Precipitate Behavior, Mechanical Properties and Corrosion Behavior of an Al-Zn-Mg-Cu Alloy during Non-Isothermal Creep Aging with Axial Tension Stress
Abstract
1. Introduction
2. Materials and Methods
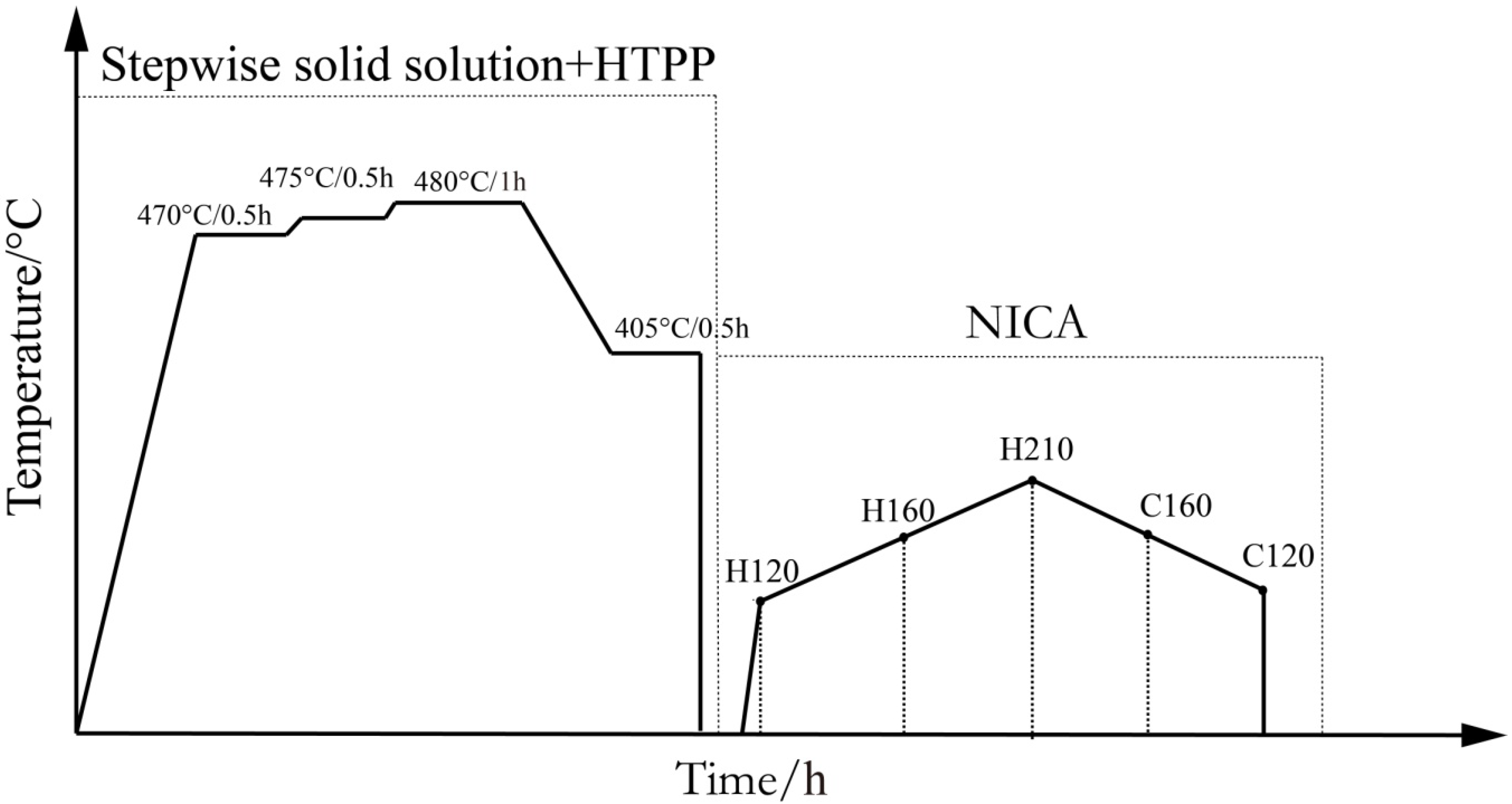
2.1. Materials and Aging Treatment
2.2. Mechanical Properties
2.3. Microstructure Characterization
2.4. Corrosion Test
3. Resultsand Discussion
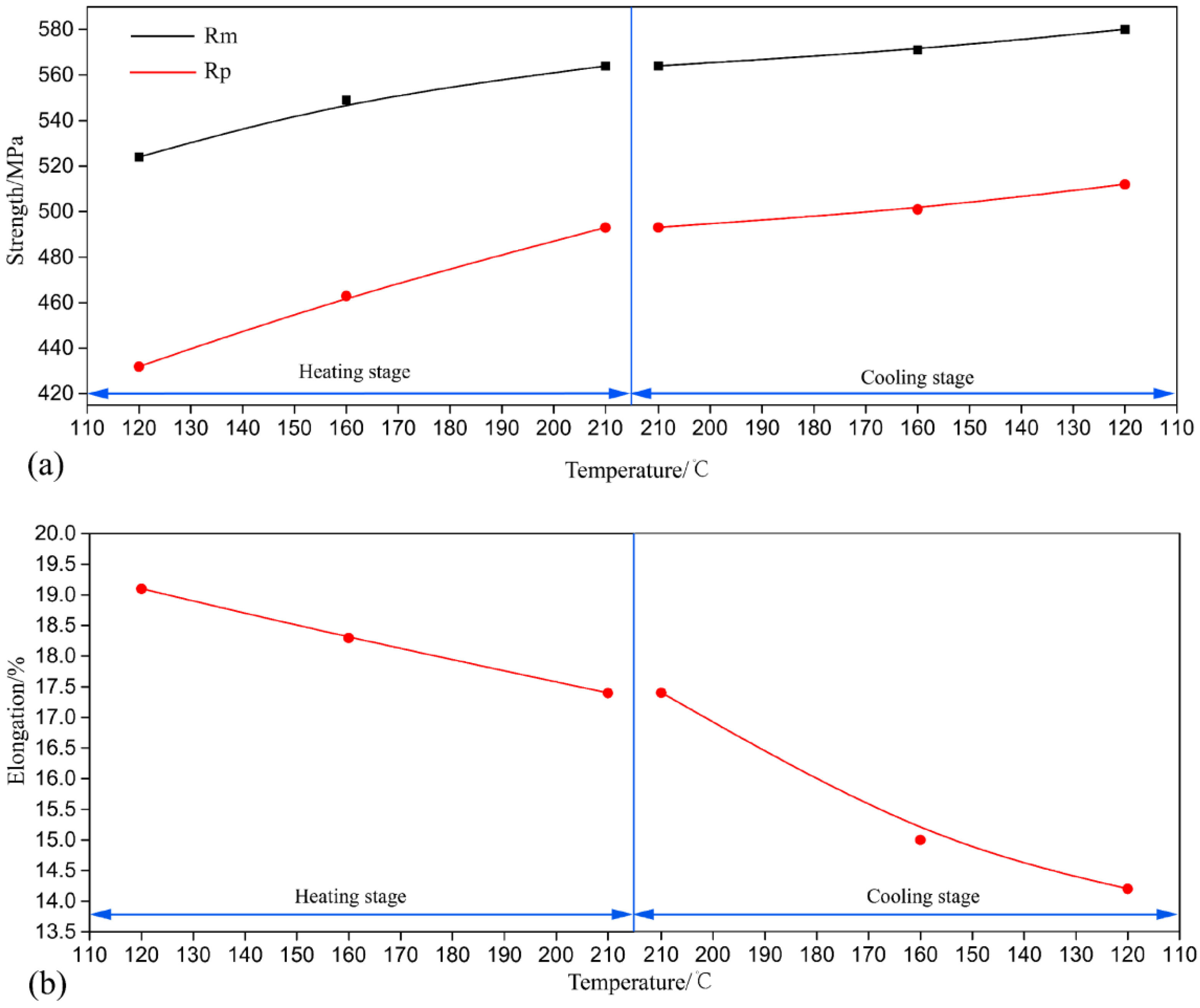
3.1. Hardness and Tensile Test
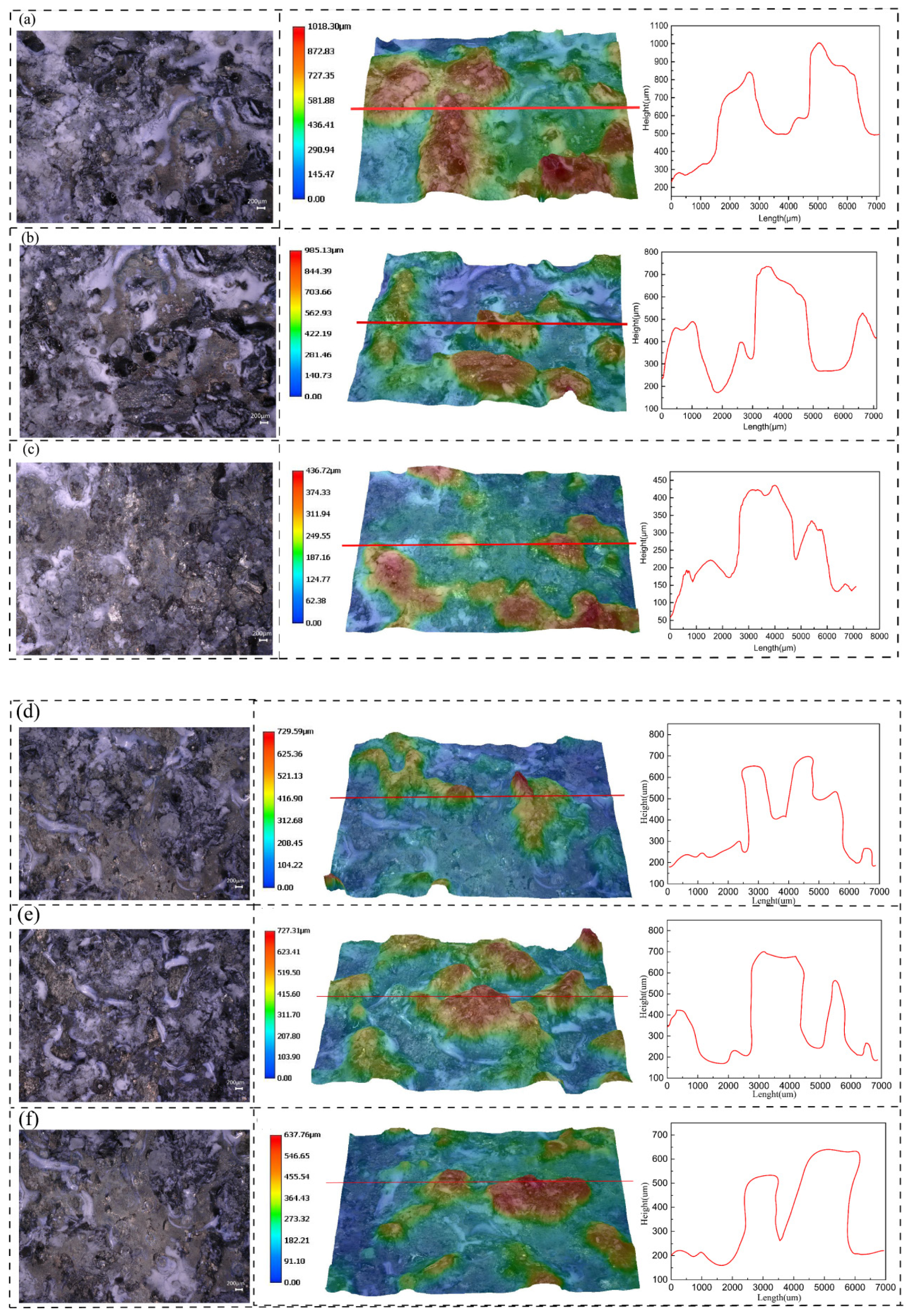
3.2. Corrosion Resistance
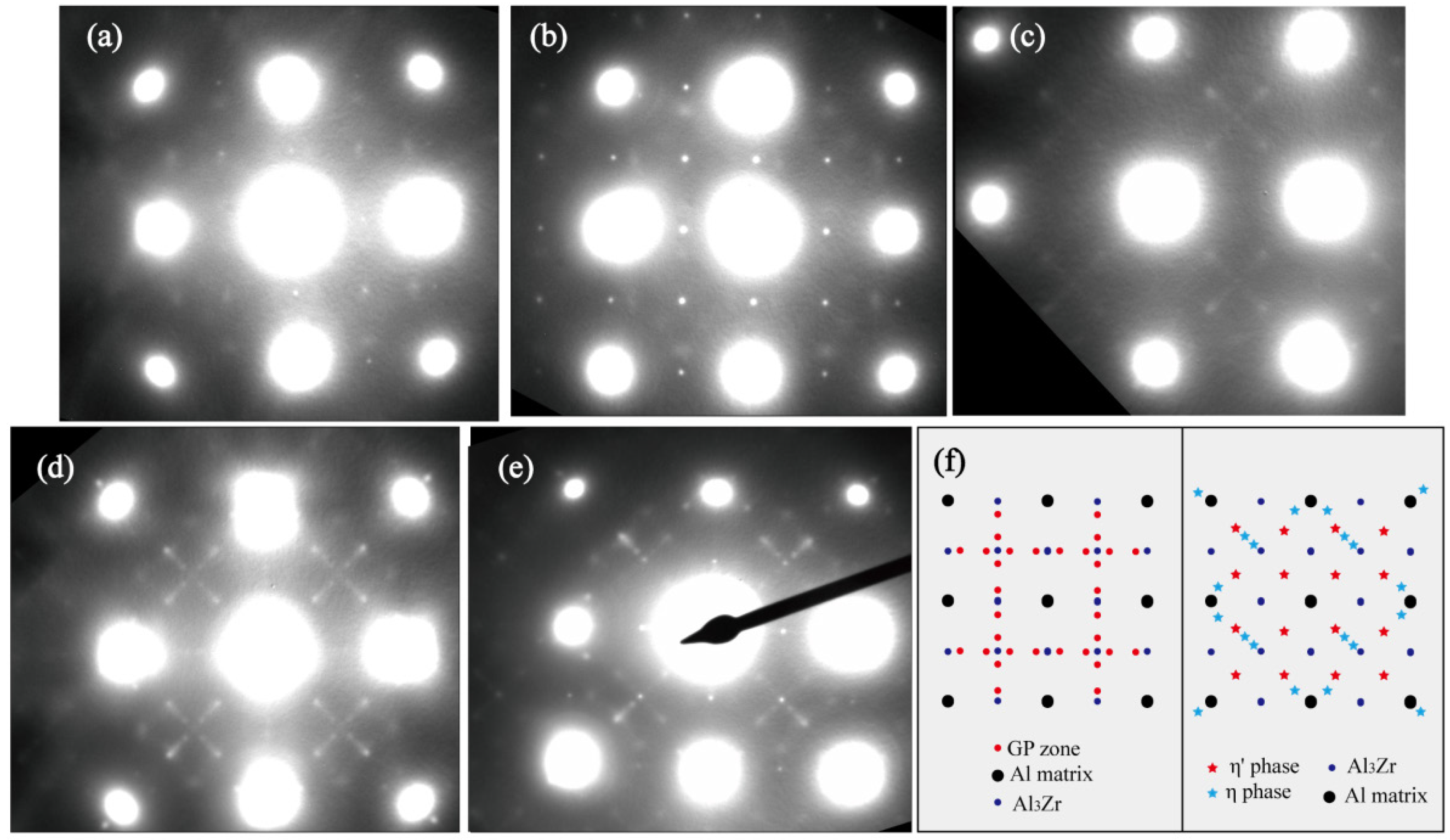
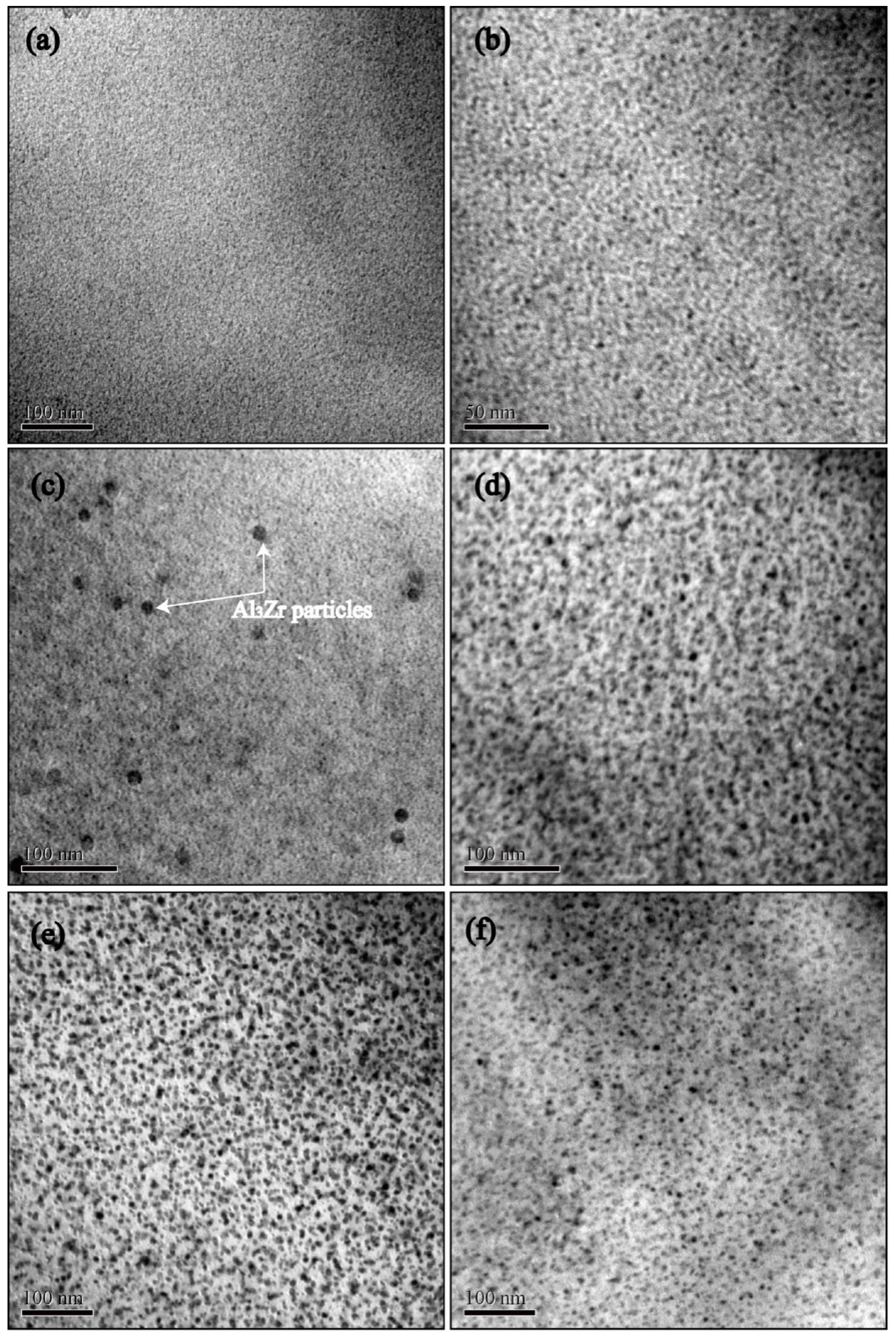
3.3. Microstructure Evolution during NICA
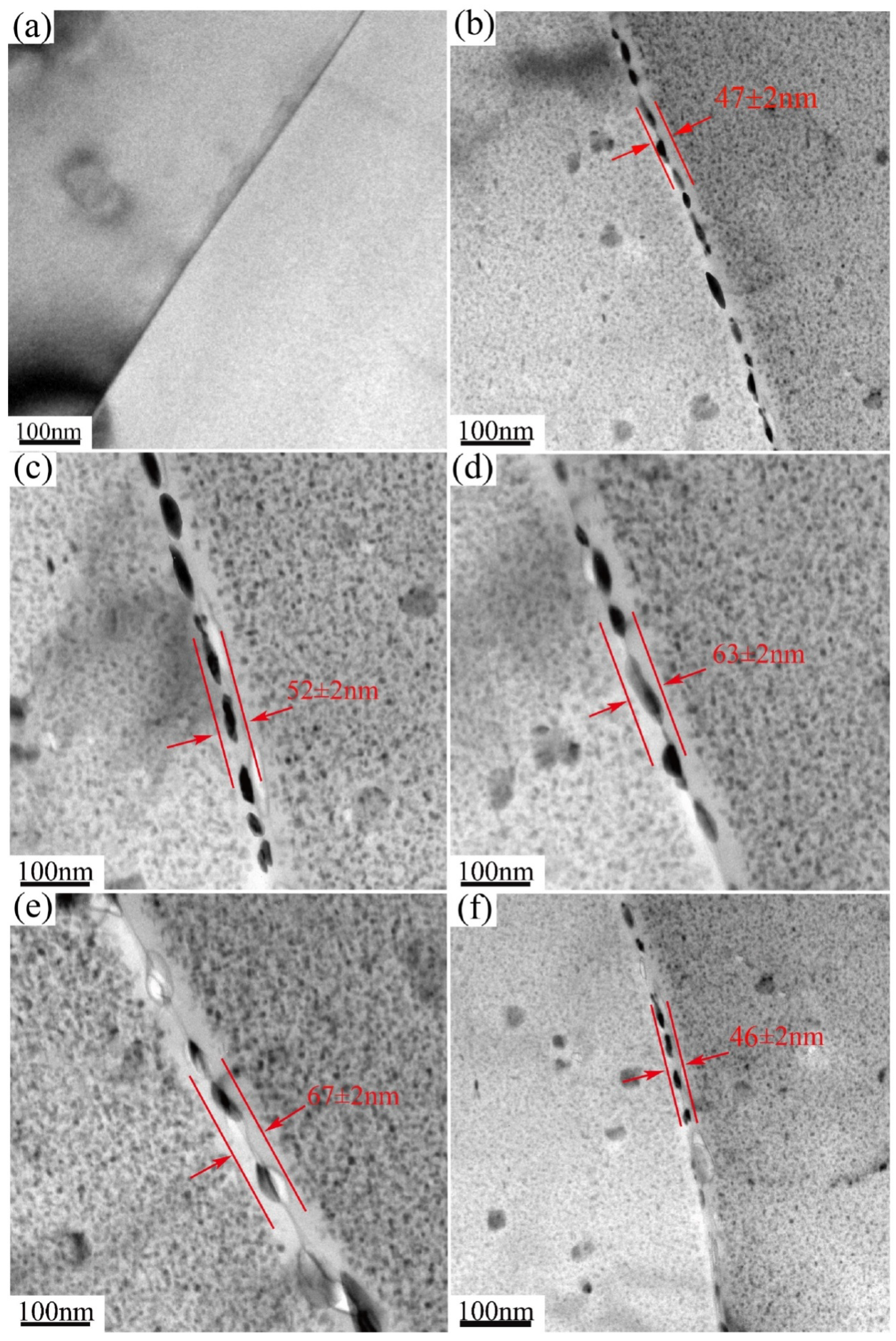
3.4. Characteristics of Grain Boundary during NICA
4. Conclusions
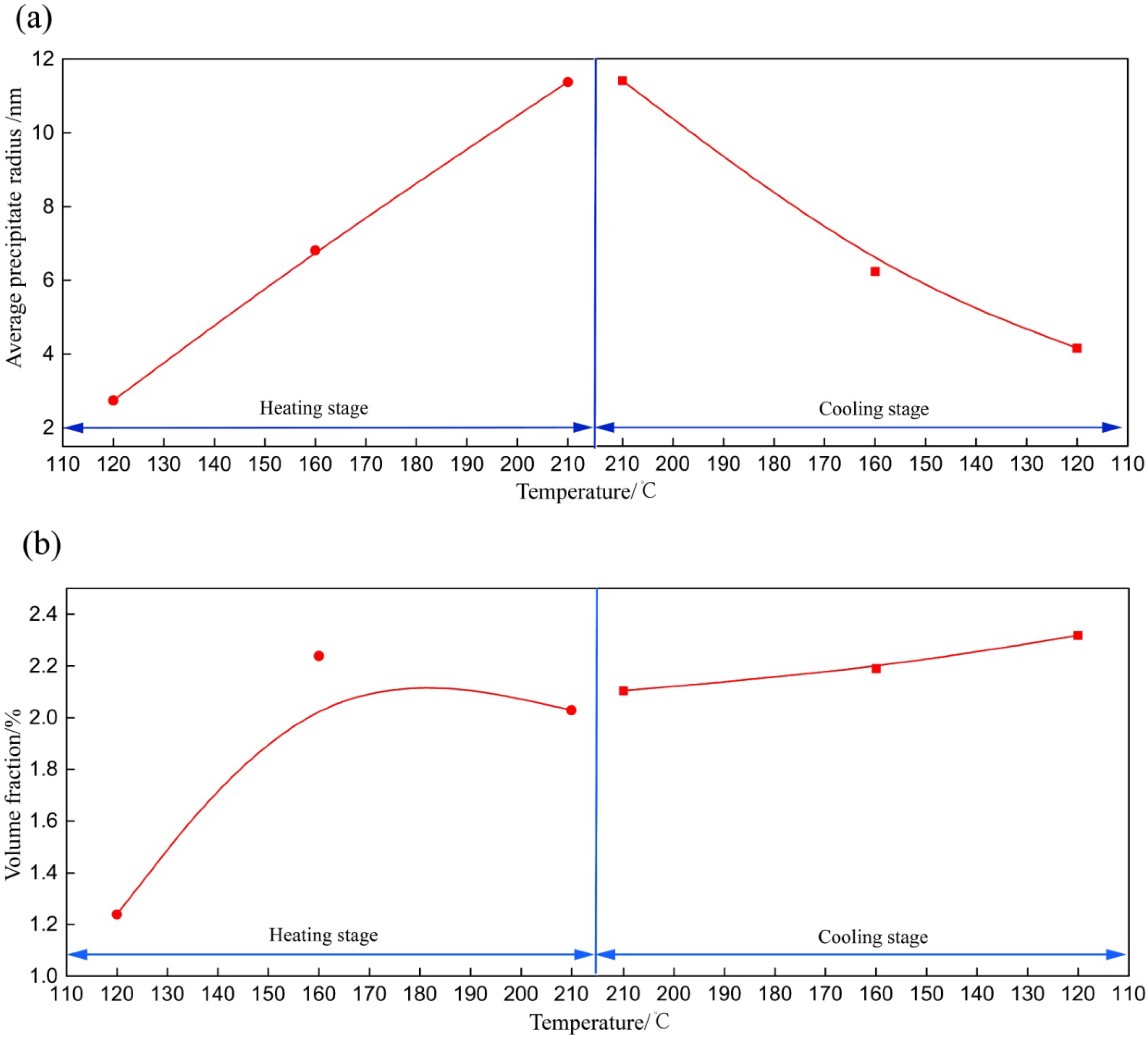
- The hardness and tensile strength generally kept growing with increasing aging time until the level of 185 HV and 580 MPa (C120). During the heating stage, GPI zones precipitated from the matrix firstly at the aging condition of H120, and then the major precipitates became η′phases with the increase of aging temperature to 210 °C (H210). Moreover, both the size and volume fraction of the precipitates increased. Hence, the tensile strength and hardness of the NICA processing technology increased during the heating stage. Furthermore, in the cooling process, coarse precipitates in the grain interior began to dissolve, and GPI zones and η′phases precipitated from the matrix secondarily. The size of the precipitates was greatly decreased, and the volume fraction reached a maximum, which led to peak hardness and tensile strength of the alloy.
- The alloy treated with NICA had the highest corrosion resistance in C120. In the cooling phase, the dissolution of precipitates caused the PFZ to narrow due to the secondary precipitation of new fine precipitates, but the coarse GBPs were not continuously distributed in the grain boundary, which further improved corrosion resistance.
- To reduce heat treatment process time and the manufacturing cost of Al-Zn-Mg-Cu alloy overall panel components, the NICA process was designed, which only lasted about 13 h without loss of alloy properties. Therefore, compared with the traditional aging process, the manufacturing cost was greatly decreased.
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Zhang, Y.D.; Jin, S.B.; Trimby, P.W.; Liao, X.Z.; Murashkin, M.Y.; Valiev, R.Z.; Liu, J.Z.; Cairney, J.M.; Ringer, S.P.; Sha, G. Dynamic precipitation, segregation and strengthening of an Al-Zn-Mg-Cu alloy (AA7075) processed by high-pressure torsion. Acta Mater. 2019, 162, 19–32. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, K.H.; Chen, S.Y.; Zhou, L.; Chang, J.Y.; Huang, L.P.; Yi, Y. Enhancing stress corrosion cracking resistance of low Cu-containing Al-Zn-Mg-Cu alloys by slow quench rate. Mater. Des. 2019, 164, 107558. [Google Scholar] [CrossRef]
- Xie, P.; Chen, S.Y.; Chen, K.H.; Jiao, H.B.; Huang, L.P.; Zhang, Z.; Yang, Z. Enhancing the stress corrosion cracking resistance of a low-Cu containing Al-Zn-Mg-Cu aluminum alloy by step-quench and aging heat treatment. Corros. Sci. 2019, 161, 108184. [Google Scholar] [CrossRef]
- Dumont, D.; Deschamps, A.; Brechet, Y. On the relationship between microstructure, strength and toughness in AA7050 aluminum alloy. Mater. Sci. Eng. A 2013, 356, 326–336. [Google Scholar] [CrossRef]
- Wang, S.S.; Jiang, J.T.; Fan, G.H.; Frankel, G.S.; Zhen, L. Effects of long-term natural aging on the altered surface layer on an Al-Zn-Mg-Cu alloy and on corrosion properties. Electrochim. Acta 2018, 266, 34–42. [Google Scholar] [CrossRef]
- Zhao, H.; De Geuser, F.; Kwiatkowski, A.; Silva, D.; Szczepaniak, A.; Gault, B.; Ponge, D.; Raabe, D. Segregation assisted grain boundary precipitation in a model Al-Zn-Mg-Cu alloy. Acta Mater. 2018, 156, 318–329. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Kinetic Monte Carlo simulation of clustering in an Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2005, 53, 907–917. [Google Scholar] [CrossRef]
- Deschamps, A.; De Geuser, F.; Horita, Z.; Lee, S.; Renou, G. Precipitation kinetics in a severely plastically deformed 7075 aluminium alloy. Acta Mater. 2014, 66, 105–117. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, K.H.; Peng, G.S.; Chen, X.H.; Ceng, Q.H. Effect of heat treatment on hot deformation behavior and microstructure evolution of 7085 aluminum alloy. J. Alloys Compd. 2012, 537, 338–345. [Google Scholar] [CrossRef]
- Berg, L.K.; Gjønnes, J.; Hansen, V.; Li, X.Z.; Knutson-Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L.R. GP-zones in Al–Zn–Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Early-stage precipitation in Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2004, 52, 4503–4516. [Google Scholar] [CrossRef]
- Stiller, K.; Warren, P.J.; Hansen, V.; Angenete, J.; Gjønnes, J. Investigation of precipitation in an Al–Zn–Mg alloy after two-step ageing treatment at 100 °C and 150 °C. Mater. Sci. Eng. A 2004, 270, 55–63. [Google Scholar] [CrossRef]
- Cao, F.H.; Zheng, J.X.; Jiang, Y.; Chen, B.; Wang, Y.R.; Hu, T. Experimental and DFT characterization of η′ nano-phase and its interfaces in AlZnMgCu alloys. Acta Mater. 2019, 164, 207–219. [Google Scholar] [CrossRef]
- Gjønnes, J.; Simensen, C.J. An electron microscope investigation of the microstructure in an aluminium-zinc-magnesium alloy. Acta Metall. 1970, 18, 881–890. [Google Scholar] [CrossRef]
- Zhan, X.; Tang, J.G.; Li, H.Z.; Liang, X.P.; Zhang, Y. Effects of non-isothermal aging on mechanical properties, corrosion behavior and microstructures of Al-Cu-Mg-Si alloy. J. Alloys Compd. 2020, 819, 152960. [Google Scholar] [CrossRef]
- Jiang, J.T.; Xiao, W.Q.; Yang, L.; Shao, W.Z.; Yuan, S.J.; Zhen, L. Ageing behavior and stress corrosion cracking resistance of a non-isothermally aged Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2014, 605, 167–175. [Google Scholar] [CrossRef]
- Jiang, J.T.; Tang, Q.J.; Yang, L.; Zhang, K.; Yuan, S.J.; Zhen, L. Non-isothermal ageing of an Al–8Zn–2Mg–2Cu alloy for enhanced properties. J. Mater. Process. Technol. 2016, 227, 110–116. [Google Scholar] [CrossRef]
- Peng, X.Y.; Guo, Q.; Liang, X.P.; Deng, X.P.; Guo, Y.; Xu, G.F.; Yin, Z.M. Mechanical properties, corrosion behavior and microstructures of a non-isothermal ageing treated Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2017, 688, 146–154. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Deng, Y.; Zhang, X. Effect of deformation degree on the creep age forming of 7475 aluminum alloy: The feasibility of the extended deformation range. Mater. Sci. Eng. A 2016, 664, 126–134. [Google Scholar] [CrossRef]
- Zheng, J.; Davies, C.M.; Lin, J.; Pan, R.; Li, C. Constitutive modelling of a T74 multi-step creep ageing behaviour of AA7050 and its application to stress relaxation ageing in age formed aluminium components. Procedia Eng. 2016, 207, 281–286. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, L.H.; Huang, M.H.; Shen, R.L.; Ma, Z.Y.; Xu, L.Z.; Wang, K.; Wang, X. Deformation behavior of Al-Cu-Mg alloy during non-isothermal creep age forming process. J. Mater. Process. Technol. 2018, 255, 26–34. [Google Scholar] [CrossRef]
- Zhan, L.H.; Lin, J.G.; Dean, T.A. A review of the development of creep age forming: Experimentation, modelling and applications. Int. J. Mach. Tools Manuf. 2011, 51, 11–17. [Google Scholar] [CrossRef]
- Jeunechamps, P.P.; Ho, K.C.; Lin, J.; Ponthot, J.P.; Dean, T.A. A closed form technique to predict springback in creep age-forming. Int. J. Mech. Sci. 2006, 48, 621–629. [Google Scholar] [CrossRef]
- Jeshvaghani, R.A.; Shahverdi, H.R.; Hadavi, S.M.M. Investigation of the age hardening and operative deformation mechanism of 7075 aluminum alloy under creep forming. Mater. Sci. Eng. A 2012, 552, 172–178. [Google Scholar] [CrossRef]
- Lin, Y.C.; Jiang, Y.Q.; Chen, X.M.; Wen, D.X.; Zhou, H.M. Effect of creep-aging on precipitates of 7075 aluminum alloy. Mater. Sci. Eng. A 2013, 588, 347–356. [Google Scholar] [CrossRef]
- Chen, J.F.; Zhen, L.; Jiang, J.T.; Yang, L.; Shao, W.Z.; Zhang, B.Y. Microstructures and mechanical properties of age-formed 7050 aluminum alloy. Mater. Sci. Eng. A 2012, 539, 115–123. [Google Scholar] [CrossRef]
- Standard, A. G34-01: Standard Test Method for Exfoliation Corrosion Susceptibility in 2XXX and 7XXX Series Al Alloys; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Zhang, W.; Xing, Y.; Jia, Z.H.; Yang, X.F.; Liu, Q.; Zhu, C.L. Effect of minor Sc and Zr addition on microstructure and properties of ultra-high strength aluminum alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 3866–3871. [Google Scholar] [CrossRef]
- Dumont, M.; Lefebvre, W.; Doisneau-Cottignies, B. Characterisation of the composition and volume fraction of η′ and η precipitates in an Al–Zn–Mg alloy by a combination of atom probe, small-angle X-ray scattering and transmission electron microscopy. Acta Mater. 2005, 53, 2881–2892. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al–Zn–Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Kinsman, K.R.; Russell, K.C. The volume free energy change associated with precipitate nucleation. Scr. Metall. 1970, 4, 101–106. [Google Scholar] [CrossRef]
- Wagner, R.; Kampmann, R. Stabilität bei geraden Stäben. J. Mater. Sci. Technol. 1991, 5, 213. [Google Scholar]
- Yang, W.C.; Ji, S.X.; Zhang, Q.; Wang, M.P. Investigation of mechanical and corrosion properties of an Al–Zn–Mg–Cu alloy under various ageing conditions and interface analysis of η′ precipitate. Mater. Des. 2015, 85, 752–761. [Google Scholar] [CrossRef]
- Li, J.H.; Li, F.G.; Ma, X.K.; Li, J.; Liang, S. Effect of grain boundary characteristic on intergranular corrosion and mechanical properties of severely sheared Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2018, 732, 53–62. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhang, B.; Lin, H.Q.; Zhou, Y.; Sun, L.; Wang, J.Q.; Han, E.H.; Ke, W. Correlations between stress corrosion cracking susceptibility and grain boundary microstructures for an Al–Zn–Mg alloy. Corros. Sci. 2013, 77, 103–112. [Google Scholar] [CrossRef]
- Qi, H.; Liu, X.Y.; Liang, S.X.; Zhang, X.L.; Cui, H.X.; Zheng, L.Y.; Gao, F.; Chen, Q.H. Mechanical properties and corrosion resistance of Al–Cu–Mg–Ag heat-resistant alloy modified by interrupted aging. J. Alloys Compd. 2016, 657, 318–324. [Google Scholar] [CrossRef]
- Huang, J.C.; Ardell, A.J. Crystal structure and stability of T 1 precipitates in aged Al–Li–Cu alloys. Mater. Sci. Technol. 1987, 3, 176–188. [Google Scholar] [CrossRef]
- Dorin, T.; Donnadieu, P.; Chaix, J.M.; Lefebvre, W.; Geuser, F.D.; Deschamps, A. Size distribution and volume fraction of T1 phase precipitates from TEM images: Direct measurements and related correction. Micron 2015, 78, 19–27. [Google Scholar] [CrossRef]

| Zn | Mg | Cu | Zr | Si | Fe | Al |
|---|---|---|---|---|---|---|
| 5.66 | 2.64 | 1.82 | 0.09 | 0.16 | 0.08 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Jiang, B.; Yi, D.; Wang, H.; Wu, G. Precipitate Behavior, Mechanical Properties and Corrosion Behavior of an Al-Zn-Mg-Cu Alloy during Non-Isothermal Creep Aging with Axial Tension Stress. Metals 2020, 10, 378. https://doi.org/10.3390/met10030378
Zhu J, Jiang B, Yi D, Wang H, Wu G. Precipitate Behavior, Mechanical Properties and Corrosion Behavior of an Al-Zn-Mg-Cu Alloy during Non-Isothermal Creep Aging with Axial Tension Stress. Metals. 2020; 10(3):378. https://doi.org/10.3390/met10030378
Chicago/Turabian StyleZhu, Junhao, Bo Jiang, Danqing Yi, Haishen Wang, and Guicheng Wu. 2020. "Precipitate Behavior, Mechanical Properties and Corrosion Behavior of an Al-Zn-Mg-Cu Alloy during Non-Isothermal Creep Aging with Axial Tension Stress" Metals 10, no. 3: 378. https://doi.org/10.3390/met10030378
APA StyleZhu, J., Jiang, B., Yi, D., Wang, H., & Wu, G. (2020). Precipitate Behavior, Mechanical Properties and Corrosion Behavior of an Al-Zn-Mg-Cu Alloy during Non-Isothermal Creep Aging with Axial Tension Stress. Metals, 10(3), 378. https://doi.org/10.3390/met10030378





