2. Materials and Methods
The chemical composition of the steels used is listed in
Table 1. The first composite (designated with A) consists of a cold work tool steel (tool steel 1) and a precipitation hardening (PH) steel. The second composite (designated with B) consists of another cold work tool steel (tool steel 2) and a maraging steel.
The two cold work tool steels have the same main alloying elements, with the exception of niobium which is only present in the first and cobalt which is only present in the second. The samples had a cylindrical shape with a diameter of 8 mm and a height of 5 mm and were ground and polished on one side.
The setup of the diffusion bonding process can be seen in
Figure 1a. Thermocouples were attached to each steel sample before placing them between the stamps of a deformation dilatometer DIL805A/D (TA Instruments, New Castle, DE, USA) with the polished surfaces at the interface. The samples were heated under a vacuum of 10
−4 to a temperature of 1150 °C and held for 45 min. The retention force of the stamps was 50 N at the beginning and was increased to 250 N after 2 min at temperature. The temperature and retention force during the diffusion bonding process can be seen in
Figure 1b. After this bonding treatment the samples were diffusion annealed at 1150 °C for 7.25 h in a vacuum furnace with a vacuum degree of 10
−5 at peak temperature.
Subsequently the heat treatment was performed without the use of a vacuum or a protective atmosphere, starting with the austenitisation at 1150 °C/1080 °C (composite A/B) for 10 min followed by an oil quench. The sheets were then tempered twice for 2 h at 540 °C.
For metallographic investigations samples were cut in half and hot mounted in PolyFast from Struers and polished up to 1 µm. Directly after polishing the samples were etched with Kalling 2 [
19] to reveal the microstructure or etched with Murakami [
19] to reveal the carbides. The etched samples were examined using an optical microscope Axio Imager M1m (Zeiss, Oberkochen, Germany). The carbide and element distribution at the interface was investigated on polished samples using a Clara scanning electron microscope (Tescan, Brünn, Czech Republic) and an X-Max energy-dispersive X-ray spectroscopy (EDX) detector (Oxford Instruments, Abingdon, United Kingdom). The acceleration voltage for these investigations were 15 and 12 kV, respectively. All EDX spot measurements were taken at a distance of a few 10 micrometers from the interface.
To obtain a deformation-free surface the samples were vibropolished with OP-S suspension from Struers for 7 h prior to EBSD measurements. In addition, the samples were plasma cleaned before being measured with an EBSD detector (Ametek, Berwyn, PA, USA) attached to a Versa 3D (FEI Company, Hillsboro, OR, USA). For the hardness mapping a Q60A+ micro hardness tester (Qness, Golling, Austria) was used to indent polished samples with HV 0.1 and a grid spacing of 100 µm.
Diffusion calculations were set up with the program MatCalc 6 (MatCalc Engineering, Vienna, Austria) to estimate the expected element distribution after diffusion bonding and heat treatment of the specimens.
3. Results
Cold work tool steels were diffusion bonded with a precipitation hardening steel using a deformation dilatometer.
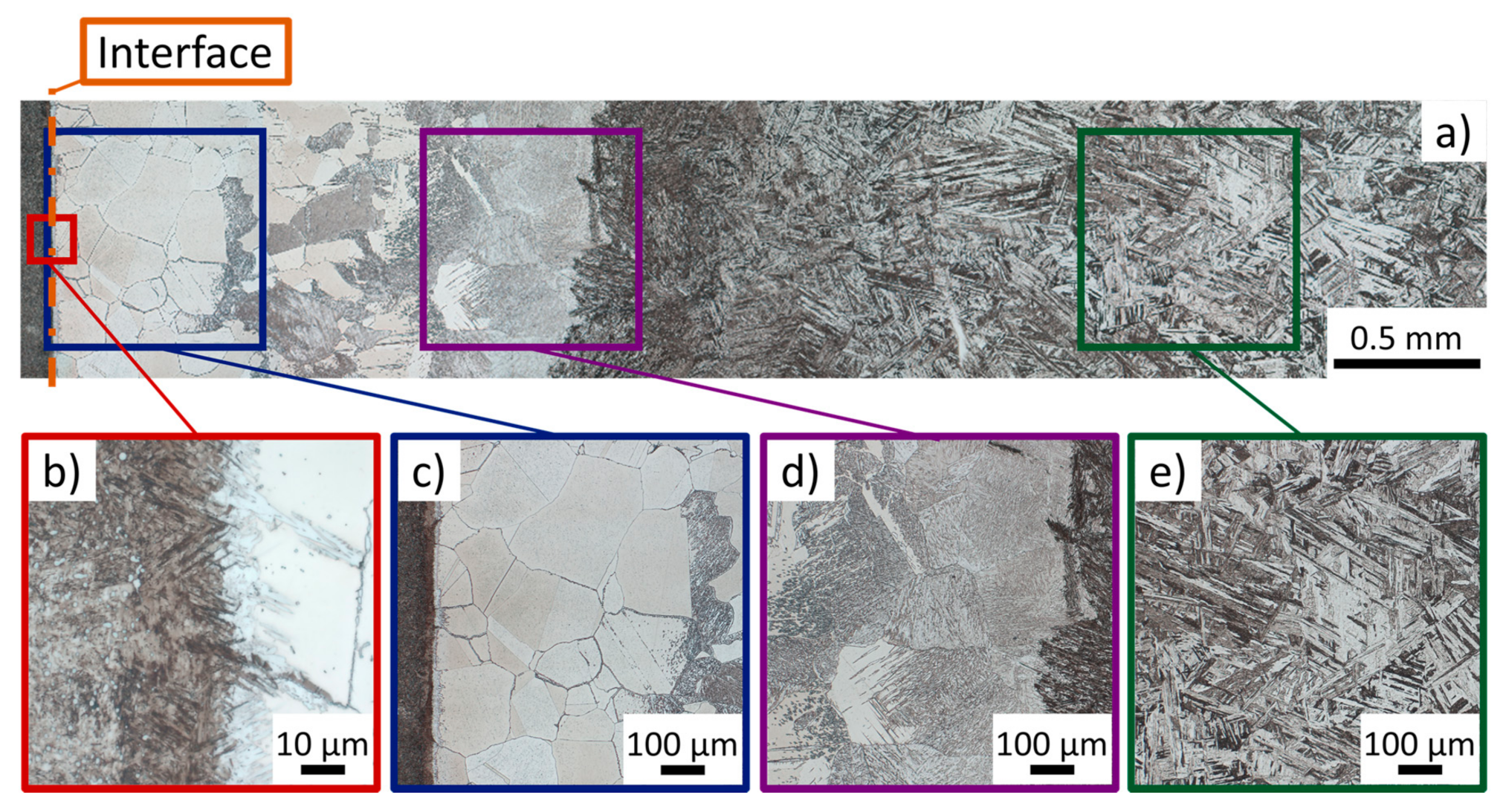
Figure 2a–e shows the final microstructure of composite A after diffusion annealing and heat treatment. Since the microstructure of the tool steel 1 did not differ from the heat treated single material, only the side of the PH steel and the interface are presented. In
Figure 2b a martensitic microstructure with globular shaped carbides on the left and an austenitic microstructure with elongated carbides on the right can be seen. In between a 20 µm thick martensitic layer can be observed. From the interface to a depth of 750 µm an austenitic structure with twins and strongly attacked grain boundaries can be seen (
Figure 2c). Further away from the interface, martensite begins to form (
Figure 2d) and the proportion increases with increasing distance up to 1.8 mm, where the microstructure consists mainly of martensite. Adjacent to the area another form of martensite is found, which was attacked more strongly by the etching and expected to be nickel martensite and which becomes coarser towards the end of the sample (
Figure 2e).
Cold work tool steels were diffusion bonded with a maraging steel using a deformation dilatometer.
Figure 3a–e shows the final microstructure of composite B after diffusion annealing and heat treatment. On the side of the carbide rich tool steel 1 there is a slightly etched zone near the interface (
Figure 3b). The microstructure of the maraging steel is martensitic with fine carbides close to the interface (
Figure 3c). In
Figure 3d no carbides are visible and further away from the interface the etching attack is getting stronger (
Figure 3e).
In order to investigate the interface in more detail, etching with Murakami [
19] of both composites was performed.
Figure 4 shows the results of this etching near the interface. Dense carbide networks can be seen in the vicinity of the interface in a distance of up to 1 mm and 650 µm for composite A and B, respectively. While small globular carbides and elongated carbides along austenite or prior austenite grain boundaries are found in both composites, rod-shaped carbides with a length of up to 12 µm are only present in composite B.
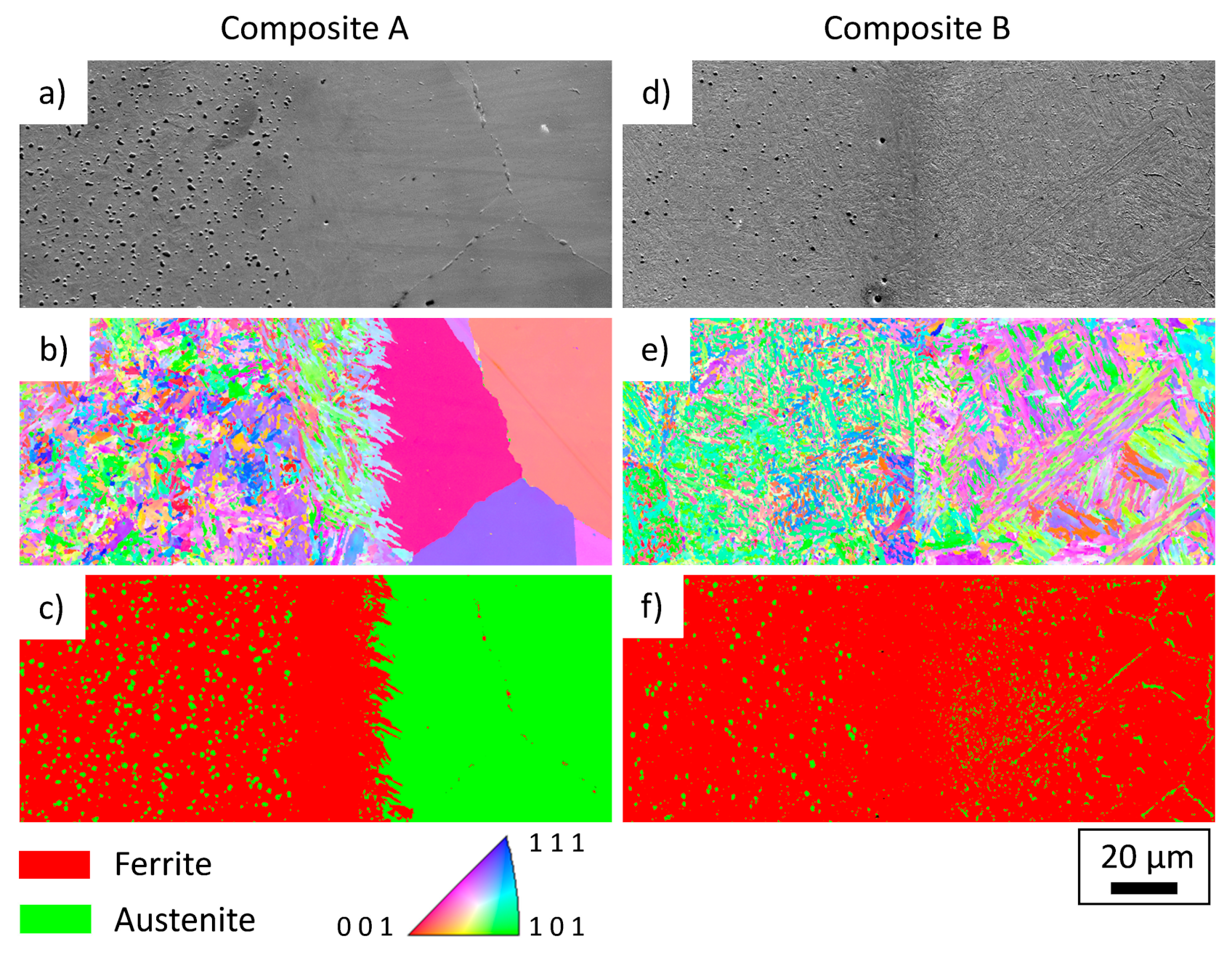
In order to investigate the present phases near the interface, EBSD measurements were carried out. In
Figure 5 the SEM images (a,d), inverse pole figure maps (b,e) and phase maps (c,f) are shown for composite A and B, respectively. Three areas can be seen for composite A, on the left side of
Figure 5a the cold work tool steel with a homogeneous distribution of globular carbides, in the middle a martensitic zone without coarse carbides and on the right an austenitic zone with large grains and carbides at the grain boundaries. Compared to composite A, composite B (
Figure 5d–f) shows a finer martensite with a lower carbide density on the left side and also a martensitic interface without coarse carbides in the middle. On the right side—the side of the maraging steel—a martensitic matrix with fine carbides near the interface can be seen. Further away from the interface, the carbides become coarser and can be observed in the matrix and at grain boundaries.
To determine the chemical composition of the carbides detected, EDX spot measurements were carried out and the related backscattered electron images are shown in
Figure 6. Since it is not possible to determine the crystallography with EDX measurements alone, only the chemical composition estimated from the strongest signals besides carbon are given. For composite A, carbides with different amounts of V, W, Mo and Nb were observed on the side of the tool steel 1. Bright and dark carbides were present in the PH steel, containing Nb and Ti, respectively (
Figure 6a). The carbides of the tool steel 2 are similar to those of tool steel 1, with the exception that they contain no Nb. In the maraging steel, both the elongated and globular carbides consist of Ti and Mo. Nevertheless, no carbides were found which contained elements of the opposing steel.
To observe the distribution of different elements and reveal their diffusion distances in the area of the interface EDX mappings were carried out, which are shown in
Figure 7 next to the SEM images. For composite A, Cr is the most abundant element on both sides. While it is evenly distributed on the cold work tool steel side, Cr carbides can be seen on the PH steel side. Nb is present in only a small percentage, but still forms both elongated and globular carbides in the PH steel. V and W, along with Mo, form carbides in the cold work tool steels of both composites, but only small amounts are dissolved in the matrix. On the PH and maraging steel side, little to no V and W was detected. Regarding the maraging steel,
Figure 7b shows a dense carbide network near the interface where Ti and Mo are present.
The changed microstructure, the formed carbides and the diffusion of the elements influence the hardness.
Figure 8 shows microhardness mappings of the two composites. Both achieve the highest hardness on the side of the cold work tool steel and the lowest hardness near the interface on the PH and maraging steel side. The width of the soft zone is approximately 1.8 mm for composite A and 0.6 mm for composite B. Although no change in the microstructure was observed with optical microscopy, a softening of the cold work tool steels can be seen up to 1 mm from the interface.
4. Discussion
The aim of the present study was to evaluate the possibility of diffusion bonding of cold work tool steels with precipitation hardening and maraging steels. The microstructural changes were investigated in detail for the composites after the applied treatment. The evolution of the microstructure at and next to the interface can be explained by the diffusion of the alloying elements. Temperature and time are the decisive factors in the diffusion process and therefore the development of the microstructure depends on this parameters during the diffusion bonding. The subsequent heat treatment only has a small influence. In total, the material was treated at 1150 °C for 8 h prior to the heat treatment, resulting in a modified microstructure and the formation of carbides for both composites. To estimate the penetration depth of different elements achieved during diffusion bonding, the simplified approach of Einstein and Smoluchowski can be used. For this purpose, the diffusion length of different elements can be estimated by using Equation (1) [
20,
21]:
where
is the mean diffusion length, D is the diffusion coefficient and t is the time. Using diffusion coefficients for the diffusion in pure iron from the literature [
22,
23], one obtains mean diffusion lengths between 10–50 µm for substitutional alloying elements. The interstitially dissolved carbon has higher mobility and has a mean diffusion length of 760 µm.
To compare this calculated mean diffusion paths, diffusion simulations were performed with MatCalc. The results of these simulations are shown in
Figure 9. The dark grey areas represent the cold work tool steels and the light grey areas represent the PH and maraging steel, respectively. For better visibility of the profiles, only the area near the interface is shown for the substitution elements. At the edge of the diagrams, i.e., at 100 µm distance from the interface, all substitution elements have reached their base composition. Therefore, the initial estimation of the mean free diffusion length with 10–50 µm is in good agreement with the simulation. The different diffusion distances of the various elements can be seen in
Figure 9a,b, but the EDX investigations were unable to verify this.
Figure 9c shows the carbon distribution of both composites over the entire sample length. The carbon values of the base alloys are marked on the left and right scale. The simulation of the carbon distribution for both composites shows a smooth transition from the high carbon content on the cold work tool steel side to the low carbon content on the PH or maraging steel side. The carbon content of 0.38 wt% at the edge of the nominally carbon-free steels shows diffusion of the interstitially dissolved carbon over the whole sample length. Compared to that, a change in the microstructure can be seen up to 3 mm from the interface in
Figure 2a and
Figure 3a. The difference between the simulation and the microstructural changes in the investigated specimen can possibly be attributed to the influence of carbide formation, which was not considered in the simulation.
The microstructure near the interface is therefore mainly influenced by the alloying elements and their diffusion. For composite A, an approximately 20 µm thick martensitic layer has formed at the interface in which only fine carbides are present (
Figure 5c). Investigations on stainless steel clad plates by Wang et al. [
18] showed martensite formation at the interface. It was concluded that at the interface, due to plastic deformation, austenite locally transforms into martensite. Controversially, Liu et al. [
12] reported that the martensite is formed due to the diffusion of ferrite stabilizing alloying elements, especially Cr. Due to the high chromium content of the PH steel in composite A, this may be a possible explanation in the present study for the martensitic layer shown in
Figure 5b. In addition, the layer thickness is in good agreement with the simulated diffusion length for substitutional elements.
In composite B the area around the interface is fully martensitic with a transition zone free of coarse carbides (
Figure 5f). Furthermore,
Figure 3b shows a light etched zone on the side of tool steel 2, which can be attributed to the diffusion of carbon. Chen et al. [
13] studied the interfaces of stainless steel plates and found decarburized zones of 75 to 91 µm for temperatures between 1100–1200 °C and a holding time of 2 h. The hardness measurements in
Figure 8 revealed that the extent of the decarburized zone is actually larger than it appears in the etched samples. The softening of the cold work tool steel has an approximate width of 1.2 and 0.8 mm for composite A and B, respectively, which is in the same order of magnitude as the calculated distance for the diffusion of carbon.
In the PH steel, coarse grained austenite is stabilized by carbon up to 1.8 mm from the interface. Comparing the microstructure in
Figure 2a with the hardness measurement in
Figure 8a, it can be seen that the low hardness of austenite is responsible for the soft zone in the PH steel. In a distance of 750 µm from the interface, an increasing amount of martensite was observed. A possible explanation for this is that the amount of carbon is no longer sufficient to stabilize the austenite completely and thus, martensite is formed once the martensite start temperature is reached. With a larger distance to the interface, the carbon content decreases further, resulting in the formation of nickel martensite at a distance of 1.8 mm from the interface, which is typical for a PH steel (
Figure 2d) [
24]. The carbon diffusion does not lead to any microstructural changes in the maraging steel of composite B, but the attack of the etchant becomes stronger with increasing distance from the interface. This can be explained by the fact that carbon refines the microstructure and thus, a lower etching attack occurs [
25].
Since PH steel and maraging steel are almost carbon free, the formation of carbides in the diffusion bonded specimen is due to above mentioned carbon diffusion. The strongest available carbide formers are Nb, followed by Cr for the PH steel and Ti, followed by Mo for the maraging steel [
26]. With the occurrence of Cr carbides along the grain boundaries near the interface of composite A, intergranular corrosion can occur locally [
6]. The investigated PH 17–4 steel forms intermetallic phases containing Cu and Ni during aging [
27]. Since none of these elements has a high affinity to carbon, carbide formation has only a minor influence on precipitation hardening. On the maraging side of composite B, Ti and Mo rich carbides occur, up to a distance of 650 µm from the interface (
Figure 6b). With Ti being the stronger carbide former, it is most likely that TiC has formed, in which Mo is dissolved [
26,
28]. As less Ti is available in the matrix due to carbide formation, there is a reduced effect of precipitation hardening, resulting in a soft zone near the interface (
Figure 8b).
Several zones have formed in the two-layered steel composite, which will have positive effects on the ballistic protection properties. The first few millimetres of the surface of the cold work steel remained unaffected by diffusion and could therefore maintain high hardness. This will have a positive effect on the destruction of the projectile on impact. The gradual hardness transition in the decarburized zone towards the well bonded interface should create a stable bond, which is essential for the ballistic testing. Although the carbides in the PH and maraging steel weaken the grain boundaries, the soft zone close to the interface helps to prevent brittle fracture [
28,
29]. The remaining carbide-free tough zone of the PH and maraging steel should absorbs the remaining energy of the projectile and thus contribute to a positive ballistic test result.
Author Contributions
Conceptualization, M.G. and R.S.; methodology, M.G.; software, M.G.; investigation, M.G.; resources, H.E. and A.P.; data curation, M.G.; writing—original draft preparation, M.G.; writing—review and editing, R.S. and I.S.; visualization, M.G.; supervision, R.S.; project administration, H.E. and A.P.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding of the Austrian BMK (846933) in the framework of the program “Production of the future” and the “BMK Professorship for Industry” is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laible, R.C. Ballistic Materials and Penetration Mechanics; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1980; ISBN 9780444601643. [Google Scholar]
- Sadanandan, S.; Hetherington, J.G. Characterisation of ceramic/steel and ceramic/aluminium armours subjected to oblique impact. Int. J. Impact Eng. 1997, 19, 811–819. [Google Scholar] [CrossRef]
- Teng, X.; Wierzbicki, T.; Huang, M. Ballistic resistance of double-layered armor plates. Int. J. Impact Eng. 2008, 35, 870–884. [Google Scholar] [CrossRef]
- Flores-Johnson, E.A.; Saleh, M.; Edwards, L. Ballistic performance of multi-layered metallic plates impacted by a 7.62-mm APM2 projectile. Int. J. Impact Eng. 2011, 38, 1022–1032. [Google Scholar] [CrossRef]
- Liu, B.X.; An, Q.; Yin, F.X.; Wang, S.; Chen, C.X. Interface formation and bonding mechanisms of hot-rolled stainless steel clad plate. J. Mater. Sci. 2019, 54, 11357–11377. [Google Scholar] [CrossRef]
- Giudice, F.; Missori, S.; Murdolo, F.; Sili, A. Metallurgical Characterization of the Interfaces in Steel Plates Clad with Austenitic Steel or High Ni Alloys by Hot Rolling. Metals 2020, 10, 286. [Google Scholar] [CrossRef]
- Husain, M.M.; Ghosh, M. Inhibition of intermetallic formation during diffusion bonding of high-carbon steel. Int. J. Adv. Manuf. Technol. 2013, 66, 1871–1877. [Google Scholar] [CrossRef]
- López, B.; Gutiérrez, I.; Urcola, J.J. Study of the microstructure obtained after diffusion bonding inconel 625 to low alloy steel by hot uniaxial pressing or hipping. Mater. Charact. 1992, 28, 49–59. [Google Scholar] [CrossRef]
- Wang, D.; Shi, Z.; Qi, R. Cladding of stainless steel on aluminum and carbon steel by interlayer diffusion bonding. Scr. Mater. 2007, 56, 369–372. [Google Scholar] [CrossRef]
- Jing, Y.A.; Qin, Y.; Zang, X.; Shang, Q.; Hua, S. A novel reduction-bonding process to fabricate stainless steel clad plate. J. Alloys Compd. 2014, 617, 688–698. [Google Scholar] [CrossRef]
- Jing, Y.A.; Qin, Y.; Zang, X.; Li, Y. The bonding properties and interfacial morphologies of clad plate prepared by multiple passes hot rolling in a protective atmosphere. J. Mater. Process. Technol. 2014, 214, 1686–1695. [Google Scholar] [CrossRef]
- Liu, B.X.; Wang, S.; Chen, C.X.; Fang, W.; Feng, J.H.; Zhang, X.; Yin, F.X. Interface characteristics and fracture behavior of hot rolled stainless steel clad plates with different vacuum degrees. Appl. Surf. Sci. 2019, 463, 121–131. [Google Scholar] [CrossRef]
- Chen, C.X.; Liu, M.Y.; Liu, B.X.; Yin, F.X.; Dong, Y.C.; Zhang, X.; Zhang, F.Y.; Zhang, Y.G. Tensile shear sample design and interfacial shear strength of stainless steel clad plate. Fusion Eng. Des. 2017, 125, 431–441. [Google Scholar] [CrossRef]
- Liu, B.X.; Wang, S.; Fang, W.; Ma, J.L.; Yin, F.X.; He, J.N.; Feng, J.H.; Chen, C.X. Microstructure and mechanical properties of hot rolled stainless steel clad plate by heat treatment. Mater. Chem. Phys. 2018, 216, 460–467. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhang, X.; Chen, C.; Fang, W.; Ji, P.; Feng, J.; Jiang, Y.; Yin, F. Microstructure and Interface Fracture Characteristics of Hot-Rolled Stainless Steel Clad Plates by Adding Different Interlayers. Steel Res. Int. 2020, 137, 1900604. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Zhang, B.; Zhang, Q. Microstructure Characterization and Mechanical Properties of Stainless Steel Clad Plate. Materials 2019, 12, 509. [Google Scholar] [CrossRef]
- Liu, B.X.; Yin, F.X.; Dai, X.L.; He, J.N.; Fang, W.; Chen, C.X.; Dong, Y.C. The tensile behaviors and fracture characteristics of stainless steel clad plates with different interfacial status. Mater. Sci. Eng. A 2017, 679, 172–182. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.X.; Chen, C.X.; Feng, J.H.; Yin, F.X. Microstructure, mechanical properties and interface bonding mechanism of hot-rolled stainless steel clad plates at different rolling reduction ratios. J. Alloys Compd. 2018, 766, 517–526. [Google Scholar] [CrossRef]
- Petzow, G. Metallographisches, Keramographisches und Plastographisches Ätzen; Gebrüder Borntraeger: Stuttgart, Germany, 2006. [Google Scholar]
- Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 1905, 549–560. [Google Scholar] [CrossRef]
- Smoluchowski, M. Zur kinetischen Theorie der Brownschen Molekularbewegung und der Suspensionen. Ann. Phys. 1906, 756–780. [Google Scholar] [CrossRef]
- Dahl, W. Eigenschaften Und Anwendungen Von Stählen. 1. Grundlagen; Verlag der Augustinus-Buchh: Aachen, Germany, 1993; ISBN 9783860731253. [Google Scholar]
- Oikawa, H. Review of lattice diffusion of substitutional impurities in iron—A summary report. Tech. Rep. Tohoku Univ. 1982, 47, 215–224. [Google Scholar]
- Schnitzer, R.; Hochfellner, R.; Nöhrer, M.; Schober, M.; Clemens, H.; Zinner, S.; Leitner, H. Microstructural Characterization of PH 13-8 Mo Maraging Steels. Prakt. Metallogr. 2009, 521–536. [Google Scholar] [CrossRef]
- Angeli, J.; Füreder, E.; Panholzer, M.; Kneissl, A.C. Etching Techniques for Characterizing the Phases of Low-Alloy Dual-Phase and TRIP Steels. Prakt. Metallogr. 2006, 43, 489–504. [Google Scholar] [CrossRef]
- Shatynski, S.R. The thermochemistry of transition metal carbides. Oxid. Met. 1979, 13, 105–118. [Google Scholar] [CrossRef]
- Hsiao, C.N.; Chiou, C.S.; Yang, J.R. Aging reactions in a 17-4 PH stainless steel. Mater. Chem. Phys. 2002, 134–142. [Google Scholar] [CrossRef]
- Eremenko, V.N.; Velikanova, T.Y. The interaction of molybdenum with titanium carbide. Poroshkovaya Met. 1963, 2, 3–9. [Google Scholar] [CrossRef]
- Briant, C.L.; Banerji, S.K. Intergranular failure in steel: The role of grain-boundary composition. Int. Met. Rev. 1978, 164–199. [Google Scholar] [CrossRef]
Figure 1.
Schematic illustration of the diffusion bonding process in a deformation dilatometer: (a) Two cylindrical samples are fitted with thermocouples and placed between two stamps. The samples are diffusion bonded by simultaneous annealing at high temperatures and applying mechanical pressure on them, (b) Temperature and retention force during the diffusion bonding process.
Figure 2.
Optical micrographs of Kalling 2 etched samples of composite A (cold work tool steel and precipitation hardening steel): (a) Overview of PH steel and the interface, (b) Martensitic zone at the interface, (c) Austenitic zone with carbides at the grain boundaries, (d) Transition from austenite and martensite to nickel martensite, (e) Nickel martensite.
Figure 3.
Optical micrographs of Kalling 2 etched samples of composite B (cold work tool steel and maraging steel): (a) Overview of maraging steel and the interface, (b) Decarburized zone of tool steel 2, (c) Slightly etched area with carbides in the matrix and at grain boundaries, (d) Slightly etched Nickel martensitic region (e) Nickel martensite.
Figure 4.
Dark field images of Murakami etched samples showing a dense carbide distribution in the matrix and at grain boundaries for (a) the PH steel of composite A and (b) the maraging steel of composite B.
Figure 5.
EBSD images showing the area near the interface of composite A (a–c) and composite B (d–f). (a,d) SEM images, (b,e) inverse pole figure maps, (c,f) phase maps. Both cold work tool steels showing a martensitic microstructure with a homogeneous distribution of globular carbides on the left side. The martensitic interface is free of coarse carbides for both composites. The PH steel (composite A) shows an austenitic microstructure with carbides at the grain boundary and the maraging steel (composite B) shows a martensitic microstructure with carbides in the matrix and at grain boundaries.
Figure 6.
Backscattered electron images close to the interface with marked positions of the EDX spot measurements for (a) composite A and (b) composite B. Carbides rich in V, W and Mo were found for both cold work tool steels. In the PH steel both Cr and Nb rich carbides were detected. For the maraging steel only Ti and Mo rich carbides were observed.
Figure 7.
Backscattered electron images of the interface with EDX mappings of the interface for various element distributions: (a) composite A, (b) composite B. The distribution of carbides in the vicinity of the interface can be observed. No diffusion of substitutionally dissolved elements can be detected.
Figure 8.
HV0.1 microhardness maps of the interface: (a) composite A, (b) composite B. For both composites, the area around the interface was softer than the respective base material.
Figure 9.
Simulated concentration profile of alloying elements near the interface after diffusion bonding and heat treatment for (a) composite A and (b) composite B. The diffusion of substitution solutes is in the range of 10–50 µm. The carbon distribution of the whole sample for both composites is displayed in (c). The carbon concentration of the base alloys is marked on the left side for the cold work tool steels and on the right for the PH and maraging steel.
Table 1.
Composition of the alloys. Composite A consists of the cold work tool steel 1 and a precipitation hardening (PH) steel. Composite B consists of the cold work tool steel 2 and a maraging steel.
| Composite | (wt%) | C | Si | Mn | Cr | Ni | V | Cu | Nb | Mo | W | Co | Ti | Fe |
|---|
| A | Cold Work Tool Steel 1 | 1.39 | 0.62 | 0.35 | 6.35 | 0.12 | 3.54 | 0.11 | 0.25 | 1.39 | 4.12 | − | − | bal. |
| PH 17–4 | 0.05 | 0.27 | 0.37 | 15.04 | 4.38 | 0.07 | 3.01 | 0.31 | 0.19 | − | − | − | bal. |
| B | Cold Work Tool Steel 2 | 0.90 | 0.51 | 0.34 | 4.23 | 0.21 | 1.90 | 0.09 | − | 2.55 | 2.32 | 4.51 | − | bal. |
| Maraging 300 | 0.01 | 0.03 | 0.03 | 0.11 | 18.36 | 0.04 | 0.09 | − | 4.18 | 0.02 | 9.31 | 0.83 | bal. |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).