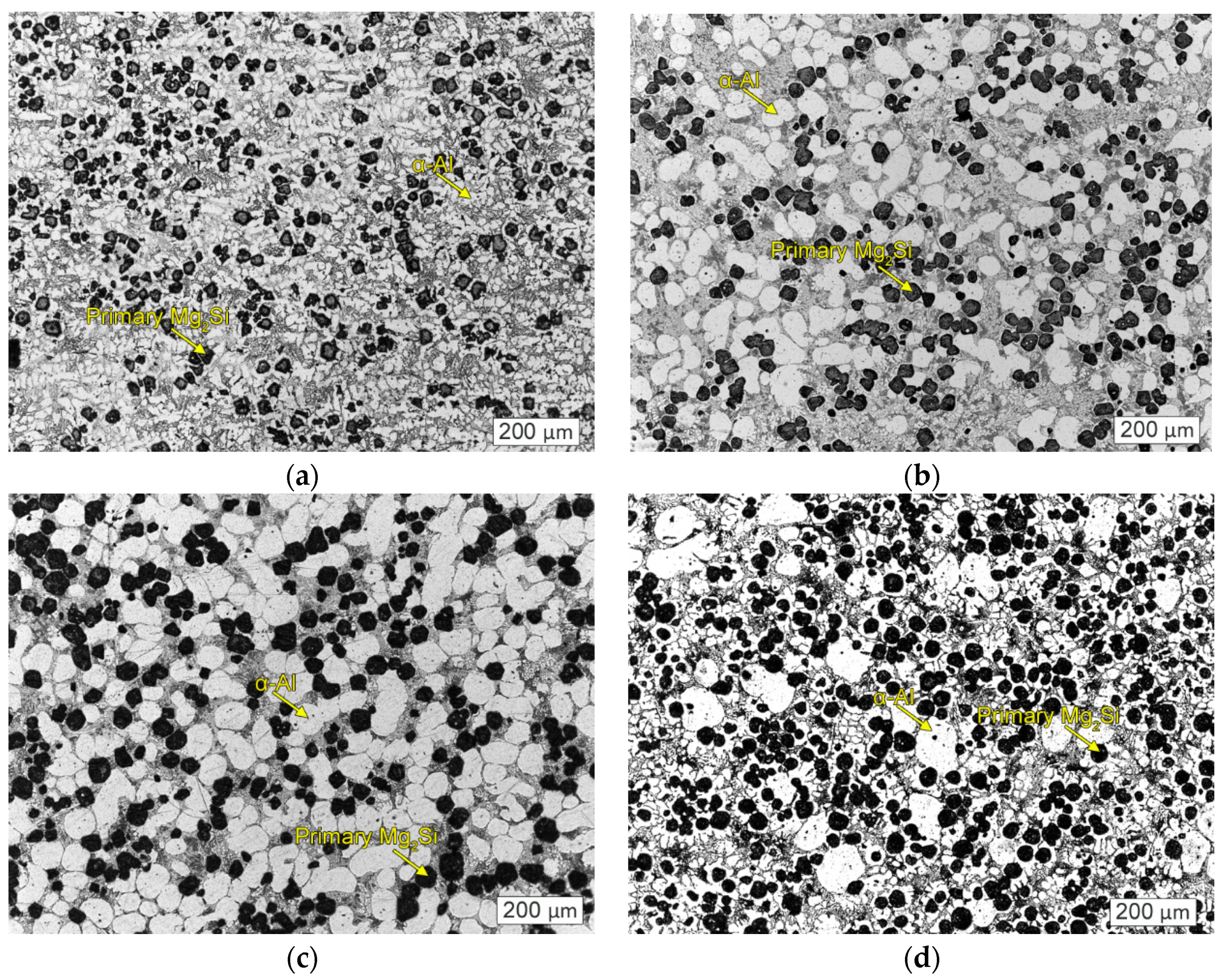
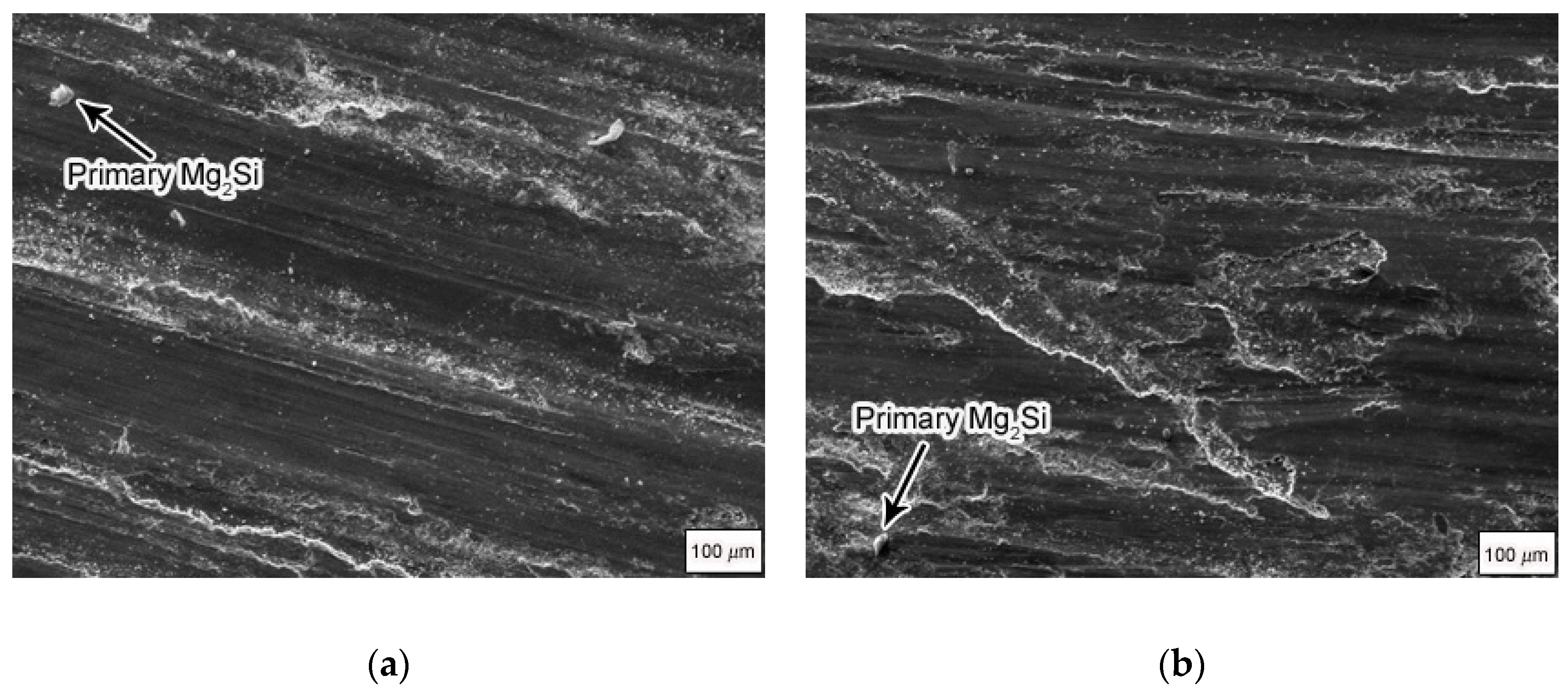
Figure 1 shows the as-cast microstructure of the Mg
2Si–Al composites modified by phosphorus, fabricated by using gravity casting and semi-solid extrusion with different isothermal heat treatment holding times. It can be seen that the Mg
2Si reinforcing phase and the α-Al matrix were spheroidized after isothermal heat treatment for 50 min, 60 min, and 160 min, as compared with ordinary gravity casting. The morphology of the primary Mg
2Si phase of gravity-cast Mg
2Si–Al composites was polygonal or quadrilateral, with an average size of about 35 μm, and the α-Al phase was dendritic, as shown in
Figure 1a. When the holding time was 50 min, the rose-like characteristics of α-Al phase disappeared almost entirely, showing obvious spherical or ellipsoidal shapes with different particle sizes and uneven distribution. At the same time, the Mg
2Si reinforced phase was also converted into spherical particles, but the roundness and size were not uniform, and the size was only 20 μm in part, while the other part was increased to 50 μm, as shown in
Figure 1b. As the holding time increased to 60 min, the α-Al phase was spheroidized and the size was relatively uniform, showing a regular spherical or ellipsoidal shape, but its size reached 85 μm, an increase compared to at 50 min. Mg
2Si exhibited a fine spherical shape which was more rounded than that at 50 min, and the individual dimensions were increased to 60 μm, as shown in
Figure 1c. As the holding time was further extended to 160 min, the spherical α-Al increased to 120 μm and the morphology gradually became non-rounded, while the primary Mg
2Si phase became more rounded and uniform in size but remained spherical. The size was about 30 μm, as shown in
Figure 1d. In addition, the eutectic structure in the semi-solid structure was refined compared to the composite material prepared by gravity casting. When the temperature was maintained for 50 min, the eutectic structure was relatively small, and was more refined as the holding time was extended to 60 min, while the eutectic structure was coarsened when the holding time was extended to 160 min.
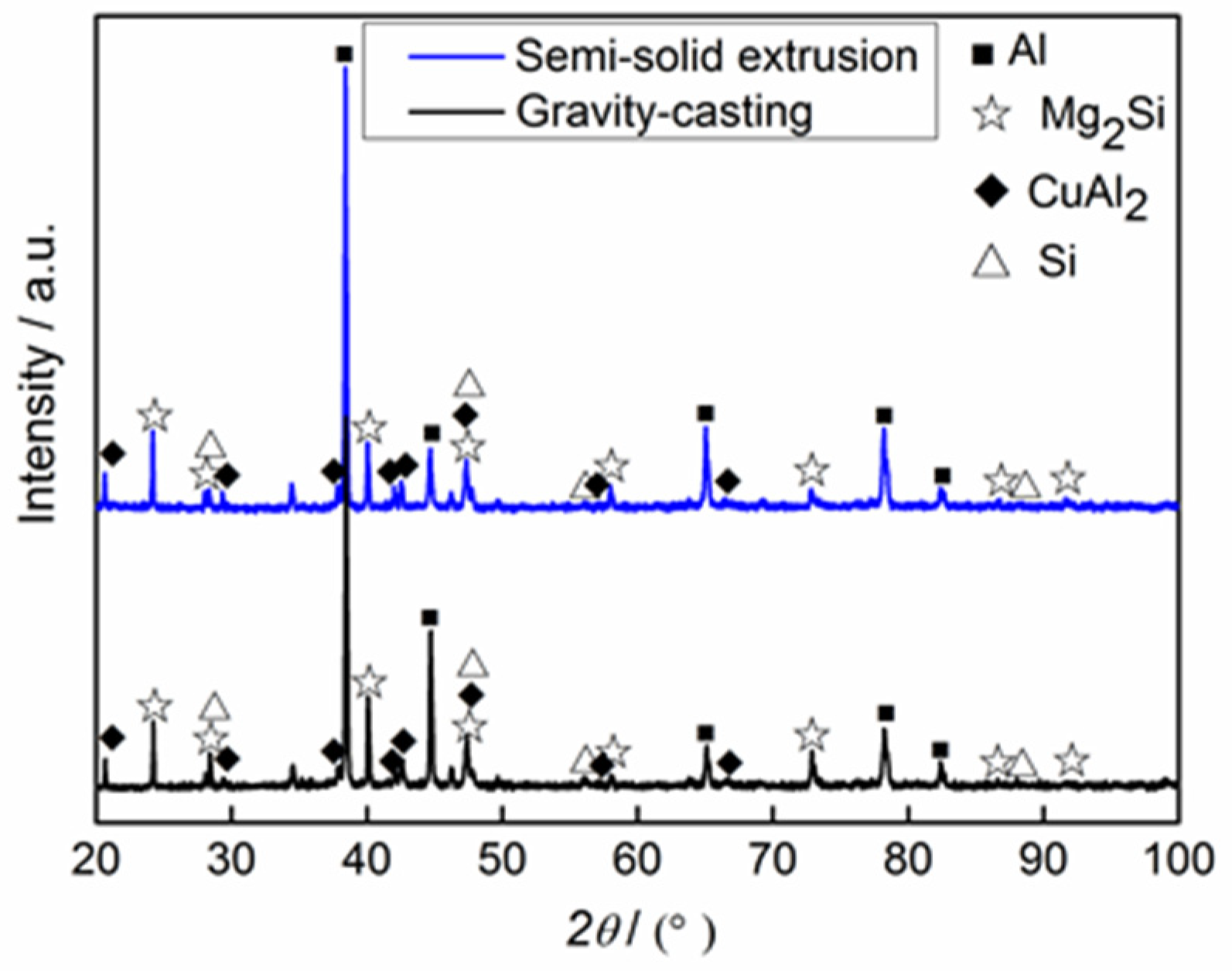
Figure 2 shows the XRD patterns of Mg
2Si–Al composites. Both of the Mg
2Si–Al composites fabricated by using gravity casting and semi-solid extrusion were composed of Al, Mg
2Si, CuAl
2, and Si phases. It was found that the morphologies of their corresponding phases were different, but the composition phases were the same.
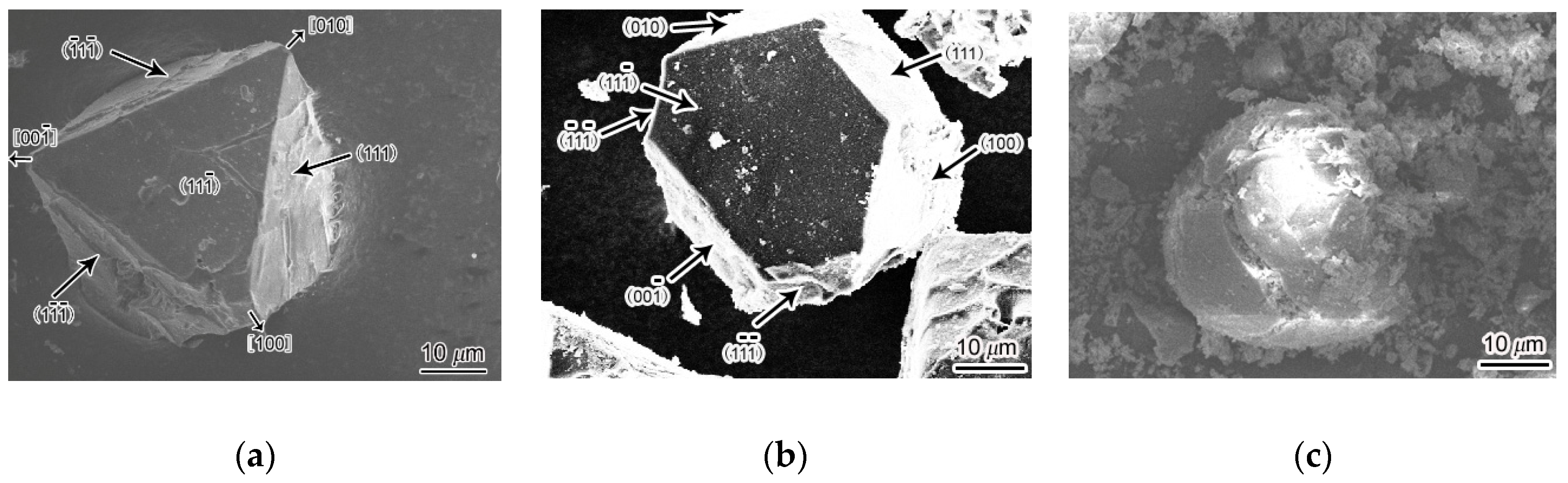
Figure 3a,b show the octahedral morphology and tetrakaidecahedral morphology of Mg
2Si crystals extracted from Mg
2Si–Al composites fabricated by using gravity casting after P metamorphism.
Figure 3c shows spherical morphology of an Mg
2Si crystal extracted from Mg
2Si–Al composite prepared by semi-solid extrusion, with an isothermal heat treatment holding time of 160 min. It shows that the Mg
2Si particles became rounded and had a regular spherical shape when the isothermal heat treatment holding time was increased to 160 min.
During solidification of the alloy, the primary Mg
2Si usually appeared as coarse dendrites without the corresponding microstructure control (such as modification treatment). These were actually formed by connecting Mg
2Si octahedral crystals together due to instability of the interface. In this study, Cu–14 wt.% P master alloy was used as a modifying agent of the Mg
2Si–Al composites. After P modification, the primary M
g2Si transformed from dendrite to a polygonal block, as shown in
Figure 1a. Because Mg
2Si has a face-centered cubic crystal structure and the growth mode is typical of facet growth, under ideal conditions, Mg
2Si will grow into an octahedron, as shown in
Figure 3a. Its growth surface was composed of densely packed crystal planes (111), and the growth direction was <100>. In addition, adding phosphorus formed a heterogeneous core Mg
3(PO
4)
2 compound [
20], which was located at the center of the Mg
2Si crystal. The existence of heterogeneous cores increased the number of crystalline cores and generated more crystal grains, which caused the supersaturation of solutes to increase rapidly during the crystal growth process. As a result, the growth velocity of preferentially grown <100> crystal orientation decreased, so that the <100> crystal orientation changed to the {100} plane, thereby forming a tetrahedron as shown in
Figure 3b. This is consistent with the research results of Qin et al. [
21].
After semi-solid isothermal heat treatment, the morphology of the enhanced phase Mg
2Si changed from a polygonal block to a sphere, as shown in
Figure 1b–d and
Figure 3c. Studies have shown that, from the perspective of thermodynamics, the spheroidization of grains is a spontaneous process during the heat treatment process. However, in the actual heat treatment process, even after a reasonable isothermal heat treatment, an ideal perfect sphere cannot be achieved. Mg
2Si is the primary phase of the alloy, has a higher melting point, and the spheroidization process is relatively simple; that is, sharp corners are dissolved and passivated to become round. With the extension of the holding time, the diffusion of most of the Mg
2Si phase and the liquid phase slightly reduced the size of the crystal grains, while a few of them increased in size due to coarsening and maturation.
3.2. Wear Resistance
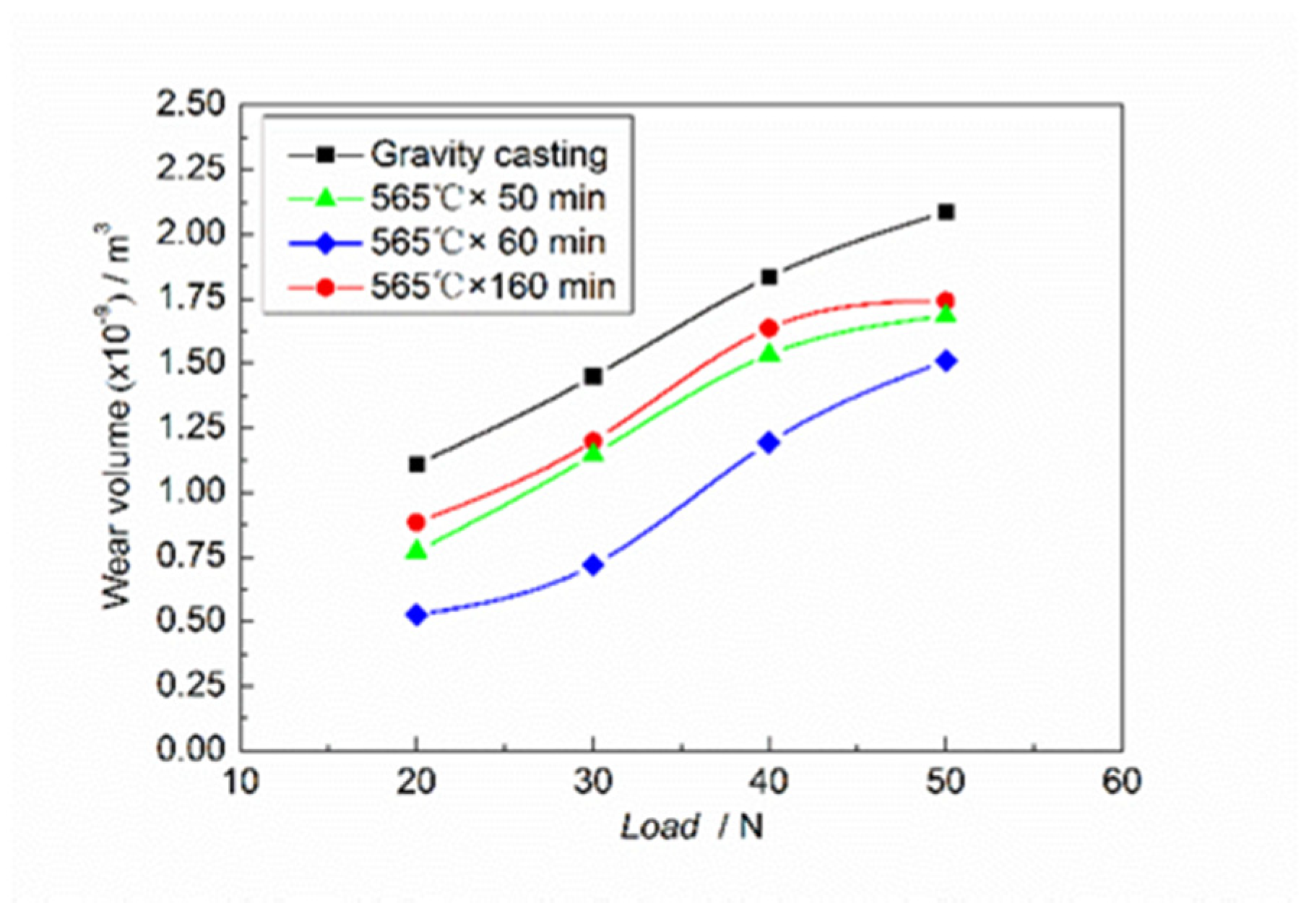
The friction and wear curves of Mg
2Si–Al composites under different loads at a fixed sliding rate of 700 r/min are shown in
Figure 4. The curves show that the wear volume gradually increased as the applied load increased when the sliding rate was constant. According to the change law of wear volume, the relationship between wear volume and load can be divided into three areas: the low-load area (20–30 N), medium-load area (30–40 N), and high-load area (40–50 N). In the low-load region, which is called the mild wear state [
22], the wear volume showed a slow linear increasing trend as the load increased. In the medium-load area, the wear volume increased non-linearly with the increase of the load, while in the high-load area, the increase in the wear volume was gentle. These two stages are called severe wear conditions [
22]. It was also found in the experiment that a more severe vibration was generated during the high loading stage. In the low-load area, alumina was generated due to frictional heat generation on the wear surface. This oxide film was very hard and sat between the abrasive disc and the wear surface, so it had a very large protective effect on the wear surface. After being worn away, this layer of oxide film could be quickly formed again, thus reducing the wear on the material surface of the grinding disc and delaying the wear failure process. At the same time, work hardening also played a certain protective role. The wear method was relatively simple; there was only slight adhesive wear and slight cutting of the wear surface by hard points, so the wear surface peeled off slowly, resulting in less increase in wear volume. In the medium-load area, as the load increased, the wear increased, and the peeling rate of the wear surface was much faster than the rate of oxide film formation and work hardening, so the wear volume increased rapidly. The primary Mg
2Si particles had high hardness and excellent abrasion resistance. In this experiment, the Mg
2Si particles were small in size and dispersed in the soft aluminum matrix, forming a good combination of hard points and soft matrix, which improved the carrying capacity of Mg
2Si–Al composites. In the high-load area, the small Mg
2Si particles were prominent, and their anti-wear effect gradually became prominent, which caused the increase in the wear volume of the composite material to slow down.
In addition, the curves also show that the wear resistances of Mg2Si–Al composites fabricated by using semi-solid extrusion were better than those of Mg2Si–Al composites fabricated by using gravity casting. Among them, the wear resistances of Mg2Si–Al composite fabricated by using semi-solid extrusion with an isothermal heat treatment holding time of 60 min were best, followed by Mg2Si–Al composites fabricated by using semi-solid extrusion with an isothermal heat treatment holding time of 50 min and 160 min. Compared with Mg2Si–Al composites fabricated by using gravity casting, under high-load (50 N) conditions, the wear volumes of Mg2Si–Al composites fabricated by using semi-solid extrusion with isothermal heat treatment holding times of 50 min, 60 min, and 160 min were 80.7%, 72.4%, and 83.5% of the former rates, respectively. Under the conditions of semi-solid extrusion, the α-Al phase and eutectic structure were not only refined but their distribution became more uniform, and the spheroidization phenomenon of the α-Al phase and Mg2Si appeared. This double spheroidizing effect reduced the cracking of the enhanced matrix and effectively improved the wear resistance of the composites.
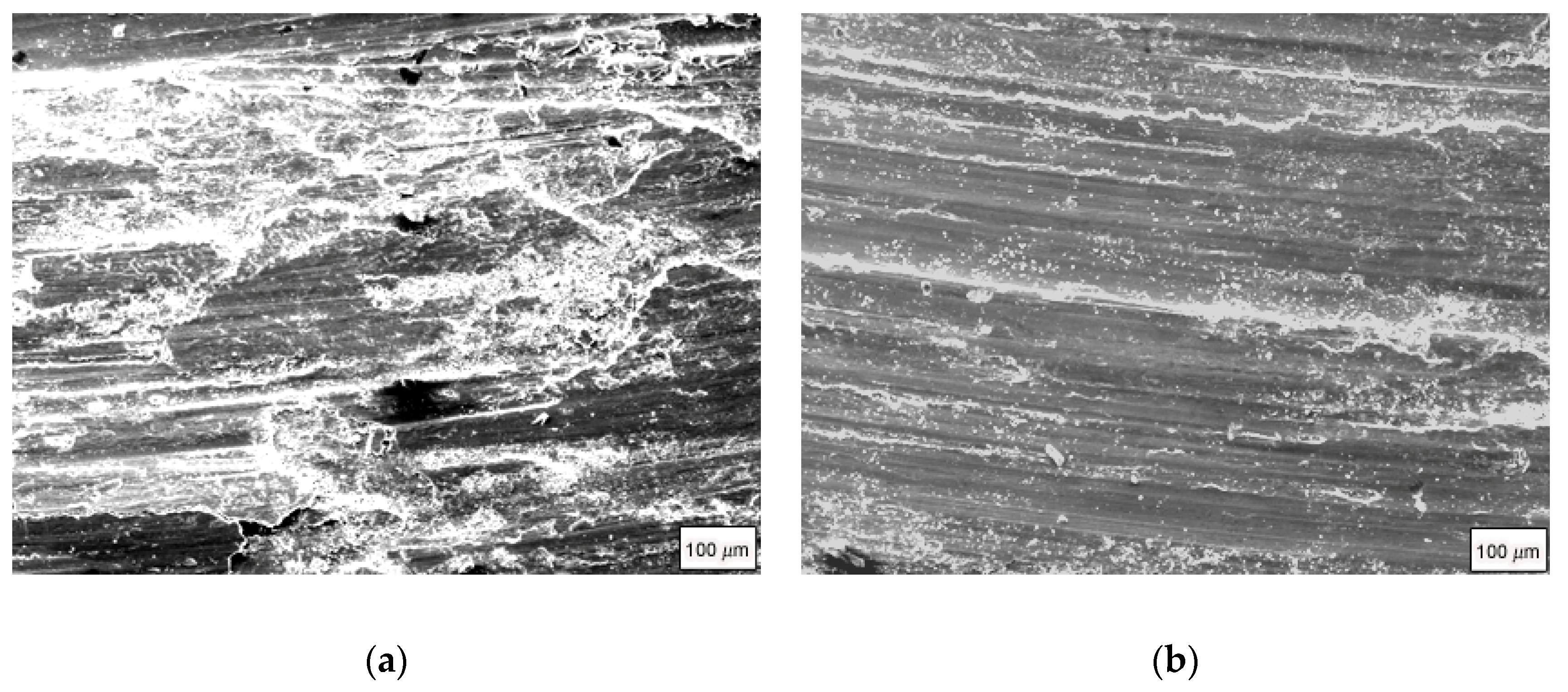
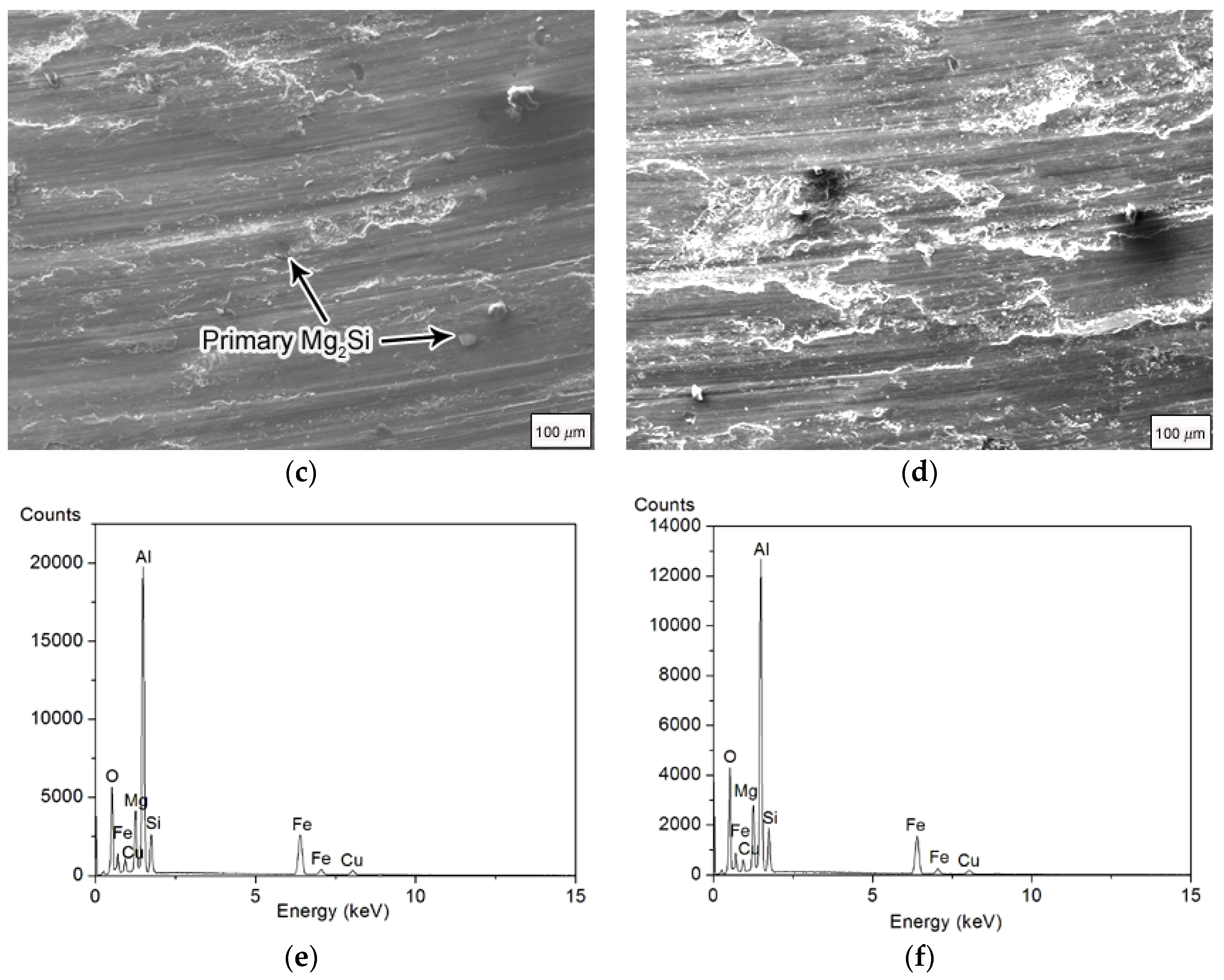
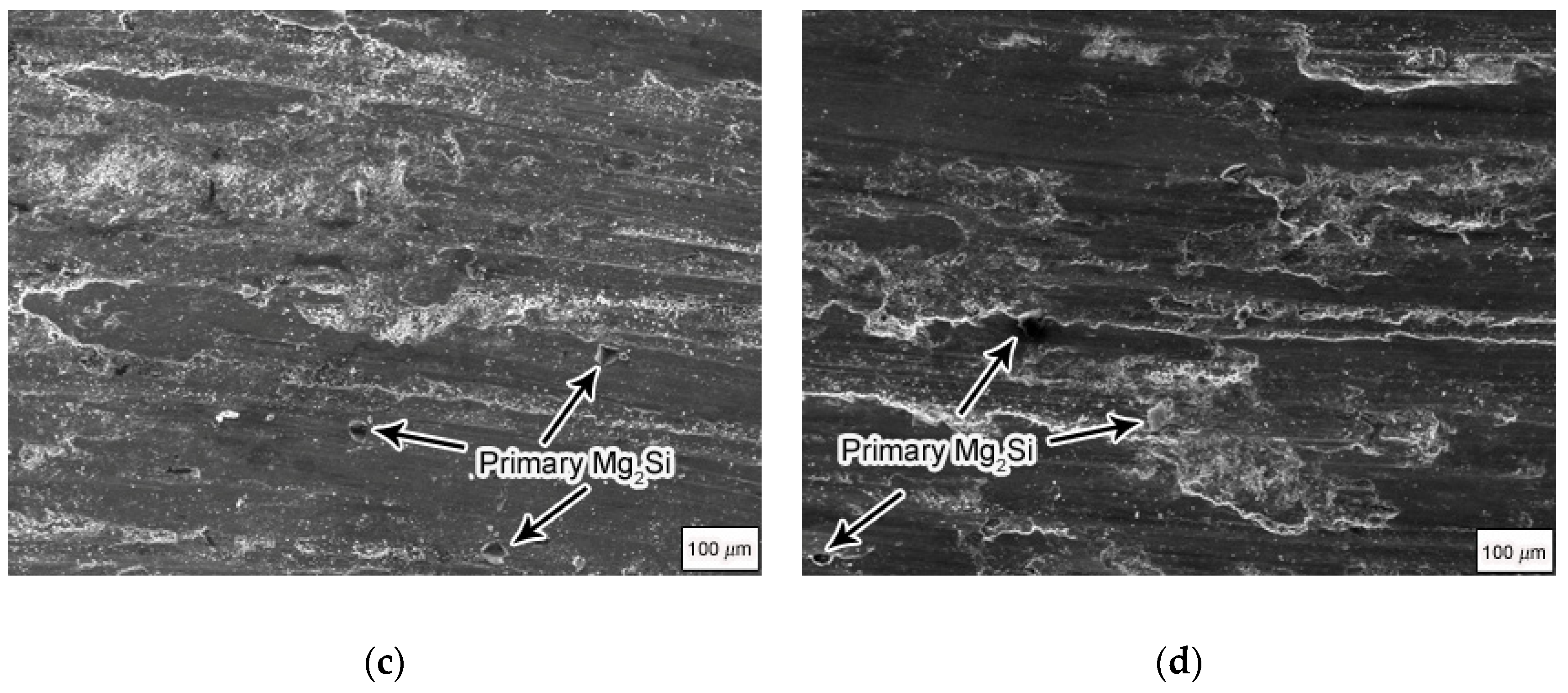
Figure 5 shows the SEM and EDS results of the wear surfaces of Mg
2Si–Al composites fabricated by using gravity casting and semi-solid extrusion with 50 N applied load. It can be seen that the wear surface of the Mg
2Si–Al composite fabricated by using gravity casting was very rough; there were obviously deep furrows, fine cracks, and spalling on the wear surface, as shown in
Figure 5a. In contrast, only shallow furrows existed on the wear surfaces of Mg
2Si–Al composites fabricated by using semi-solid extrusion, and occasionally slight spalling was seen, as shown in
Figure 5b–d. The furrows were the shallowest when the isothermal heat treatment holding time was 60 min, while the furrows were the deepest when the holding time was 160 min. At the same time, there were still some protruding Mg
2Si particles and some accumulations on the wear surface. This was because the temperature of the wear surface was higher under severe wear conditions, which caused a strong plastic flow and wear surface. Some parts of the slab were piled up or plowed off to form abrasive dust, so the Mg
2Si particles in the bottom layer were exposed. In addition, some Mg
2Si particles fell off after being pulled out, and there were still marks remaining on the worn surface after their falling off. It can be inferred that severe wear generated a large amount of heat. This heat accumulation caused the local temperature of the wear surface to be very high, causing a flash temperature phenomenon and even melting. As a result, the bonding strength of the matrix and Mg
2Si particles was reduced; Mg
2Si particles were no longer constrained by the matrix to be fixed in the corresponding position, and finally the reinforced particles fell off from the matrix, forming a trace after falling off.
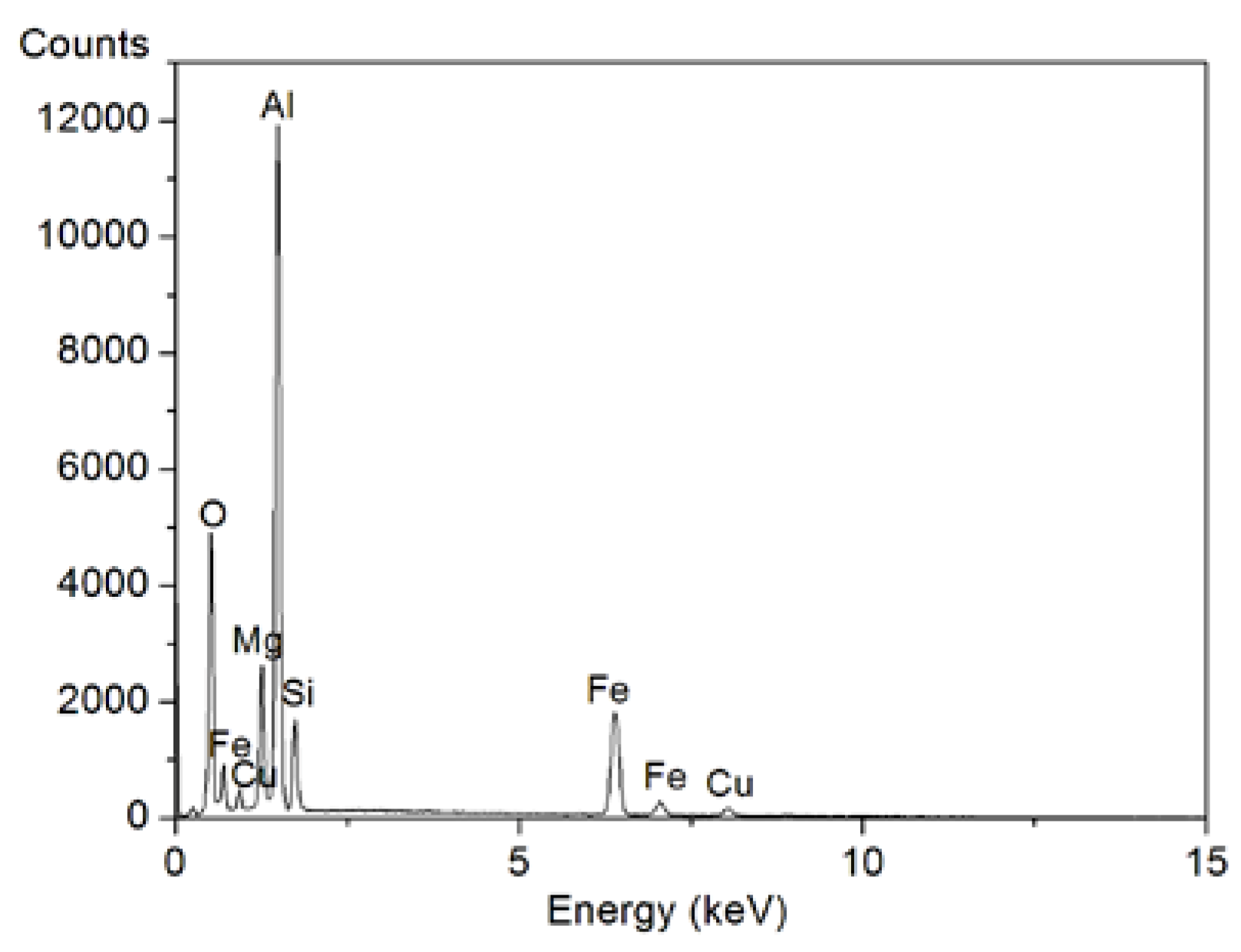
Figure 5e,f show the EDS results of the wear surfaces of Mg
2Si–Al composites. The results showed that in addition to aluminum, magnesium, silicon, and copper, the wear surfaces of the two composites also contained iron and oxygen. The main component of the composite material did not contain iron. The iron came from a counter-mill disc made of 45 steel, indicating that iron element transfer occurred during the wear process. The presence of oxygen indicated the presence of Al
2O
3 on the worn surface. Qin et al. [
20] found the wear debris was mainly composed of Mg
2Si, Al, and Al
2O
3 phases by XRD analysis of the abrasive debris of the Mg
2Si–Al composite. Generally, under the condition of mild wear (low load), the Al
2O
3 oxide film can protect the wear surface to a certain extent, delay the wear failure, and reduce the wear rate. However, during the severe wear stage, although an oxide film is produced, the surface is strongly softened due to the high load, severe wear, and increased temperature of the friction interface. At this time, the peeling rate of the surface layer is dominant, and the oxide film will be quickly worn away. Its formation rate may be much slower than the peeling rate of the surface layer, thereby limiting the protective effect of the oxide film. Therefore, even if the anti-wear effect of Mg
2Si particles is very prominent in the high-load stage, which makes the change of the wear curve slow, the wear volume of the composite material is still large.
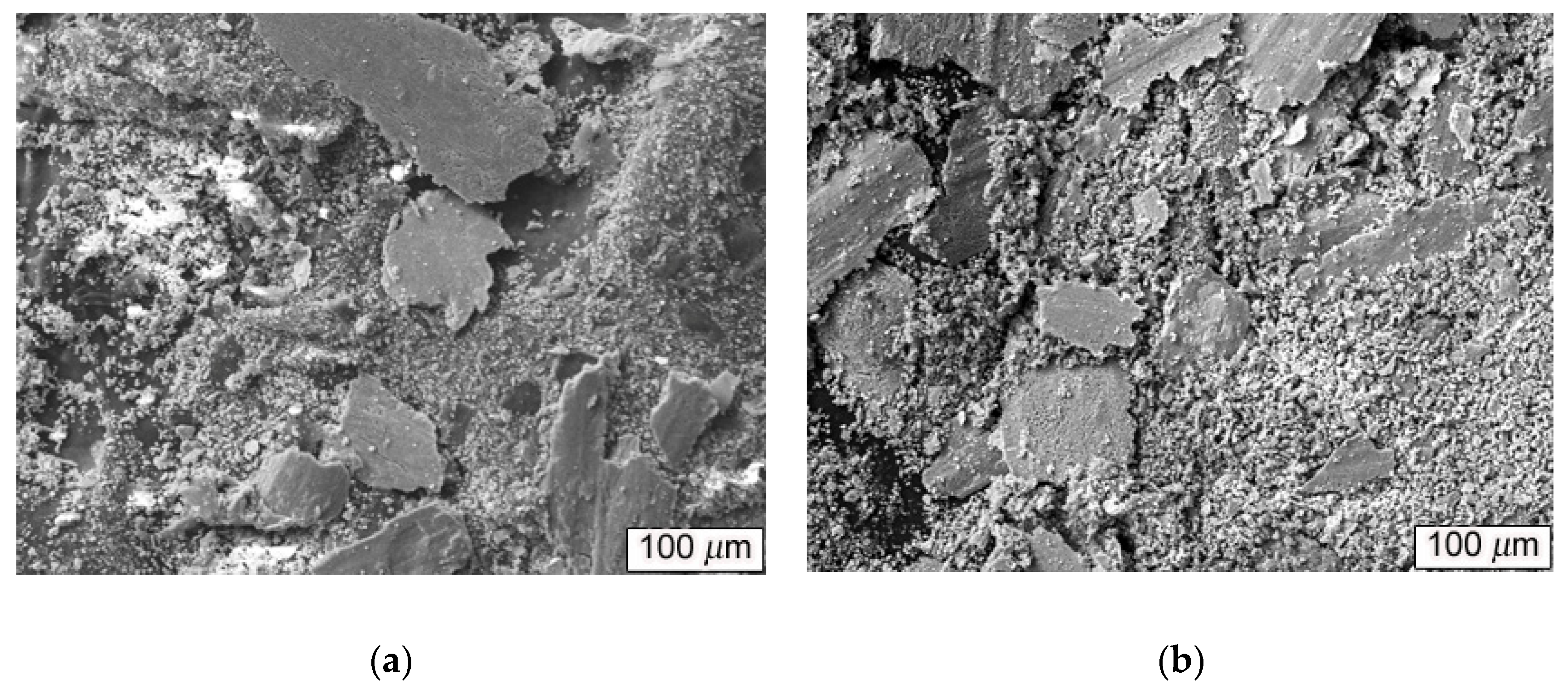
Figure 6 shows the SEM image of abrasive debris from Mg
2Si–Al composite by using gravity casting and semi-solid extrusion with isothermal heat treatment holding time of 60 min with 50 N applied load. Under severe abrasion, the abrasive debris of the two composites consisted of large layers of lamellar debris and small particle agglomerates. Among them, the composition of layered debris was mainly matrix and Mg
2Si particles.
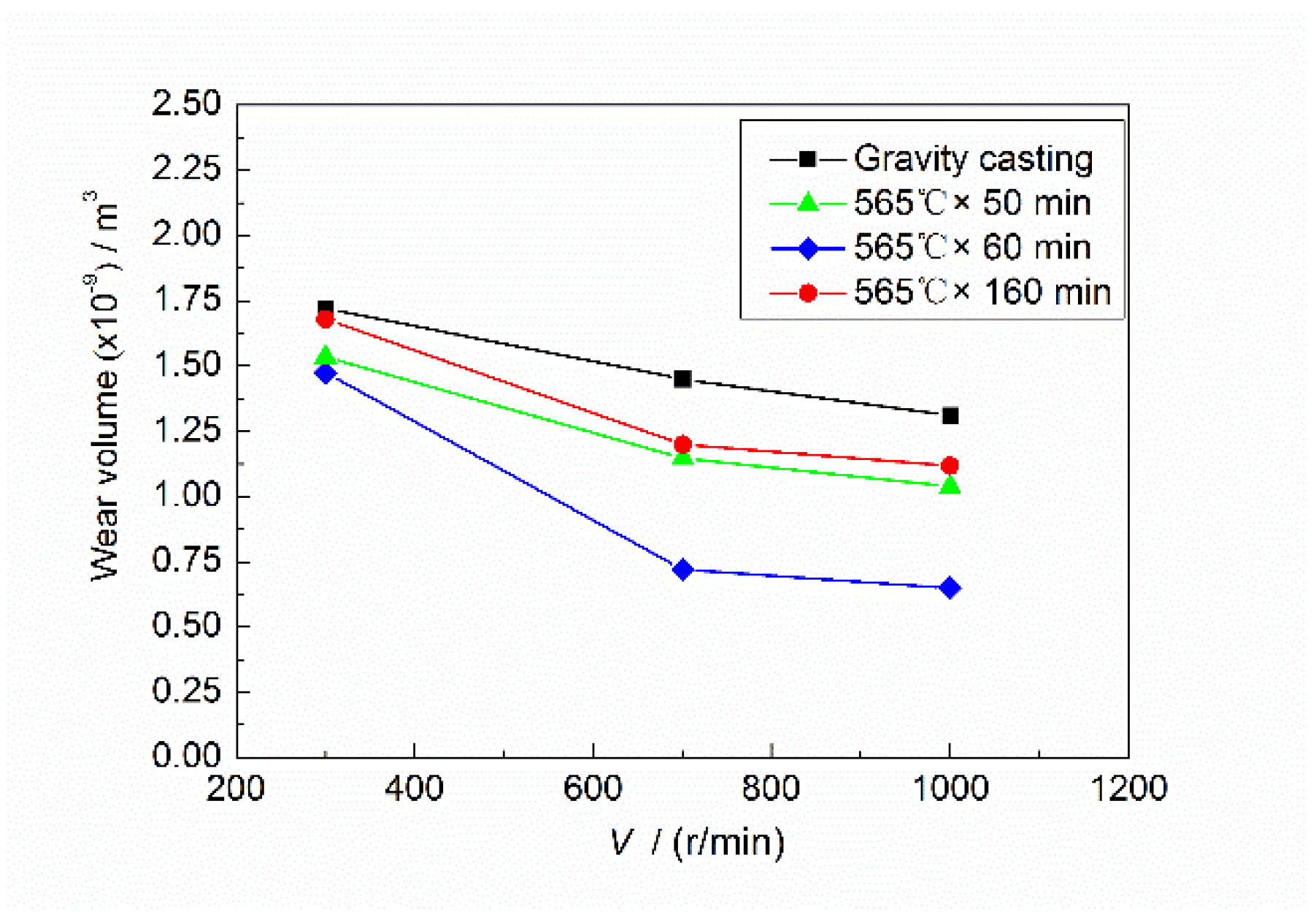
Figure 7 shows the wear curve of Mg
2Si–Al composites fabricated by using gravity casting and semi-solid extrusion with 30 N applied load. It can be seen that the wear volume of Mg
2Si–Al composites decreased with increasing sliding rate. The wear volume decreased faster between 300 and 700 r/min, and the decreasing trend slowed down between 700 and 1000 r/min. This was because between 300 and 700 r/min, the wear was a mild state of wear due to low load (30 N) and a slow sliding rate. Al
2O
3 on the wear surface played a certain protective role, and its comprehensive protection effect with work hardening was much greater than the peeling rate of the wear surface, coupled with the anti-wear effect of the Mg
2Si particles, so the wear volume was significantly reduced. In the range of 700–1000 r/min, a large amount of frictional heat was generated because of the high sliding rate, which resulted in a local high temperature of the friction interface and a reduced binding effect of the substrate on the surface and subsurface layers, although the load was still low. In addition, the increased surface temperature will also lead to a decrease in the shear strength of the subsurface layer [
22], resulting in a strong softening of the worn surface, an increase in the peeling rate of the surface layer, and an acceleration of the failure of the wear material, so the reduction trend of the wear volume becomes slow.
Figure 8 shows SEM of wear surfaces of Mg
2Si–Al composites fabricated by using gravity casting and semi-solid extrusion with a sliding velocity of 1000 r/min. The figure shows that plastic flow occurred on the wear surface of the Mg
2Si–Al composite, and craters and furrows remained after the plastic flow. The furrows on the wear surface of Mg
2Si–Al composite fabricated by using gravity casting were deep and wide (
Figure 8a), while the furrows of Mg
2Si–Al composites fabricated by using semi-solid extrusion were shallow and thin (
Figure 8b–d). When the holding times were 50 min and 160 min, the width and depth of the furrows of semi-solid extruded Mg
2Si–Al composites were not significantly different. When the holding time was 60 min, the furrows were the shallowest and hardly visible. In addition, prominent Mg
2Si particles, an accumulation phenomenon, and some peeling pits remaining after Mg
2Si fell off were still visible.
Figure 9 shows the wear surfaces of Mg
2Si–Al composites fabricated by semi-solid extrusion at high magnification. It can be seen from the figure that Mg
2Si reinforced particles and deposits existed on the worn surface. This was because the increase of the sliding rate promoted the plastic flow of the composite material. When the sliding rate was 1000 r/min, the friction surface generated heat and the temperature of the wear surface was high, resulting in a strong plastic flow. Some parts of the wear surfaces were pushed forward during the wear process, forming a pile, and at the same time, the Mg
2Si reinforced particles in the bottom layer of this part were also exposed.
Figure 10 shows the EDS result of wear surface of Mg
2Si–Al composite fabricated by using semi-solid extrusion with isothermal heat treatment holding time of 160 min, with sliding velocity of 1000 r/min. Similar to the EDS analysis of the wear surface under high load, iron and oxygen were also present on the worn surface, indicating that at a high sliding rate, the iron element in the grinding disc (45 steel) was transferred to the worn surface of the composite material, and there was also a protective layer of Al
2O
3 on the worn surface.
Kwork et al. [
23] studied the friction behavior of SiC
p–Al composites at high speeds and found that the Al surface melted when the speed reached a certain value. Qin et al. [
20] also believed that because of the influence of frictional heat, the worn surface locally reached a higher temperature and the surface melted, thereby reducing the binding effect of the Al matrix on the Mg
2Si reinforced particles, causing the reinforced particles to fall off and a residual mark to form. In this experiment, the hard Al
2O
3 oxide film was continuously worn away and constantly produced. The oxide film and work hardening had a good protective effect on the composite material, which was a factor that delayed the wear failure. However, as the sliding rate increased to 1000 r/min, the temperature of the wear surface increased, the shear strength of the alloy was reduced, and the plastic flow capacity of the surface layer and sub-surface layer was greatly increased, which accelerated the material failure rate. This was a factor contributing to wear failure. Under the condition of a low sliding rate, the favorable factors that delay the wear failure played a leading role, resulting in a rapid reduction in the volume of wear. With the increase of the sliding rate, the factors of accelerated wear failure played an increasingly important role. The combined effect of the two slowed down the trend of reducing the wear volume, and the characteristics shown in the wear curve appeared.
For Mg
2Si–Al composites, the dry sliding wear process is affected by both external and internal factors [
24]. External factors include sliding distance, ambient temperature, form of motion, sliding rate, load, surface state, and grinding disc. Among many factors, load and sliding rate are two more important parameters, which have a greater impact on the wear failure process. The material of the grinding disc also has a certain effect on the dry sliding wear process. Since dry sliding friction and wear require a pair of friction pairs, the wear behavior of the grinding disc will also affect the wear resistance of the tested material. If the sample material is close to the microstructure of the disc material, then a conjugate friction pair will be formed during the pairing process due to the large mutual solubility of metals. This kind of friction pair has strong vibration and noise during the wear process, which results in severe wear of the tested materials and the grinding disc. Conversely, if the microstructure of the sample material is different from that of the disc material, the mutual solubility of the metal is small, resulting in a stable friction and wear process and less wear. In this experiment, 45 steel (composed of ferrite and pearlite) was used as the paired friction pair. Obviously, the microstructures of Mg
2Si–Al composites and the grinding disc are completely different, and the metal mutual solubility between the two is small; therefore, the material of the grinding disc has less influence on the wear behaviors of the composites.
Internal factors, such as the matrix and reinforcement (type, size, micromorphology, distribution, volume fraction, etc.) also affect the friction and wear behaviors of the composites. The matrix hardness of the composite material also greatly affects its wear resistance. According to the hardness test, the Brinell hardness of Mg
2Si–Al composite fabricated by using gravity casting was 115 HB, and the Brinell hardnesses of Mg
2Si–Al composite fabricated by using semi-solid extrusion with isothermal heat treatment holding times of 50 min, 60 min and 160 min were 153 HB, 160 HB, and 134 HB, respectively. Since the hardnesses of Mg
2Si–Al composites fabricated by using semi-solid extrusion were significantly improved, plastic flow on the worn surface did not easily occur, which reduced the wear volume of the wear sample, so their wear resistances were improved. Reinforcement particles also play a role in the wear process [
24]. Studies have shown [
25,
26] that, for alloys, if there are fine hard particles in the matrix, their wear resistance will be correspondingly good. In general, harder particles have wear abrasion resistance [
24,
27]. The greater the volume fraction, the more uniform the distribution of the reinforcement, and the better its wear resistance. There is no obvious law on the effect of reinforcement size on wear resistance. In addition, during the wear process, stress concentration, micro-deformation, holes, and micro-cracks are more likely to breed at the interface of the reinforcement and the matrix due to the large difference in plastic deformation capacity between the reinforcement and the matrix, thereby reducing the properties of the composites. The microstructure directly affects the wear properties of alloys. Therefore, the micromorphology of the reinforcement has a greater impact on the wear behaviors. For Mg
2Si–Al composites fabricated by using semi-solid extrusion, the Mg
2Si reinforced phase is spherical, small, and round, and the stress concentration around the particles is weak, so crack generation and propagation are delayed. Because the spherical Mg
2Si particles have a higher bonding strength with the matrix, they do not easily fall off the matrix. Once the spherical Mg
2Si particles come off the matrix, they only act as spherical abrasive particles, so the cutting and abrasion of the composite material is relatively small, forming a shallow furrow. In short, semi-solid extrusion changed the morphology of the primary Mg
2Si, which contributed significantly to the better wear resistance of the composite.