3.1. Microscopy
Slag samples were collected from the AOD converter at the end of the decarburization period. An example of a typical slag sample is seen in
Figure 2. As can be seen, the sample consists of separate slag particles of approximately a 100 mm size. Here, it should be noted that the sizes of the slag particles were approximately the same as the size of the lime particles that were added. However, a study of the influence of size on the petrography results was not the focus of this study. Also, data for five heats are discussed in the present study.
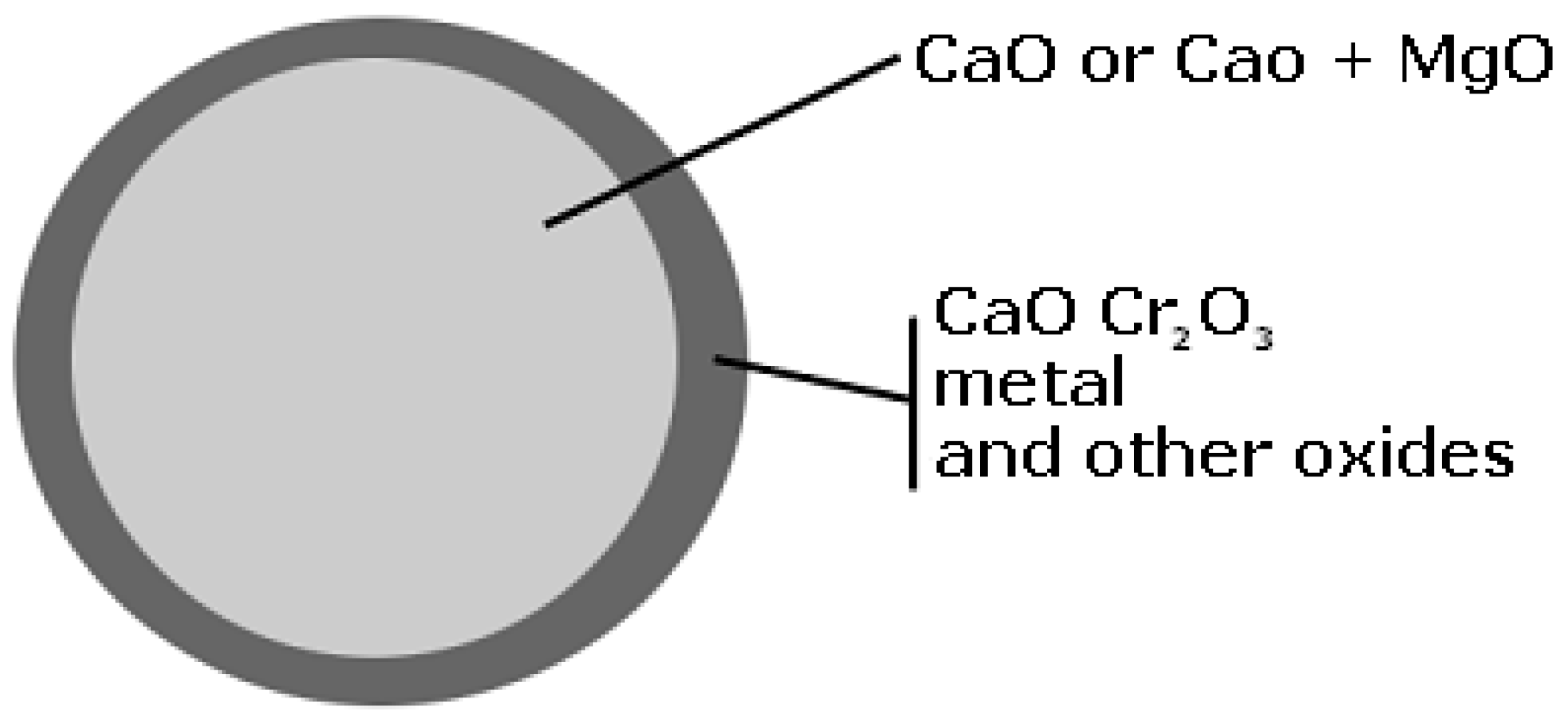
One particle of the slag sample is schematically shown in
Figure 3. It contained a core of lime surrounded by a shell consisting of different oxides and metals. Furthermore, this is found as the mechanism for lime dissolution in liquid silicate slags [
26,
27] and experimentally found for AOD slags [
28,
29].
It should be mentioned that the polished specimen surface decomposed gradually over a period of 3–4 weeks. Thus, it was important to keep the samples in a vacuum container and to study them as soon as possible after finishing the sample preparation in order to obtain accurate results.
A typical result of an XRF determination of a slag sample is shown in
Table 2. The main components in the slag were CaO (
mass%) and Cr
2O
3 (
mass%), as was expected. However, the slag also contained FeO, SiO
2, MnO and MgO. In addition, smaller amounts of elements and components such as F, S and NiO were detected. However, these were neglected in the remaining evaluation of the slag data. The reasons are that they are deemed not to affect the results, since the contents are low. Moreover, if the number of components is fewer it is easier to determine the phases and melting points using phase diagrams.
After removing the elements and components, the XRF analyses for the remaining six components were normalized. The amounts of each component for all studied samples are given in weight percent in
Table 3. Data are given for five heats denoted A–E. In addition, three sets of data are given for heat B where repetitive determinations were done. It can be seen that the amounts of the two main components in the slag CaO and Cr
2O
3 showed a variation of 49.6–71.0 mass% and 20.0–32.0 mass%, respectively.
It was mentioned earlier that it is generally believed that the slag samples from the AOD process are inhomogeneous with respect to the chemical composition. Thus, three repeated XRF determinations were made for one heat number (B-1 to B-3). It was seen that the Cr2O3 content varied between 20.0–32.0 mass% and the CaO content varied from 54.1–71.0 mass%. However, for a statistical significance an expanded study of more samples is needed. When interpreting these results it was discussed whether the slag is so in-homogeneous at this stage of the AOD process, so that it was too hard to determine a reliable composition using XRF. Thus, it was decided to study if alternative analysis methods could be developed to give more reliable and homogeneous data of the chemical slag compositions.
When studying the samples in a light optical microscope it is obvious that the samples contain both slag and “free” lime, as is shown in
Figure 3. From this figure it is seen that the lime is concentrated in the center. Therefore, the data from
Table 3 were further processed. The CaO and MgO contents were removed, since they are believed to represent the lime phase of the slag samples. Thereafter, the compositions of the remaining four components (Cr
2O
3, FeO, SiO
2 and MnO) were normalized, as illustrated in
Table 4. Similar to
Table 3, this table presents data for five heats including repetitive determinations for heat B. Overall, the results show that the composition of the main component Cr
2O
3 varies from 49.0–72.7 mass% in the different slag samples taken from different heats (A–E). Thus, there is still a variation in chemical composition between the studied heats.
The results in
Table 4 also show that when CaO and MgO are excluded from the XRF results and the remaining data are normalized, the Cr
2O
3 content only varies between 71.3–71.6 mass% for the repetitive composition determinations for heat B. Thus, the difference in composition determination is highly improved compared to when CaO and MgO are not excluded from the results (20.0–32.0 mass%). In addition, the results also show that the variation in composition determination is very small for the oxides FeO, SiO
2 and MnO. Thus, based on these results it is concluded that the XRF method is suitable to use when determining the chemical composition of AOD slag samples. However, one needs to be careful when interpreting the results. It is especially important to separate the solid lime-phase (CaO and MgO) from the rest of the slag phase before presenting the data. From our experience the free lime is not homogenously distributed in the slag sample and also varies with sampling time and from process conditions.
3.3. Summarized Discussion
As the sampling from the converter must be performed manually, the specimens were checked regarding variable appearance. A fairly good reproducibility was obtained based on ocular inspections of the samples. However, the high content of lime leads to difficulties in the sample preparation as well as in examination of the samples. More specifically, samples with large CaO contents will disintegrate during storage and sample preparation. Thus, to get the best performance when examining the different slag phases the specimens with high CaO contents were sorted out. Overall, these findings made it clear that it is important to discuss how it is possible to estimate the CaO- and MgO contents in a reliable manner, when making an overall evaluation of the results.
The procedure of using normalizations of the XRF data in the present study seems to be useful when comparing the slag components between different heats. However, there are some uncertainties in the XRF determinations. For example, the accuracy in determining the Cr-content using XRF is affected by the lack of high chromium-containing reference materials for determinations of chemical compositions of slags. Further, an in-homogeneous distribution of CaO/MgO causes a scatter in the chromium determinations.
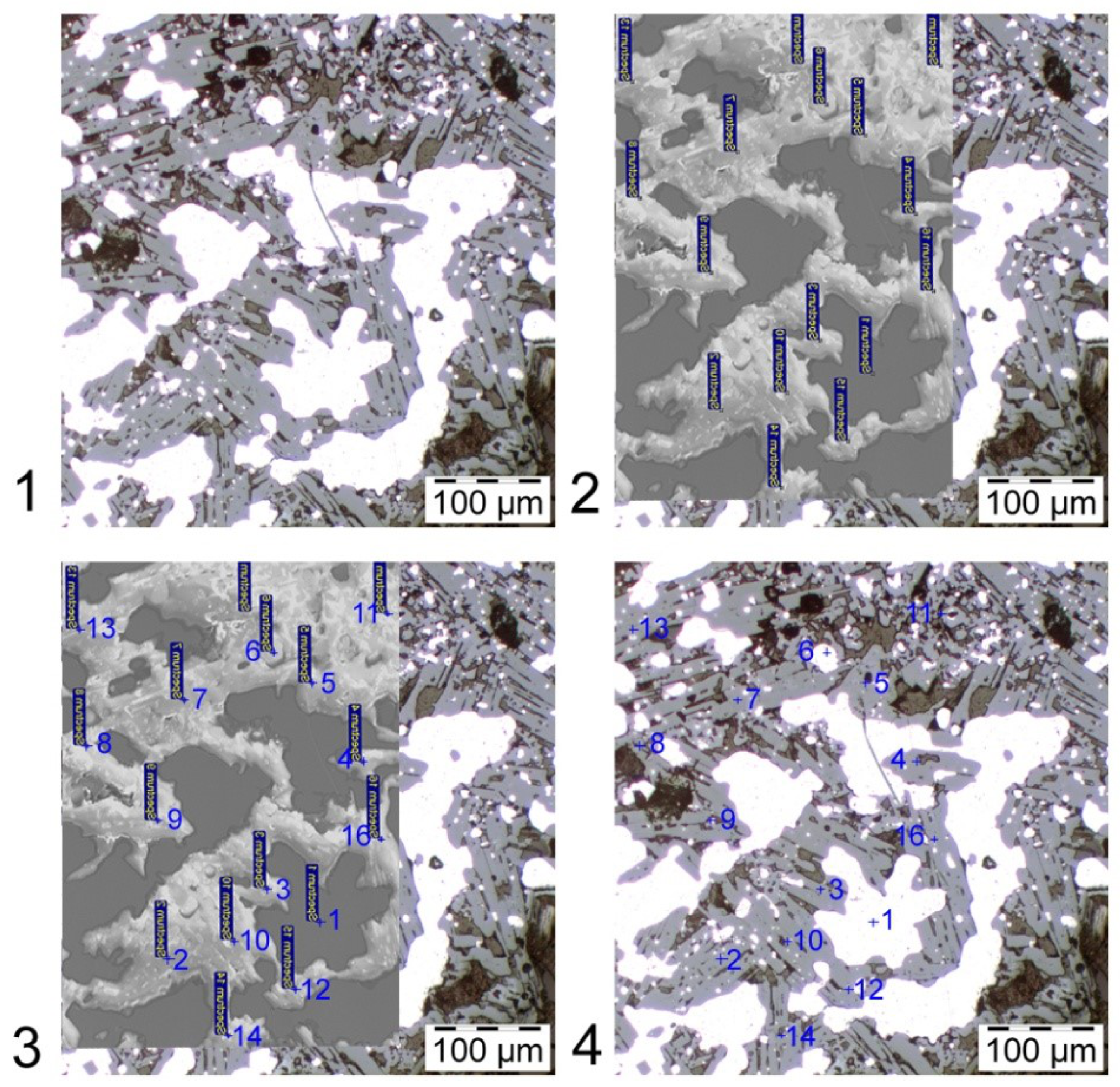
Errors can also occur in the SEM-EDS determination of the oxides, due to charging of non-conductive phases. However, these expected errors seem to be small and negligible, as shown by the low standard deviation values within a specimen and between specimens in
Table 6. Specifically, the measurements for Ca and Cr were (
) at% and (
) at%, respectively.
The possible phases in the slag samples were evaluated based on data of the chemical composition of the slag samples (
Table 3). In addition, some XRD measurements were done to verify that the slag contained the following phases: CaO·Cr
2O
3, 3CaO·2SiO
2 and 3CaO·Cr
2O
3·3SiO
2. Thereafter, the percentage of Ca, Cr, Si and O were determined based on stoichiometry, se
Table 6. Overall, the agreement between the stoichiometric and measured values was found to be good. More specifically, as is shown in
Table 5 and
Table 6 the SEM-EDS determination show that the CaO·Cr
2O
3 phase contained
at% chromium and the stoichiometric calculations in
Table 6 suggest that the chromium content should be
at%. Thus, the difference between the calculated value based on stoichiometry and measured data for Ca and Cr are
% (14.3/12.8) and
% (28.6/25.7), respectively. This is considered to be a good agreement for industrial samples. The differences from stoichiometry is likely due to solubility of other elements in the oxides.
Overall, it is believed that the current procedure of determining the slag characteristics based on petrography is suitable to use for AOD converter decarburization slags.
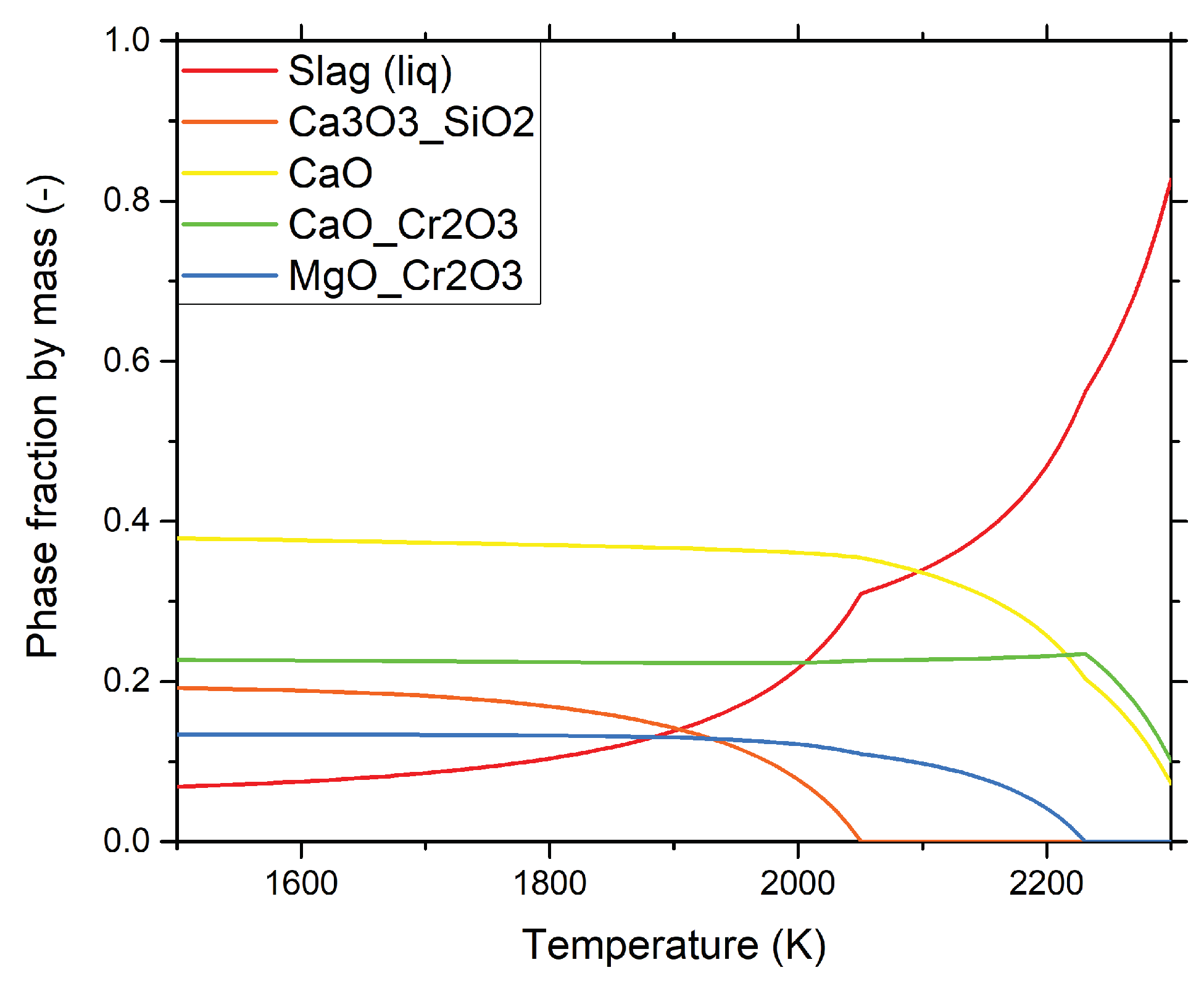
Figure 5 illustrates the phase fractions of different chemical compounds found in a typical AOD slag as a function of the temperature calculated using Thermo-Calc. The calculations were done as follows, based on the SLAG4 database. First, the average slag composition for sample B-1 in
Table 3 was used as input to the calculations. This was to include the major chemical driving forces in the process and what phases that are expected to be stable. Thereafter, simulations were done at different temperatures ranging from 1500–2300 K in order to simulate what phases that will be present during a lowering of the temperature. A similar approach was used to good agreement with experiments where slow cooling of slags was studied [
33].
At 2300 K, the fraction of liquid slag is approximately 0.82 and the other two phases are a solid CaO phase 0.1 and a CaO·Cr
2O
3 phase 0.08. At 2250
, a solid MgO·Cr
2O
3 phase is formed. Finally, the 3CaO·SiO
2 phase will precipitate as a solid phase at a temperature of 2040
. The predicted slag component compositions were found to have a reasonable good stoichiometric agreement with data found in the slag atlas literature [
24]. The phases present are also in accordance with the experimental findings [
28,
32]. In contrast, the predicted liquid fraction is much less than [
8]. This is most likely due to a much higher CaO content used for the calculations in this study.
Figure 5 also shows that the molten slag fraction decreases rapidly from 2300–2040 K and that then it continues to decease until it reaches a value of 0.07 at 1500
. During steelmaking conditions the slag temperatures in an AOD are typically 1800–2100 K. Specifically, the solid fraction in a slag is approximately 90% at 1800
and 57% at 2100
. Thus, the fraction of liquid phase is very low in comparison to the majority of the slags found in other steel making reactors.
It should be pointed out that only the dark gray edges of a sample shown in
Figure 3 were studied. This is due to the fact that it is mainly this region that will take part in the decarburization reactions. Moreover, this surface may also be in contact with the steel melt under the formation of a liquid/solid interface. This was also observed by Münchberg et al. [
28]. It is also of interest to mention that CaO or MgO compounds were only detected in the center of the petrographic samples.
Figure 6 shows a sketch over the methodology to study slag characteristics in AOD slag samples, which was developed based on the results in this study. First, a slag sample is taken using a scoop from the desired part of the AOD process. From this sample, a slag piece is selected which does not contain a lot of undissolved CaO pieces. Thereafter, the slag piece is baked into Bakelite. During the final sample preparation, a careful grinding is carried out in order to obtain a plain surface which is suitable for accurate microscopy determinations.
The initial microscopy studies are carried out by using LOM. For each sample, 30 views are selected. These are saved before image analysis determinations are made. Here, SEM in combination with EDS is used for a verification of the phases identified using LOM. During the image analysis studies, the different phases in the samples are studied. In the end, the purpose is to get reproducible data with respect to the phases in the slag samples.