Compound Layer Design for Deep Nitrided Gearings
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference State
2.2. Investigated Material
2.3. Nitriding Treatments
2.4. Characterization of the Compound Layer
3. Results and Discussion
3.1. Short-Term Nitriding Treatments
3.2. One-Stage Deep Nitriding
3.3. Two-Stage Deep Nitriding
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conrado, E.; Gorla, C.; Davoli, P.; Boniardi, M. A comparison of bending fatigue strength of carburized and nitrided gears for industrial applications. Eng. Fail. Anal. 2017, 78, 41–54. [Google Scholar] [CrossRef]
- König, J.; Hoja, S.; Tobie, T.; Hoffmann, F.; Stahl, K. In Increasing the Load Carrying Capacity of Highly Loaded Gears by Nitriding. In Proceedings of the MATEC Web of Conferences, Varna, Bulgaria, 19–22 June 2019. [Google Scholar] [CrossRef]
- Niemann, G.; Winter, H. Band 2: Getriebe allgemein, Zahnradgetriebe-Grundlagen. In Maschinenelemente; Springer: Heidelberg/Berlin, Germany, 2003. [Google Scholar]
- Hoja, S.; Hoffmann, F.; Zoch, H.W.; Schurer, S.; Tobie, T.; Stahl, K. Entwicklung von Prozessen zum Tiefnitrieren von Zahnrädern. HTM J. Heat Treatm. Mat. 2015, 70, 276–285. [Google Scholar] [CrossRef]
- Hoja, S.; Hoffmann, F.; Steinbacher, M.; Zoch, H.W. Untersuchung des Anlasseffekts beim Nitrieren. HTM J. Heat Treatm. Mat. 2018, 73, 335–343. [Google Scholar] [CrossRef]
- Bräutigam, F. Nitrieren im Ammoniakgasstrom: Ein Oberflächenhärte-Verfahren mit großer Zukunft. Industrie Anzeiger 1981, 103, 36–39. [Google Scholar]
- Limodin, N.; Verreman, Y. Fatigue strength improvement of a 4140 steel by gas nitriding: Influence of notch severity. Mater. Sci. Eng. A 2006, 435–436, 460–467. [Google Scholar] [CrossRef]
- Zlatanović, M.; Popović, N.; Mitrić, M. Plasma processing in carbon containing atmosphere for possible treatment of wind turbine components. Thin Solid Films 2007, 516, 228–232. [Google Scholar] [CrossRef]
- Wu, K.; Liu, G.Q.; Wang, L.; Xu, B.F. Research on new rapid and deep plasma nitriding techniques of AISI 420 martensitic stainless steel. Vacuum 2010, 84, 870–875. [Google Scholar] [CrossRef]
- Floe, C.F. A study of the nitriding process. Effect of ammonia dissociation on case depth and structure. Trans. ASM 1944, 32, 44–171. [Google Scholar]
- Floe, C.F. Method of Nitriding. U.S. Patent No. 2,437,249, 17 April 1946. [Google Scholar]
- Hassani-Gangaraj, S.M.; Moridi, A.; Guagliano, M.; Ghidini, A.; Boniardi, M. The effect of nitriding, severe shot peening and their combination on the fatigue behavior and micro-structure of a low-alloy steel. Int. J. Fatigue 2014, 62, 67–76. [Google Scholar] [CrossRef]
- Bossy, E.; Noyel, J.P.; Kleber, X.; Ville, F.; Sidoroffd, C.; Thibault, S. Competition between surface and subsurface rolling contact fatigue failures of nitrided parts: A Dang Van approach. Tribol. Int. 2019, 140, 105888. [Google Scholar] [CrossRef]
- Hoja, S.; Schurer, S.; Hoffmann, F.; Tobie, T. Tiefnitrieren von Zahnrädern. In FVA-Nr. 615 II, FVA-Forschungsheft Nr. 1147; Forschungsvereinigung Antriebstechnik e. V.: Frankfurt, Germany, 2015. [Google Scholar]
- Ochoa, E.A.; Wisnivesky, D.; Minea, T.; Ganciu, M.; Tauziede, C.; Chapon, P.; Alvarez, F. Microstructure and properties of the compound layer obtained by pulsed plasma nitriding in steel gears. Surf. Coat. Technol. 2009, 203, 1457–1461. [Google Scholar] [CrossRef]
- Zornek, B.; Hoja, S.; Tobie, T.; Hoffmann, F. Tribologische Tragfähigkeit nitrierter Innen- und Außenverzahnungen bei geringen Umfangsgeschwindigkeiten. In FVA-Forschungsheft Nr. 1206; Forschungsvereinigung Antriebstechnik e. V.: Frankfurt, Germany, 2017. [Google Scholar]
- Hoffmann, F.; Bujak, I.; Mayr, P.; Löffelbein, B.; Gienau, M. Verschleißwiderstand nitrierter und nitrocarburierter Stähle. HTM-Härterei-Techn. Mitt. 1997, 52, 376–386. [Google Scholar]
- Liedtke, D. Beitrag zum Technisch-Wirtschaftlichen Optimieren des Nitrocarburierens von Bauteilen. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 1986. [Google Scholar]
- Binder, C.; Bendo, T.; Hammes, G.; Klein, A.N.; de Mello, J.D.B. Effect of nature of nitride phases on sliding wear of plasma nitrided sintered iron. Wear 2015, 332–333, 995–1005. [Google Scholar] [CrossRef]
- Hollomon, J.H.; Jaffe, L.D. Ferrous Metallurgical Design; Wiley: Hoboken, NJ, USA, 1947. [Google Scholar]
- Huchel, U.; Klümper-Westkamp, H.; Liedtke, D. Lichtmikroskopische Bestimmung der Dicke und Porigkeit der Verbindungsschichten nitrierter und nitrocarburierter Werkstücke. In Prüfvorschrift des AWT-FA3; Arbeitsgemeinschaft Wärmebehandlung + Werkstofftechnik e. V.: Bremen, Germany, 2008. [Google Scholar]
- Hoffmann, R.; Mittemeijer, E.J.; Somers, M.A.J. Verbindungsschichtbildung beim Nitrieren und Nitrocarburieren. HTM-Härterei-Techn. Mitt. 1996, 51, 162–169. [Google Scholar]
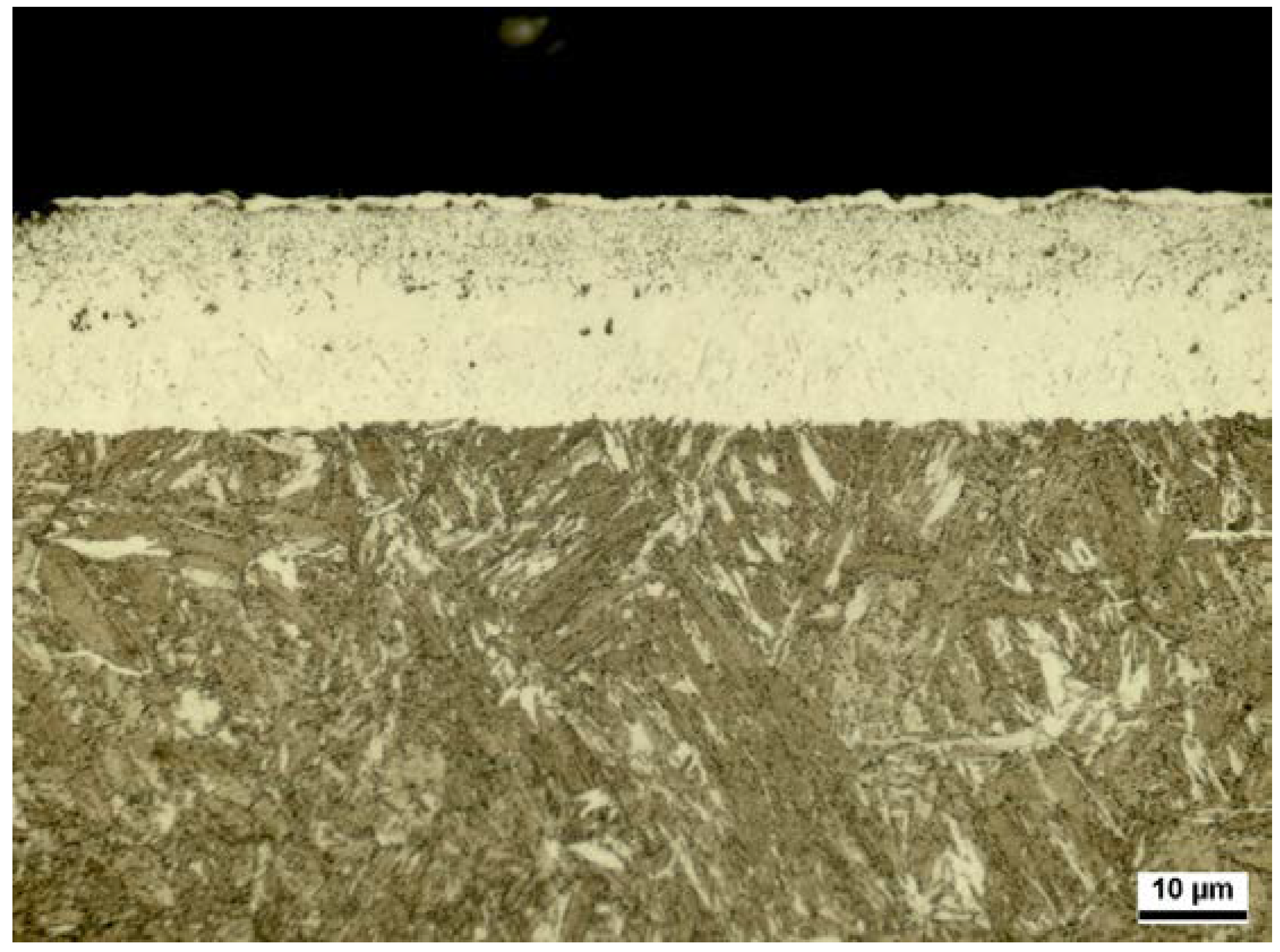



| Reference 32CDV13 | CLT | CLTP | NHD |
| 15–17 µm | 5–7 µm | ca. 1 mm |
| Short-Term Treatments | Long-Term Treatments | ||
|---|---|---|---|
| Nitriding | Nitrocarburizing | One-Stage | Two-Stage |
| 520 °C 15 h KN = 1 | 520 °C 15 h KN = 1 KCB = 0.1 | 530 °C 170 h KN=0.5 | 520 °C 15 h KN = 5 530 °C 170 h KN = 0.5 |
| 520 °C 15 h KN = 3 | 520 °C 15 h KN = 5 KCB = 0.1 | 530 °C 170 h KN = 1 | 530 °C 170 h KN = 0.5 520 °C 15 h KN = 5 |
| 520 °C 15 h KN = 5 | 550 °C 15 h KN = 5 KCB = 0.1 | 530 °C 170 h KN = 3 | 530 °C 170 h KN = 0.5 550 °C 15 h KN = 5 |
| 550 °C 120 h KN = 1 | 530 °C 170 h KN = 0.5 550 °C 15 h KN = 5 KCB = 0.1 | ||
| Nitriding | Nitrocarburizing | ||||
|---|---|---|---|---|---|
| 520 °C 15 h KN = 1 | 520 °C 15 h KN = 3 | 520 °C 15 h KN = 5 | 520 °C 15 h KN = 1 KCB = 0.1 | 520 °C 15 h KN = 5 KCB = 0.1 | 550 °C 15 h KN = 5 KCB = 0.1 |
 |  |  |  |  |  |
| CLT = 2 µm | CLT = 7 µm | CLT = 12 µm | CLT = 3 µm | CLT = 6 µm | CLT = 19 µm |
| CLTP = 1 µm | CLTP = 3 µm | CLTP = 5 µm | CLTP = 2 µm | CLTP = 5 µm | CLTP = 7 µm |
| Nitriding | γ’-Nitride in % | ε-Nitride in % | Nitro-Carburizing | γ’-Nitride in % | ε-(Carbo-) Nitride in % |
|---|---|---|---|---|---|
| 520 °C 15 h KN = 1 | ca. 74 | ca. 26 | 520 °C 15 h KN = 1 KCB = 0.1 | ca. 54 | ca. 46 |
| 520 °C 15 h KN = 3 | ca. 79 | ca. 21 | 520 °C 15 h KN = 5 KCB = 0.1 | ca. 23 | ca. 77 |
| 520 °C 15 h KN = 5 | ca. 89 | ca. 11 | 550 °C 15 h KN = 5 KCB = 0.1 | ca. 68 | ca. 32 |
| 530 °C 170 h KN = 0.5 | 530 °C 170 h KN = 1 | 530 °C 170 h KN = 3 | 550 °C 120 h KN = 0.5 |
|---|---|---|---|
 |  | 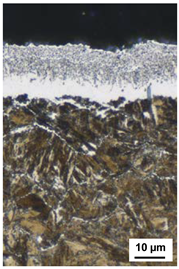 | 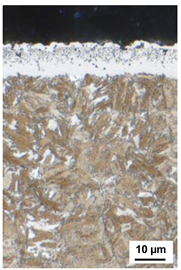 |
| CLT = 6 µm | CLT = 7 µm | CLT = 18 µm | CLT = 10 µm |
| CLTP = 3 µm | CLTP = 4 µm | CLTP = 12 µm | CLTP = 6 µm |
| NHD = 0.75 mm | NHD = 0.83 mm | NHD = 0.88 mm | NHD = 0.85 mm |
| 520 °C 15 h KN = 5 530 °C 170 h KN = 0.5 | 530 °C 170 h KN = 0.5 520 °C 15 h KN = 5 | 530 °C 170 h KN = 0.5 550 °C 15 h KN = 5 | 530 °C 170 h KN = 0.5 550 °C 15 h KN = 5 KCB = 0.1 |
|---|---|---|---|
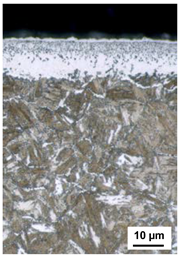 |  | 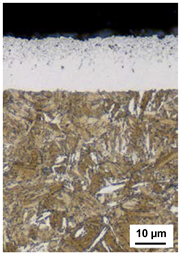 |  |
| CLT = 12 µm | CLT = 13 µm | CLT = 17 µm | CLT = 20 µm |
| CLTP = 7 µm | CLTP = 6 µm | CLTP = 8 µm | CLTP = 8 µm |
| NHD = 0.75 mm | NHD = 0.82 mm | NHD = 0.81 mm | NHD = 0.85 mm |
| Two-Stage Nitriding | γ’-Nitride in % | ε-Nitride in % |
|---|---|---|
| 520 °C 15 h KN = 5 530 °C 170 h KN = 0.5 | ca. 88 | ca. 12 |
| 530 °C 170 h KN = 0.5 520 °C 15 h KN = 5 | ca. 85 | ca. 15 |
| 530 °C 170 h KN = 0.5 550 °C 15 h KN = 5 | ca. 88 | ca. 12 |
| 530 °C 170 h KN = 0.5 550 °C 15 h KN = 5 KCB = 0.1 | ca. 60 | ca. 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoja, S.; Steinbacher, M.; Zoch, H.-W. Compound Layer Design for Deep Nitrided Gearings. Metals 2020, 10, 455. https://doi.org/10.3390/met10040455
Hoja S, Steinbacher M, Zoch H-W. Compound Layer Design for Deep Nitrided Gearings. Metals. 2020; 10(4):455. https://doi.org/10.3390/met10040455
Chicago/Turabian StyleHoja, Stefanie, Matthias Steinbacher, and Hans-Werner Zoch. 2020. "Compound Layer Design for Deep Nitrided Gearings" Metals 10, no. 4: 455. https://doi.org/10.3390/met10040455
APA StyleHoja, S., Steinbacher, M., & Zoch, H.-W. (2020). Compound Layer Design for Deep Nitrided Gearings. Metals, 10(4), 455. https://doi.org/10.3390/met10040455





