1. Introduction
High market demands related to material quality and properties strongly influence redesigning of common safety loaded aluminum alloy castings. The quality of aluminum components and associated obtained mechanical properties are strongly dependent on the casting process and parameters, as well as on the chemical composition. Therefore, the redesigning of chemical composition of high-strength aluminum alloys becomes significant for safety critical structural components in automotive industry.
Common AlSi7Mg alloy represents a frequent choice for complex geometry castings with high properties demand due to excellent castability and favorable relation between strength and weight, especially in the heat-treated state. Synergy of alloying and trace elements contributes to the wide range of intermetallic phase evolution [
1,
2,
3,
4,
5,
6]. The content of secondary alloying elements (Mg, Cu) significantly changes the solidification path of the Al-Si alloy [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. The addition of secondary elements, melt treatment, and solidification conditions have a synergy effect on the crystallization kinetic and therefore on the properties’ development as well [
20,
21,
22,
23,
24,
25,
26]. Intermetallic phase development could be attributed to the constitutional undercooling and its equilibrium partition coefficient,
k. When compared to existing solid phase, bulk liquid becomes enriched in solute elements and can originate different intermetallic phases and microsegregation during solidification process. Coexistence of number of complex intermetallic phases and their solidification sequence in correlation to cooling/solidification conditions influence on achieved final properties.
The solidification sequence of hypoeutectic AlSi7Mg alloys begins with development of primary aluminum dendrites αAl and formation of dendritic network, followed by the eutectic reaction (α
Al + β
Si) on the primary grains α
Al or independently on a present nucleant rich in iron and/or other impurities with different crystallographic orientation [
8]. The primary and most important alloying element in conventional AlSi7Mg alloy is Si, which is characterized by high fluidity and reduction in shrinkage, followed by Mg, responsible for strength increase especially in heat-treated state [
8,
9,
10,
11].
Magnesium addition up to a 0.7 wt.% has a strengthening effect through the precipitation of α
Al + Mg
2Si eutectic phase and/or Mg-rich intermetallic with other alloying elements mostly due to transformation of the deleterious Al
5FeSi platelets into a ramified morphology with stoichiometry Al
8Mg
3FeSi
6. [
9,
12,
13]. High Mg content comprehends to the Al
8Mg
5 phase precipitation at the grain boundaries [
14]. The yield strength, tensile strength, and elongation of the as-cast Al-Si-Mg alloys can vary with the Mg content [
15]. The yield strength increases with increasing of Mg content, although no significant influence with increasing the Mg
2Si phase content has been determined. Conversely, the elongation decreases with an increase in the Mg and Mg
2Si ratio [
15].
Additionally, Cu is a frequent addition as a secondary alloying element in order to increase the strength of cast alloys, especially when heat treatment is applied. In Al-Si alloys, Cu is usually added in levels between 1.5–3.5 wt.%, thus forming the intermetallic phase Al
2Cu [
16,
17,
18]. Copper significantly decreases the solidus and eutectic temperature of an alloy. Therefore, the copper enlarges the solidification interval of an alloy and facilitates the condition of porosity formation [
19,
26,
27]. The literature survey reveals a number of investigations related to the influence of alloying elements on the AlSi7Mg alloy properties [
28,
29,
30]. A correlation of equilibrium phase diagram modelling, thermal analysis results, and microconstituent identification allows the determination of the thermodynamic stability of an alloy and its behavior [
5,
31,
32,
33,
34]. The conventional AlSi7Mg alloy, corresponding to numerous standards (EN 1706, IDM 4234) [
35,
36], has been investigated previously [
37]. Requested chemical composition is indicated in
Table 1.
Automotive producers’ standard [
36] narrows the requested content interval of particular elements such as Mg, while at the same time, it widens the Fe content range, whose phases in corresponding morphologies can contribute to the aluminum alloys strength increase. Demand for mechanical properties of AlSi7Mg alloy, in particular yield strength (R
p0.2), tensile strength (R
m), and elongation relating to 50 mm test length for separately cast samples in as-cast state (A
50), shown in
Table 2, is strictly prescribed by norm (EN 1706) [
35,
38].
An innovative approach in this investigation was pointed into the redesigning the chemical composition of common AlSi7Mg alloy in order to improve its mechanical properties. With this aim, an extra addition of Cu (1.435 wt.%) as a secondary alloying element, has been added. The hypothesis of this investigation was that higher content of Cu will modify the solidification sequence of innovative AlSi7MgCu alloy due to numerous additional interactions. The consequence of presumed interactions reflects in the development of complex but morphologically favorable intermetallic phases [
39,
40]. Innovative approach obtained with higher Cu content in AlSi7MgCu alloy reveals an improvement in the requested tensile strength already in as-cast state.
2. Materials and Methods
Characterization of solidification sequence of the newly designed AlSi7MgCu alloy has been performed through the correlation of equilibrium phase diagram modelling, the results of simultaneous thermal analysis, XRD analysis, and microstructural analysis.
Thermodynamic calculations of equilibrium and Scheil–Gulliver non-equilibrium phase diagram of AlSi7MgCu alloy have been performed by Thermo-Calc software TCW 5.0 (Thermo-Calc Software, Stockholm, Sweden), with database TTAL7.
The newly designed AlSi7MgCu alloy melt was prepared in an induction furnace ABB IMTK 2000 (ABP Induction Systems GmbH, Dortmund, Germany). Charge material and return material ratio was 1:1. After melt preparation (770 ± 5 °C), degassing was performed with the nitrogen (N2) using an MTS 1500—Foseco melt treatment system (Vesuvius GmbH - Foseco Foundry Division, Borken, Germany). Melt treatment consists of inoculation with AlTi5B master alloy and modification with AlSr10 master alloy. Chemical composition analysis was performed using optical emission spectrometer ARL-3460 (Thermo ScientificTM, Waltham, MA, USA). Uncertainty of measurements obtained by certification with reference material has been indicated in results for every particular element.
Metallographic investigation samples were prepared by using standard metallographic preparation procedure by grinding and polishing, followed by etching in 0.5% HF. Metallographic analyses cover optical microscopy analysis (Olympus GX 51; Olympus Europa SE & Co. KG, Hamburg, Germany) and microstructural investigations using a standard method with a scanning electron microscope equipped with an energy dispersive X-ray spectrometer (SEM-EDS, Tescan Vega TS 5136 MM; TESCAN ORSAY HOLDING, a.s., Brno, Czech Republic). Standard SEM-EDS analysis allows mapping analysis of the characteristic region using EDS detector. Focused ion beam scanning electron microscopes (FIB-SEM, Helios NanoLab G4 DualBeamTM; Thermo Fisher Scientific, Materials & Structural Analysis Division, Hillsboro, OR, USA) were equipped with 3D slicing/imaging technology. The 3D data were reconstructed using Avizo software, and analysis was subsequently performed in order to visualize 3D distribution of intermetallic phases in the corresponding region of interest. Slices with 15 nm thickness, obtained by FIB milling (V = 19 × 12 × 12 μm3), were analyzed. The number of SE images was analyzed by FEI AVIZO software in order to obtain 3D reconstruction of phases’ distribution. EDS analysis was applied in order to identify chemical composition of particular phases and to predict the resulted stoichiometry in correlation to corresponding morphologies.
Transformation temperatures were obtained by simultaneous thermal analysis, differential scanning calorimetry/thermogravimetry, with instrument STA DSC/TG, NETZSCH Jupiter 449 F1 (NETZSCH Holding, Selb, Germany). Temperature and sensitivity calibration files were created with measurements of 8 different pure materials: In, Sn, Bi, Pb, Zn, Al, Ag, and Ni. The resolution of DSC instrument was 1 µm and temperature resolution 0.001 K. Base line was determined with alumina pans. Dynamic measurements were performed in temperature interval from 25 to 710 °C, in argon atmosphere, with a heating/cooling rate of 10 °C/min. Measurements were repeated 2 times, and the results of the second measurement were presented as referent obtained values.
X-ray diffraction (XRD) technique was used for additional identification of the phases present in investigated alloys. PANalytical X’Pert PRO diffractometer (Malvern Panalytical Ltd, Malvern, UK) with non-monochromated X-rays produced by Empyrean Cu anode tube operating at 45 kV and 40 mA were used in this investigation.
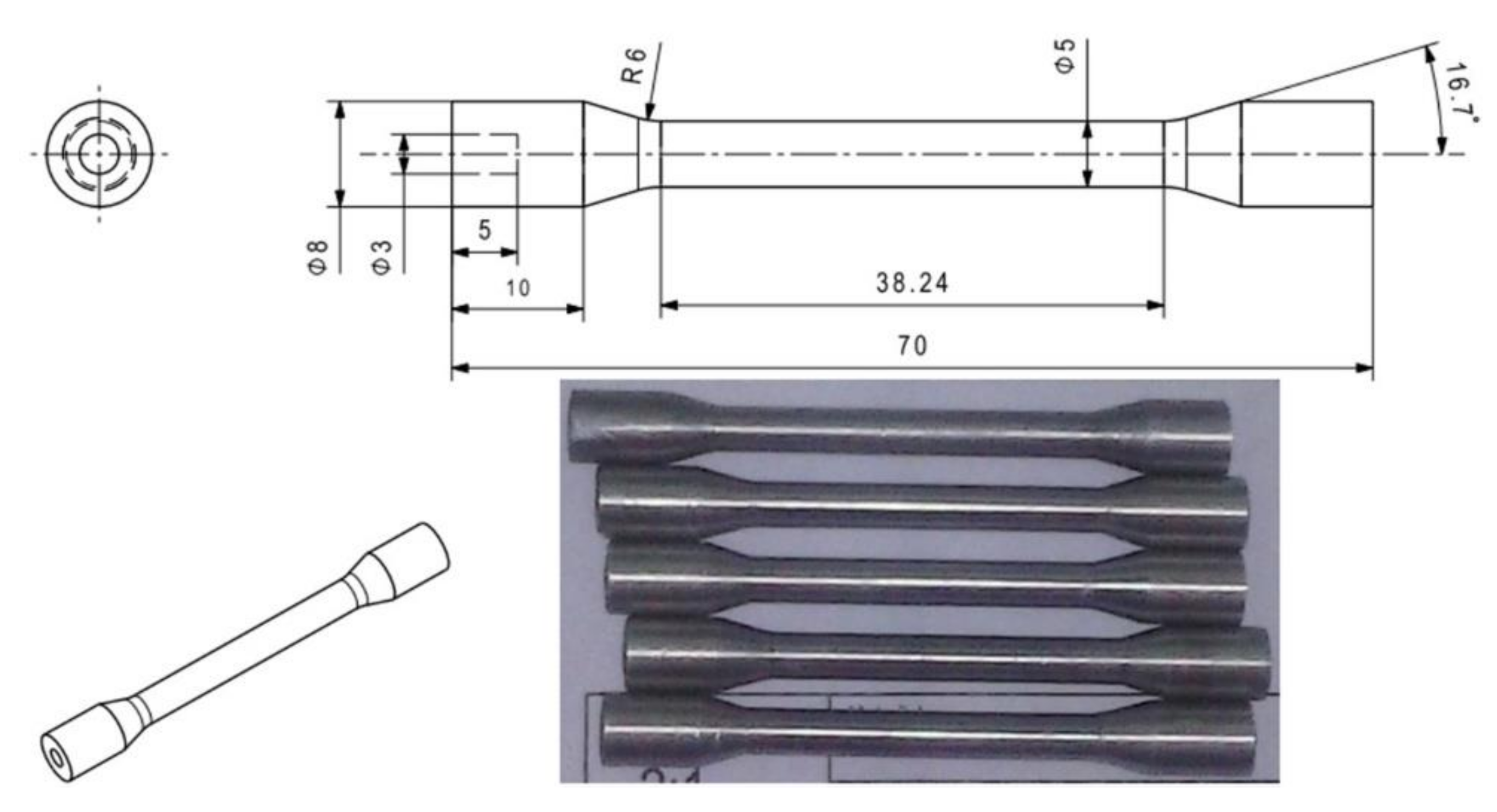
Mechanical tensile properties investigations were performed on testing machine MTS 810 (MTS, Eden Prairie, Minneapolis, MN, USA), at room temperature T = 20 °C in accordance with EN 10002-1:1998 [
38]. Five samples for tensile testing were prepared with a diameter of 5 mm and a measuring length of 38.24 mm, as presented in
Figure 1.
3. Results
New chemical composition of AlSi7MgCu alloy with the extra addition of copper has been designed and compared with those prescribed by EN norm [
35] indicated in
Table 1 and previously investigated conventional AlSi7Mg alloy [
38], as shown in
Table 3. Chemical composition analysis was performed using optical emission spectrometry with indicated measurement uncertainty.
Both alloys, commercial AlSi7Mg and the new AlSi7MgCu, comply with the EN 42000 AC standard for AlSi7Mg alloy relating to the content of the base alloying elements (Si and Mg) and trace elements such as Fe and Mn. Deviation has been implemented with significant increase in Cu content.
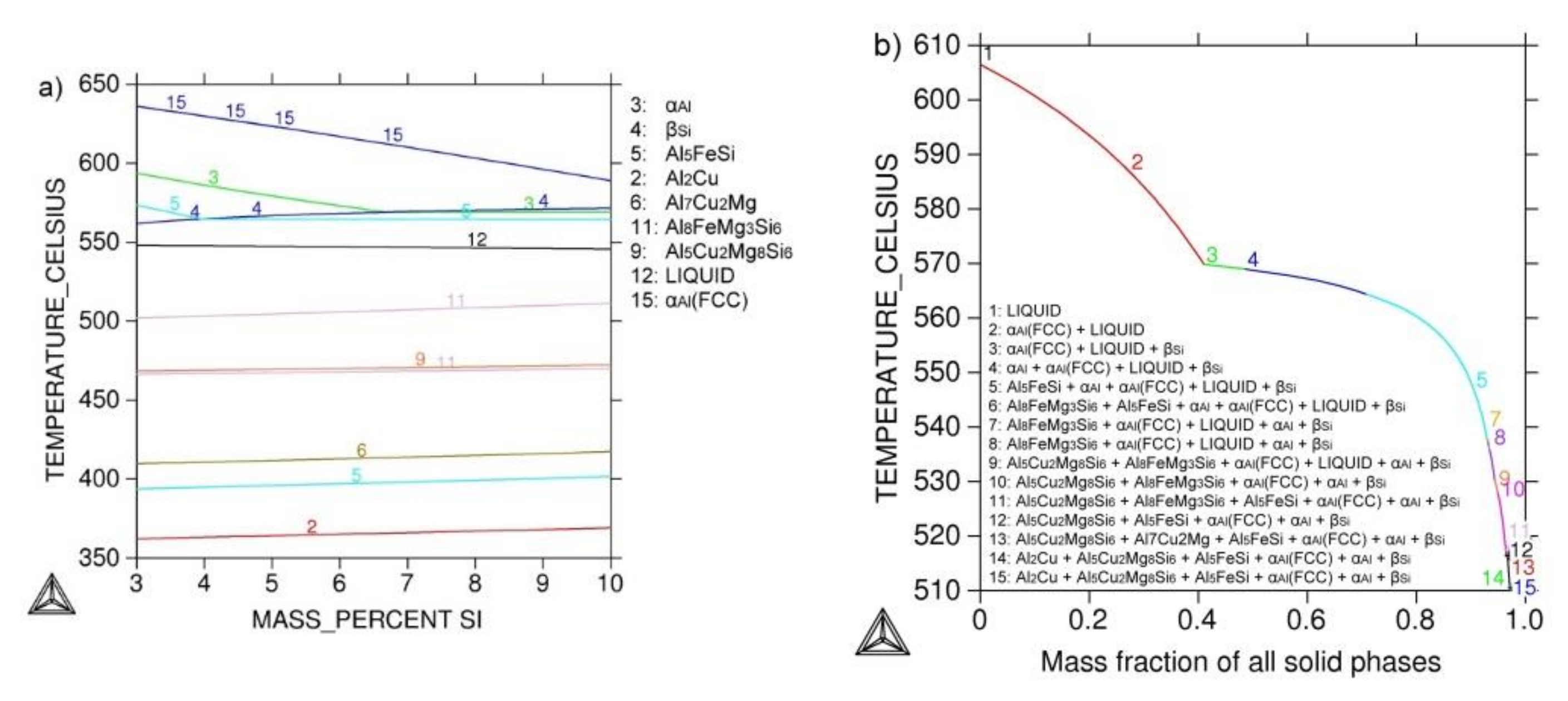
Thermodynamic calculation of the newly designed AlSi7MgCu alloy by TCW 5.0 program resulted in equilibrium and Scheil–Gulliver non-equilibrium phase diagram. Interaction of alloying and trace elements reveals a wide range of intermetallic phases. Calculation of AlSi7MgCu alloy solidification sequence is shown in
Figure 2.
Equilibrium condition calculation enabled an overview of stable equilibrium phases in aluminum corner of phase diagram. Non-equilibrium calculation revealed solidification sequence of AlSi7MgCu alloy with temperature stability of present phases. It reveals narrow solidification interval of ΔTL-S = 96.4 °C. The calculated solidification sequence is as follows:
- (1)
606.5 °C L→L1 + αAl
- (2)
569.0 °C L1→L2 + βSi
- (3)
564.4 °C L2→L3 + (αAl+βSi)
- (4)
537.4 °C L3→L4 + Al5FeSi
- (5)
537.4 °C L4→L5 + Al8FeMg3Si6
- (6)
528.0 °C L5→L6 + Al5Cu2Mg8Si6
- (7)
510.1 °C L6→L7 + Al7Cu2Mg
- (8)
510.1 °C L7→Al2Cu
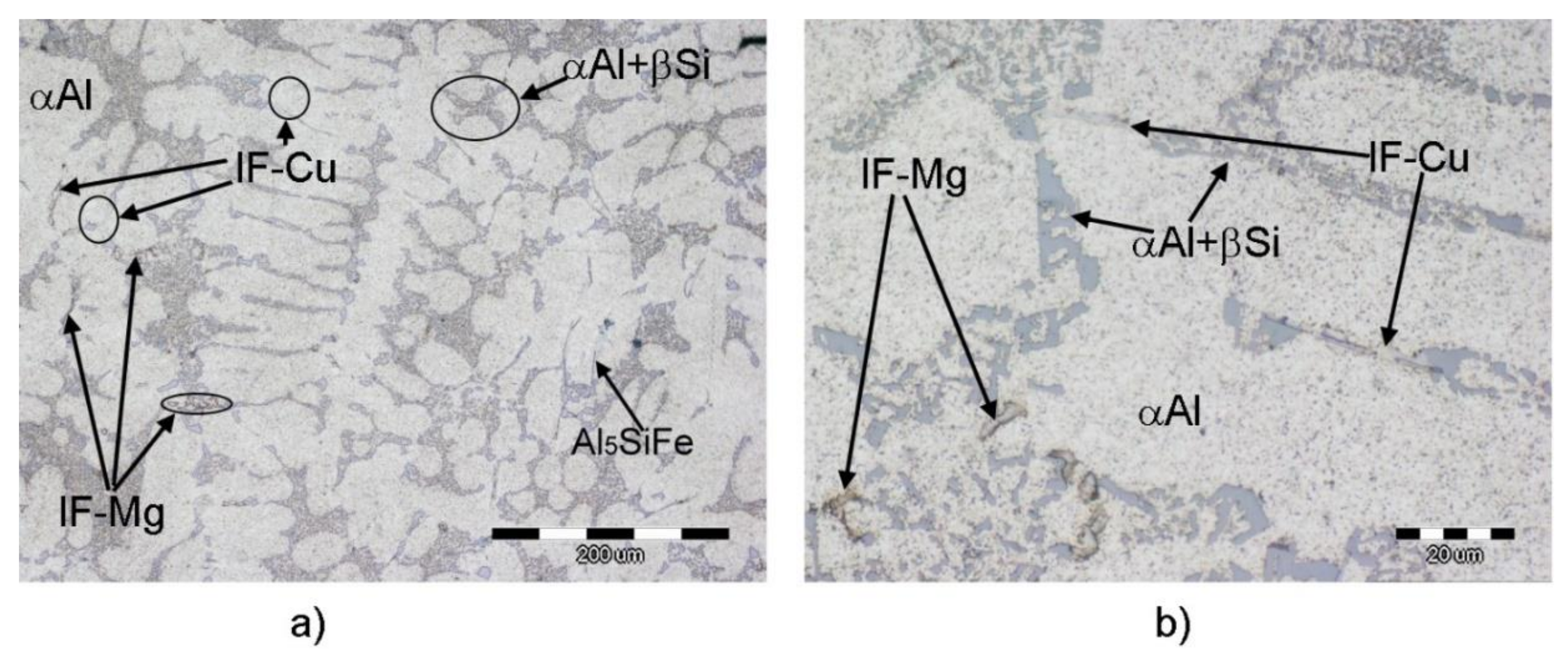
Microstructure of AlSi7MgCu alloy was investigated using optical microscopy to get an overview of microstructure development, as shown in
Figure 3.
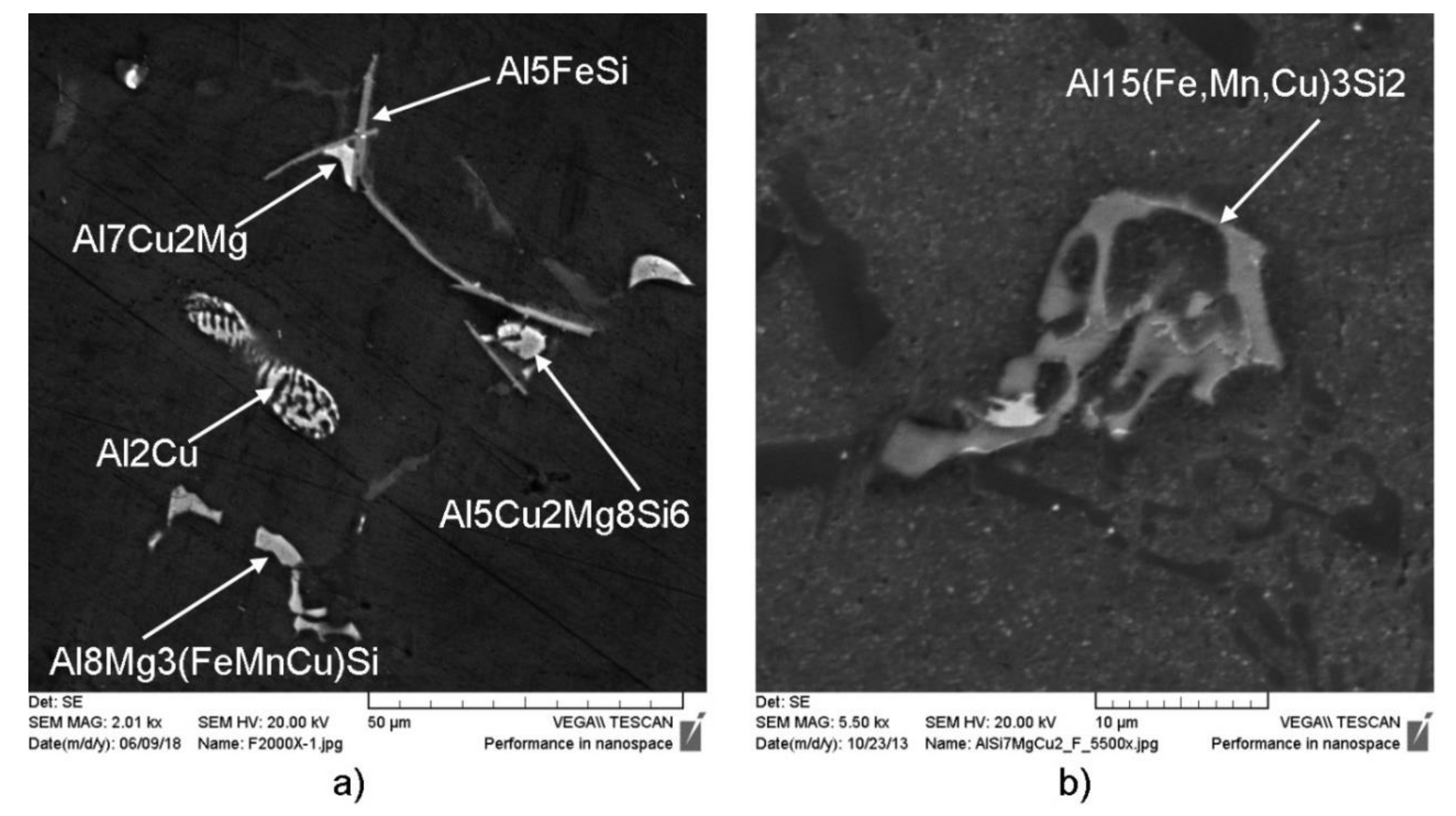
Identification of present phases at micrographs was performed using visual assessment to its morphologies and comparison with literature survey of similar alloys. A smaller magnification reveals uniform distribution of primary dendritic network surrounded by evenly distributed eutectic cells. Higher magnification indicates rougher and broken dendritic branches dotted with Fe-bearing intermetallic phases with needle-like morphology (Al5SiFe) and Chinese script (Al15(Fe,Mn)3Si2); light-grey to white coarse intermetallic phases, which correspond to Cu-bearing phases (IF-Cu) in interdendritic spaces and at grain boundaries; and fine dark intermetallic phases, which correspond to Mg-bearing phases (IF-Mg) placed at grain boundaries. High magnification micrographs also reveal mixed morphology of main eutectic (αAl + βSi), both fiber and lamella. On the base of visual recognition of particular phases and their micro-locations, it can be stated that a modelled solidifying sequence has been followed. The IF-Cu phases seem to occur through the whole solidification sequence—near the high-temperature needle-like Fe-bearing phases, in interdendritic spaces and at grain boundaries—whereas the IF-Mg phases occur only at the grain boundaries as an intermetallic phase in combination with Fe, Mn, and/or Cu.
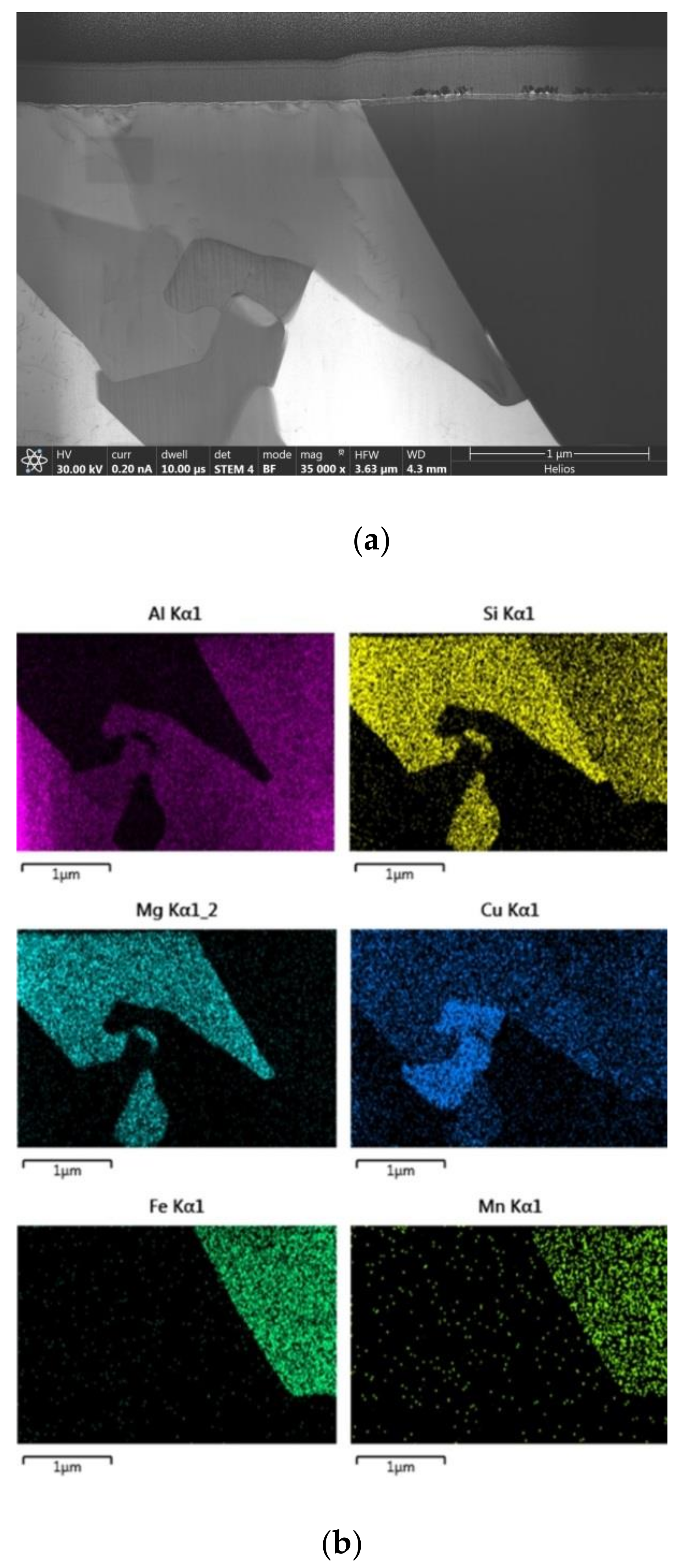
EDS mapping analysis of AlSi7MgCu alloy sample is performed in order to determine the distribution of significant chemical elements and their mutual interaction. The elements of interest were Al, Si, Fe, Mn, Mg, and Cu, as shown in
Figure 4.
EDS analysis reveals strong interaction of present elements forming following systems: Al-Fe-Mn-Cu-Si, Al-Mg-Cu-Si, Al-Cu, and Mg-Si. The obtained results indicate that Mn and non-equilibrium solidification played an important role in formation of Al15(Fe,Mn)3Si2 phase instead of Al5FeSi, as predicted in calculation of the equilibrium phase diagram. Additionally, Cu distribution proofs its infiltration in high-temperature intermetallic phase with modified chemical composition corresponding to the Al-(Fe-Mn-Cu)-Si system.
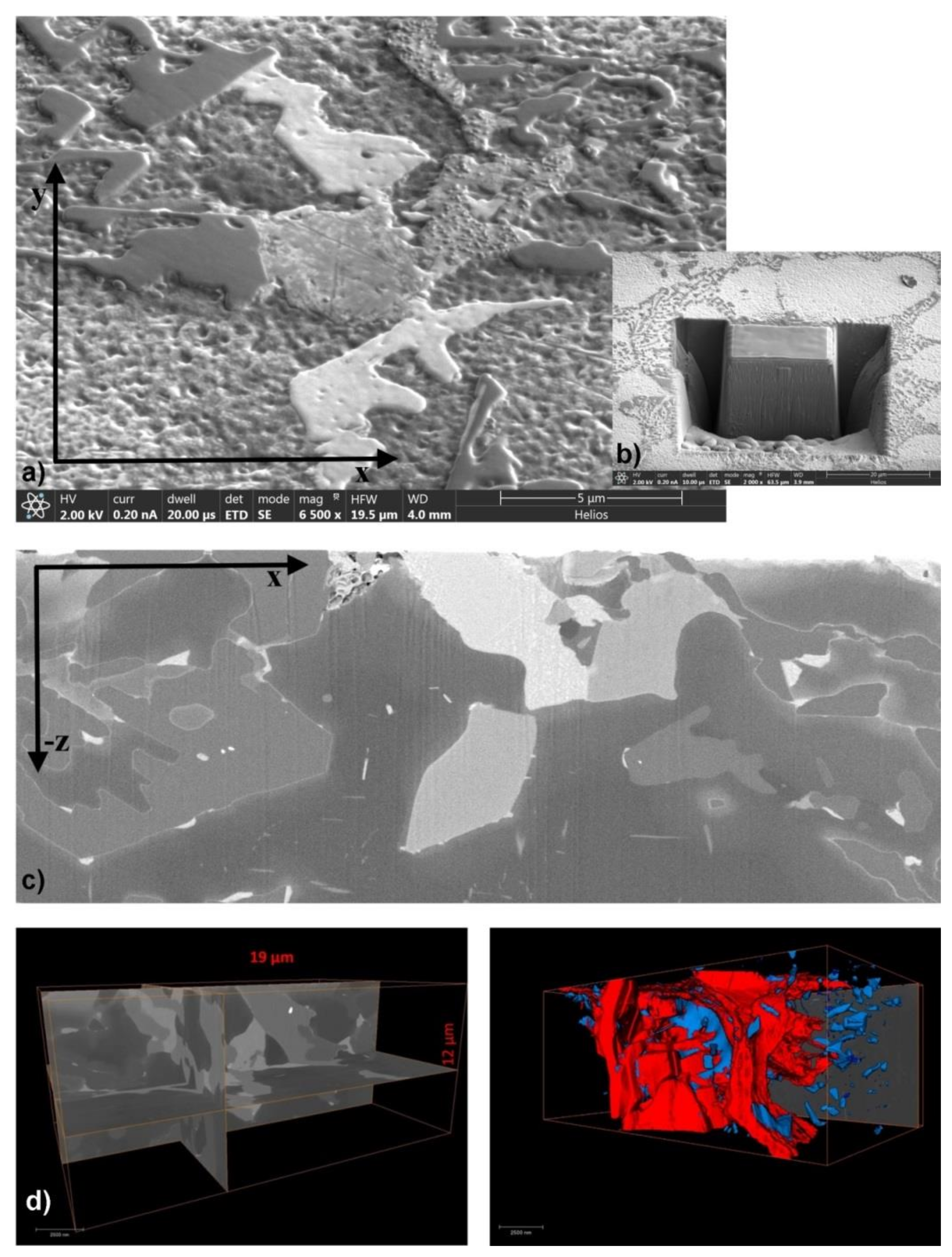
Since data acquired from the sample surface do not provide information about the phases’ size, distribution and interaction, FIB-SEM analysis on 782 slices was applied. The obtained SE images enable 3D reconstruction of microstructure and distribution of each phase. A FIB-SEM slice-and-view study of three-dimensional phase distribution in AlSi7MgCu alloy is shown in
Figure 5.
Among all systems previously identified by regular mapping analysis, volume distribution of phases of interest on the base, the Al-Si-Mg-Cu (red) and Al-(Fe-Mn-Cu)-Si (blue) systems enable visualization of their distribution and interaction. Orthoslices in the order of appearance of each of the imaging plane provide an insight in the solidification sequence and visual estimation of phases’ coherency and/or congruency. The dense arrangement of present phases, their size, and their distribution determine the order of appearance. First to appear is dendrite network (α-Al), followed by fine, but ramified Al-(Fe-Mn-Cu)-Si phases and main eutectic. The bulk of the volume was filled with Al-Si-Mg-Cu phase and other intermetallic phases on the base of Al-Fe-Mg-Si, Al-Cu-Mg, and Al-Cu systems. Strong connections and uniform distribution of observed phases comprise complement precipitation and compact microstructure and, therefore, represent the base for achieving higher mechanical properties. Chemical composition of particular phases has been determined by EDS point analysis, as indicated at two characteristic details in
Figure 6.
Quantitative chemical analysis of characteristic intermetallic phases in AlSi7MgCu alloy obtained by EDS analysis at typical intermetallic phases, shown in
Figure 5, has been given as an average weight percent in
Table 4.
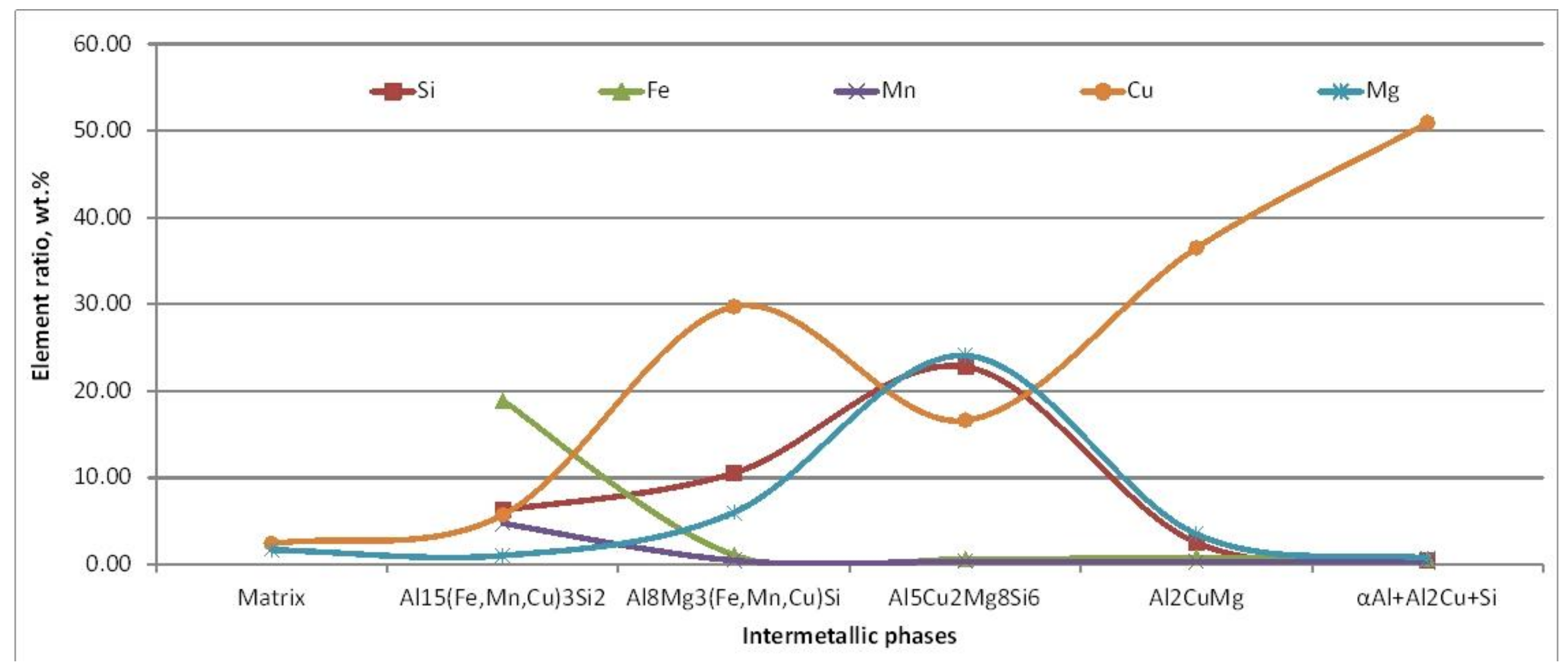
EDS analysis indicates significant Cu presence in all present intermetallic phases. Change in chemical composition of particular phases has been noticed. Matrix (α
Al) and eutectic phase (α
Al + β
Si) indicate high solubility of Cu. Intermetallic phases with recognizable morphology such as Chinese script (Al
15(Fe,Mn,Cu)
3Si
2) and π-phase (Al
8Mg
3(Fe,Mn,Cu)Si) reveal the infiltration of Cu. The amount of Cu is increasing with solidification progress. Simultaneous thermal analysis performed at heating/cooling rate at 10 K/min rate allowed further identification of significant thermodynamic changes in AlSi7MgCu alloy during the solidification process and determination of solidification sequence, as shown in
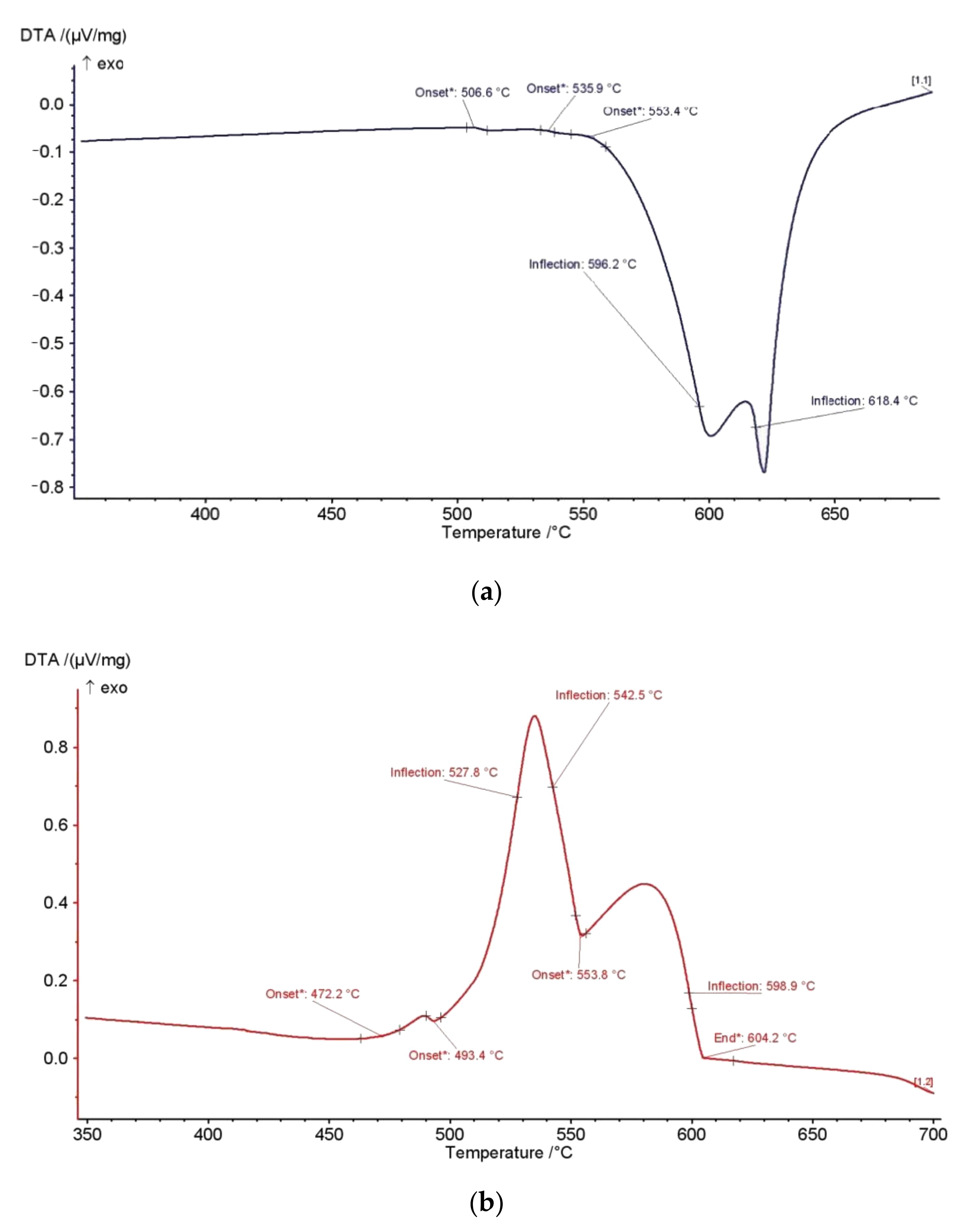
Figure 7.
The heating and cooling curves of AlSi7MgCu alloy resulted in establishing the exact temperatures of phase transformations and precipitation, as shown in
Table 5. Heating and cooling curve temperatures differ slightly due to energy output dedicated to different melting and solidification phenomena. Additionally, the heating curve did not indicate energy changes connected to all phases identified by microstructural analysis. The only strong signal related to phase transition was detected at liquidus temperature corresponding to dendrite network development. Other signals were diffused, indicating the strong interaction of a number of elements during the solidification process. Comparison of the solidification path predicted based on the equilibrium phase diagram modelling with those from simultaneous thermal analysis and microstructural investigations allowed the solidification sequence determination of AlSi7MgCu alloy listed in
Table 5.
After dendrite network development, needle-like Al
5FeSi and complex Chinese script formation Al
15(Fe,Mn,Cu)
3Si
2 enriched in Cu develop from the bulk melt. The corresponding amount of Cu and Mg form metal matrix solid solution, while the complex intermetallic phases, such as Al
8Mg
3(Fe,Mn,Cu)Si
6 also enriched in Cu, followed by Al
5Cu
2Mg
8Si
6 and Al
7Cu
2Mg, cohesively precipitate from the bulk melt. Solidification ends with secondary eutectic α
Al + Al
2Cu + β
Si. Dense distribution of fine complex intermetallic phases mainly with ramified morphology comprehends to the matrix coherency and also rigidity. The XRD technique was used for additional determination of the phases present in AlSi7MgCu alloys, as shown in
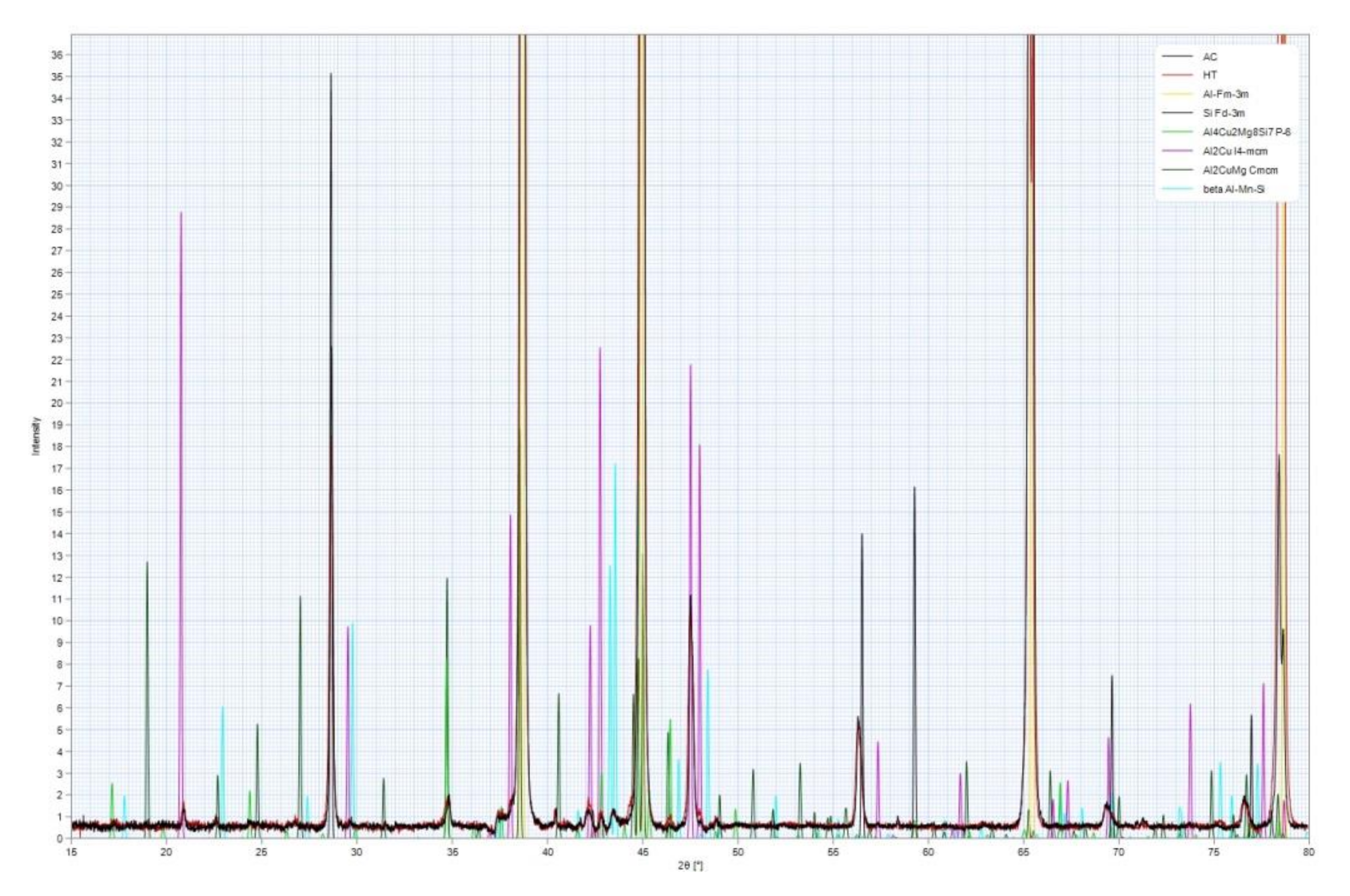
Figure 8.
Complex interaction of elements and the number of particular phases indicated some difference in determined phases. XRD analysis reveals the presence of α-Al, β-Si, Al2Cu, Al2CuMg, Al4Cu2Mg8Si7, and Al-Mn-Si phases. Ratio of other present complex intermetallic phases was undetectably low.
Mechanical properties of AlSi7MgCu alloy have been investigated in order to determine the influence of higher amount of Cu and, therefore, altered the solidification sequence of an alloy. Comparison of tensile strength obtained for new AlSi7MgCu and previously investigated conventional AlSi7Mg [
38] is shown in
Table 6 with indicated measurement uncertainty.
A comparison of obtained results including standard deviation data as a result of measurement dispersion and example of obtained stress–strain diagram are presented in
Figure 9.
Significant increase in yield strength and tensile strength of innovative AlSi7MgCu alloy has been noticed. Determined intermetallic phases show more complexity in chemical composition and important role of Cu in whole solidification process. Microstructure analysis indicated number of phases and good coherency between precipitated intermetallics. Improvement of mechanical properties is related to the change in solidification mechanism due to Cu addition resulting in complex interactions of Al15(Fe,Mn,Cu)3Si2 phase modified with Cu addition as well as formation of compacted Al5Cu2Mg8Si6 and Al8(Fe,Mn,Cu)Mg3Si6 phases. Elongation of AlSi7MgCu alloy was decreased, which confirms the rigid effect of complex intermetallic phases enriched on Cu. A densely developed microstructure with a number of fine complex intermetallic phases comprehended to obtaining higher values of tensile strength already in an as-cast state. The characterization of the solidification path for a new AlSi7MgCu alloy with high Cu content (1.435 wt.%) and correlation with obtained mechanical properties is essentially important for the automotive industry. Beside light-weighting and higher mechanical properties of automotive parts obtained already in an as-cast state, avoiding expensive heat treatment plays an important role in economic production.
4. Discussion
The design of an innovative chemical composition was based on classic composition of commercial AlSi7Mg alloy according to the EN norm. The addition of a higher amount of Cu resulted in its increase to 1.436 wt.% in the chemical composition of the alloy.
Introduction of additional amount of Cu revealed its significant interaction in solidification sequence especially below 528 °C. According to new chemical composition thermodynamic modelling of the phase diagram in equilibrium and non-equilibrium, the influence of Cu encourages the number of intermetallic phase developments. Phases such as Al5Cu2Mg8Si6, Al7Cu2Mg, and Al2Cu are found responsible for the increase in tensile strength in a number of other commercial alloys. Narrow solidification interval indicates a potential of an alloy for casting of thin-walled sections.
Nevertheless, microstructure investigation revealed significant role of Cu even in early stages of solidification. The EDS investigation widened the palette of phases in which it occurs during the whole solidification process: Al
15(Fe,Mn,Cu)
3Si
2 at the begging of the solidification after dendrite network development, in intermetallic phase Al
5Cu
2Mg
8Si
6, Al
8Mg
3(Fe,Mn,Cu)Si, and Al
7Cu
2Mg developed after main eutectic reaction, and finally, in a secondary eutectic Al + Al
2Cu + Si as a last solidifying phase. Sophisticated FIB-SEM analysis performed on a determined volume allowed 3D reconstruction of microstructure and size and distribution of each phase present in AlSi7Mg alloy. FIB-SEM microstructure evaluation enables solidification sequence as follows: first to appear is dendrite network (α-Al), followed by fine, but ramified Al-(Fe-Mn-Cu)-Si phases and main eutectic. The bulk of the volume was filled with Al-Si-Mg-Cu phase and other intermetallic phases on the base of Al-Fe-Mg-Si, Al-Cu-Mg, and Al-Cu systems. Uniform distribution of observed phases and their coherency comprises complement precipitation and compact microstructure and therefore represent the base for update of properties. The impact in
Supplementary Material was placed on Al-Si-Mg-Cu (red) and Al-(Fe-Mn-Cu)-Si (blue) systems due to infiltration of Cu in the early stages of the solidification phases. Behavior of all affected elements during the solidification process is shown in
Figure 10.
STA analysis enabled the determination of all previously indicated phase evolution events. Due to the energy output dedicated to different melting and solidification phenomena and, therefore, overlapping of particular signals, some of the phase were identified during cooling, i.e., solidification process.
A strong connection between the microstructure constituent, XRD pattern, and characteristic temperatures of phase transformation and precipitation revealed the exact solidification sequence of AlSi7MgCu alloy.
The hypothesis of this investigation was that introduction of higher content of Cu in commercial AlSi7Mg will influence the solidification sequence change. Numerous interactions of elements and therefore phase development in innovative AlSi7MgCu alloy resulted in the diversification of microstructure development but still coherent and compact microstructure due to infiltration of Cu in intermetallic phases and high amount of Cu (1.435 wt.%) in matrix as a solid solution. The obtained microstructural constituents and their distribution due to higher Cu content had an impact on the increase in yield strength and tensile strength values already in the as-cast state, when compared to commercial AlSi7Mg alloy. Achieved improvement of mechanical properties is accomplished with the redesign of chemical composition due to Cu addition. Redesign of the microstructure manifests through complex composition of Al15(Fe,Mn,Cu)3Si2 phase enriched in Cu addition as well as due to the formation of compacted Al5Cu2Mg8Si6 and Al8(Fe,Mn,Cu)Mg3Si6 phases.
Author Contributions
Conceptualization of idea, Z.Z.B. and D.S.; literature survey and comparison Z.Z.B.; design of methodology, Z.Z.B., D.S., and L.L.; experimental setup Z.Z.B. and D.S.; casting, D.S.; sample preparation, D.S. and Z.Z.B.; mechanical properties investigation, D.S.; microstructure investigation, Z.Z.B. and L.L.; STA analysis, Z.Z.B.; validation of results, Z.Z.B., D.S., and L.L.; formal analysis, Z.Z.B., D.S., and L.L.; resources, D.S. and L.L.; writing—original draft preparation, Z.Z.B.; writing—review and editing, Z.Z.B.; visualization, Z.Z.B., D.S., and L.L.; supervision, Z.Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Laboratory equipment has been provided by collaborative companies and Faculty of Metallurgy within research project IP-124 University of Zagreb Faculty of Metallurgy, Centre for Foundry Technology—SIMET, KK.01.1.1.02.0020 and VIRTULAB—Integrated laboratory for primary and secondary raw materials, KK.01.1.1.02.0022.
Acknowledgments
Investigations have been performed in collaboration with companies CIMOS-P.P.C. Buzet, d.o.o., Buzet, Croatia and FEI Netherlands, Eindhoven, The Netherlands. Investigations were performed within the Institutional project IP-124 funded by University of Zagreb Faculty of Metallurgy, topic “Design and Characterization of Innovative Engineering Alloys”, Infrastructural scientific project: Centre for Foundry Technology—SIMET, KK.01.1.1.02.0020 funded by European Regional Development Fund, Operational Programme Competitiveness and Cohesion 2014–2020, and Infrastructural Scientific Project: VIRTULAB—Integrated laboratory for primary and secondary raw materials, KK.01.1.1.02.0022 funded by European Regional Development Fund, Operational Programme Competitiveness and Cohesion 2014–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raghavan, V. Al-Fe-Mn-Si (Aluminum-Iron-Manganese-Silicon). J. Phase Equilibria Diffus. 2007, 28, 215–217. [Google Scholar] [CrossRef]
- Tillova, E.; Chalupova, M.; Hurtalova, L. Evolution of Phases in a Recycled Al-Si Cast Alloy During Solution Treatment. In Scanning Electron Microscopy; Kazmiruk, V., Ed.; IntechOpen: London, UK, 2012; Volume 21, pp. 411–438. [Google Scholar]
- Tanski, T.; Labisz, K.; Krupińska, B.; Krupiński, M.; Król, M.; Maniara, R.; Borek, W. Analysis of crystallization kinetics of cast aluminum–silicon alloy. J. Therm. Anal. Calorim. 2015, 123, 63–74. [Google Scholar] [CrossRef][Green Version]
- Iqbal, N.; Van Dijk, N.; Offerman, S.; Geerlofs, N.; Moret, M.; Katgerman, L.; Kearley, G. In situ investigation of the crystallization kinetics and the mechanism of grain refinement in aluminum alloys. Mater. Sci. Eng. A 2006, 416, 18–32. [Google Scholar] [CrossRef]
- Chang, K.; Liu, S.; Zhao, D.; Du, Y.; Zhou, L.; Chen, L. Thermodynamic description of the Al–Cu–Mg–Mn–Si quinary system and its application to solidification simulation. Thermochim. Acta 2011, 512, 258–267. [Google Scholar] [CrossRef]
- Heugue, P.; Larouche, D.; Breton, F.; Massinon, D.; Martinez, R.; Chen, X.-G. Precipitation Kinetics and Evaluation of the Interfacial Mobility of Precipitates in an AlSi7Cu3.5Mg0.15 Cast Alloy with Zr and V Additions. Metals 2019, 9, 777. [Google Scholar] [CrossRef]
- Dahle, A.; Arnberg, L. Development of strength in solidifying aluminium alloys. Acta Mater. 1997, 45, 547–559. [Google Scholar] [CrossRef]
- Davis, J.R. (Ed.) ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International: Ohio, OH, USA, 1993. [Google Scholar]
- Bäckerund, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminium Alloys: Foundry Alloys; AFS/Skanaluminium: Stockhlom, Sweden, 1999; Volume 2. [Google Scholar]
- Dispinar, D.; Campbell, J. Effect of melting and casting conditions on aluminium metal quality. Foundry Trade J. 2006, 180, 328–331. [Google Scholar]
- Santos, J.; Dahle, A.K.; Jarfors, A.E. Magnesium Solubility in Primary α-Al and Heat Treatment Response of Cast Al-7Si-Mg. Metals 2020, 10, 614. [Google Scholar] [CrossRef]
- McGregor, R.R. Structure and Properties. Ind. Eng. Chem. 1954, 46, 2323–2325. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams, Applications for Commercial Aluminum Alloys; Elsevier: London, UK, 2005. [Google Scholar]
- Brodarac, Z.Z.; Unkic, F.; Medved, J.; Mrvar, P. Determination of solidification sequence of the AlMg9 alloy. Met. Mater. 2012, 50, 59–67. [Google Scholar] [CrossRef]
- Ji, S.; Yan, F.; Fan, Z. A High Strength Aluminium Alloy for High Pressure Die Casting. In Light Metals—The Minerals, Metals & Materials Society; Williams, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bäckman, J. Processing Aspects for Improving Mechanical Properties in Aluminium Castings. Ph.D. Thesis, Linköping University, Jönköping, Sweden.
- Wang, E.; Hui, X.; Wang, S.; Zhao, Y.; Chen, G. Improved mechanical properties in cast Al–Si alloys by combined alloying of Fe and Cu. Mater. Sci. Eng. A 2010, 527, 7878–7884. [Google Scholar] [CrossRef]
- Brodarac, Z.; Dolic, N.; Unkic, F. Influence of copper content on microstructure development of AlSi9Cu3 alloy. J. Min. Met. Sect. B Met. 2014, 50, 53–60. [Google Scholar] [CrossRef]
- Samuel, F.H.; Samuel, A.M.; Doty, H.W. Factors controlling the type and morphology of Cu-containing phases in 319 Al alloy. AFS Trans. 1996, 104, 893–901. [Google Scholar]
- Schöbel, M.; Baumgartner, G.; Gerth, S.; Bernardi, J.; Hofmann, M. Microstresses and crack formation in AlSi7MgCu and AlSi17Cu4 alloys for engine components. Acta Mater. 2014, 81, 401–408. [Google Scholar] [CrossRef]
- Pabel, T.; Bozorgi, S.; Kneissl, C.; Faerber, K.; Schumacher, P. Hot cracking susceptibility of AlSi7MgCu alloys and effects of alloying elements magnesium and copper. China Foundry 2013, 10, 69–74. [Google Scholar]
- Huter, P.; Oberfrank, S.; Grün, F.; Stauder, B. Thermo-mechanical fatigue influence of copper and silicon on hypo-eutectic Al–Si–Cu and Al–Si–Mg cast alloys used in cylinder heads. Int. J. Fatigue 2016, 88, 142–155. [Google Scholar] [CrossRef]
- Huter, P.; Renhart, P.; Oberfrank, S.; Schwab, M.; Grün, F.; Stauder, B.J. High- and low-cycle fatigue influence of silicon, copper, strontium and iron on hypo-eutectic Al–Si–Cu and Al–Si–Mg cast alloys used in cylinder heads. Int. J. Fatigue 2016, 82, 588–601. [Google Scholar] [CrossRef]
- Huter, P.; Winter, G.; Schwab, M.; Renhart, P.; Oberfrank, S.; Grün, F.; Stauder, B.J. Comparison of microstructural crack paths between hypo-eutectic Al–Si–Cu and Al–Si–Mg cast alloys in high plasticity regimes under rotating bending. Mater. Sci. Eng. A 2014, 618, 578–585. [Google Scholar] [CrossRef]
- Dietz, S.; Djurdjevic, M.B.; Gutiérrez, R.F.; Rafetzeder, M. Solidification Path of NemAlloy. In Vehicle and Automotive Engineering 2: Proceedings of the 2nd VAE2018 Miskolc Hungary; Jarmai, K., Bollo, B., Eds.; Springer: Cham, Switzerland, 2018; pp. 209–219. [Google Scholar] [CrossRef]
- Cáceres, C.; Djurdjevic, M.B.; Stockwell, T.; Sokolowski, J. The effect of Cu content on the level of microporosity in Al-Si-Cu-Mg casting alloys. Scr. Mater. 1999, 40, 631–637. [Google Scholar] [CrossRef]
- Shabestari, S.; Moemeni, H. Effect of copper and solidification conditions on the microstructure and mechanical properties of Al–Si–Mg alloys. J. Mater. Process. Technol. 2004, 153, 193–198. [Google Scholar] [CrossRef]
- Dugić, I.; Henriksson, F.; Strebel, C.; Kosmaz, Ö; Seifeddine, S. On the Effect of Alloying Element Range on the Mechanical Properties of Recycled Aluminium Alloy EN AB-46000. In Light Metals 2016—The Minerals, Metals & Materials Society; Williams, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zamani, M.; Seifeddine, S.; Ghassemali, E. Effect of cooling rate and eutectic modification on texture and grain structure of Al-Si-Cu-Mgdie cast alloy. Metall. Ital. 2016, 108, 29–32. [Google Scholar]
- Tocci, M.; Pola, A.; Raza, L.; Armellin, L.; Afeltra, U. Optimization of heat treatment parameters for a nonconventional Al-Si-Mg alloy with Cr addition by DoE method. Metall. Ital. 2016, 108, 141–144. [Google Scholar]
- Brodarac, Z.Z.; Grgurić, T.H.; Burja, J. Thermodynamic stability of AlSi11 alloy microconstituents. J. Therm. Anal. Calorim. 2016, 127, 431–438. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Huber, G.; Odanovic, Z. Synergy between thermal analysis and simulation. J. Therm. Anal. Calorim. 2012, 111, 1365–1373. [Google Scholar] [CrossRef]
- Mahfoud, M.; Rao, A.K.P.; Emadi, D. The role of thermal analysis in detecting impurity levels during aluminum recycling. J. Therm. Anal. Calorim. 2010, 100, 847–851. [Google Scholar] [CrossRef]
- Zuo, L.; Ye, B.; Feng, J.; Kong, X.; Jiang, H.; Ding, W. Effect of Q-Al 5 Cu 2 Mg 8 Si 6 phase on mechanical properties of Al-Si-Cu-Mg alloy at elevated temperature. Mater. Sci. Eng. A 2017, 693, 26–32. [Google Scholar] [CrossRef]
- EN 1706:2010. Aluminum and Aluminum Alloys—Castings—Chemical Compositions and Mechanical Properties; CEN—European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- IDM 4234. Honeywell—Garret, Industrial Division Specification, Aluminium Alloy Castings 356-F; Garrett Motion France SAS: Thaon Les Vosges, France, 2008. [Google Scholar]
- Stanić, D.; Zovko Brodarac, Z.; Unkić, F. Mikrostrukturna i mehanička svojstva kokilno lijevanih uzoraka AlSi7Mg legure (Microstructure and mechanical properties of AlSi7Mg alloy samples cast in permanent mould, in Croatian). In Proceedings Book of 9th International Foundrymen Conference; Unkić, F., Ed.; University of Zagreb Faculty of Metallurgy: Sisak, Croatia, 2009; CD_ROM 17-2009. [Google Scholar]
- EN 10002-1:2001. Metallic Materials—Tensile Testing—Part 1: Method of Test (at Ambient Temperature); CEN—European Committee for Standardization: Brussels, Belgium, 2001. [Google Scholar]
- Zovko Brodarac, Z.; Stanić, D.; Holjevac Grgurić, T. Solidification sequence of innovative AlSi7MgCu alloy. In Proceedings of the 48th International October Conference on Mining and Metallurgy, Belgrade, Serbia, 28 September–1 October 2016; Štrbac, N., Živković, D., Eds.; University of Belgrade Technical Faculty in Bor: Zaječar, Serbia, 2016; pp. 375–378. [Google Scholar]
- Zovko Brodarac, Z.; Stanić, D.; Li, L. Study of AlSi7MgCu alloy with improved properties in as-cast state. In Proceedings of the 73rd World Foundry Congress “Creative Foundry”, Krakow, Poland, 23–27 September 2018; Sobczak, N., Asthana, R., Szajnar, J., Eds.; World Foundry Organization Ltd: Herefordshire, UK, 2018; p. 39. [Google Scholar]
- Stanić, D.; Zovko Brodarac, Z.; Li, L. Influence of Microstructure Development on Mechanical Properties of AlSi7MgCu Alloy. In Light Metals 2019—The Minerals, Metals & Materials Series; Chesonis, C., Ed.; Springer: Cham, Switzerland, 2019; pp. 199–207. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).