Effect of Film-Forming Amines on the Acidic Stress-Corrosion Cracking Resistance of Steam Turbine Steel
Abstract
1. Introduction
2. Materials and Methods
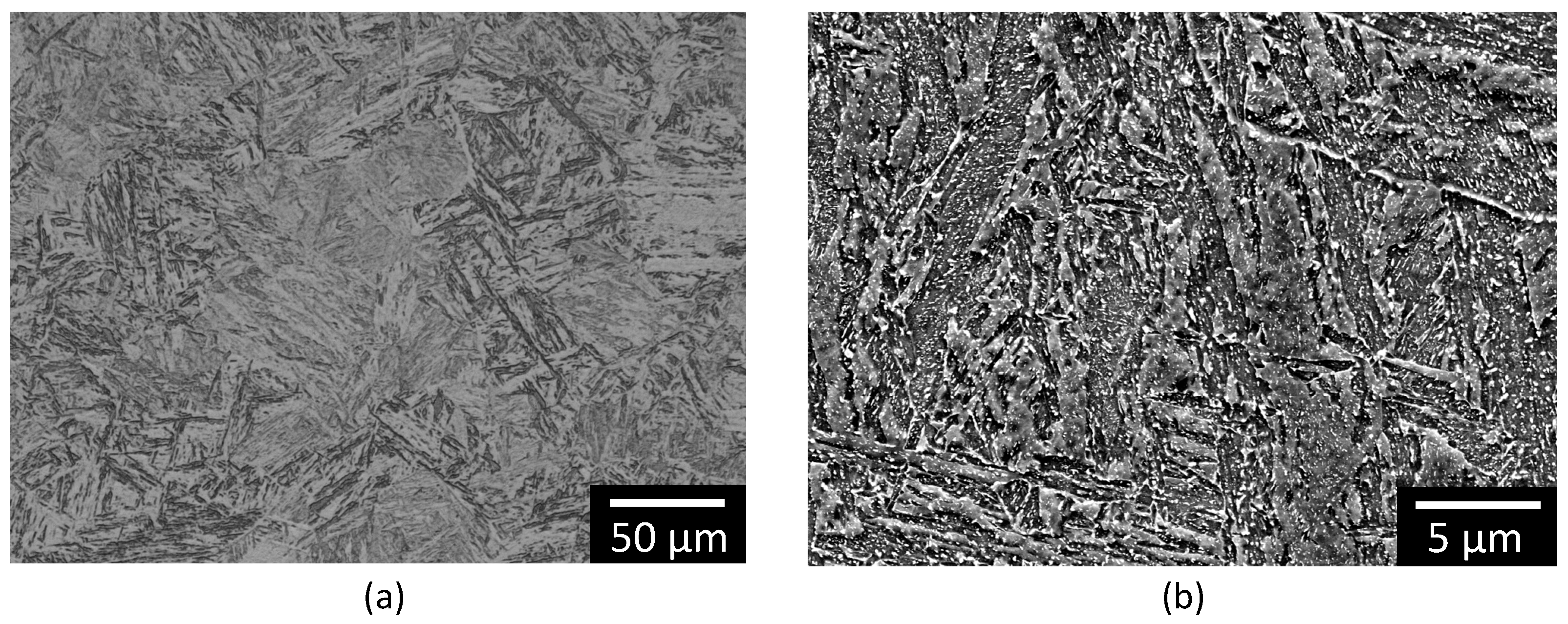
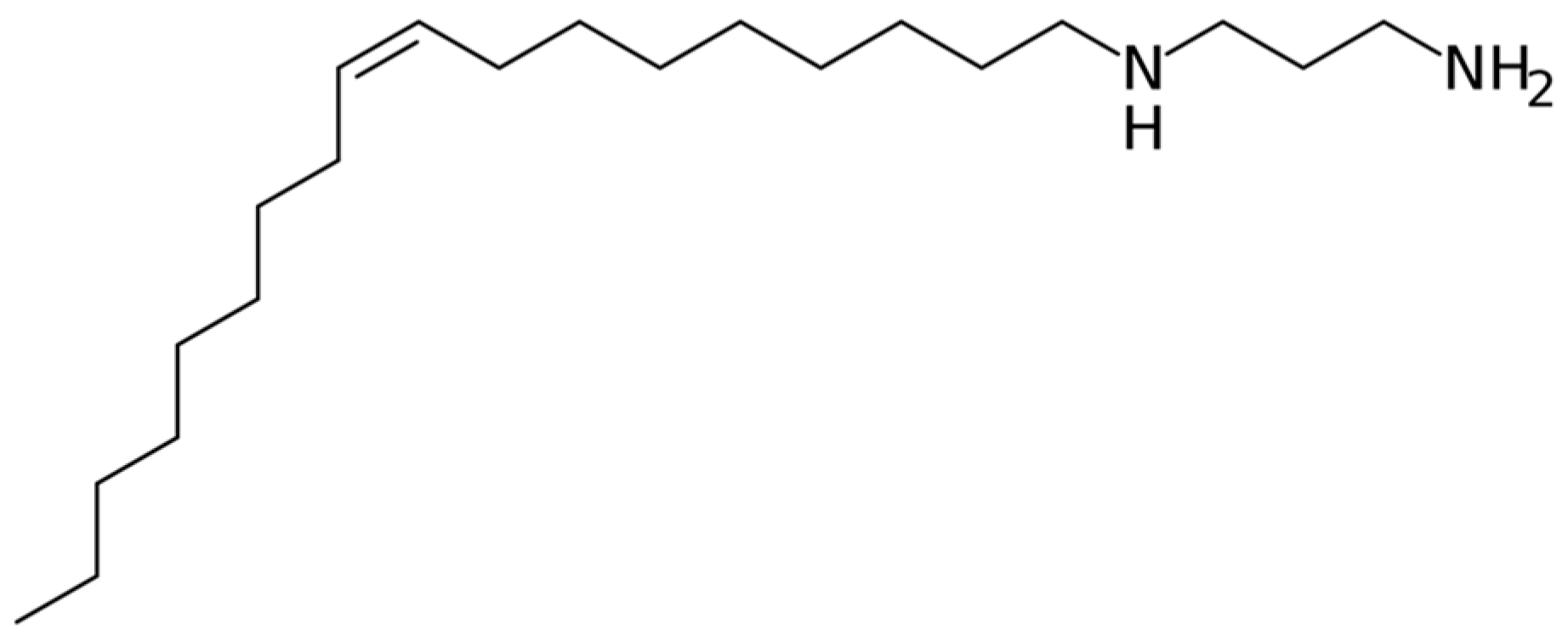
2.1. Material Characterisation and FFA Adsorption
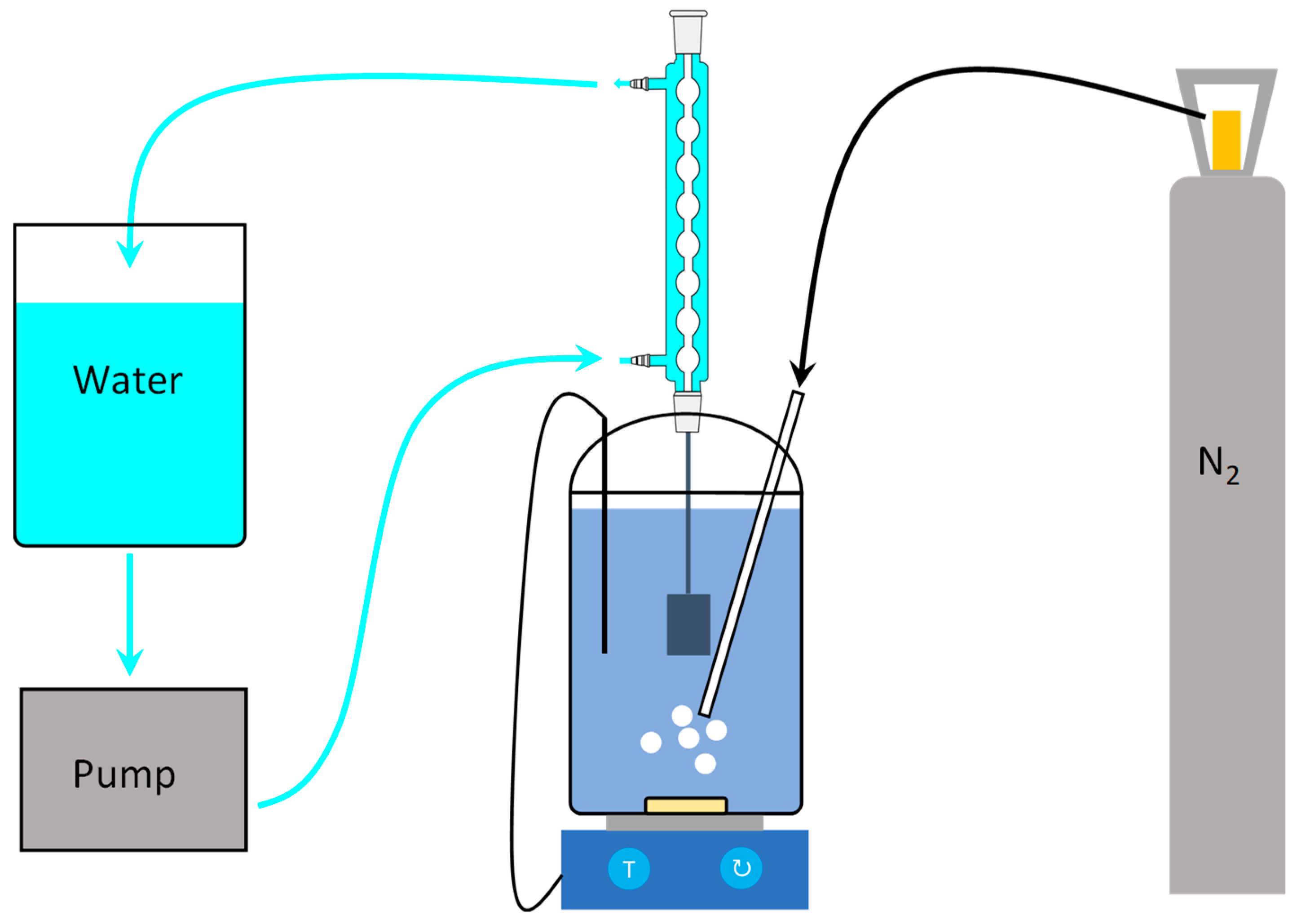
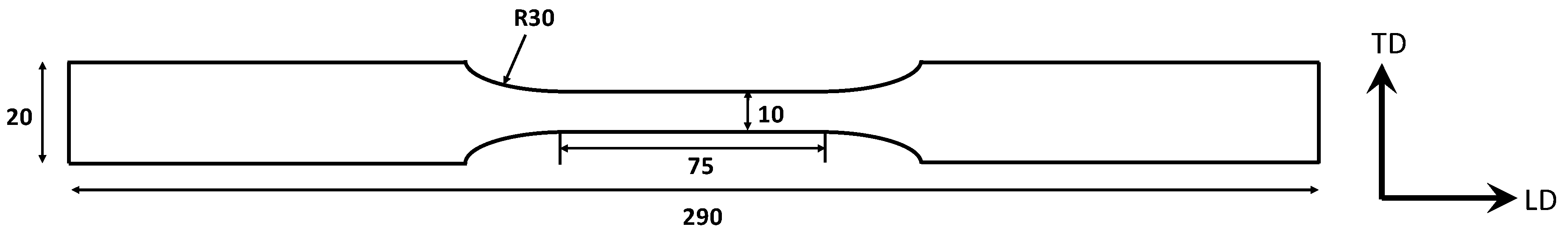
2.2. SCC-Induced Mechanical Behaviour
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Surface Analysis
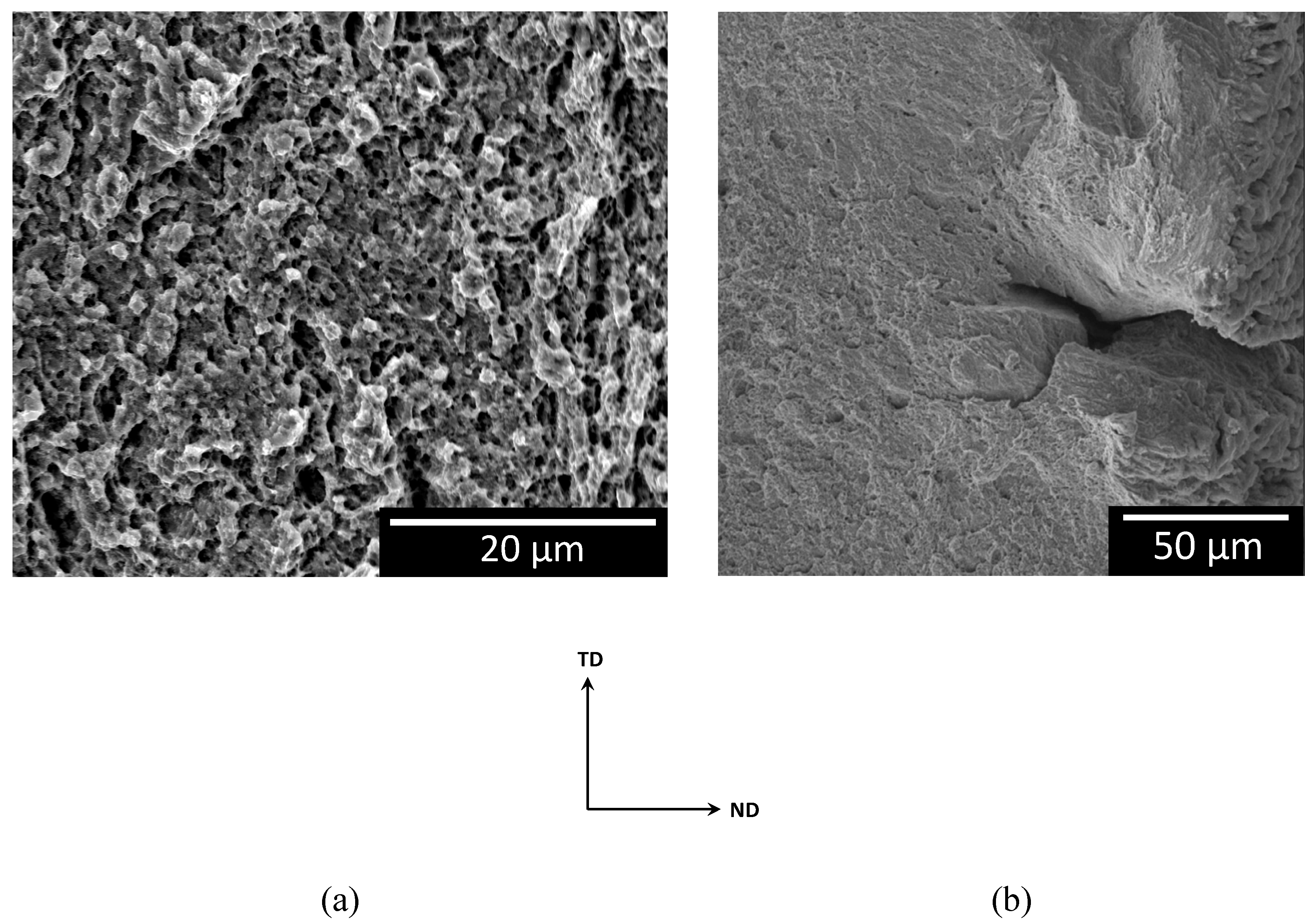
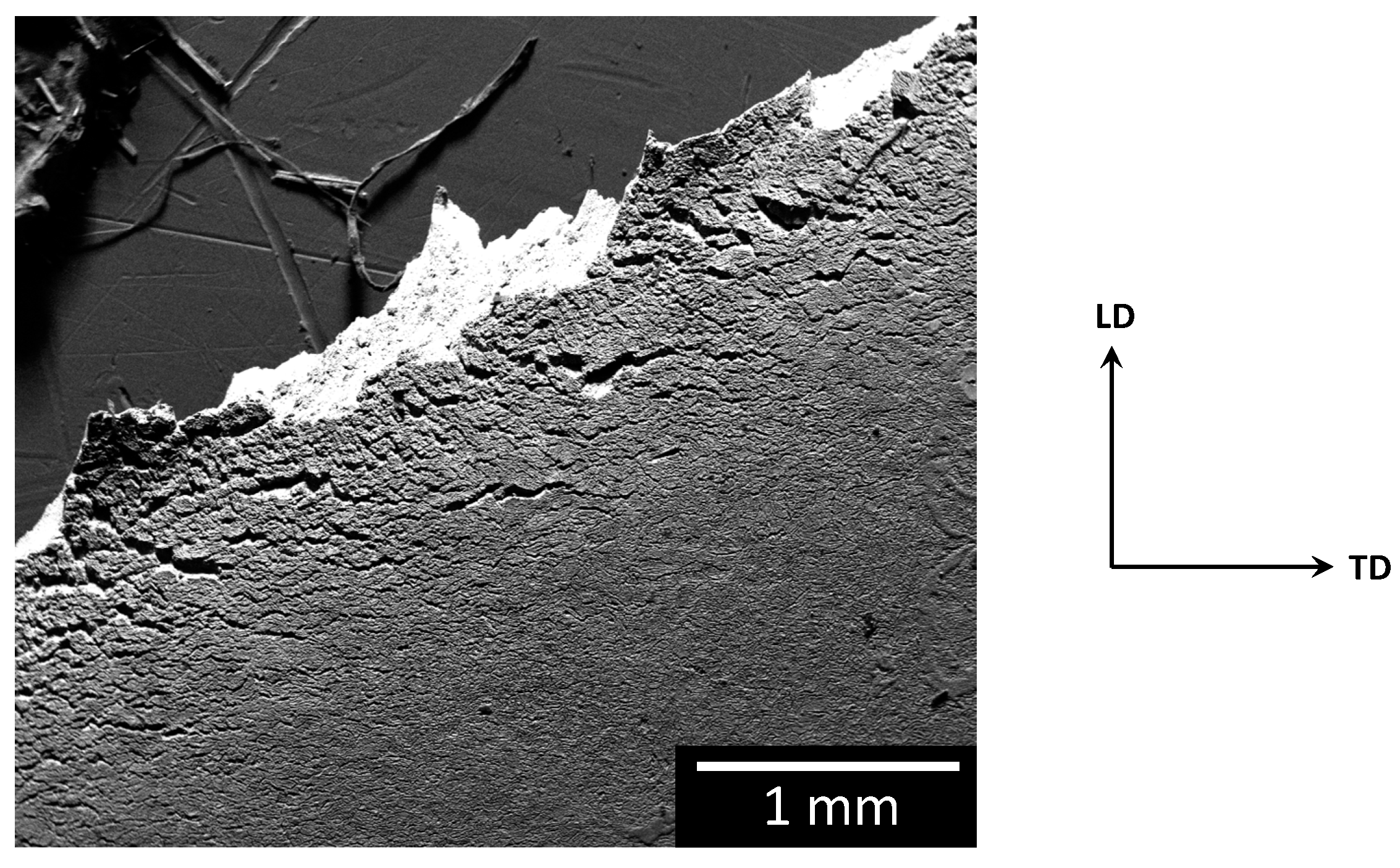
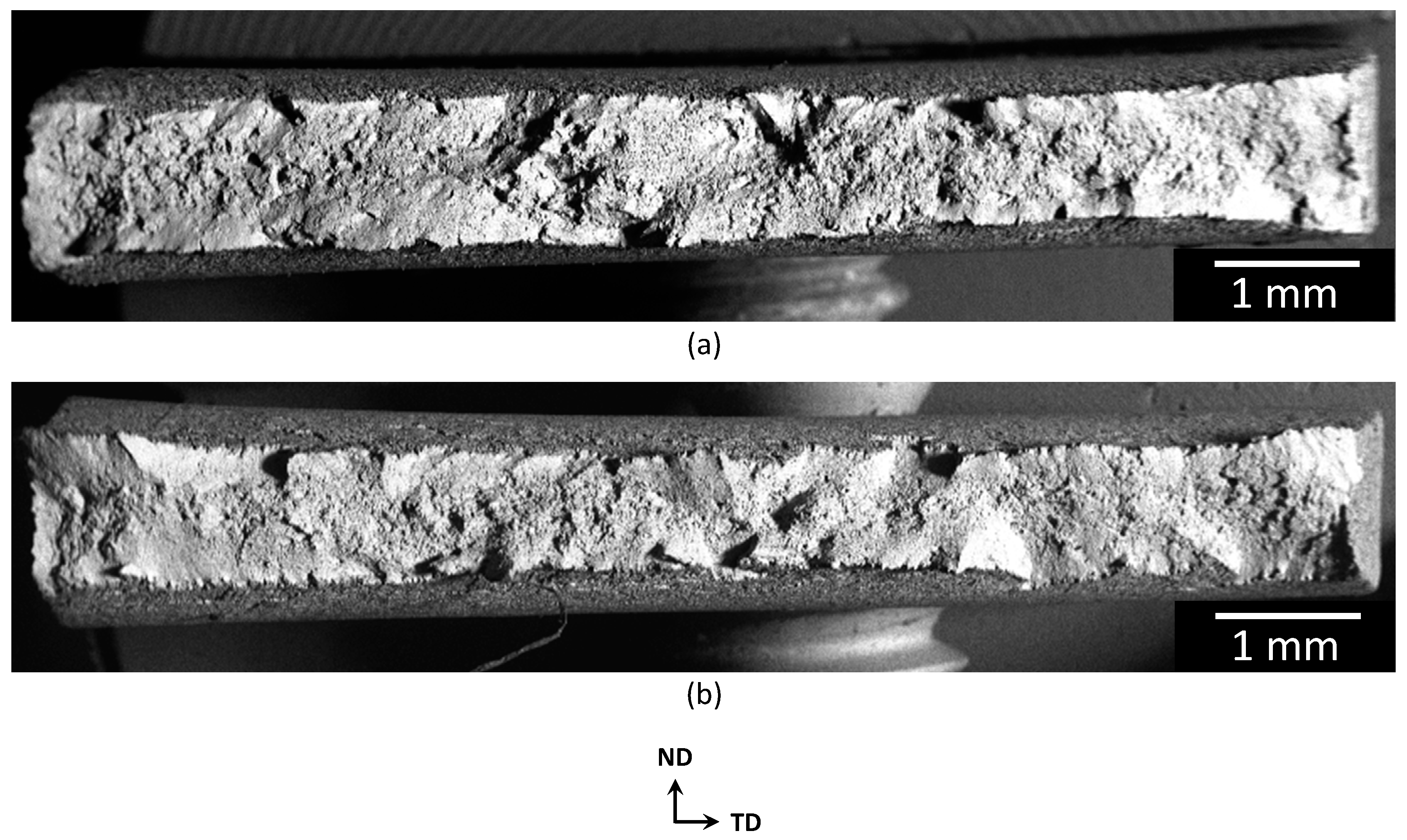
3.2. SCC-Induced Mechanical Behaviour
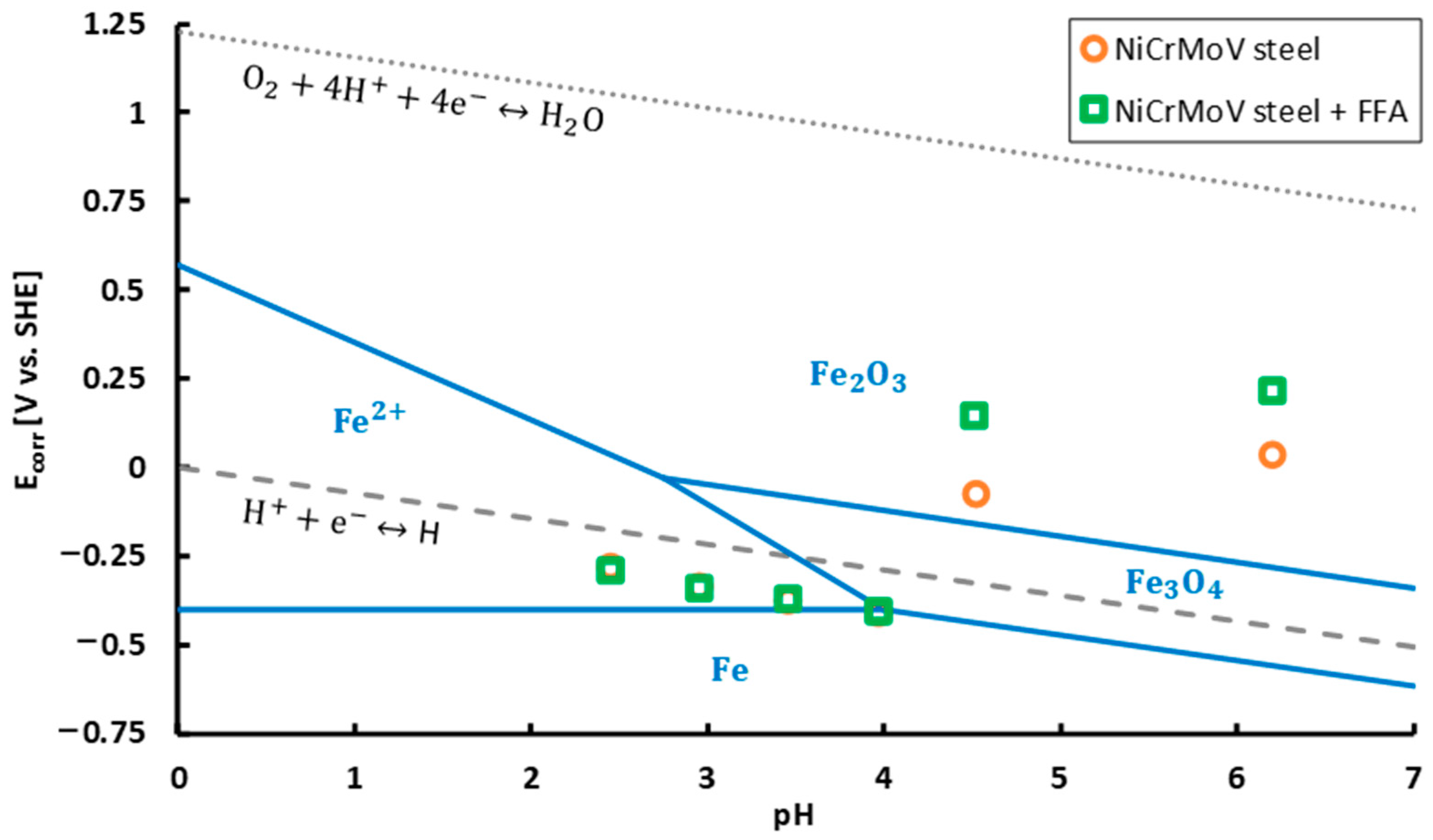
3.3. Evaluation on the Active Mechanism of Corrosion
4. Conclusions
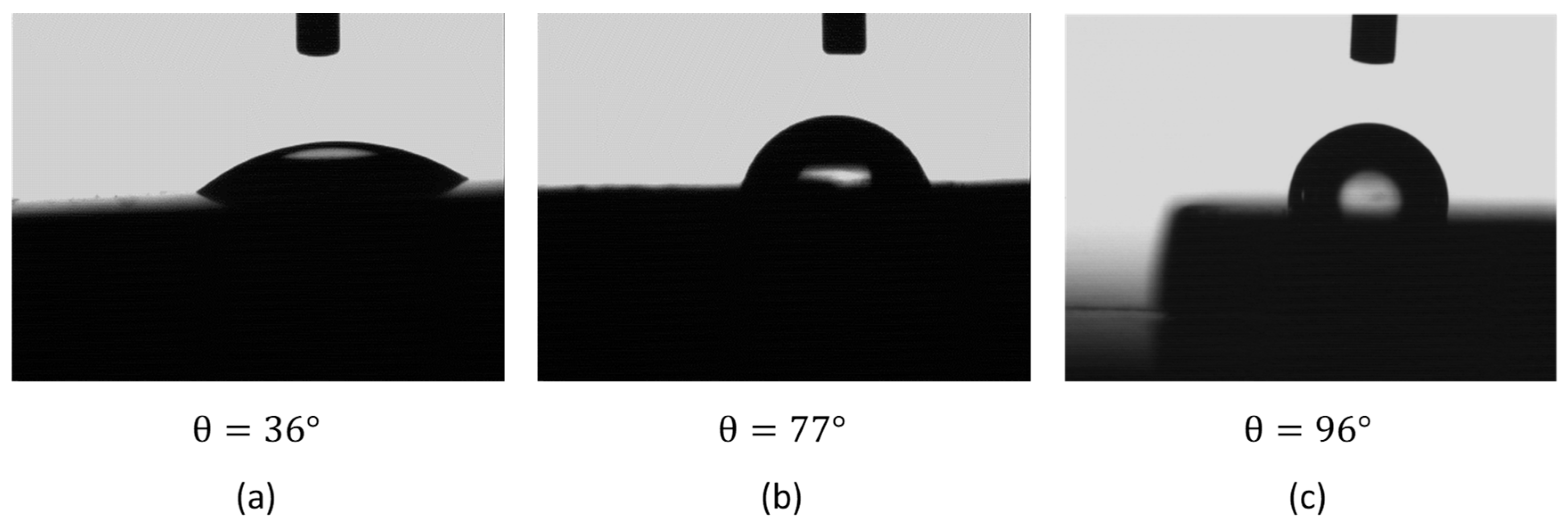
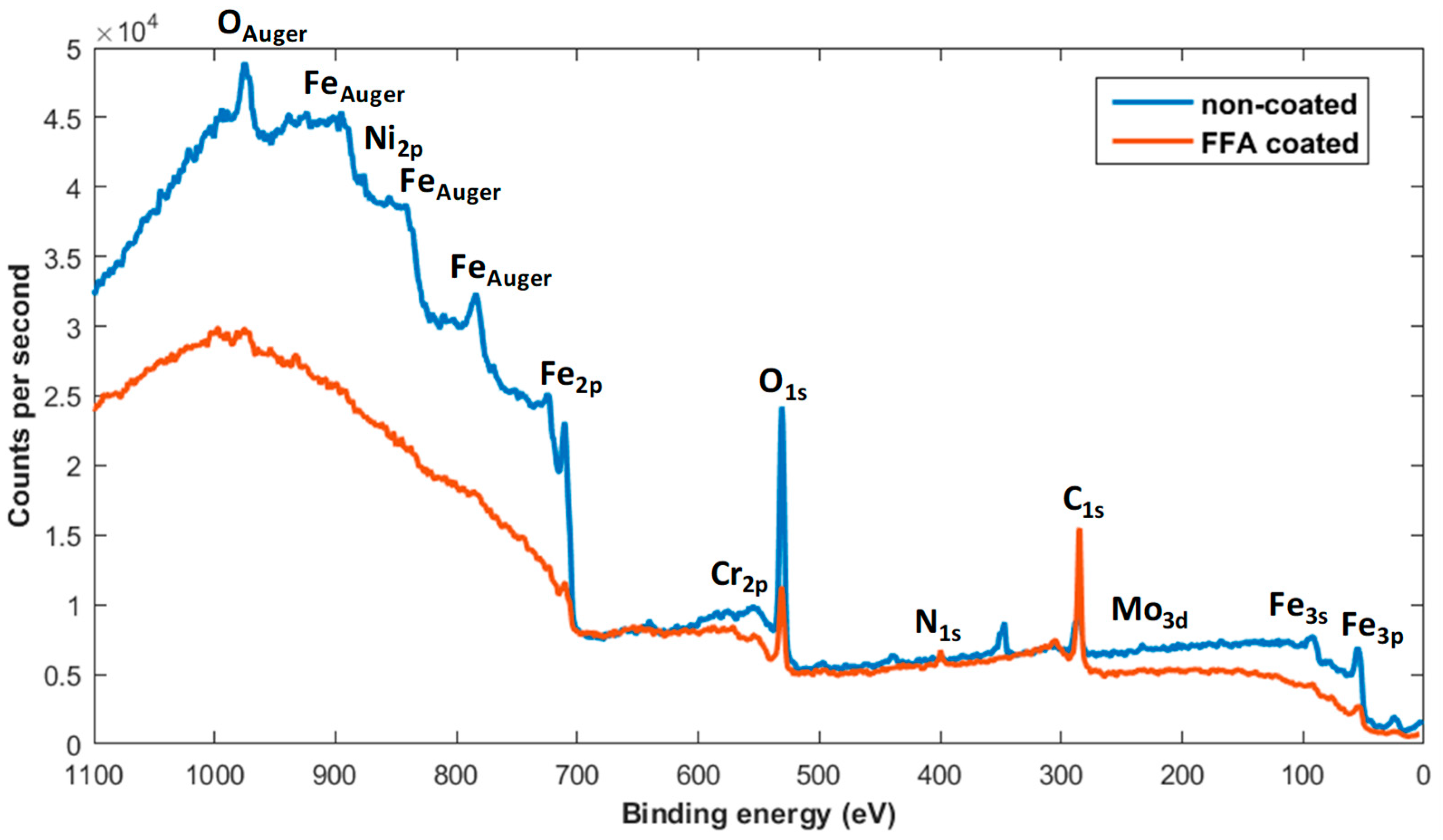
- Applying an FFA coating on the steel by inserting it in a 5-ppm OLDA solution for one day at 90 °C resulted in a contact angle of 96°, compared to 36° without coating, as such showing the expected hydrophobic behaviour when coated. XPS measurements further confirmed the presence of an FFA layer after applying the coating.
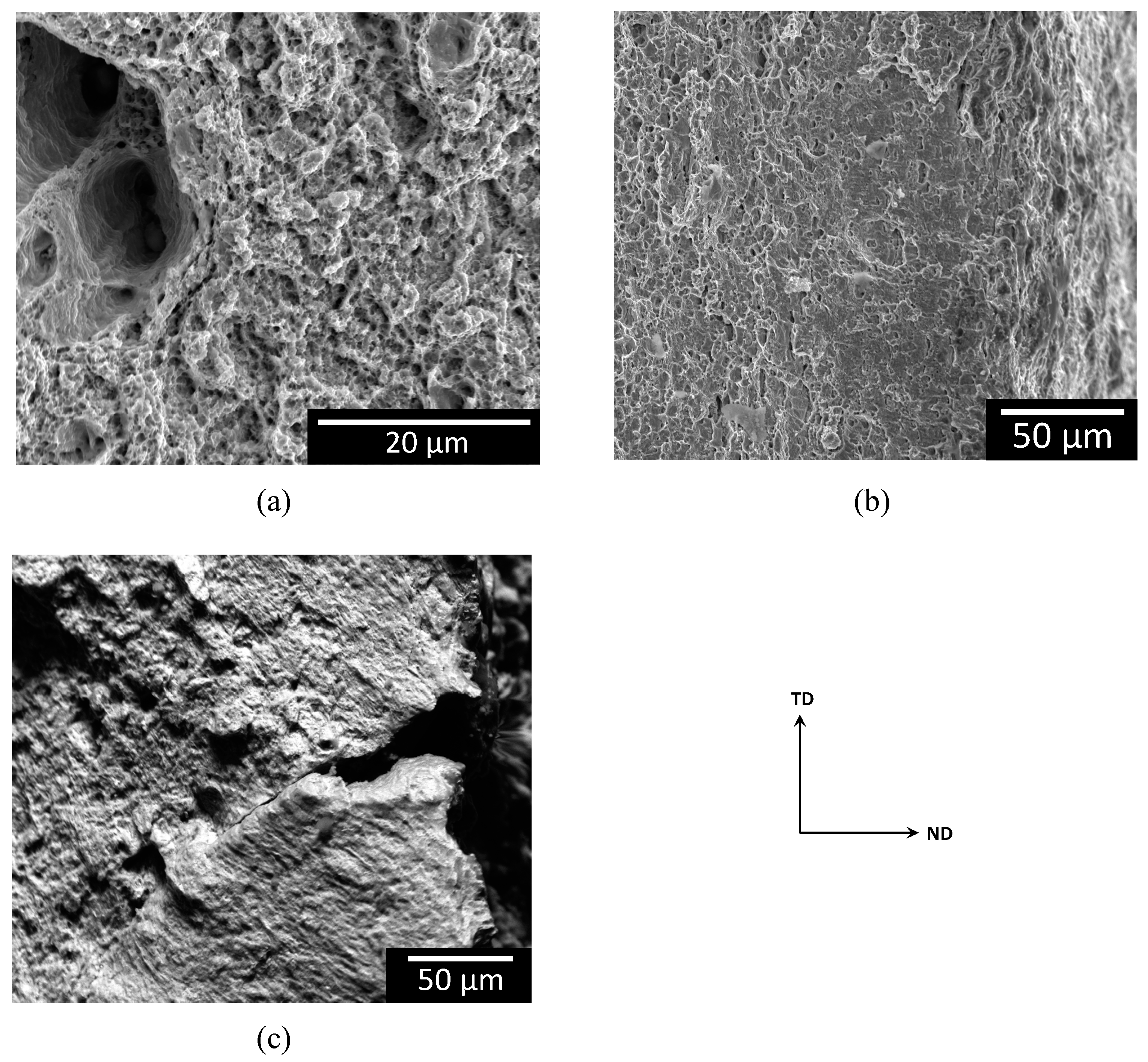
- Based on potentiodynamic measurements, coating the steel with FFA had no significant influence on the thermodynamically stable iron states, neither on the type of active redox reactions, i.e., iron oxidation reaction, oxygen reduction reaction and hydrogen proton reduction reaction. Clearly, both coated and non-coated steel suffer from anodic dissolution and hydrogen embrittlement. In accordance, in addition to ductile dimples, embrittled regions on the fracture surface as well as stress-corrosion cracks on the top surface, which both are linked with hydrogen uptake and embrittlement, were observed both on the non-coated steel and the steel coated with FFA.
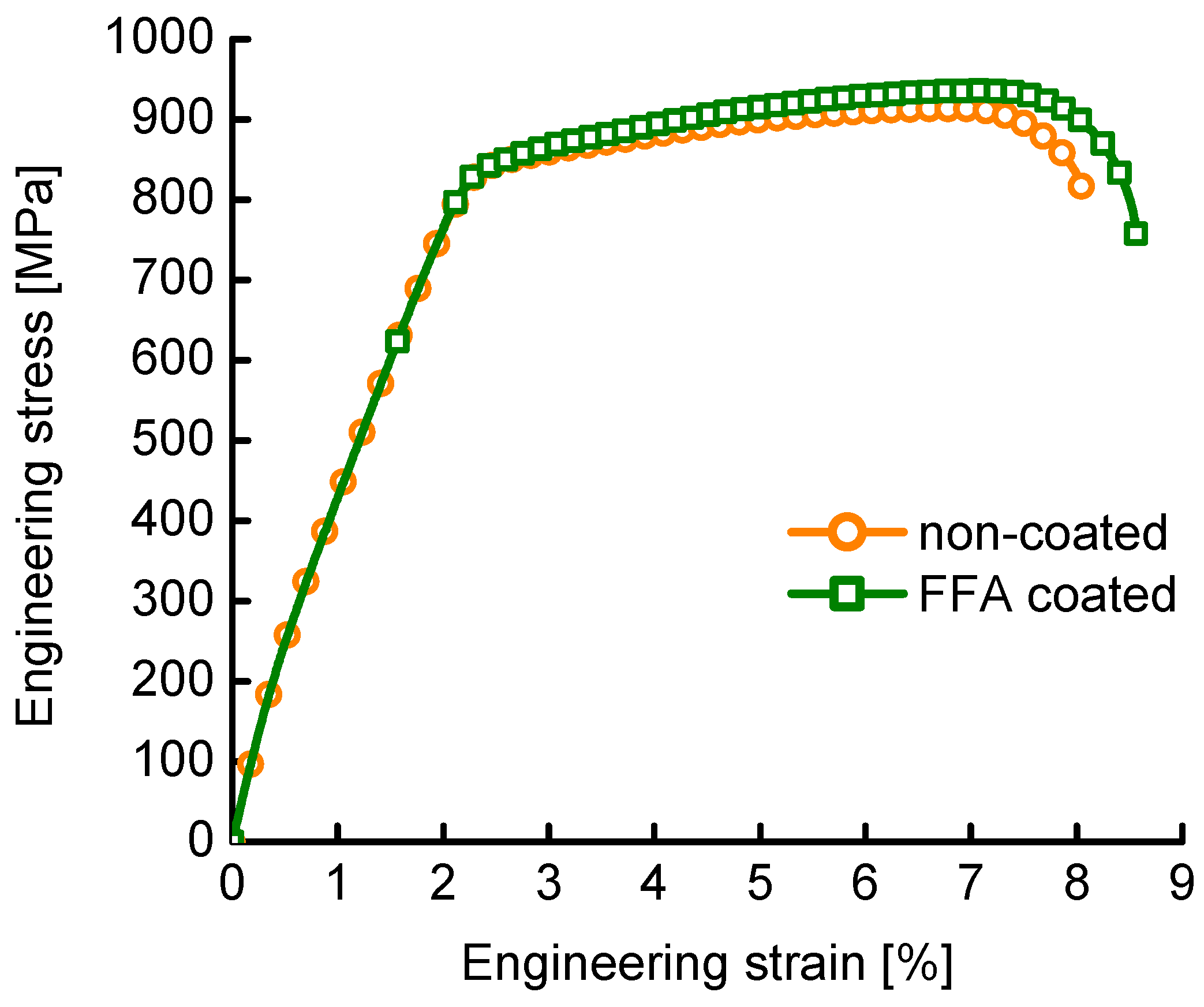
- However, a ductility gain of 13% for FFA-coated steel with respect to non-coated steel when in situ CERT in 0.01 M acetic acid at 90 °C (pH = 3.5) was observed. This ductility gain was explained by the decrease in both corrosion rate and hydrogen uptake when covering the steel with an FFA layer. The FFA is hypothesised to disturb the accessibility of active sites for redox reactions to take place, as such lowering both anodic dissolution and hydrogen embrittlement.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kluck, R.; Torres, J.; Antompietri, A.; Rivera, J. Experiences Using Neutralizing Amines to Control pH and Minimize FAC in a Combined-Cycle Power Plant. Power Plant Chem. 2010, 13, 94–103. [Google Scholar]
- Tsubakizaki, S.; Takada, M.; Suto, T.; Kawashima, H.; Ichihara, T.; Yoshida, A. Achievement on OT (Oxygenated Feed-Water Treatment) Application and Introduction of Countermeasures for Powdered Scale Deposit. Mitsubishi Heavy Ind. Tech. Rev. 2012, 49, 24–28. [Google Scholar]
- United States General Accounting Office. Nuclear Regulation: Action Needed to Ensure That Utilities Monitor and Repair Pipe Damage. GAO/RCED-88-73 Pipe Degradation; 1988. Available online: https://www.gao.gov/assets/150/146211.pdf (accessed on 20 April 2020).
- Buecker, B. Flow-Accelerated Corrosion: A Critical Issue Revisited. Power Eng. 2007, 111, 20–23. [Google Scholar]
- Buecker, B. The Evolution of Power Plant Water/Steam Chemistry. Power Eng. 2012, 116. Available online: https://www.power-eng.com/2012/10/01/the-evolution-power-plant-water-steam-chemistry/#gref (accessed on 19 July 2019).
- Dooley, R.B. Flow-Accelerated Corrosion in Fossil and Combined Cycle/HRSG Plants. Power Plant Chem. 2008, 10, 68–89. [Google Scholar]
- Uchida, S.; Naitoh, M.; Uehara, Y.; Okada, H.; Hiranuma, N.; Sugino, W.; Koshizuka, S.; Lister, D.H. Evaluation Methods for Corrosion Damage of Components in Cooling Systems of Nuclear Power Plants by Coupling Analysis of Corrosion and Flow Dynamics (III): Evaluation of Wall Thinning Rate with the Coupled Model of Static Electrochemical Analysis and Dynamic Double Oxide Layer Analysis. J. Nucl. Sci. Technol. 2009, 46, 31–40. [Google Scholar] [CrossRef]
- Uchida, S.; Naitoh, M.; Okada, H.; Uehara, Y.; Koshizuka, S. Evaluation of flow accelerated corrosion by coupled analysis of corrosion and flow dynamics. Relationship of oxide film thickness, hematite/magnetite ratio, ECP and wall thinning rate. Nucl. Eng. Des. 2011, 241, 4585–4593. [Google Scholar] [CrossRef]
- Jack, M.; Weerakul, S.; Lister, D.H. The Interaction of a Film-Forming Amine with Surfaces of a Recirculating Experimental Water Loop. In Proceedings of the International Conference on Heat Exchanger Fouling and Cleaning, Dublin, Ireland, 7–12 June 2015; pp. 112–118. [Google Scholar]
- Gasnier, C.; Lister, D.H. The Effects of Chemical Additives on Magnetite Deposition in Boiling Heat Transfer. In Proceedings of the International Conference on Heat Exchanger Fouling and Cleaning, Budapest, Hungary, 9–14 June 2013; pp. 85–93. [Google Scholar]
- Gasnier, C. The Effect of Chemical Additives on the Deposition of Magnetite onto Alloy-800 under Nucleate Boiling Heat Transfer, Master of Science in Engineering. Master’s Thesis, The University of New Brunswick, Fredericton, NB, Canada, 2014. [Google Scholar]
- Phosphate and NaOH Treatments for the Steam-Water Circuits of Drum Boilers of Fossil and Combined Cycle/HRSG Power Plants; International Association for the Properties of Water and Steam: Stockholm, Sweden, 2015.
- Baghni, I.M.; Zwebek, A.I. Using of congruent phosphate as equilibrium phosphate boilers water treatment. In Disaster Management and Human Health Risk: Reducing Risk, Improving Outcomes; WIT Press: Southampton, UK, 2009; pp. 159–169. [Google Scholar] [CrossRef]
- Selby, A. Caustic Treatment of Electric Utility Drum Boilers. Mater. Perform. 2009, 48, 39–43. [Google Scholar]
- Dooley, R.B.; Bursik, A. Caustic Gouging. Power Plant Chem. 2010, 12, 188–192. [Google Scholar]
- Nalco Chemical Company. Phosphate Hideout. 1996. Available online: https://www.steamforum.com/pictures/B-268.pdf (accessed on 15 July 2019).
- Dooley, R.B.; Bursik, A. Acid Phosphate Corrosion. Power Plant Chem. 2010, 12, 368–372. [Google Scholar]
- Cooper, J.R.; Dooley, R.B. Procedures for the Measurement of Carryover of Boiler Water into Steam; International Association for the Properties of Water and Steam: Berlin, Germany, 2008. [Google Scholar]
- Jonas, O.; Machemer, L. Steam Turbine Corrosion and Deposits Problems and Solutions. In Proceedings of the 37th Turbomachinery Symposium, Houston, TX, USA, 8–11 September 2008; pp. 211–228. [Google Scholar]
- Hater, W.; Smith, B.; McCann, P.; de Bache, A. Experience with the Application of a Film Forming Amine in the Connah’s Quay Triple Stage Combined Cycle Gas Turbine Power Plant Operating in Cycling Mode. Power Plant Chem. 2018, 20, 136–144. [Google Scholar]
- Asano, T.; Levine, A.D. Wastewater reclamation, recycling and reuse: Past, present, and future. Water Sci. Technol. 1996, 33, 1–14. [Google Scholar] [CrossRef]
- Ma, H.; Allen, H.E.; Yin, Y. Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent. Water Res. 2001, 35, 985–996. [Google Scholar] [CrossRef]
- Anderson, J. The environmental benefits of water recycling and reuse. Water Supply 2003, 3, 1–10. [Google Scholar] [CrossRef]
- Van Lipzig, N.; Willems, P. Actualisatie en Verfijning Klimaatscenario’s Tot 2100 voor Vlaanderen; MIRA: Mechelen, Belgium, 2015. [Google Scholar]
- Unesco. Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017. [Google Scholar]
- Parker, J.G.; Sadler, M. Stress corrosion cracking of a low alloy steel in high purity steam. Corros. Sci. 1975, 15, 57–63. [Google Scholar] [CrossRef]
- McMinn, A.; Lyle, F.F.; Leverant, G.R. Mechanical and metallurgical properties of retired 3.5NiCrMoV low pressure steam turbine discs. J. Mater. Energy Syst. 1984, 6, 184–199. [Google Scholar] [CrossRef]
- Lyle, F.F.; McMinn, A.; Leverant, G.R. Low-Pressure Steam Turbine Disc Cracking—An Update. Proc. Inst. Mech. Eng. Part A Power Process. Eng. 1985, 199, 59–67. [Google Scholar] [CrossRef]
- Liu, C.; Macdonald, D.D. Prediction of Failures of Low-Pressure Steam Turbine Disks. J. Press. Vessel. Technol. 1997, 119, 393–400. [Google Scholar] [CrossRef]
- McCloskey, T.H. Troubleshooting Turbine Steam Path Damage Mechanisms. In Turbomachinery Laboratories; Texas A&M University: College Station, TX, USA, 2002; pp. 105–144. [Google Scholar] [CrossRef]
- Dooley, R.B.; Bursik, A. Proceedings of the International Conference on the Interaction of Organics and Organic Cycle Treatment Chemicals with Water, Steam and Materials; Stuttgart, Germany, 4–6 October 2005; Electric Power Research Institute: Palo Alto, CA, USA, 2005. [Google Scholar]
- Savelkoul, J.; van Lier, R. Operational Experience with Organics in Industrial Steam Generation. Power Plant Chem. 2005, 7, 733–739. [Google Scholar]
- Zhou, S.; Turnbull, A. Steam Turbine Operating Conditions, Chemistry of Condensates, and Environment Assisted Cracking—A Critical Review; NPL Report MATC (A). 95; National Physical Laboratory: Teddington, Middlesex, UK, 2002. [Google Scholar]
- Huijbregts, W.M.M.; Leferink, R.G.I. Latest advances in the understanding of acid dewpoint corrosion: Corrosion and stress corrosion cracking in combustion gas condensates. Anti-Corros. Methods Mater. 2004, 51, 173–188. [Google Scholar] [CrossRef]
- Technical Guidance Document: Steam Purity for Turbine Operation; International Association for the Properties of Water and Steam: London, UK, 2013.
- Svoboda, R.; Gabrielli, F.; Hehs, H.; Seipp, H.-G.; Leidich, F.-U.; Roberts, B. Organic Impurities and Organic Conditioning Agents in the Steam/Water Cycle: A Power Plant Manufacturer’s Point of View. Power Plant Chem. 2006, 8, 502–509. [Google Scholar]
- Palmer, D.A.; Marshall, S.L.; Simonson, J.M.; Gruszkiewicz, M.S. The Partitioning of Acetic, Formic, and Phosphoric Acids between Liquid Water and Steam; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2008. [Google Scholar]
- Moed, D.H.; Verliefde, A.; Heijman, S.G.; Rietveld, L.C. Organic acid formation in steam–water cycles: Influence of temperature, retention time, heating rate and O2. Appl. Therm. Eng. 2014, 65, 194–200. [Google Scholar] [CrossRef]
- Moed, D.H.; Verliefde, A.R.D.; Rietveld, L.C. Effects of Temperature and Pressure on the Thermolysis of Morpholine, Ethanolamine, Cyclohexylamine, Dimethylamine, and 3-Methoxypropylamine in Superheated Steam. Ind. Eng. Chem. Res. 2015, 54, 2606–2612. [Google Scholar] [CrossRef]
- De Meyer, E.; Peeters, B.; Vanoppen, M.; Verbeken, K.; Verliefde, A.R.D. Organic Matter Composition More Important than Concentration in Ion Exchange Demineralization of Different Water Qualities for the Production of Steam. Ind. Eng. Chem. Res. 2018, 57, 3742–3752. [Google Scholar] [CrossRef]
- De Meyer, E.; Van Overstraeten, T.; Heyse, J.; Uddin, M.R.; Vanoppen, M.; Boon, N.; De Gusseme, B.; Verbeken, K.; Verliefde, A.R.D. Organic Matter and Microbial Cell Density Behavior during Ion Exchange Demineralization of Surface Water for Boiler Feedwater. Ind. Eng. Chem. Res. 2019, 58, 14368–14379. [Google Scholar] [CrossRef]
- Betova, I.; Bojinov, M.; Saario, T. Film-Forming Amines in Steam/Water Cycles—Structure, Properties, and Influence on Corrosion and Deposition Processes; VTT Technical Research Centre of Finland: Espoo, Finland, 2014. [Google Scholar]
- Pensini, E.; Van Lier, R.; Cuoq, F.; Hater, W.; Halthur, T. Enhanced corrosion resistance of metal surfaces by film forming amines: A comparative study between cyclohexanamine and 2-(diethylamino)ethanolbased formulations. Water Resour. Ind. 2018, 20, 93–106. [Google Scholar] [CrossRef]
- Van Lier, R.; Cuoq, F.; Peters, R.; Savelkoul, J. Ten Years of Experience with Polyamines in the High-Pressure Steam System of a Naphta Cracker. Power Plant Chem. 2015, 17, 356–363. [Google Scholar]
- Hater, W.; Olivet, D. The chemistry and properties of organic boiler feed water additives based on film-forming amines, and their use in steam generators. In Proceedings of the ICPWS XV, Berlin, Germany, 7–11 September 2008; p. 9. [Google Scholar]
- Stiller, K.; Wittig, T.; Urschey, M. The Analysis of Film-Forming Amines—Methods, Possibilities, Limits and Recommendations. Power Plant Chem. 2011, 13, 602–611. [Google Scholar]
- Odar, S. Use of Film Forming Amines (FFA) in Nuclear Power Plants for Lay-Up and Power Operation; Advanced Nuclear Technology International: Molnlycke, Sweden, 2017. [Google Scholar]
- Smith, B.; McCann, P.; Uchida, K.; Mori, S.; Jasper, J.; Hater, W. Determination of Oleyl Propylenediamine, a Commonly Used Film Forming Amine, on the Surfaces of Water-Steam Cycles. Power Plant Chem. 2017, 19, 129–140. [Google Scholar]
- Riznic, J. Steam Generators for Nuclear Power Plants; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Technical Guidance Document: Application of Film Forming Substances in Fossil and Combined Cycle Plants; International Association for the Properties of Water and Steam: Banff, AB, Canada, 2019.
- Steam Chemistry: Interaction of Chemical Species with Water, Steam, and Materials during Evaporation, Superheating, and Condensation; Electric Power Research Institute: Freiburg, Germany, 1999.
- Tomarov, G.V.; Mikhailov, A.V.; Velichko, E.V.; Budanov, V.A. Extending the erosion-corrosion service life of the tube system of heat-recovery boilers used as part of combined-cycle plants. Therm. Eng. 2010, 57, 22–27. [Google Scholar] [CrossRef]
- Bursik, A.; Hater, W. All-Volatile Treatment with Film Forming Amines—A First Suggestion for an Application Guidance. Power Plant Chem. 2015, 17, 342–353. [Google Scholar]
- International Association for the Properties of Water and Steam. Technical Guidance Document: Application of Film Forming Amines in Fossil and Combined Cycle Plants; International Association for the Properties of Water and Steam: Dresden, Germany, 2016. [Google Scholar]
- Dooley, B.; Lister, D. Flow-Accelerated Corrosion in Steam Generating Plants. Power Plant Chem. 2018, 20, 194–242. [Google Scholar]
- Lister, D. Flow-accelerated corrosion in power plants: The influence of corrosion-product oxides. In Proceedings of the EUROCORR 2019 The Annual Congress of the European Federation of Corrosion, Seville, Spain, 9–13 September 2019; p. 20. [Google Scholar]
- Weerakul, S.; Leaukosol, N.; Lister, D.H.; Mori, S.; Hater, W. Effects on Flow-Accelerated Corrosion of Oleylpropanediamine Under Single-Phase Water Conditions Pertinent to Power Plant Feedwater. Corrosion 2020, 76, 217–230. [Google Scholar] [CrossRef]
- Foret, C.; Stoianovici, G.; Chaussec, G.; de Bache, A.; Kolk, C.Z.; Hater, W. Study of Efficiency and Stability of Film Forming Amines (FFA) for the Corrosion Protection of the Carbon Steel in Water Circuits. In Proceedings of the Edinburgh International Conference Centre, Edinburgh, UK, 7–11 September 2008; p. 12. [Google Scholar]
- De Meyer, E.; De Seranno, T.; Hater, W.; Vanoppen, M.; Verbeken, K.; Verliefde, A.R.D. Degradation of oleyl-propylenediamine under steam generator conditions. 2020. submitted. [Google Scholar]
- De Seranno, T.; Lambrechts, E.; Depover, T.; Verliefde, A.R.D.; Verbeken, K. SCC of steam turbine steel in acidic aqueous environment. In Proceedings of the EUROCORR 2019 The Annual Congres of the European Federation of Corrosion, Seville, Spain, 9–13 September 2019; p. 10. [Google Scholar]
- De Seranno, T.; Lambrechts, E.; Verliefde, A.; DePover, T.; Verbeken, K. Mechanistic interpretation on acidic stress-corrosion cracking of NiCrMoV steam turbine steel. Mater. Sci. Eng. A 2020, 140433. [Google Scholar] [CrossRef]
- Bezzoli, P.; Cramer, K. Organic Plant Cycle Treatment Chemicals—A Power Plant Chemistry Interview. Power Plant Chem. 2009, 11, 45–47. [Google Scholar]
- De Meyer, E. The Behavior of Organic Matter in Industrial Demineralization and Steam-Water Cycles. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2020. [Google Scholar]
- Gras, J.M.; Vaillant, F.; Dordonat, M.; Dury, J.P. Stress Corrosion Cracking of Turbine Disc Steels: A Study of Mechanism; Electricité de France: Direction Des Etudes et Recherches; Service Réacteurs Nucléaires et Echangeurs: Département Etude Des Matériaux, 1993; Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/27/000/27000428.pdf?r=1 (accessed on 21 November 2019).
- Ghobeira, R.; Philips, C.; Liefooghe, L.; Verdonck, M.; Asadian, M.; Cools, P.; Declercq, H.; De Vos, W.H.; De Geyter, N.; Morent, R. Synergetic effect of electrospun PCL fiber size, orientation and plasma-modified surface chemistry on stem cell behavior. Appl. Surf. Sci. 2019, 485, 204–221. [Google Scholar] [CrossRef]
- De Wispelaere, M. Early Condensate in a Fossil Power Plant using organic treatment. In Proceedings of the 14th International Conference on the Properties of Water and Steam, Kyoto, Japan, 29 August–3 September 2004; pp. 602–605. [Google Scholar]
- Iannuzzi, M. Environmentally assisted cracking (EAC) in oil and gas production. In Stress Corrosion Cracking; Elsevier: Amsterdam, The Netherlands, 2011; pp. 570–607. [Google Scholar] [CrossRef]
- De Seranno, T.; Vandewalle, L.; DePover, T.; Verliefde, A.R.; Verbeken, K. Mechanical degradation of Fe-C-X steels by acidic stress-corrosion cracking. Corros. Sci. 2020, 167, 108509. [Google Scholar] [CrossRef]
- De Seranno, T.; DePover, T.; Verliefde, A.R.; Verbeken, K. Evaluation of the active mechanism for acidic SCC induced mechanical degradation: A methodological approach. Mater. Sci. Eng. A 2020, 790, 139645. [Google Scholar] [CrossRef]
- DePover, T.; Escobar, D.M.P.; Wallaert, E.; Zermout, Z.; Verbeken, K. Effect of hydrogen charging on the mechanical properties of advanced high strength steels. Int. J. Hydrogen Energy 2014, 39, 4647–4656. [Google Scholar] [CrossRef]
- Baux, J.; Caussé, N.; Esvan, J.; Delaunay, S.; Tireau, J.; Roy, M.; You, D.; Pébère, N. Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochim. Acta 2018, 283, 699–707. [Google Scholar] [CrossRef]
- Weinhold, F.; Klein, R.A. What is a hydrogen bond? Resonance covalency in the supramolecular domain. Chem. Educ. Res. Pract. 2014, 15, 276–285. [Google Scholar] [CrossRef]
- Vinutha, M.R.; Venkatesha, T.V. Review on Mechanistic Action of Inhibitors on Steel Corrosion in Acidic Media. Port. Electrochim. Acta 2016, 34, 157–184. [Google Scholar] [CrossRef]
- Jäppinen, E.; Ikäläinen, T.; Järvimäki, S.; Saario, T.; Sipilä, K.; Bojinov, M. Corrosion Behavior of Carbon Steel Coated with Octadecylamine in the Secondary Circuit of a Pressurized Water Reactor. J. Mater. Eng. Perform. 2017, 26, 6037–6046. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Vanderleyden, E.; Dubruel, P.; Morent, R. Surface Analysis of Titanium Cleaning and Activation Processes: Non-thermal Plasma Versus Other Techniques. Plasma Chem. Plasma Process. 2014, 34, 917–932. [Google Scholar] [CrossRef]
- Topp, H. On the Interaction of Chemically Conditioned Water with Steel Heating Surfaces during Saturated Pool Boiling—An Experimental Thermotechnical Approach. Ph.D. Thesis, University of Rostock, Rostock, Germany, 2010. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Van Gorp, A.C. Influence of Heat Treatment on the Fracture Toughness of AA6061 and AA6061 mmc. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 1999. [Google Scholar]
- Beachem, C.; Meyn, A. Fracture by microscopic plastic deformation processes. In Electron Fractography; Beachem, C., Ed.; ASTM International: Conshohocken, PA, USA, 1968; pp. 59–88. [Google Scholar]
- Atrens, A.; Venezuela, J.; Liu, Q.; Zhou, Q.; Verbeken, K.; Tapia-Bastidas, C.; Gray, E.; Christien, F.; Wolski, K. Electrochemical and Mechanical Aspects of Hydrogen Embrittlement Evaluation of Martensitic Steels. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 201–225. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions. 1974. Available online: https://linkinghub.elsevier.com/retrieve/pii/0022072867800597 (accessed on 6 January 2020).
- Jones, R.H. Stress-Corrosion Cracking; ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
- Was, G.S. Fundamentals of Radiation Materials Science: Metals and Alloys; Springer: Berlin, Germany; New York, NY, USA, 2007. [Google Scholar]
- Lynch, S.P. Mechanistic and fractographic aspects of stress-corrosion cracking (SCC). In Stress Corrosion Cracking: Theory and Practice; Woodhead Publishing: Sawston, UK, 2011; pp. 3–89. [Google Scholar] [CrossRef]
- Cheng, Y.F. Fundamentals of Stress Corrosion Cracking. In Stress Corrosion Cracking of Pipelines; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 7–41. [Google Scholar] [CrossRef]
- Newman, J.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Alonso-Vante, N.; Roldán, C.A.C.; de Guadalupe González Huerta, R.; Sánchez, G.R.; Robledo, A.M. Fundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental Protection; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Santos, E.; Hindelang, P.; Quaino, P.; Schmickler, W. A model for the Heyrovsky reaction as the second step in hydrogen evolution. Phys. Chem. Chem. Phys. 2011, 13, 6992–7000. [Google Scholar] [CrossRef]
- Djukic, M.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- De Seranno, T.; Vander Vennet, S.; Claeys, L.; Verliefde, A.R.D.; Depover, T.; Verbeken, K. Experimental confirmation of the role of hydrogen in acidic stress-corrosion cracking of low carbon steel. 2020. submitted. [Google Scholar]
- Bäßler, R.; Uhlemann, M.; Mummert, K. Inhibiting effect of octadecylamine on pitting corrosion behaviour of stainless steel type 1.4541 up to 250 °C. Mater. Corros. 1999, 50, 146–153. [Google Scholar] [CrossRef]



| C | Ni | Cr | Mo | V | Fe |
|---|---|---|---|---|---|
| 0.27 | 3.70 | 1.50 | 0.35 | 0.10 | Balance |
| Fe | C | N | O | Ni | Cr | Mo | V | |
|---|---|---|---|---|---|---|---|---|
| non-coated | 15.9 | 30.9 | 0.5 | 51.8 | 0.4 | 0.4 | 0.1 | Trace element |
| FFA coated | 3.1 | 71.5 | 3.6 | 21.4 | 0.2 | 0.1 | 0.1 | Trace element |
| FFA Coating | Environment | ||
|---|---|---|---|
| no | demi water | 0.040 | 0.4 |
| no | 10−4 M acetic acid | −0.056 | 2.0 |
| no | 10−3 M acetic acid | −0.405 | 5.2 |
| no | 10−2 M acetic acid | −0.374 | 13.9 |
| no | 10−1 M acetic acid | −0.342 | 44.3 |
| no | 1 M acetic acid | −0.299 | 135.7 |
| yes | demi water | 0.214 | 0.3 |
| yes | 10−4 M acetic acid | 0.147 | 1.6 |
| yes | 10−3 M acetic acid | −0.405 | 4.4 |
| yes | 10−2 M acetic acid | −0.372 | 12.9 |
| yes | 10−1 M acetic acid | −0.339 | 32.0 |
| yes | 1 M acetic acid | −0.291 | 113.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Seranno, T.; Lambrechts, E.; De Meyer, E.; Hater, W.; De Geyter, N.; Verliefde, A.R.D.; Depover, T.; Verbeken, K. Effect of Film-Forming Amines on the Acidic Stress-Corrosion Cracking Resistance of Steam Turbine Steel. Metals 2020, 10, 1628. https://doi.org/10.3390/met10121628
De Seranno T, Lambrechts E, De Meyer E, Hater W, De Geyter N, Verliefde ARD, Depover T, Verbeken K. Effect of Film-Forming Amines on the Acidic Stress-Corrosion Cracking Resistance of Steam Turbine Steel. Metals. 2020; 10(12):1628. https://doi.org/10.3390/met10121628
Chicago/Turabian StyleDe Seranno, Tim, Ellen Lambrechts, Evelyn De Meyer, Wolfgang Hater, Nathalie De Geyter, Arne R. D. Verliefde, Tom Depover, and Kim Verbeken. 2020. "Effect of Film-Forming Amines on the Acidic Stress-Corrosion Cracking Resistance of Steam Turbine Steel" Metals 10, no. 12: 1628. https://doi.org/10.3390/met10121628
APA StyleDe Seranno, T., Lambrechts, E., De Meyer, E., Hater, W., De Geyter, N., Verliefde, A. R. D., Depover, T., & Verbeken, K. (2020). Effect of Film-Forming Amines on the Acidic Stress-Corrosion Cracking Resistance of Steam Turbine Steel. Metals, 10(12), 1628. https://doi.org/10.3390/met10121628








