Influence of HPT Deformation on the Structure and Properties of Amorphous Alloys
Abstract
1. Introduction
2. Atomic Structure of Amorphous Alloys Subjected to SPD
3. Transformation of the Properties of Amorphous Alloys as a Result of SPD Processing
4. Influence of HPT on the Behaviors of Amorphous Alloys during Annealing
4.1. The Strain Achieved by HPT and Special Monitoring Schemes for the Formation of Shear Bands
4.2. Accumulative HPT Procedure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greer, A.L.; Ma, E. Bulk Metallic Glasses: At the Cutting Edge of Metals Research. MRS Bull. 2007, 32, 611–619. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Abrosimova, G.E. Evolution of the structure of amorphous alloys. Physics-Uspekhi 2011, 54, 1227–1242. [Google Scholar] [CrossRef]
- Goncharova, E.V.; Konchakov, R.A.; Makarov, A.S.; Kobelev, N.P.; Khonik, V.A. On the nature of density changes upon structural relaxation and crystallization of metallic glasses. J. Non. Cryst. Solids 2017, 471, 396–399. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic Glasses. Science 1995, 267, 1947–1953. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, N. New Bulk Metallic Glasses for Applications as Magnetic-Sensing, Chemical, and Structural Materials. MRS Bull. 2007, 32, 651–658. [Google Scholar] [CrossRef]
- Axinte, E. Metallic glasses from “alchemy” to pure science: Present and future of design, processing and applications of glassy metals. Mater. Des. 2012, 35, 518–556. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. Bulk Metallic Glasses. In Handbook of Magnetic Materials; Elsevier: Sendai, Japan, 2013; pp. 131–171. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xie, X.H.; Li, H.F.; Wang, X.L.; Zhao, M.Z.; Zhang, E.W.; Bai, Y.J.; Zheng, Y.F.; Qin, L. Biodegradable CaMgZn bulk metallic glass for potential skeletal application. Acta Biomater. 2011, 7, 3196–3208. [Google Scholar] [CrossRef]
- Cao, Q.P.; Liu, J.W.; Yang, K.J.; Xu, F.; Yao, Z.Q.; Minkow, A.; Fecht, H.J.; Ivanisenko, J.; Chen, L.Y.; Wang, X.D.; et al. Effect of pre-existing shear bands on the tensile mechanical properties of a bulk metallic glass. Acta Mater. 2010, 58, 1276–1292. [Google Scholar] [CrossRef]
- Park, K.-W.; Lee, C.-M.; Kim, H.-J.; Lee, J.-H.; Lee, J.-C. A methodology of enhancing the plasticity of amorphous alloys: Elastostatic compression at room temperature. Mater. Sci. Eng. A 2009, 499, 529–533. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Zhang, W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A. Stable flowing of localized shear bands in soft bulk metallic glasses. Acta Mater. 2010, 58, 904–909. [Google Scholar] [CrossRef]
- Ma, E.; Ding, J. Tailoring structural inhomogeneities in metallic glasses to enable tensile ductility at room temperature. Mater. Today 2016, 19, 568–579. [Google Scholar] [CrossRef]
- Ketov, S.V.; Sun, Y.H.; Nachum, S.; Lu, Z.; Checchi, A.; Beraldin, A.R.; Bai, H.Y.; Wang, W.H.; Louzguine-Luzgin, D.V.; Carpenter, M.A.; et al. Rejuvenation of metallic glasses by non-affine thermal strain. Nature 2015, 524, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zechetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Bulk Nanostructured Materials; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2013; ISBN 9781118742679. [Google Scholar]
- Zhilyaev, A.; Langdon, T. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Kovács, Z.; Henits, P.; Zhilyaev, A.P.; Révész, Á. Deformation induced primary crystallization in a thermally non-primary crystallizing amorphous Al85Ce8Ni5Co2 alloy. Scr. Mater. 2006, 54, 1733–1737. [Google Scholar] [CrossRef]
- Kovács, Z.; Henits, P.; Zhilyaev, A.P.; Chinh, N.Q.; Révész, Á. Microstructural characterization of the crystallization sequence of a severe plastically deformed Al-Ce-Ni-Co amorphous alloy. Mater. Sci. Forum 2006, 519–521, 1329–1334. [Google Scholar] [CrossRef]
- Henits, P.; Révész, Á.; Schafler, E.; Szabó, P.J.; Lábár, J.L.; Varga, L.K.; Kovács, Z. Correlation between microstructural evolution during high-pressure torsion and isothermal heat treatment of amorphous Al 85 Gd 8 Ni 5 Co 2 alloy. J. Mater. Res. 2010, 25, 1388–1397. [Google Scholar] [CrossRef]
- Boucharat, N.; Hebert, R.J.; Rösner, H.; Wilde, G. Deformation-Induced Nanocrystallization in Al-Rich Metallic Glasses. Solid State Phenom. 2006, 114, 123–132. [Google Scholar] [CrossRef]
- Sarac, B.; Spieckermann, F.; Rezvan, A.; Gammer, C.; Krämer, L.; Kim, J.T.; Keckes, J.; Pippan, R.; Eckert, J. Annealing-assisted high-pressure torsion in Zr55Cu30Al10Ni5 metallic glass. J. Alloys Compd. 2019, 784, 1323–1333. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhu, Y.T.; Liao, X.Z.; Valiev, R.Z. Amorphization of TiNi induced by high-pressure torsion. Philos. Mag. Lett. 2004, 84, 183–190. [Google Scholar] [CrossRef]
- Meng, F.; Tsuchiya, K.; Seiichiro; Yokoyama, Y. Reversible transition of deformation mode by structural rejuvenation and relaxation in bulk metallic glass. Appl. Phys. Lett. 2012, 101, 121914. [Google Scholar] [CrossRef]
- Glezer, A.M.; Sundeev, R.V.; Shalimova, A.V. The cyclic character of phase transformations of the crystal ⇔ amorphous state type during severe plastic deformation of the Ti50Ni25Cu25 alloy. Dokl. Phys. 2011, 56, 476–478. [Google Scholar] [CrossRef]
- Sundeev, R.V.; Glezer, A.M.; Shalimova, A.V. Are the abilities of crystalline alloys to amorphization upon melt quenching and severe plastic deformation identical or different? Mater. Lett. 2016, 175, 72–74. [Google Scholar] [CrossRef]
- Sundeev, R.V.; Shalimova, A.V.; Glezer, A.M.; Pechina, E.A.; Gorshenkov, M.V.; Nosova, G.I. In situ observation of the “crystalline⇒amorphous state” phase transformation in Ti 2 NiCu upon high-pressure torsion. Mater. Sci. Eng. A 2017, 679, 1–6. [Google Scholar] [CrossRef]
- Edalati, K.; Yokoyama, Y.; Horita, Z. High-pressure torsion of machining chips and bulk discs of amorphous Zr50Cu30Al10Ni10. Mater. Trans. 2010, 51, 23–26. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A.; Matveev, D.; Pershina, E. Nanocrystal formation, structure and magnetic properties of Fe–Si–B amorphous alloy afterdeformation. Mater. Lett. 2013, 97, 15–17. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S.; Dobatkin, S.V.; Kaloshkin, S.D.; Matveev, D.V.; Rybchenko, O.G.; Tatyanin, E.V.; Zverkova, I.I. The Formation of Nanocrystalline Structure in Amorphous Fe-Si-B Alloy by Severe Plastic Deformation. J. Metastable Nanocrystalline Mater. 2005, 24–25, 69–72. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A. Nanocrystal formation in Al- and Ti-based amorphous alloys at deformation. J. Alloys Compd. 2018, 747, 26–30. [Google Scholar] [CrossRef]
- Czeppe, T.; Korznikova, G.; Morgiel, J.; Korznikov, A.; Chinh, N.Q.; Ochin, P.; Sypień, A. Microstructure and properties of cold consolidated amorphous ribbons from (NiCu)ZrTiAlSi alloys. J. Alloys Compd. 2009, 483, 74–77. [Google Scholar] [CrossRef]
- Korznikova, G.F.; Korznikova, E.A. Production of bulk samples from Ni based melt-spun ribbons by consolidation on Bridgman anvils. Lett. Mater. 2012, 2, 25–28. [Google Scholar] [CrossRef][Green Version]
- Korznikova, G.F.; Czeppe, T.H.; Korznikov, A.V. On plastic deformation of bulk metallic glasses in Bridgman anvils. Lett. Mater. 2014, 4, 117–120. [Google Scholar] [CrossRef]
- Révész, Á.; Kovács, Z. Severe Plastic Deformation of Amorphous Alloys. Mater. Trans. 2019, 60, 1283–1293. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Krasilnikov, N.A.; Tsenev, N.K. Plastic deformation of alloys with submicron-grained structure. Mater. Sci. Eng. A 1991, 137, 35–40. [Google Scholar] [CrossRef]
- Valiev, R. Materials science: Nanomaterial advantage. Nature 2002, 419, 887, 889. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Raab, G.I.; Sharafutdinov, A.V.; Stolyarov, V.V.; Sellers, C. Cold consolidation of nanocrystalline NdFeB powders via a severe plastic deformation method. In Proceedings of the Fifteenth International Workshop on Rare-Earth Magnets and Their Applications, Dresden, Germany, 30 August–3 September 1998; Schultz, L., Mueller, K.H., Eds.; pp. 359–362. [Google Scholar]
- Popov, A.G.; Ermolenko, A.S.; Gaviko, V.S.; Schegoleva, N.N.; Stolyarov, V.V.; Gunderov, D.V. Magnetic Hysteresis Properties and Structural Features of Nanocrystalline Nd9Fe84B7 Alloy Prepared by Melt-spinning and Severe Plastic Deformation. In Proceedings of the Sixteenth International Workshop on Rare-Earth Magnets and Their Applications, Sendai, Japan, 2000; Kaneko, H., Homma, M., Okada, M., Eds.; The Japanese Institute of Metals: Sendai, Japan, 2000; pp. 621–630. [Google Scholar]
- Valiev, R.; Gunderov, D.; Zhilyaev, A.P.; Popov, A.G.; Pushin, V. Nanocrystallization Induced by Severe Plastic Deformation of Amorphous Alloys. J. Metastable Nanocrystalline Mater. 2004, 22, 21–26. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, Y.; Gunderov, D.V.; Zhang, X. Phase evolution, microstructure and magnetic properties of bulk α-Fe/Nd2Fe14B nanocomposite magnets prepared by severe plastic deformation and thermal annealing. J. Alloys Compd. 2015, 651, 434–439. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Gunderov, D.V.; López, G.A.; Baretzky, B. Amorphization of crystalline phases in the Nd–Fe–B alloy driven by the high-pressure torsion. Mater. Lett. 2015, 161, 735–739. [Google Scholar] [CrossRef]
- Teitel’, E.I.; Metlov, L.S.; Gunderov, D.V.; Korznikov, A.V. On the structural and phase transformations in solids induced by severe plastic deformation. Phys. Met. Metallogr. 2012, 113, 1162–1168. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Stolyarov, V.V. Bulk α-Fe/Nd2Fe14B nanocomposite magnets produced by severe plastic deformation combined with thermal annealing. J. Appl. Phys. 2010, 108, 053901. [Google Scholar] [CrossRef]
- Korolev, A.V.; Kourov, N.I.; Pushin, V.G.; Gunderov, D.V.; Boltynjuk, E.V.; Ubyivovk, E.V.; Valiev, R.Z. Paramagnetic susceptibility of the Zr62Cu22Al10Fe5Dy1 metallic glass subjected to high-pressure torsion deformation. J. Magn. Magn. Mater. 2017, 437, 67–71. [Google Scholar] [CrossRef]
- Gunderov, D.; Boltynjuk, E.; Ubyivovk, E.; Lukyanov, A.; Churakova, A.; Zamula, Y.; Batyrshin, E.; Kilmametov, A.; Valiev, R.Z. Atomic Force Microscopy Studies of Severely Deformed Amorphous TiNiCu Alloy. Defect Diffus. Forum 2018, 385, 200–205. [Google Scholar] [CrossRef]
- Zhang, N.; Gunderov, D.; Yang, T.T.; Cai, X.C.; Jia, P.; Shen, T.D. Influence of alloying elements on the thermal stability of ultra-fine-grained Ni alloys. J. Mater. Sci. 2019, 54, 10506–10515. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Popov, A.G.; Schegoleva, N.N.; Stolyarov, V.V.; Yavary, A.R. Phase Transformation in Crystalline and Amorphous Rapidly Quenched Nd-Fe-B Alloys under SPD. In Nanomaterials by Severe Plastic Deformation; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Gemany, 2005; pp. 165–169. [Google Scholar]
- Wang, X.D.; Cao, Q.P.; Jiang, J.Z.; Franz, H.; Schroers, J.; Valiev, R.Z.; Ivanisenko, Y.; Gleiter, H.; Fecht, H.-J. Atomic-level structural modifications induced by severe plastic shear deformation in bulk metallic glasses. Scr. Mater. 2011, 64, 81–84. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Pushin, V.G.; Gunderov, D.V.; Popov, A.G. The use of severe deformations for preparing bulk nanocrystalline materials from amorphous alloys. Dokl. Phys. 2004, 49, 519–521. [Google Scholar] [CrossRef]
- Popov, A.G.; Gaviko, V.S.; Shchegoleva, N.N.; Shreder, L.A.; Gunderov, D.V.; Stolyarov, V.V.; Li, W.; Li, L.L.; Zhang, X.Y. Effect of High-Pressure Torsion Deformation and Subsequent Annealing on Structure and Magnetic Properties of Overquenched Melt-Spun Nd9Fe85B6 Alloy. J. Iron Steel Res. Int. 2006, 13, 160–165. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Nan, Y.; Xu, Z.; Zhang, X.; Popov, A.G.; Gunderov, D.V.; Stolyarov, V.V. Nanocrystallization and magnetic properties of amorphous Nd9Fe85B6 subjected to high-pressure torsion deformation upon annealing. J. Appl. Phys. 2008, 104, 023912. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Guo, D.; Sato, K.; Gunderov, D.V.; Stolyarov, V.V.; Zhang, X. Atomic-scale structural evolution in amorphous Nd9Fe85B6 subjected to severe plastic deformation at room temperature. Appl. Phys. Lett. 2009, 94, 231904. [Google Scholar] [CrossRef]
- Gunderov, D.; Slesarenko, V.; Lukyanov, A.; Churakova, A.; Boltynjuk, E.; Pushin, V.; Ubyivovk, E.; Shelyakov, A.; Valiev, R. Stability of an Amorphous TiCuNi Alloy Subjected to High-Pressure Torsion at Different Temperatures. Adv. Eng. Mater. 2015, 17, 1728–1732. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Slesarenko, V.Y.; Churakova, A.A.; Lukyanov, A.V.; Soshnikova, E.P.; Pushin, V.G.; Valiev, R.Z. Evolution of the amorphous structure in melt-spun Ti50Ni25Cu25 alloy subjected to high pressure torsion deformation. Intermetallics 2015, 66, 77–81. [Google Scholar] [CrossRef]
- Boltynjuk, E.V.; Gunderov, D.V.; Ubyivovk, E.V.; Lukianov, A.V.; Kshumanev, A.M.; Bednarz, A.; Valiev, R.Z. The structural properties of Zr-based bulk metallic glasses subjected to high pressure torsion at different temperatures. AIP Conf. Proc. 2016, 1748, 6. [Google Scholar] [CrossRef]
- Gunderov, D.V. Some regularities in the amorphization and nanocrystallization under severe plastic deformation of crystalline and amorphous multicomponent alloys (In Russian). Investig. Russ. 2006, 151, 1404–1413. [Google Scholar]
- Gunderov, D.; Boltynjuk, E.; Ubyivovk, E.; Churakova, A.; Kilmametov, A.; Valiev, R. Consolidation of the Amorphous Zr 50 Cu 50 Ribbons by High-Pressure Torsion. Adv. Eng. Mater. 2019, 1900694. [Google Scholar] [CrossRef]
- Gunderov, D.; Boltynjuk, E.; Churakova, A.; Batirshin, E.; Mullayanov, A.; Titov, V.; Ivanisenko, J. Effect of high-pressure torsion on the mechanical behavior of a Zr-based BMG. IOP Conf. Ser. Mater. Sci. Eng. 2019, 672, 012028. [Google Scholar] [CrossRef]
- Shao, H.; Xu, Y.; Shi, B.; Yu, C.; Hahn, H.; Gleiter, H.; Li, J. High density of shear bands and enhanced free volume induced in Zr70Cu20Ni10 metallic glass by high-energy ball milling. J. Alloys Compd. 2013, 548, 77–81. [Google Scholar] [CrossRef]
- Argon, A.S.; Kuo, H.Y. Plastic flow in a disordered bubble raft (an analog of a metallic glass). Mater. Sci. Eng. 1979, 39, 101–109. [Google Scholar] [CrossRef]
- Yokoyama, Y. Ductility improvement of Zr–Cu–Ni–Al glassy alloy. J. Non. Cryst. Solids 2003, 316, 104–113. [Google Scholar] [CrossRef]
- Rösner, H.; Peterlechner, M.; Kübel, C.; Schmidt, V.; Wilde, G. Density changes in shear bands of a metallic glass determined by correlative analytical transmission electron microscopy. Ultramicroscopy 2014, 142, 1–9. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Peterlechner, M.; Binkowski, I.; Zadorozhnyy, M.Y.; Golovin, I.S.; Divinski, S.V.; Wilde, G. The impact of elastic and plastic strain on relaxation and crystallization of Pd–Ni–P-based bulk metallic glasses. Acta Mater. 2015, 90, 318–329. [Google Scholar] [CrossRef]
- Jiang, W.H.; Pinkerton, F.E.; Atzmon, M. Deformation-induced nanocrystallization in an Al-based amorphous alloy at a subambient temperature. Scr. Mater. 2003, 48, 1195–1200. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Shiflet, G.J.; Poon, S.J. Deformation-induced nanocrystal formation in shear bands of amorphous alloys. Nature 1994, 367, 541–543. [Google Scholar] [CrossRef]
- Lee, S.-W.; Huh, M.-Y.; Fleury, E.; Lee, J.-C. Crystallization-induced plasticity of Cu–Zr containing bulk amorphous alloys. Acta Mater. 2006, 54, 349–355. [Google Scholar] [CrossRef]
- Kim, J.J.; Choi, Y.; Suresh, S.; Argon, A.S. Nanocrystallization during nanoindentation of a bulk amorphous metal alloy at room temperature. Science 2002, 295, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.G.; Gaviko, V.S.; Shchegoleva, N.N.; Shreder, L.A.; Stolyarov, V.V.; Gunderov, D.V.; Zhang, X.Y.; Li, W.; Li, L.L. High-pressure-torsion deformation of melt-spun Nd9Fe85B6 alloy. Phys. Met. Metallogr. 2007, 104, 238–247. [Google Scholar] [CrossRef]
- Buschow, K.H.J. New permanent magnet materials. Mater. Sci. Reports 1986, 1, 1–63. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Gunderov, D.V.; Valiev, R.Z.; Popov, A.G.; Gaviko, V.S.; Ermolenko, A.S. Metastable states in R2Fe14B-based alloys processed by severe plastic deformation. J. Magn. Magn. Mater. 1999, 196–197, 166–168. [Google Scholar] [CrossRef]
- Gaviko, V.S.; Popov, A.G.; Ermolenko, A.S.; Shchegoleva, N.N.; Stolyarov, V.V.; Gunderov, D.V. Decomposition of the Nd2Fe14B intermetallic compound upon severe plastic deformation by shear under pressure. Phys. Met. Metallogr. 2001, 92, 58–66. [Google Scholar]
- Stolyarov, V.V.; Gunderov, D.V.; Popov, A.G.; Gaviko, V.S.; Ermolenko, A.S. Structure evolution and changes in magnetic properties of severe plastic deformed Nd(Pr)–Fe–B alloys during annealing. J. Alloys Compd. 1998, 281, 69–71. [Google Scholar] [CrossRef]
- Popov, A.G.; Gaviko, V.S.; Shchegoleva, N.N.; Puzanova, T.Z.; Ermolenko, A.S.; Stolyarov, V.V.; Gunderov, D.V.; Raab, G.I.; Valiev, R.Z. Severe plastic deformation of R-Fe-B (R = Pr or Nd) hard magnetic alloys. Phys. Met. Metallogr. 2002, 94, S75–S81. [Google Scholar]
- Popov, A.G.; Gaviko, V.S.; Shchegoleva, N.N.; Shreder, L.A.; Gunderov, D.V.; Stolyarov, V.V.; Li, W.; Li, L.L.; Zhang, X.Y. Effect of high-pressure torsion deformation and subsequent annealing on structure and magnetic properties of overquenched melt-spun Nd9Fe85B6 alloy. In Proceedings of the 19th International Workshop on Rare Earth Permanent Magnets and Their Applications, Beijing, China, 30 August–2 September 2006. [Google Scholar]
- Glezer, A.M.; Plotnikova, M.P.; Shalimova, A.V.; Dobatkin, S.V. Severe plastic deformation of amorphous alloys: I. Structure and mechanical properties. Bull. Russ. Acad. Sci. Phys. 2009, 73, 1233–1239. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A. On decomposition of amorphous phase in metallic glasses. Rev. Adv. Mater. Sci. 2017, 50, 55–61. [Google Scholar]
- Gapontsev, V.L.; Kesarev, A.G.; Kondrat’ev, V.V.; Ermakov, A.E. Phase separation in nanocrystalline alloys upon generation of nonequilibrium vacancies at grain boundaries. Phys. Met. Metallogr. 2000, 89, 430–434. [Google Scholar]
- Handrich, K.; Kobe, S. Amorphe Ferro- und Ferrimagnetika; Physik-Verlag: Weinheim, Germany, 1980. [Google Scholar]
- Gryaznov, V.G.; Kaprelov, A.M.; Romanov, A.E. Size effect of dislocation stability in small particles and microcrystallites. Scr. Metall. 1989, 23, 1443–1448. [Google Scholar] [CrossRef]
- Yavari, A.R.; Le Moulec, A.; Inoue, A.; Nishiyama, N.; Lupu, N.; Matsubara, E.; Botta, W.J.; Vaughan, G.; Di Michiel, M.; Kvick, Å. Excess free volume in metallic glasses measured by X-ray diffraction. Acta Mater. 2005, 53, 1611–1619. [Google Scholar] [CrossRef]
- Gleiter, H. Nanoglasses: A new kind of noncrystalline materials. Beilstein J. Nanotechnol. 2013, 4, 517–533. [Google Scholar] [CrossRef]
- Gleiter, H.; Schimmel, T.; Hahn, H. Nanostructured solids–From nano-glasses to quantum transistors. Nano Today 2014, 9, 17–68. [Google Scholar] [CrossRef]
- Joo, S.-H.; Pi, D.-H.; Setyawan, A.D.H.; Kato, H.; Janecek, M.; Kim, Y.C.; Lee, S.; Kim, H.S. Work-Hardening Induced Tensile Ductility of Bulk Metallic Glasses via High-Pressure Torsion. Sci. Rep. 2015, 5, 9660. [Google Scholar] [CrossRef]
- Jing, J.; Krämer, A.; Birringer, R.; Gleiter, H.; Gonser, U. Modified atomic structure in a PdFeSi nanoglass. J. Non. Cryst. Solids 1989, 113, 167–170. [Google Scholar] [CrossRef]
- Gleiter, H. Our thoughts are ours, their ends none of our own: Are there ways to synthesize materials beyond the limitations of today? Acta Mater. 2008, 56, 5875–5893. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline solids. J. Appl. Crystallogr. 1991, 24, 79–90. [Google Scholar] [CrossRef]
- Weissmüller, J.; Schubert, P.; Franz, H.; Birringer, R.; Gleiter, H. The physics of non-crystalline solids. In Proceedings of the VII National Conference on the Physics of Non-Crystalline Solids, Cambridge, UK, 4–9 August 1991. [Google Scholar]
- Fang, J.X.; Vainio, U.; Puff, W.; Würschum, R.; Wang, X.L.; Wang, D.; Ghafari, M.; Jiang, F.; Sun, J.; Hahn, H.; et al. Atomic Structure and Structural Stability of Sc 75 Fe 25 Nanoglasses. Nano Lett. 2012, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Śniadecki, Z.; Wang, D.; Ivanisenko, Y.; Chakravadhanula, V.S.K.; Kübel, C.; Hahn, H.; Gleiter, H. Nanoscale morphology of Ni50Ti45Cu5 nanoglass. Mater. Charact. 2016, 113, 26–33. [Google Scholar] [CrossRef]
- Cao, Q.P.; Li, J.F.; Zhou, Y.H.; Horsewell, A.; Jiang, J.Z. Effect of rolling deformation on the microstructure of bulk Cu60Zr20Ti20 metallic glass and its crystallization. Acta Mater. 2006, 54, 4373–4383. [Google Scholar] [CrossRef]
- Aronin, A.; Matveev, D.; Pershina, E.; Tkatch, V.; Abrosimova, G. The effect of changes in Al-based amorphous phase structure on structure forming upon crystallization. J. Alloys Compd. 2017, 715, 176–183. [Google Scholar] [CrossRef]
- Ubyivovk, E.V.; Boltynjuk, E.V.; Gunderov, D.V.; Churakova, A.A.; Kilmametov, A.R.; Valiev, R.Z. HPT-induced shear banding and nanoclustering in a TiNiCu amorphous alloy. Mater. Lett. 2017, 209, 327–329. [Google Scholar] [CrossRef]
- Boltynjuk, E.V.; Gunderov, D.V.; Ubyivovk, E.V.; Monclús, M.A.; Yang, L.W.; Molina-Aldareguia, J.M.; Tyurin, A.I.; Kilmametov, A.R.; Churakova, A.A.; Churyumov, A.Y.; et al. Enhanced strain rate sensitivity of Zr-based bulk metallic glasses subjected to high pressure torsion. J. Alloys Compd. 2018, 747, 595–602. [Google Scholar] [CrossRef]
- Churyumov, A.Y.; Bazlov, A.I.; Zadorozhnyy, V.Y.; Solonin, A.N.; Caron, A.; Louzguine-Luzgin, D.V. Phase transformations in Zr-based bulk metallic glass cyclically loaded before plastic yielding. Mater. Sci. Eng. A 2012, 550, 358–362. [Google Scholar] [CrossRef]
- Kilmametov, A.; Gröger, R.; Hahn, H.; Schimmel, T.; Walheim, S. Bulk Density Measurements of Small Solid Objects Using Laser Confocal Microscopy. Adv. Mater. Technol. 2017, 2, 1600115. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Boltynjuk, E.V.; Sitdikov, V.D.; Abrosimova, G.E.; Churakova, A.A.; Kilmametov, A.R.; Valiev, R.Z. Free volume measurement of severely deformed Zr 62 Cu 22 Al 10 Fe 5 Dy 1 bulk metallic glass. J. Phys. Conf. Ser. 2018, 1134, 012010. [Google Scholar] [CrossRef]
- Jiang, W.H.; Atzmon, M. Rate dependence of serrated flow in a metallic glass. J. Mater. Res. 2003, 18, 755–757. [Google Scholar] [CrossRef]
- Glezer, A.M.; Louzguine-Luzgin, D.V.; Khriplivets, I.A.; Sundeev, R.V.; Gunderov, D.V.; Bazlov, A.I.; Pogozhev, Y.S. Effect of high-pressure torsion on the tendency to plastic flow in bulk amorphous alloys based on Zr. Mater. Lett. 2019, 256, 126631. [Google Scholar] [CrossRef]
- Glezer, A.M.; Potekaev, A.I.; Cheretaeva, A.O. Thermal and Time Stability of Amorphous Alloys; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315158112. [Google Scholar]
- Gunderov, D.V.; Churakova, A.A.; Lukyanov, A.V.; Prokofiev, E.A.; Khasanova, D.A.; Zamanova, G.I. Thin microstructure of amorphous Ti-Ni-Cu alloy subjected to high pressure torsion. Bull. Bashkir Univ. 2015, 20, 406–407. [Google Scholar]
- Bhowmick, R.; Raghavan, R.; Chattopadhyay, K.; Ramamurty, U. Plastic flow softening in a bulk metallic glass. Acta Mater. 2006, 54, 4221–4228. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, D.H.; Zhang, L.C.; Zhang, Z.B.; He, L.; Sun, J.; Zhang, Z.F. Microstructure evolution and mechanical properties of Cu46Zr47Al7 bulk metallic glass composite containing CuZr crystallizing phases. Mater. Sci. Eng. A 2007, 467, 139–145. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, J.; Sun, J.; Zhang, Z. Enhanced strength and plasticity of a Ti-based metallic glass at cryogenic temperatures. Mater. Sci. Eng. A 2008, 498, 203–207. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Churakova, A.A.; Boltynjuk, E.V.; Ubyivovk, E.V.; Astanin, V.V.; Asfandiyarov, R.N.; Valiev, R.Z.; Xioang, W.; Wang, J.T. Observation of shear bands in the Vitreloy metallic glass subjected to HPT processing. J. Alloys Compd. 2019, 800, 58–63. [Google Scholar] [CrossRef]
- Kovács, Z.; Schafler, E.; Szommer, P.; Révész, Á. Localization of plastic deformation along shear bands in Vitreloy bulk metallic glass during high pressure torsion. J. Alloys Compd. 2014, 593, 207–212. [Google Scholar] [CrossRef]
- Dmowski, W.; Yokoyama, Y.; Chuang, A.; Ren, Y.; Umemoto, M.; Tsuchiya, K.; Inoue, A.; Egami, T. Structural rejuvenation in a bulk metallic glass induced by severe plastic deformation. Acta Mater. 2010, 58, 429–438. [Google Scholar] [CrossRef]
- Adachi, N.; Todaka, Y.; Yokoyama, Y.; Umemoto, M. Cause of hardening and softening in the bulk glassy alloy Zr50Cu40Al10 after high-pressure torsion. Mater. Sci. Eng. A 2015, 627, 171–181. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Pereira, P.H.R.; Aguilar, M.T.P.; Cetlin, P.R.; Langdon, T.G. Using finite element modeling to examine the temperature distribution in quasi-constrained high-pressure torsion. Acta Mater. 2012, 60, 3190–3198. [Google Scholar] [CrossRef]
- Edalati, K.; Miresmaeili, R.; Horita, Z.; Kanayama, H.; Pippan, R. Significance of temperature increase in processing by high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 7301–7305. [Google Scholar] [CrossRef]
- Edalati, K.; Hashiguchi, Y.; Pereira, P.H.R.; Horita, Z.; Langdon, T.G. Effect of temperature rise on microstructural evolution during high-pressure torsion. Mater. Sci. Eng. A 2018, 714, 167–171. [Google Scholar] [CrossRef]
- Yamaguchi, D.; Horita, Z.; Nemoto, M.; Langdon, T.G. Significance of adiabatic heating in equal-channel angular pressing. Scr. Mater. 1999, 41, 791–796. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; García-Infanta, J.M.; Carreño, F.; Langdon, T.G.; Ruano, O.A. Particle and grain growth in an Al-Si alloy during high-pressure torsion. Scr. Mater. 2007, 57, 763–765. [Google Scholar] [CrossRef]
- Todaka, Y.; Umemoto, M.; Yamazaki, A.; Sasaki, J.; Tsuchiya, K. Influence of High-Pressure Torsion Straining Conditions on Microstructure Evolution in Commercial Purity Aluminum. Mater. Trans. 2008, 49, 7–14. [Google Scholar] [CrossRef]
- Zhilyaev, A. Energy Stored during High Pressure Torsion of Pure Metals. Lett. Mater. 2019, 9, 142–146. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Greer, A.L. Temperature rise at shear bands in metallic glasses. Nat. Mater. 2006, 5, 15–18. [Google Scholar] [CrossRef]
- Hóbor, S.; Kovács, Z.; Révész, Á. Macroscopic thermoplastic model applied to the high pressure torsion of metallic glasses. J. Appl. Phys. 2009, 106, 023531. [Google Scholar] [CrossRef]
- Slipenyuk, A.; Eckert, J. Correlation between enthalpy change and free volume reduction during structural relaxation of Zr55Cu30Al10Ni5 metallic glass. Scr. Mater. 2004, 50, 39–44. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, Y.; Mathaudhu, S.N.; Valiev, R.Z.; Tsao, C.Y.A.; Schoenung, J.M.; Lavernia, E.J. Multiple and extended shear band formation in MgCuGd metallic glass during high-pressure torsion. Scr. Mater. 2014, 86, 24–27. [Google Scholar] [CrossRef]
- Boltynjuk, E.; Ubyivovk, E.; Gunderov, D.; Mikhalovskii, V.; Valiev, R.Z. Multiple Shear Bands in Zr-Based Bulk Metallic Glass Processed by Severe Plastic Deformation. Defect Diffus. Forum 2018, 385, 319–324. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Boltynjuk, E.V.; Ubyivovk, E.V.; Churakova, A.A.; Abrosimova, G.E.; Sitdikov, V.D.; Kilmametov, A.R.; Valiev, R.Z. High pressure torsion induced structural transformations in Ti- and Zr-based amorphous alloys. IOP Conf. Ser. Mater. Sci. Eng. 2018, 447, 012052. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. R Rep. 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Pan, J.; Chen, Q.; Liu, L.; Li, Y. Softening and dilatation in a single shear band. Acta Mater. 2011, 59, 5146–5158. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Churakova, A.A.; Astanin, V.V.; Asfandiyarov, R.N.; Hahn, H.; Valiev, R.Z. Accumulative HPT of Zr-based bulk metallic glasses. Mater. Lett. 2020, 261, 127000. [Google Scholar] [CrossRef]
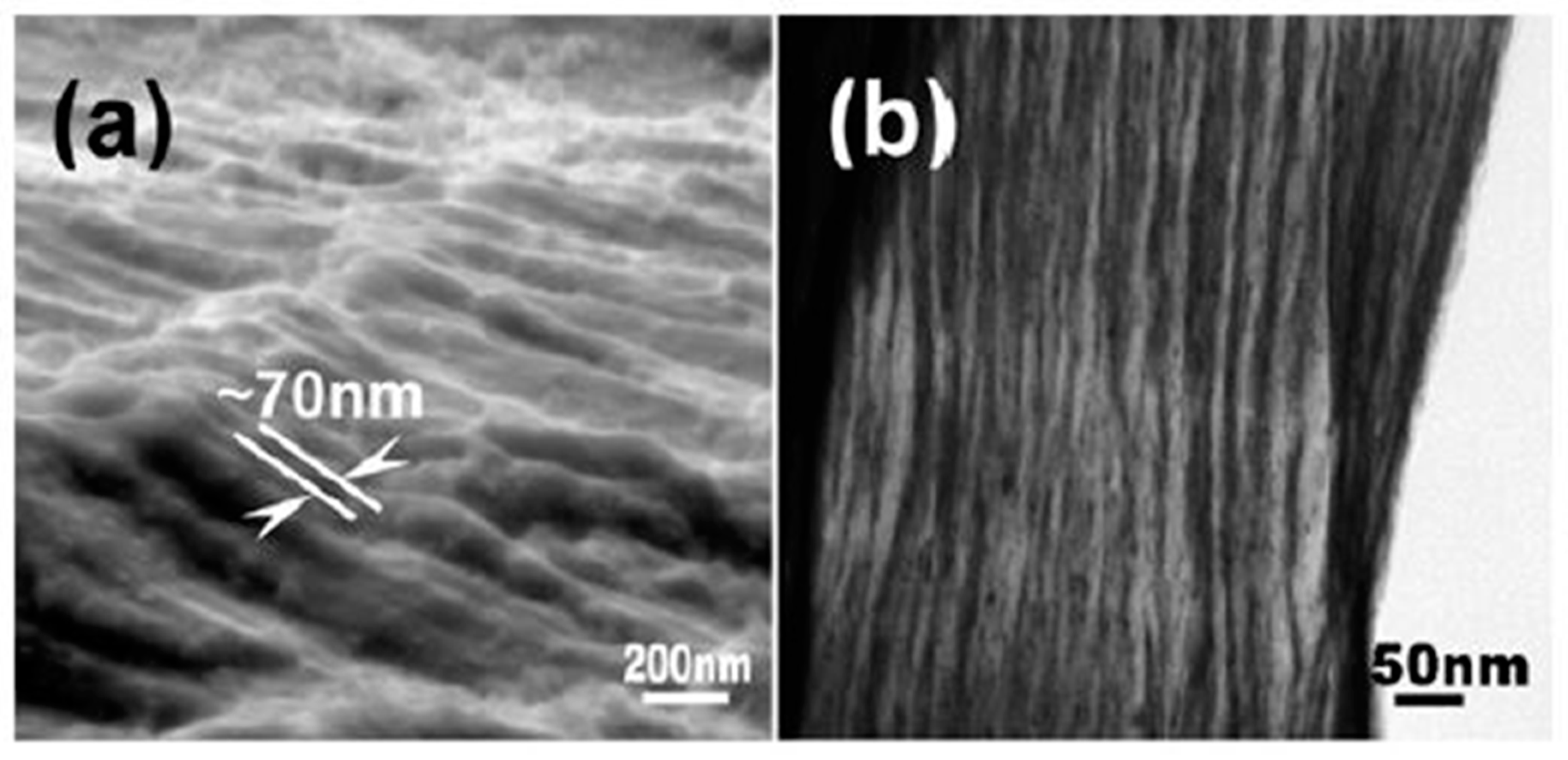
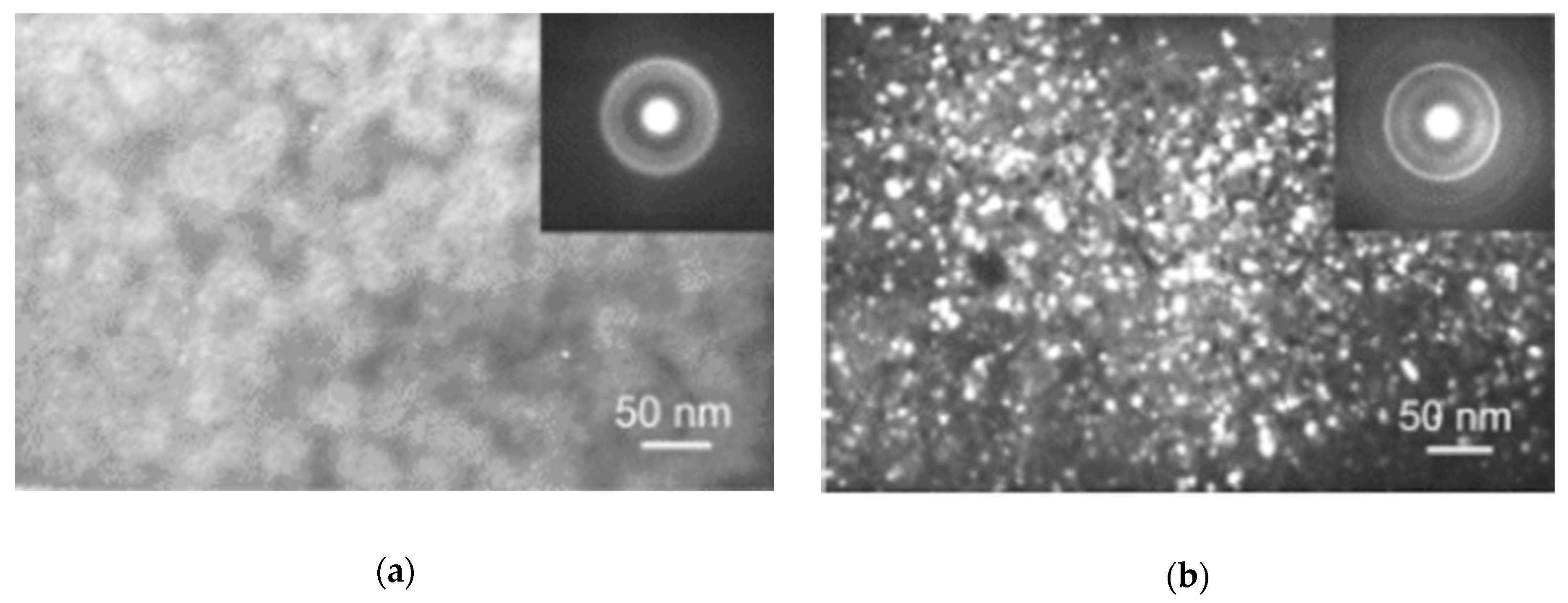
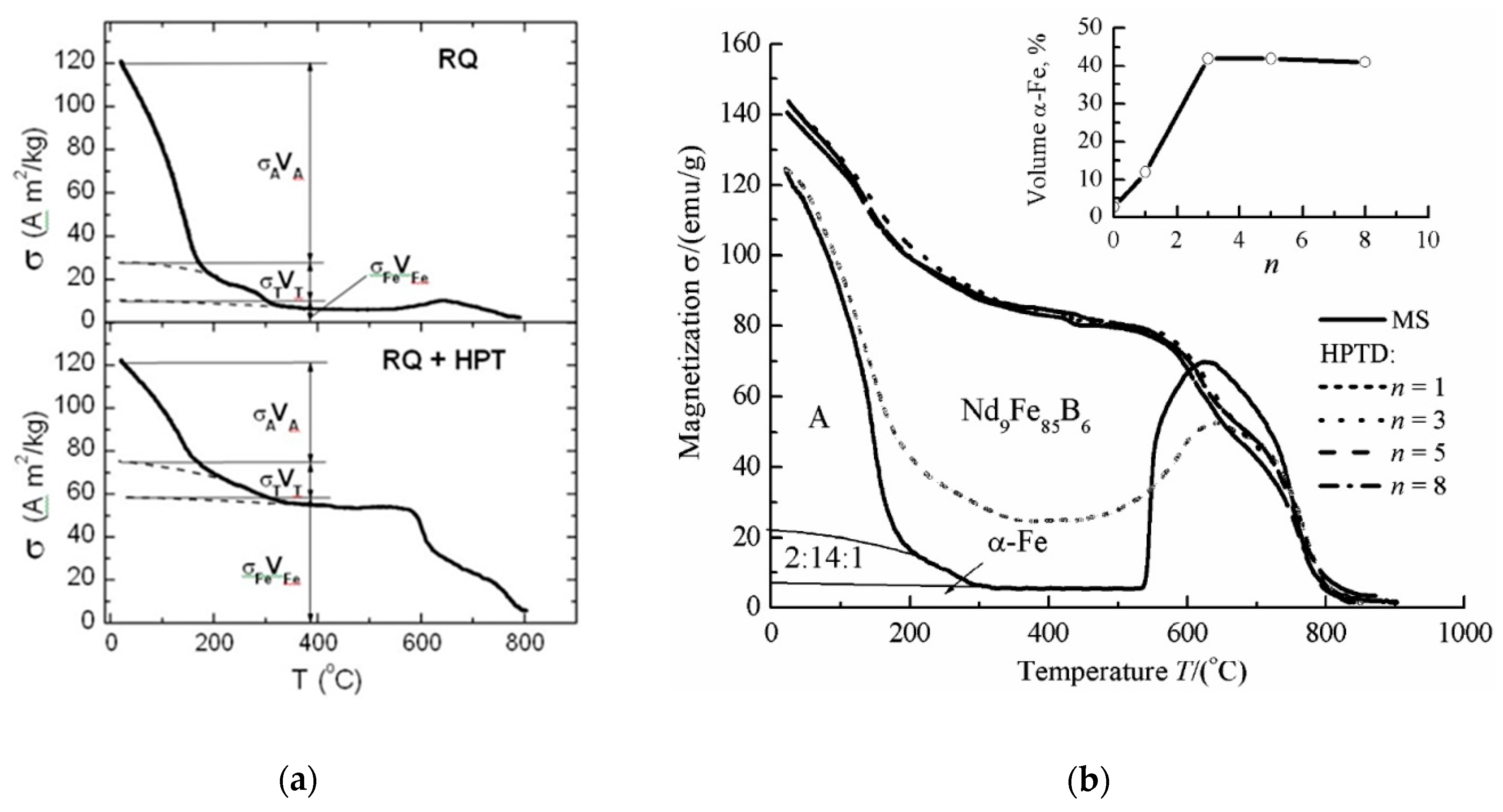
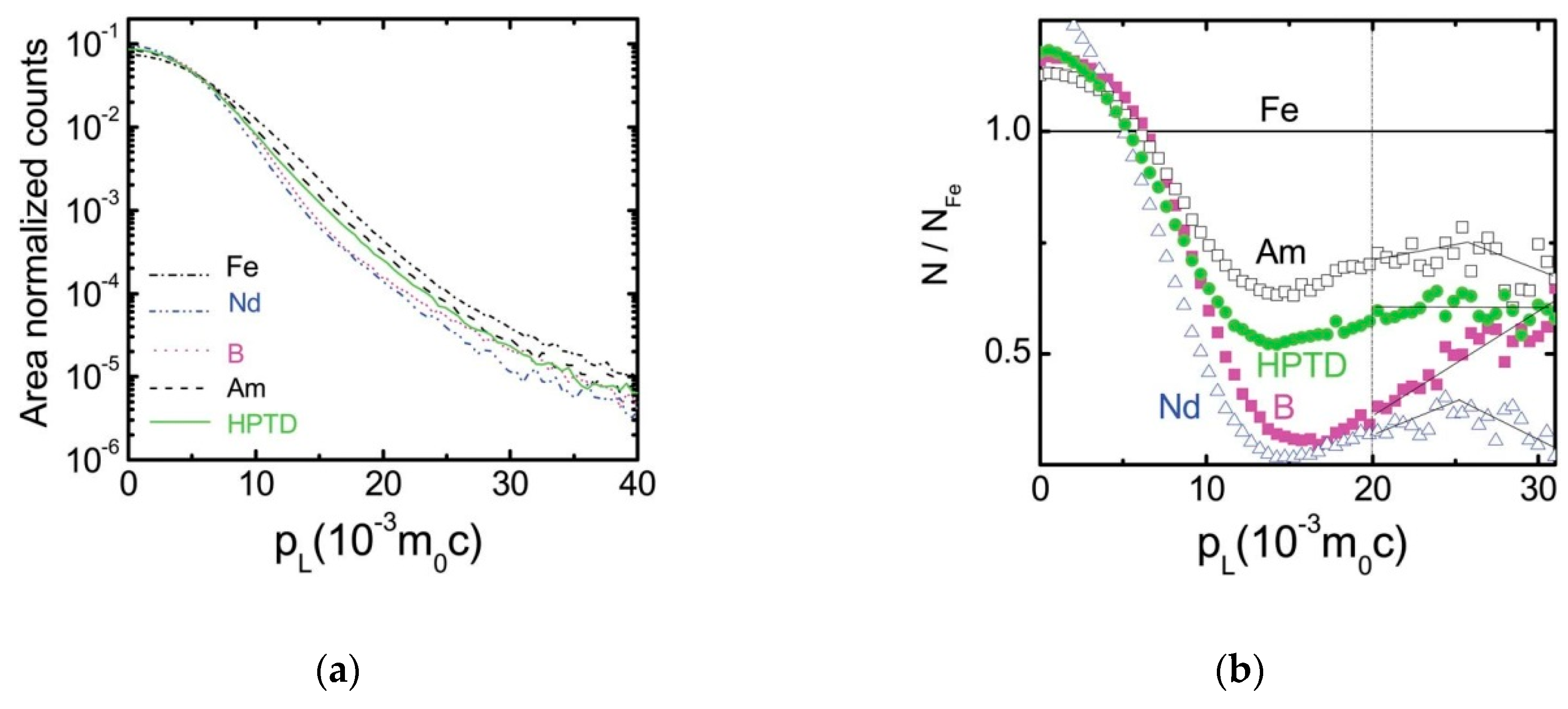
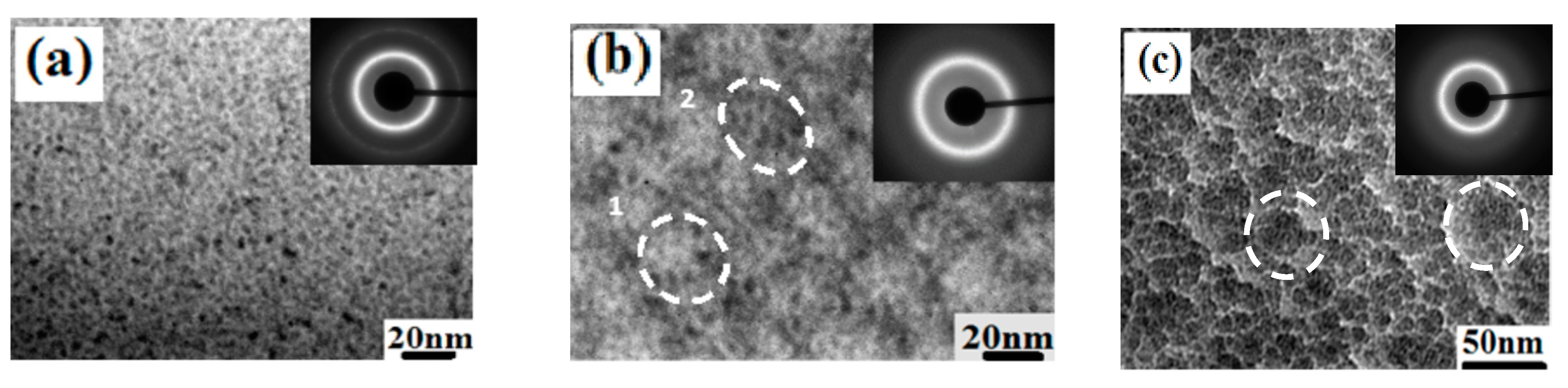

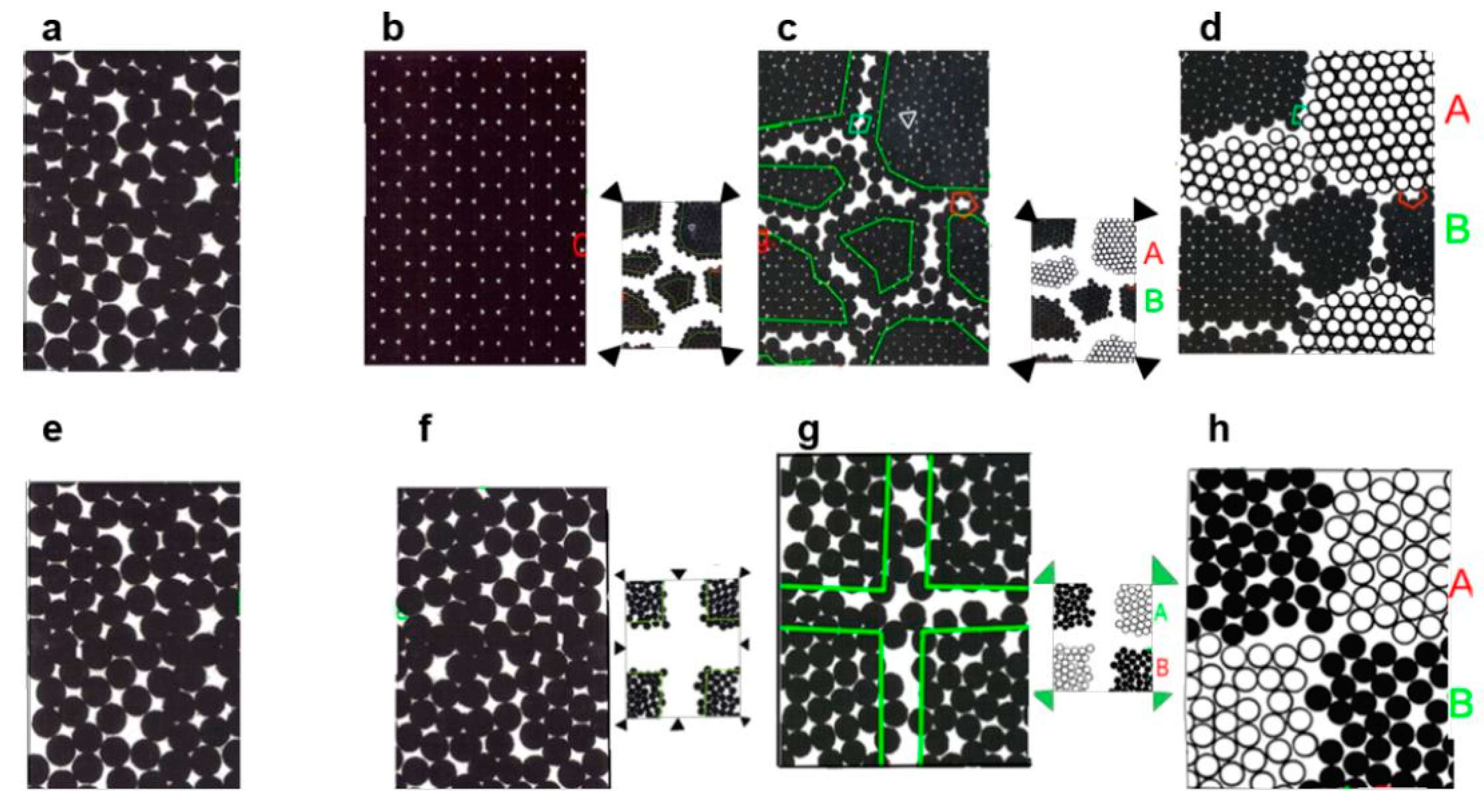
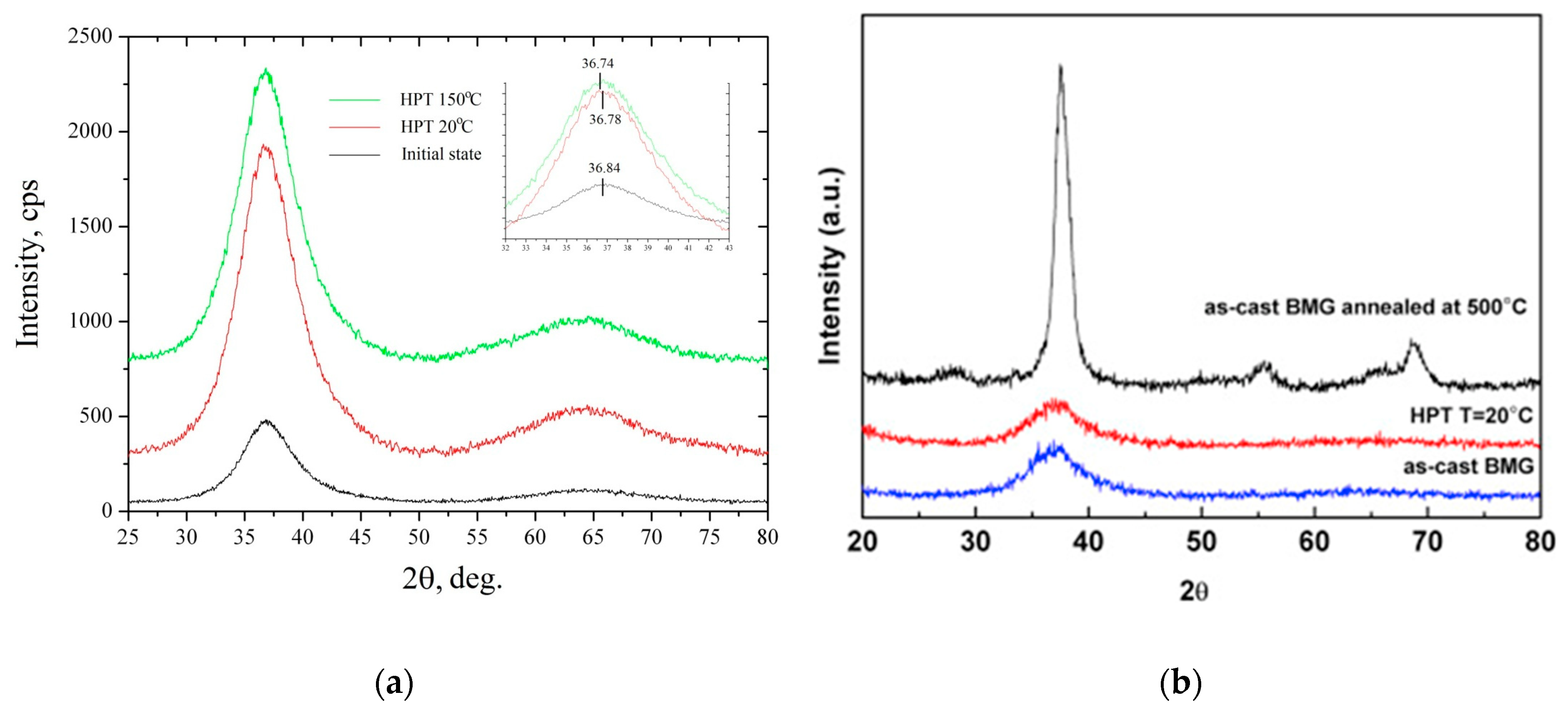
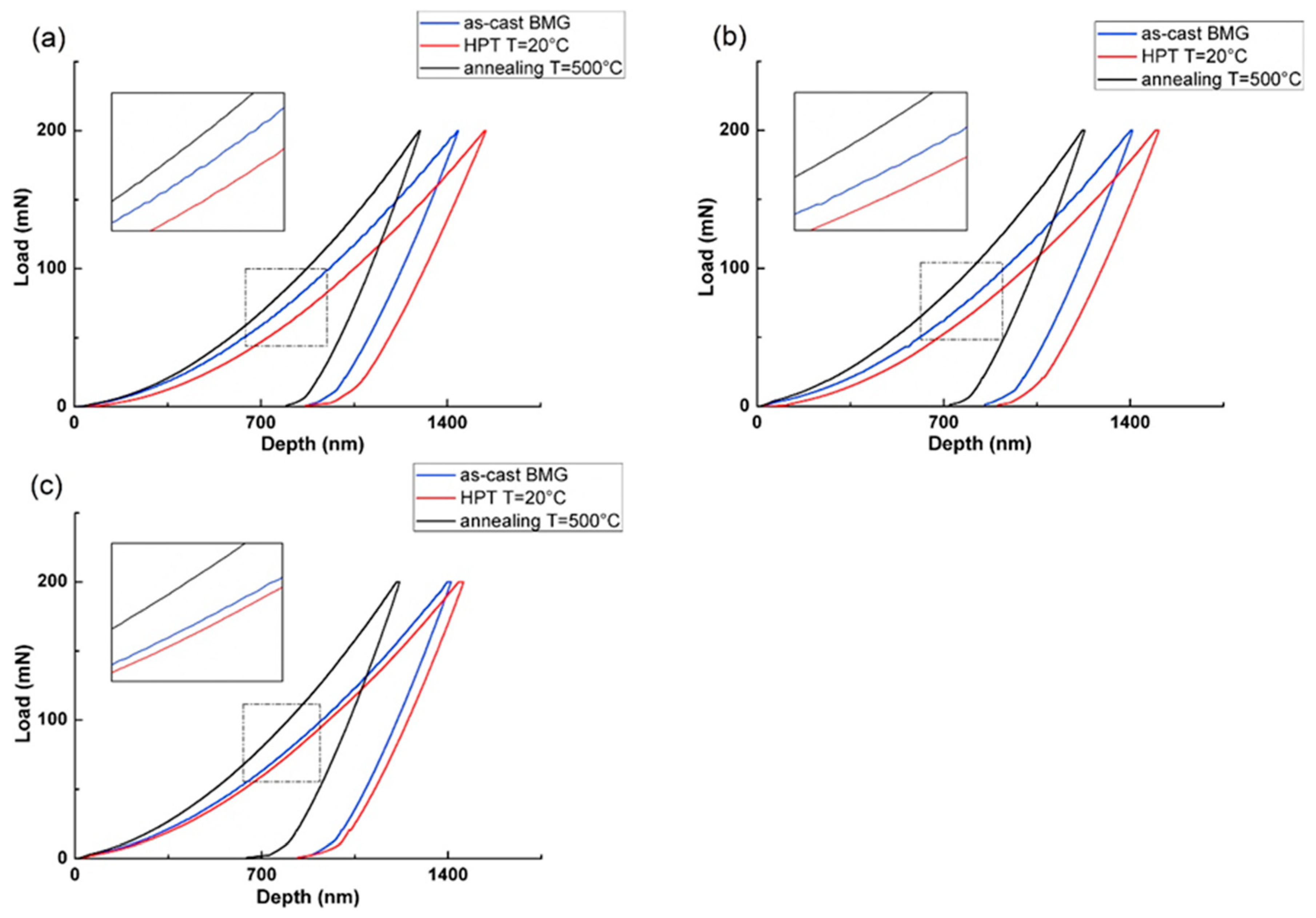
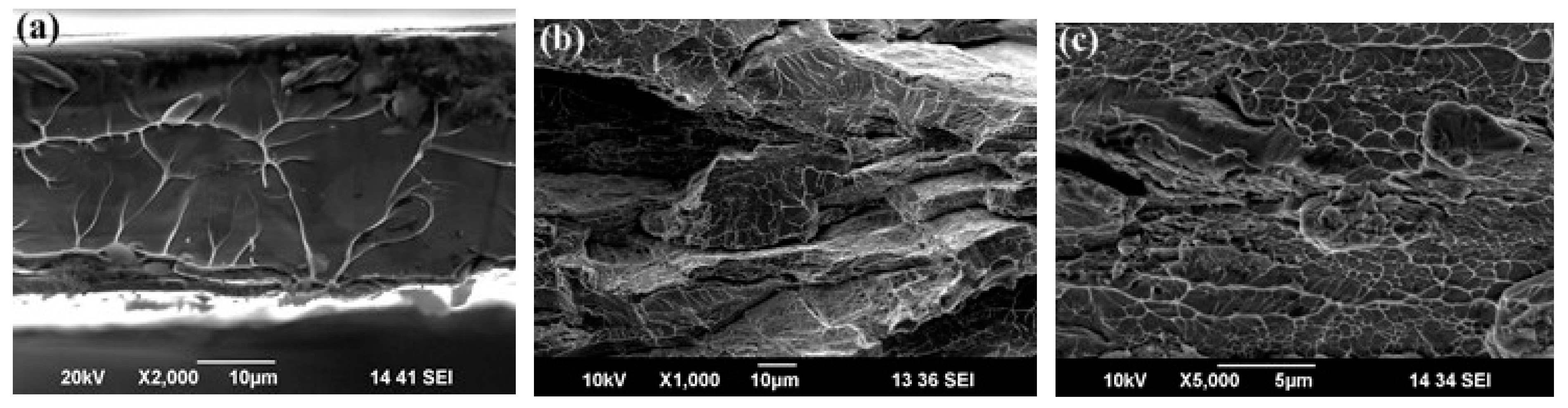
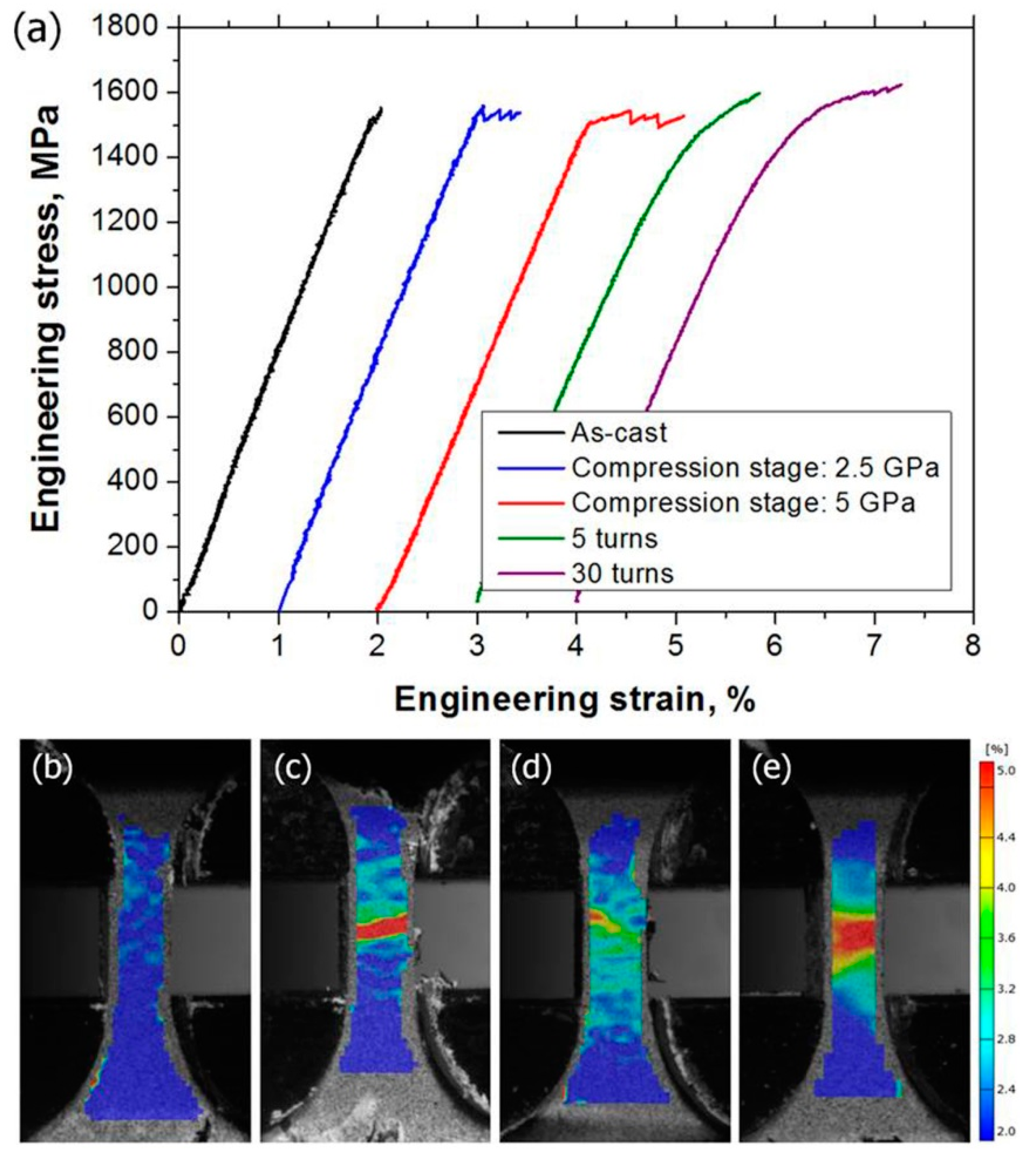
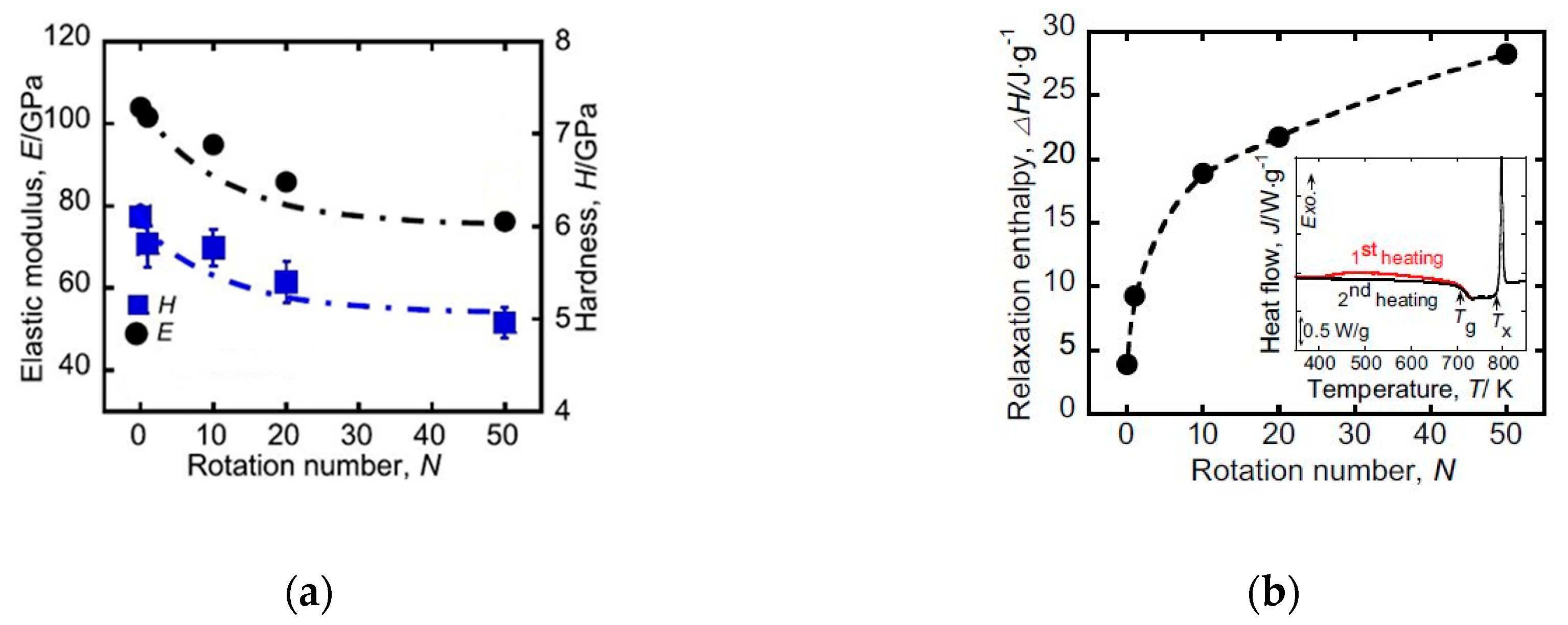
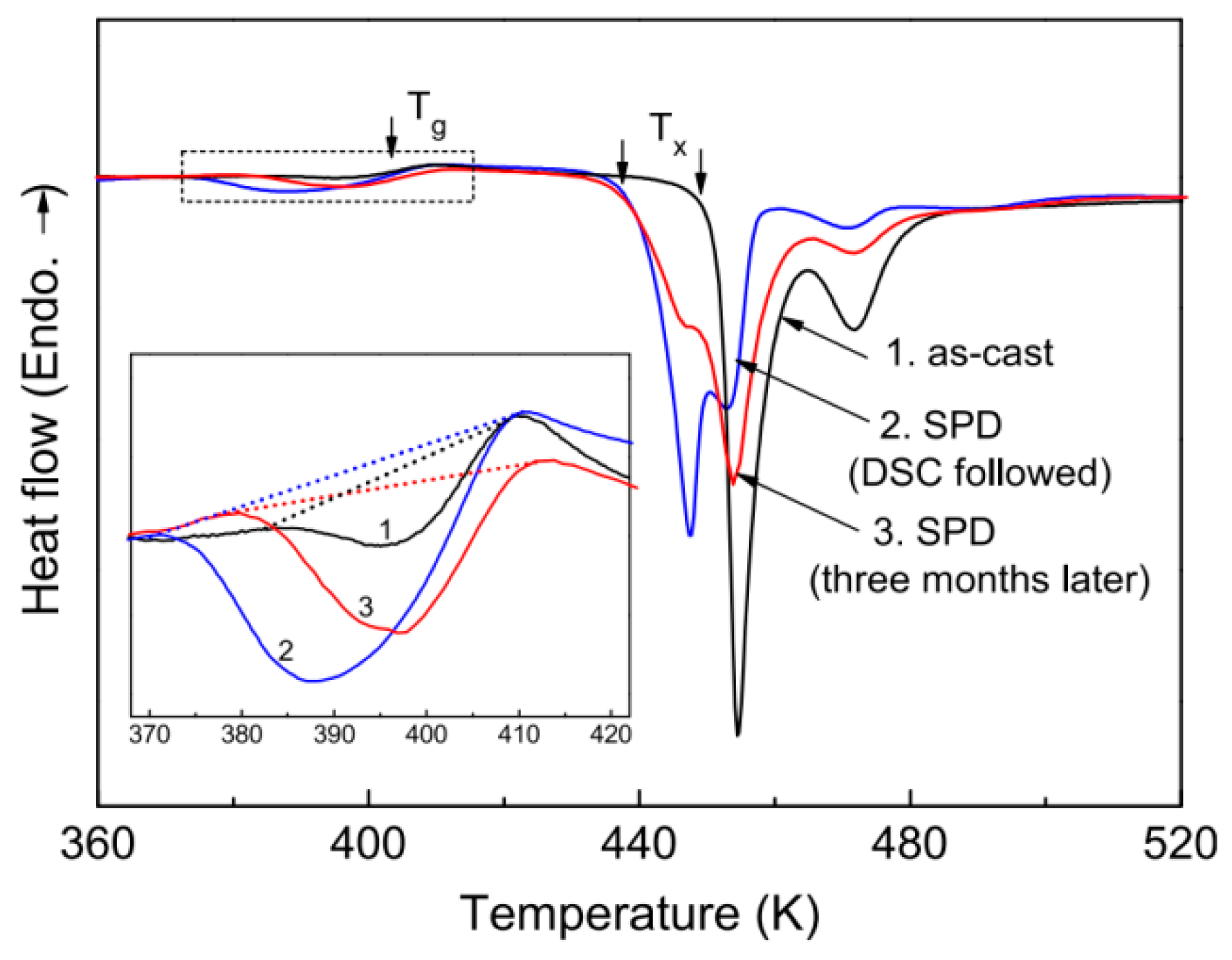
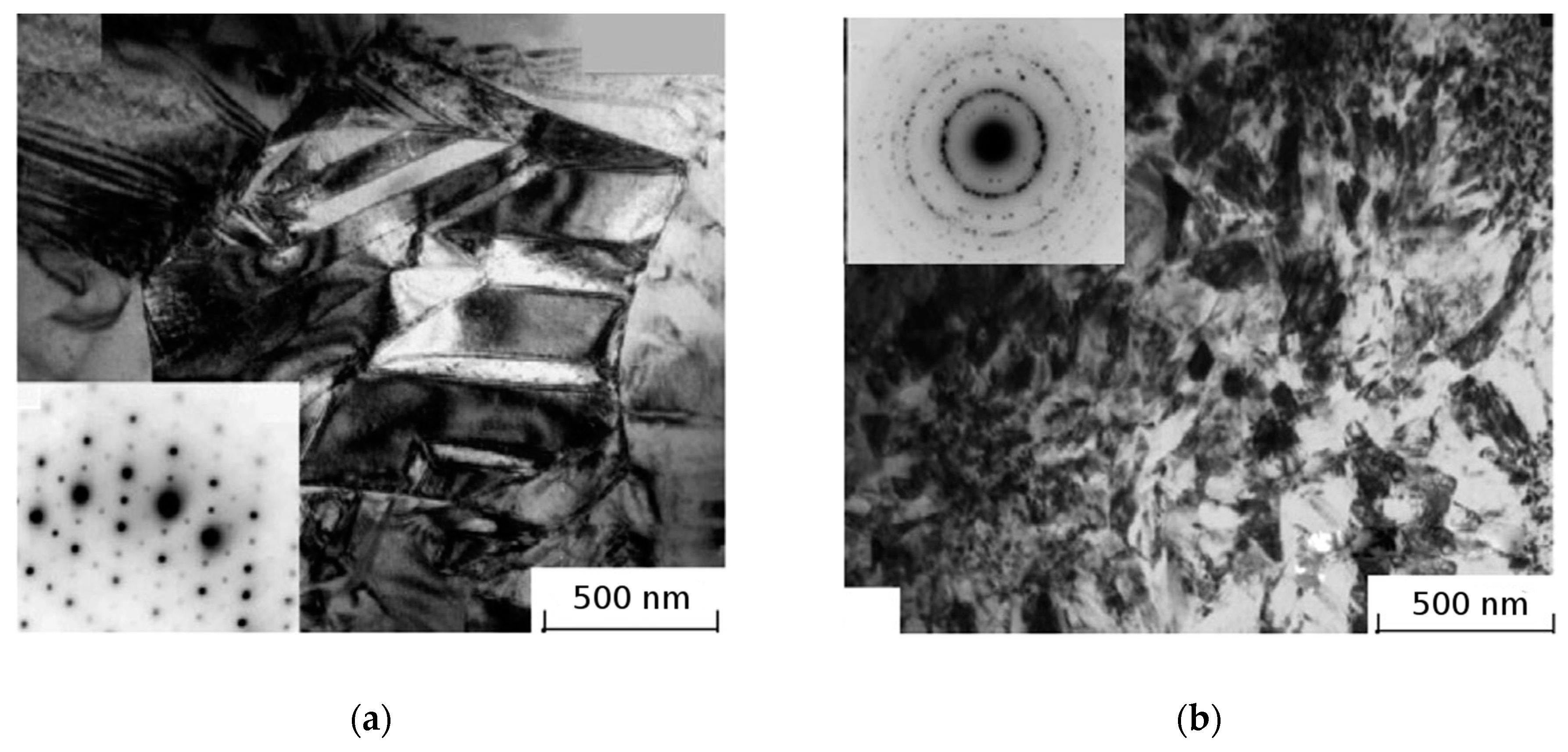

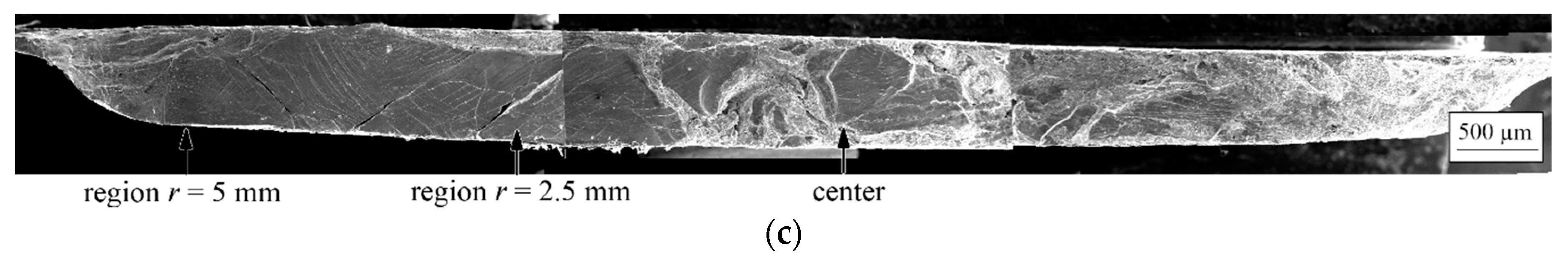

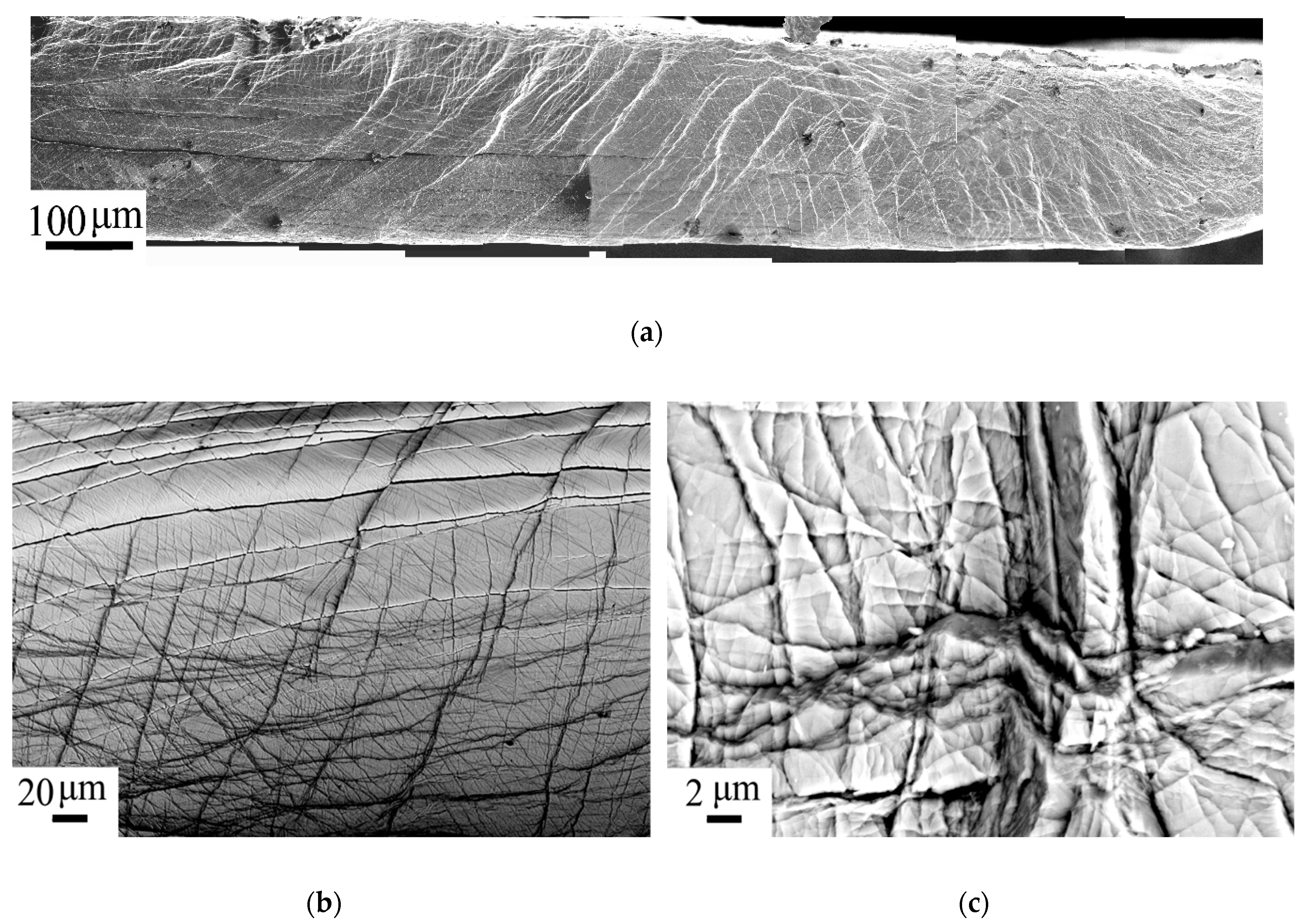
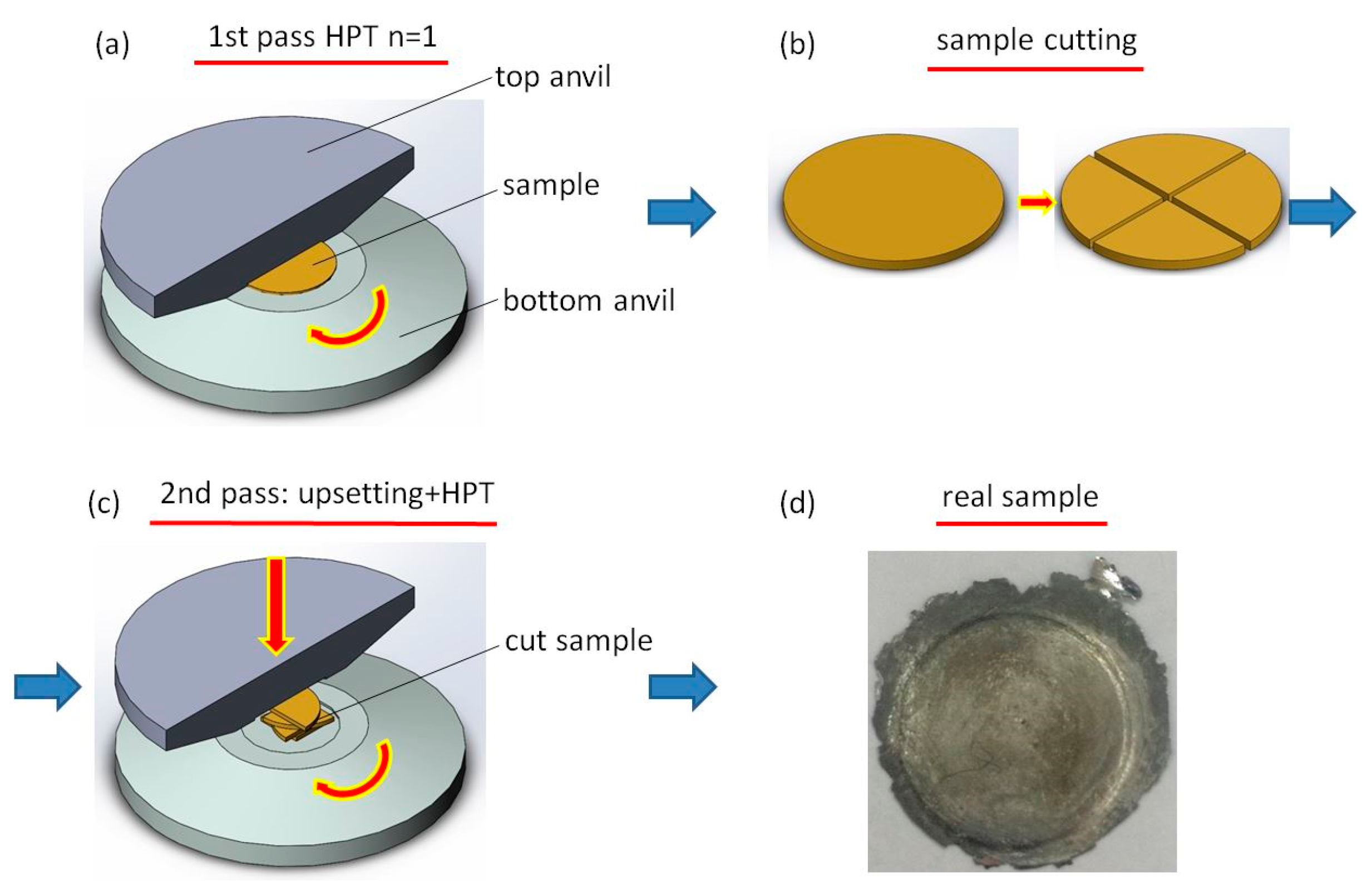
| State | Amorphous Phase, vol. % | Nd2Fe14B, vol. % | α-Fe, vol. % |
|---|---|---|---|
| Nd12Fe82B6 alloy | |||
| MS | 83 | 14 | 3 |
| MS + HPT n = 5 | 65 | 10 | 25 |
| Nd9Fe85B6 alloy | |||
| MS | 83 | 15 | 2 |
| MS + HPT n = 1 | 74 | 14 | 12 |
| MS + HPT n = 3 | 45 | 13 | 42 |
| MS + HPT n = 5 | 45 | 13 | 42 |
| MS + HPT n = 8 | 47 | 12 | 41 |
| State | 2θ, deg | R1, Å | FWHM, deg | ΔVXRD, % | ρ, g/cm3 | Δρ, % |
|---|---|---|---|---|---|---|
| Initial BMG | 36.84(3) | 2.999 | 5.50 | - | 6.98 | - |
| HPT at 20 °C | 36.780(16) | 3.003 | 6.106 | 0.44 | 6.83 | 2.14 |
| HPT at 150 °C | 36.743(15) | 3.006 | 6.309 | 0.74 | 6.90 | 1.07 |
| Sample | Crystallization Peak, °C | Crystallization Start, °C | Crystallization Finish, °C | Relaxation Energy, J/g |
|---|---|---|---|---|
| as-spun | 460 | 444 | 463 | 41 |
| HPT20 | 432 | 401 | 450 | 37 |
| HPT50 | 437 | 408 | 453 | 37 |
| HPT100 | 437 | 414 | 455 | 35 |
| HPT150 | 441 | 421 | 455 | 24 |
| State | Hc (kA/m) | σr (Am2/kg) |
|---|---|---|
| MS + annealing | 336 | 72 |
| MS + HPT + annealing | 528 | 86.5 |
| BMG state | 2θ (deg) | R1 (ang.) | FWHM (deg) | ΔV |
|---|---|---|---|---|
| Initial | 37.57(4) | 2.942 | 6.34(4) | - |
| conventional HPT n = 5 | 37.42(4) | 2.953 | 6.69(4) | 1.1 |
| accumulative HPT | 37.22(4) | 2.969 | 7.25(4) | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunderov, D.; Astanin, V. Influence of HPT Deformation on the Structure and Properties of Amorphous Alloys. Metals 2020, 10, 415. https://doi.org/10.3390/met10030415
Gunderov D, Astanin V. Influence of HPT Deformation on the Structure and Properties of Amorphous Alloys. Metals. 2020; 10(3):415. https://doi.org/10.3390/met10030415
Chicago/Turabian StyleGunderov, Dmitry, and Vasily Astanin. 2020. "Influence of HPT Deformation on the Structure and Properties of Amorphous Alloys" Metals 10, no. 3: 415. https://doi.org/10.3390/met10030415
APA StyleGunderov, D., & Astanin, V. (2020). Influence of HPT Deformation on the Structure and Properties of Amorphous Alloys. Metals, 10(3), 415. https://doi.org/10.3390/met10030415





