Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Thermodynamic Simulation
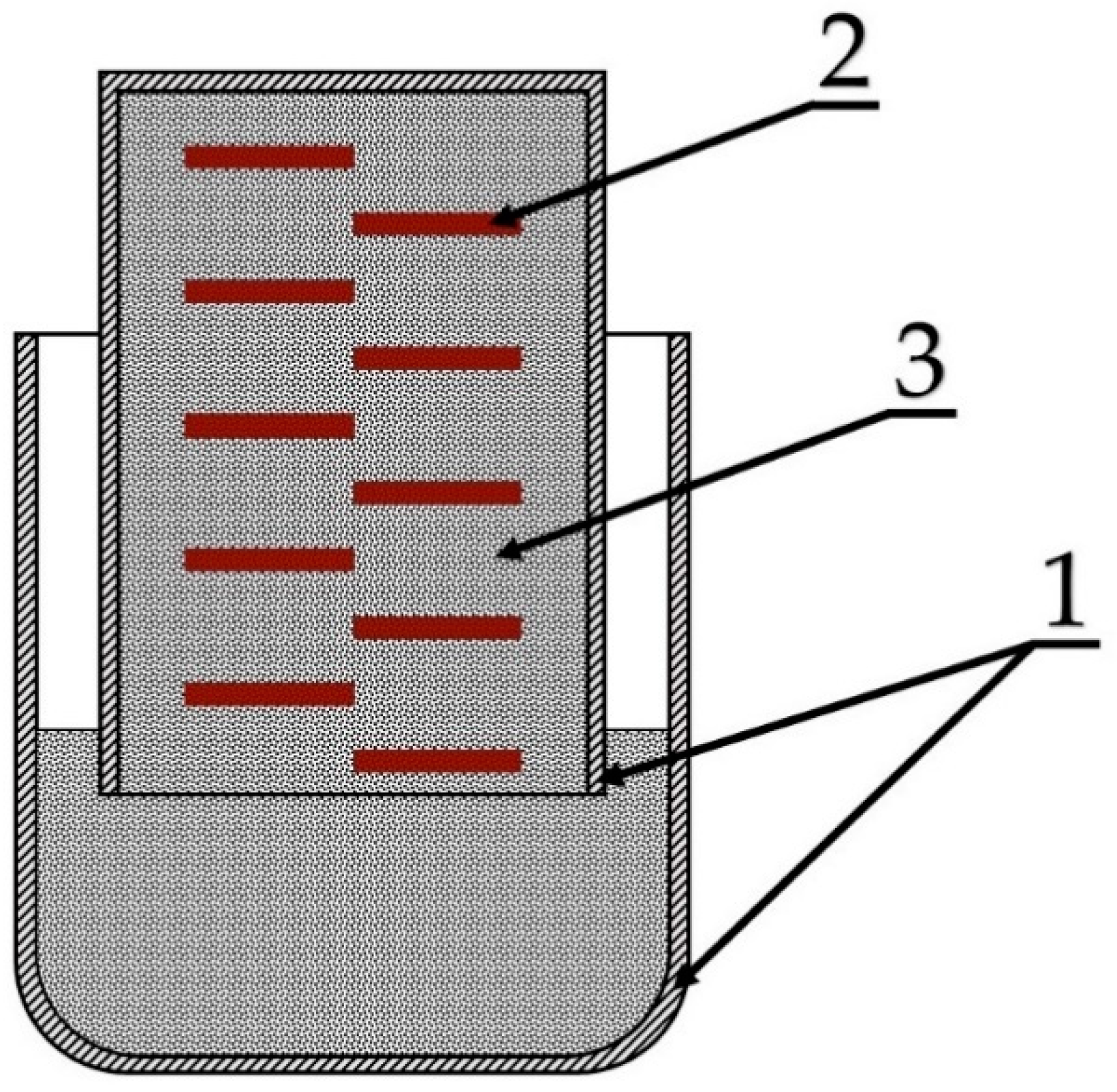
2.3. Experimental Procedure
2.4. Calculation of Iron Grain Size
2.5. Magnetic Separation
3. Results
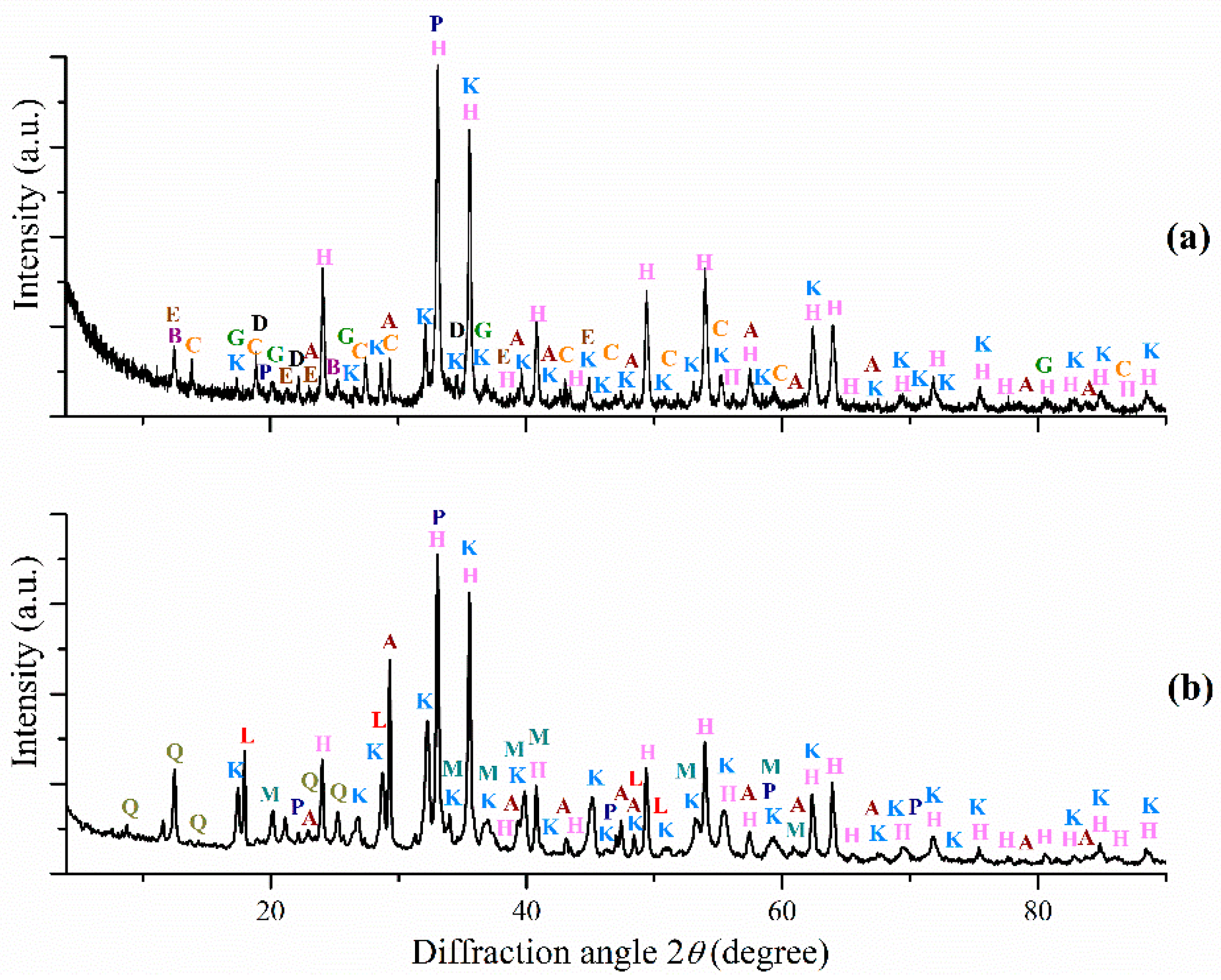
3.1. Red Mud Samples Characterization
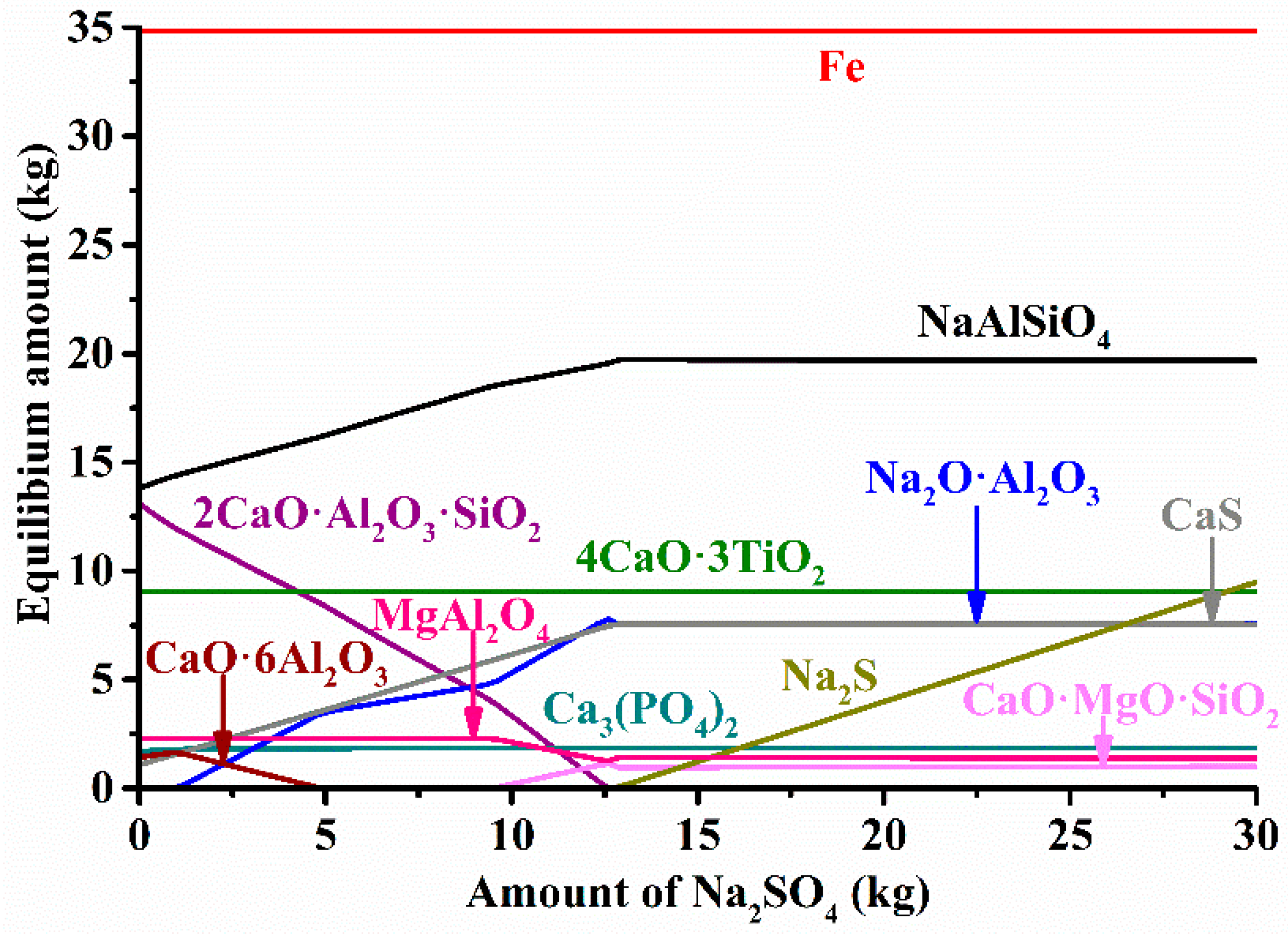
3.2. Thermodynamic Calculations
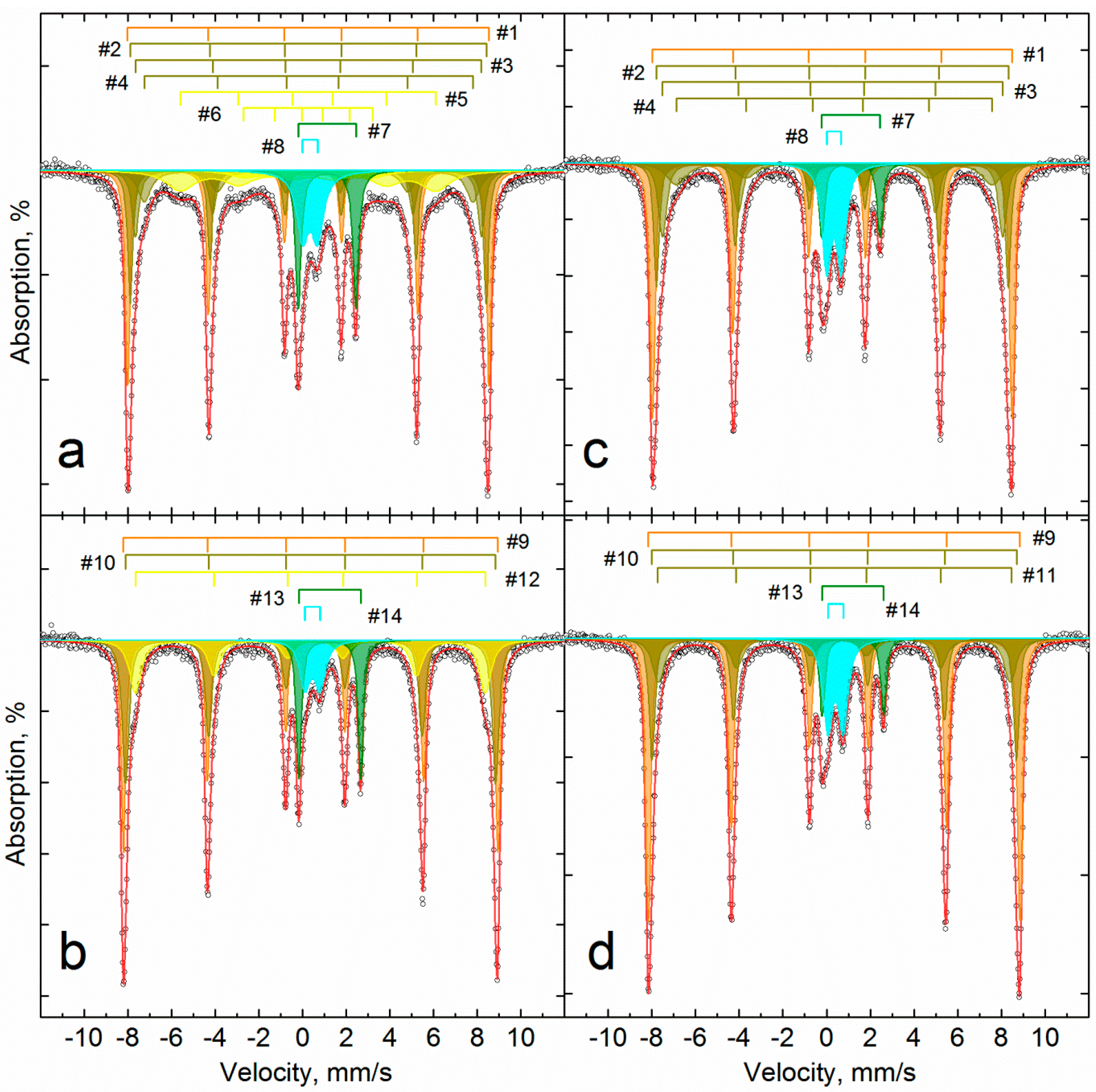
3.3. Investigation of Iron Reduction in the Roasted Samples
3.4. Experimental Results of the Carbothermic Roasting Followed by Magnetic Separation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alumina Production. Available online: http://www.world-aluminium.org/statistics/alumina-production/#data (accessed on 16 March 2020).
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Xue, S.G.; Wu, Y.J.; Li, Y.W.; Kong, X.F.; Zhu, F.; William, H.; Li, X.F.; Ye, Y.Z. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review. J. Cent. South Univ. 2019, 26, 268–288. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Tian, K.; Peng, D.; Liao, X.; Wu, X. Geochemical characteristics and toxic elements in alumina refining wastes and leachates from management facilities. Int. J. Environ. Res. Public Health 2019, 16, 1297. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk-Sokulska, E.; Kulczycka, J. Impact of landfilling of red mud waste on local environment—The case of Górka. Gospod. Surowcami Min. Min. Resour. Manag. 2015, 31, 137–156. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, Y.; Peng, D.; Huang, T.; Sun, C. pH-Dependent Leaching Characteristics of Major and Toxic Elements from red mud. Int. J. Environ. Res. Public Health 2019, 16, 2046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, K.; Liu, Y.; Lyu, G.; Li, X.; Chen, X. A review of comprehensive utilization of high-iron red mud of China. In Light Metals 2020; The Minerals, Metals & Materials Series; Tomsett, A., Ed.; Springer: Cham, Switzerland, 2020; pp. 65–71. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Extraction of Iron values from red mud. Mater. Today Proc. 2018, 5, 17064–17072. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, J.P. Utilization of iron values of red mud for metallurgical applications. In Environmental and Waste Management; Bandopadhyay, A., Goswami, N.G., Rao, P.R., Eds.; National Metallurgical Laboratory: Jamshedpur, India, 1998; pp. 108–119. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Met. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Miner. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.X.; Liu, X.M. Recovery of scandium from bauxite residue—Red mud: A review. Rare Met. 2016, 35, 887–900. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Meshram, A.; Meshram, P. Overview on extraction and separation of rare earth elements from red mud: Focus on scandium. Min. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Lima, M.S.S.; Thives, L.P.; Haritonovs, V.; Bajars, K. Red mud application in construction industry: Review of benefits and possibilities. Iop Conf. Ser. Mater. Sci. Eng. 2017, 251, 012033. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Venkatesh, C.; Chand, M.S.R.; Nerella, R. A state of the art on red mud as a substitutional cementitious material. Ann. Chim. Sci. Des Mater. 2019, 43, 99–106. [Google Scholar] [CrossRef]
- Kumar, M.; Senapati, B.; Kumar, C.S. Management of industrial waste: The case of effective utilization of red mud and fly ash at Vedanta Aluminium Limited—Lanjigarh. In Light Metals 2013; The Minerals, Metals & Materials Series; Sadler, B., Ed.; Springer: Cham, Switzerland, 2016; pp. 119–123. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Maurina, S.; Conci, A.; Salviati, A.; Carturan, G.; Cocco, G. Bauxite “red mud” in the ceramic industry. Part 2: Production of clay-based ceramics. J. Eur. Ceram. Soc. 2000, 20, 245–252. [Google Scholar] [CrossRef]
- Hertel, T.; Pontikes, Y. Geopolymers, inorganic polymers, alkali-activated materials and hybrid binders from bauxite residue (red mud)—Putting things in perspective. J. Clean. Prod. 2020, 258, 120610. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B Environ. 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Itskov, Y. Red Mud: Problem and Solutions. Available online: https://www.metalbulletin.com/events/download.ashx/document/speaker/7453/a0ID000000X0k8RMAR/Presentation (accessed on 19 March 2020).
- Keskinkilic, E.; Pournaderi, S.; Geveci, A.; Topkaya, Y.A. Solid-state reduction studies for recovery of iron from red mud. In 11th International Symposium on High-Temperature Metallurgical Processing; The Minerals, Metals & Materials Series; Peng, Z., Ed.; Springer: Cham, Switzerland, 2020; pp. 511–519. [Google Scholar] [CrossRef]
- Keskinkilic, E.; Pournaderi, S.; Geveci, A.; Topkaya, Y.A. Smelting studies for recovery of iron from red mud. In 10th International Symposium on High-Temperature Metallurgical Processing; The Minerals, Metals & Materials Series; Jiang, T., Hwang, J.-Y., Gregurek, D., Peng, Z.W., Downey, J.P., Zhao, B.J., Eds.; Springer: Cham, Switzerland, 2019; pp. 489–499. [Google Scholar] [CrossRef]
- Long, H.; Meng, Q.; Chun, T.; Wang, P.; Li, J. Preparation of metallic iron powder from copper slag by carbothermic reduction and magnetic separation. Can. Metall. Q. 2016, 55, 338–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yu, X. Recovery of iron from cyanide tailings with reduction roasting-water leaching followed by magnetic separation. J. Hazard. Mater. 2012, 213–214, 167–174. [Google Scholar] [CrossRef]
- Park, J.W.; Ahn, J.C.; Song, H.; Park, K.; Shin, H.; Ahn, J.S. Reduction characteristics of oily hot rolling mill sludge by direct reduced iron method. Resour. Conserv. Recycl. 2002, 34, 129–140. [Google Scholar] [CrossRef]
- Khaki, J.V.; Shalchian, H.; Rafsanjani-Abbasi, A.; Alavifard, N. Recovery of iron from a high-sulfur and low-grade iron ore. Thermochim. Acta 2018, 662, 47–54. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.; Kou, J.; Wei, Y.; Xu, C.; Liu, Z. The function of Ca(OH)2 and Na2CO3 as additive on the reduction of high-phosphorus oolitic hematite-coal mixed pellets. ISIJ Int. 2013, 53, 427–433. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chun, T.J.; Pan, J.; Lu, L.M.; He, Z. Upgrading and dephosphorization of Western Australian iron ore using reduction roasting by adding sodium carbonate. Int. J. Min. Met. Mater. 2013, 20, 505–513. [Google Scholar] [CrossRef]
- Qu, G.; Zhou, S.; Wang, H.; Li, B.; Wei, Y. Production of ferronickel concentrate from low-grade nickel laterite ore by non-melting reduction magnetic separation process. Metals 2019, 9, 1340. [Google Scholar] [CrossRef]
- Ma, B.; Xing, P.; Yang, W.; Wang, C.; Chen, Y.; Wang, H. Solid-state metalized reduction of magnesium-rich low-nickel oxide ores using coal as the reductant based on thermodynamic analysis. Met. Mater. Trans. B Process Met. Mater. Process. Sci. 2017, 48, 2037–2046. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, L.; Guo, Z.; Pan, J.; Zhang, F. Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv. Powder Technol. 2019, 30, 451–460. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Cardenia, C.; Balomenos, E.; Panias, D. Iron recovery from bauxite residue through reductive roasting and wet magnetic separation. J. Sustain. Metall. 2019, 5, 9–19. [Google Scholar] [CrossRef]
- Zhu, D.; Chun, T.; Pan, J.; He, Z. Recovery of iron from high-iron red mud by reduction roasting with adding sodium salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef]
- Suprapto, S.; Istiqomah, Z.; Santoso, E.; Dawam, A.A.; Prasetyoko, D. Alumina extraction from red mud by magnetic separation. Indones. J. Chem. 2018, 18, 331–336. [Google Scholar] [CrossRef]
- Rao, M.; Zhuang, J.; Li, G.; Zeng, J.; Jiang, T. Iron recovery from red mud by reduction roasting-magnetic separation. In Light Metals 2013; The Minerals, Metals & Materials Series; Sadler, B.A., Ed.; Springer: Cham, Switzerland, 2016; pp. 125–130. [Google Scholar] [CrossRef]
- Grudinskii, P.I.; Zinoveev, D.V.; Semenov, A.F.; Zakunov, A.S.; Dyubanov, V.G.; Petelin, A.L. Advanced method for recycling red mud by carbothermal solid-phase reduction using sodium sulfate. Metallurgist 2020, 63, 15–21. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, J.-H.; Peng, Y.; Shen, S.-Y.; Chen, T. Iron extraction from red mud using roasting with sodium salt. Min. Process. Extr. Met. Rev. 2019. [Google Scholar] [CrossRef]
- Chun, T.; Li, D.; Di, Z.; Long, H.; Tang, L.; Li, F.; Li, Y. Recovery of iron from red mud by hightemperature reduction of carbonbearing briquettes. J. S. Afr. Inst. Min. Metall. 2017, 117, 361–364. [Google Scholar] [CrossRef]
- Zinoveev, D.; Grudinsky, P.; Zakunov, A.; Semenov, A.; Panova, M.; Valeev, D.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Influence of Na2CO3 and K2CO3 addition on iron grain growth during carbothermic reduction of red mud. Metals 2019, 9, 1313. [Google Scholar] [CrossRef]
- Ding, W.; Peng, Y.; Wu, Q.; Shen, S.; Xiao, J.; Liang, G.; Huang, W. Increase of iron concentration and reduction of impurities in red mud from the Wenshan area of Yunnan Province by segregation roasting-low intensity magnetic separation. J. Mines Met. Fuels 2019, 67, 377–384. [Google Scholar]
- Huang, Z.-C.; Cai, L.-B.; Zhang, Y.; Yang, Y.-B.; Jiang, T. Reduction of iron oxides of red mud reinforced by Na2CO3 and CaF2. Zhongnan Daxue Xuebao Ziran Kexue Ban J. Cent. South Univ. Sci. Technol. 2010, 41, 838–844. [Google Scholar]
- Lv, W.; Bai, C.; Lv, X.; Hu, K.; Lv, X.; Xiang, J.; Song, B. Carbothermic reduction of ilmenite concentrate in semi-molten state by adding sodium sulfate. Powder Technol. 2018, 340, 354–361. [Google Scholar] [CrossRef]
- Gao, E.X.; Sun, T.C.; Liu, Z.G.; Geng, C.; Xu, C.Y. Effect of sodium sulfate on direct reduction of beach titanomagnetite for separation of iron and titanium. J. Iron Steel Res. Int. 2016, 23, 428–433. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, Z.; Pan, J.; Zhang, F. Synchronous upgrading iron and phosphorus removal from high phosphorus Oolitic hematite ore by high temperature flash reduction. Metals 2016, 6, 123. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; You, Z.; Lv, X. Kinetics study on non-isothermal carbothermic reduction of nickel laterite ore in presence of Na2SO4. Powder Technol. 2020, 362, 486–492. [Google Scholar] [CrossRef]
- Rao, M.; Li, G.; Zhang, X.; Luo, J.; Peng, Z.; Jiang, T. Reductive roasting of nickel laterite ore with sodium sulphate for Fe-Ni production. Part II: Phase transformation and grain growth. Sep. Sci. Technol. 2016, 51, 1727–1735. [Google Scholar] [CrossRef]
- Wang, X.P.; Sun, T.C.; Chen, C.; Kou, J. Effects of Na2SO4 on iron and nickel reduction in a high-iron and low-nickel laterite ore. Int. J. Min. Metall. Mater. 2018, 25, 383–390. [Google Scholar] [CrossRef]
- Grudinskii, P.I.; Dyubanov, V.G.; Zinoveev, D.V.; Zheleznyi, M.V. Solid-phase reduction and iron grain growth in red mud in the presence of alkali metal salts. Russ. Met. 2018, 2018, 1020. [Google Scholar] [CrossRef]
- Meher, S.N.; Rout, A.; Padhi, B.K. Extraction of alumina from red mud by divalent alkaline earth metal soda ash sinter process. In Light Metals 2011; Lindsay, S.J., Ed.; Springer: Cham, Switzerland, 2011; pp. 231–236. [Google Scholar]
- Anisonyan, K.G.; Kopyev, D.Y.; Olyunina, T.V.; Sadykhov, G.B. Influence of Na2CO3 and CaCO3 additions on the aluminate slag formation during a single-stage reducing roasting of red mud. Non-Ferr. Met. 2019, 17–21. [Google Scholar] [CrossRef]
- Tam, P.W.Y.; Panias, D.; Vassiliadou, V. Sintering optimisation and recovery of aluminum and sodium from greek bauxite residue. Minerals 2019, 9, 571. [Google Scholar] [CrossRef]
- Fan, D.C.; Ni, W.; Yan, A.Y.; Wang, J.Y.; Cui, W.H. Orthogonal experiments on direct reduction of carbon-bearing pellets of bayer red mud. J. Iron Steel Res. Int. 2015, 22, 686–693. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Deng, X.; Wang, K.; He, C.; Li, X.; Wei, Y. Comprehensive recovery of iron and aluminum from ordinary bayer red mud by reductive sintering–magnetic separation–digesting process. JOM 2019, 71, 2936–2943. [Google Scholar] [CrossRef]
- Shiryaeva, E.V.; Podgorodetskiy, G.S.; Malyscheva, T.Y.; Gorbunov, V.B.; Zavodyaniy, A.V.; Schapovalov, A.N. Influence of low alkaline red mud on the properties and microstructure of the agglomerates from the charge materials JSC “Ural steel”. Izv. Ferr. Met. 2014, 57, 14–19. [Google Scholar] [CrossRef][Green Version]
- HSC Chemistry, version 9.9; software for chemical reaction and equilibrium calculation; Outotec: Pori, Finland, 2019.
- Match! Version 3.10; Software for Phase Analysis Using Powder Diffraction; Crystal Impact: Bonn, Germany, 2019.
- Pankratov, D.A.; Anuchina, M.M.; Spiridonov, F.M.; Krivtsov, G.G. Fe3-δO4 nanoparticles synthesized in the presence of natural polyelectrolytes. Crystallogr. Rep. 2020, 65, 393–397. [Google Scholar] [CrossRef]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive smelting of neutralized red mud for iron recovery and produced pig iron for heat-resistant castings. Metals 2020, 10, 32. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of iron oxides commonly formed as corrosion products on steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N.; Minkina, T.M.; Kubrin, S.P.; Pankratov, D.A.; Fedorenko, A.G. Common and rare iron, sulfur, and zinc minerals in technogenically contaminated hydromorphic soil from Southern Russia. Environ. Geochem. Health 2020, 42, 95–108. [Google Scholar] [CrossRef]
- Hairi, S.N.M.; Jameson, G.N.L.; Rogers, J.J.; MacKenzie, K.J.D. Synthesis and properties of inorganic polymers (geopolymers) derived from Bayer process residue (red mud) and bauxite. J. Mater. Sci. 2015, 50, 7713–7724. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Barrero, C.A.; Da Costa, G.M.; Van San, E.; De Grave, E. Mössbauer characterization of iron oxides and (oxy)hydroxides: The present state of the art. Hyperfine Interact. 2000, 126, 247–259. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Korobov, M.S.; Pankratov, D.A.; Yurkov, G.Y.; Gubin, S.P. Catalytic conversions of chloroolefins over iron oxide nanoparticles 2. Isomerization of dichlorobutenes over iron oxide nanoparticles stabilized on the surface of ultradispersed poly(tetrafluoroethylene). Russ. Chem. Bull. 2005, 54, 1425–1432. [Google Scholar] [CrossRef]
- Jónás, K.; Solymár, K.; Zöldi, J. Some applications of mössbauer spectroscopy for the quantitative analysis of minerals and mineral mixtures. J. Mol. Struct. 1980, 60, 449–452. [Google Scholar] [CrossRef]
- Morin, F.J. Magnetic susceptibility of αFe2O3 and αFe2O3 with added titanium. Phys. Rev. 1950, 78, 819–820. [Google Scholar] [CrossRef]
- Van San, E.; De Grave, E.; Vandenberghe, R.E.; Desseyn, H.O.; Datas, L.; Barrón, V.; Rousset, A. Study of Al-substituted hematites, prepared from thermal treatment of lepidocrocite. Phys. Chem. Min. 2001, 28, 488–497. [Google Scholar] [CrossRef]
- Pankratov, D.A. Mössbauer study of oxo derivatives of iron in the Fe2O3-Na2O2 system. Inorg. Mater. 2014, 50, 82–89. [Google Scholar] [CrossRef]
- Khalil, M.I. Crystal chemistry and Mössbauer spectroscopic analysis of clays around Riyadh for brick industry. Hyperfine Interact. 2013, 218, 113–121. [Google Scholar] [CrossRef]
- Karimi, E.; Teixeira, I.F.; Ribeiro, L.P.; Gomez, A.; Lago, R.M.; Penner, G.; Kycia, S.W.; Schlaf, M. Ketonization and deoxygenation of alkanoic acids and conversion of levulinic acid to hydrocarbons using a red mud bauxite mining waste as the catalyst. Catal. Today 2012, 190, 73–88. [Google Scholar] [CrossRef]
- Ammasi, A. Effect of heating rate on decomposition temperature of goethite ore. Trans. Indian Inst. Met. 2020, 73, 93–98. [Google Scholar] [CrossRef]
- Yang, J.; Mori, T.; Kuwabara, M. Mechanism of carbothermic reduction of hematite in hematite-carbon composite pellets. ISIJ Int. 2007, 47, 1394–1400. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, T.; Liu, Z.; Kou, J.; Liu, N.; Zhang, S. Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. Int. J. Min. Process. 2013, 123, 32–38. [Google Scholar] [CrossRef]
- Rayzman, V.; Filipovich, I.; Nisse, L.; Vlasenko, Y. Sodium aluminate from alumina-bearing intermediates and wastes. JOM 1998, 50, 32–37. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M. Nature-inspired synthesis of magnetic non-stoichiometric Fe3O4 nanoparticles by oxidative in situ method in a humic medium. Mater. Chem. Phys. 2019, 231, 216–224. [Google Scholar] [CrossRef]
- Sauer, W.E.; Reynik, R.J. Electronic and magnetic structure of dilute iron-base alloys. J. Appl. Phys. 1971, 42, 1604–1605. [Google Scholar] [CrossRef]
- Vincze, I.; Campbell, I.A. Mossbauer measurements in iron based alloys with transition metals. J. Phys. F Met. Phys. 1973, 3, 647–663. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Cieslak, J. Short-range order in iron-rich Fe-Cr alloys as revealed by Mössbauer spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 10–13. [Google Scholar] [CrossRef]
- Haneda, K.; Zhou, Z.X.; Morrish, A.H.; Majima, T.; Miyahara, T. Low-temperature stable nanometer-size fcc-Fe particles with no magnetic ordering. Phys. Rev. B 1992, 46, 13832–13837. [Google Scholar] [CrossRef]
- Ding, J.; Huang, H.; Mccormick, P.G.; Street, R. Magnetic properties of martensite-austenite mixtures in mechanically milled 304 stainless steel. J. Magn. Magn. Mater. 1995, 139, 109–114. [Google Scholar] [CrossRef]
- Le Caer, G.; Dubois, J.M.; Senateur, J.P. Etude par spectrométrie Mössbauer des carbures de Fer Fe3C et Fe5C2. J. Solid State Chem. 1976, 19, 19–28. [Google Scholar] [CrossRef]
- Baskakov, A.O.; Starchikov, S.S.; Lyubutin, I.S.; Ogarkova, Y.L.; Davydov, V.A.; Kulikova, L.F.; Egorova, T.B.; Agafonov, V.N.; Starchikova, I.Y. Distribution of iron atoms in nonequivalent crystallographic Sites of Fe7C3 carbide in core–shell nanostructures. Crystallogr. Rep. 2019, 64, 331–336. [Google Scholar] [CrossRef]
- Jiraskova, Y.; Pizurova, N.; Titov, A.; Janickovic, D.; Friak, M. Phase separation in Fe-Ti-Al alloy—Structural, magnetic, and Mössbauer study. J. Magn. Magn. Mater. 2018, 468, 91–99. [Google Scholar] [CrossRef]
- Bai, S.; Wen, S.; Liu, S.; Zhang, W.; Xian, Y. Catalyzing carbothermic reduction of siderite ore with high content of phosphorus by adding sodium carbonate. ISIJ Int. 2011, 51, 1601–1607. [Google Scholar] [CrossRef]
- Sadangi, J.K.; Das, S.P.; Tripathy, A.; Biswal, S.K. Investigation into recovery of iron values from red mud dumps. Sep. Sci. Technol. 2018, 53, 2186–2191. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Iron Carbide for Electric Arc Furnaces. Available online: http://iicarbide.com/archives/IIC_iron_carbide_for_EAFs_rev_02.pdf (accessed on 20 August 2020).
- Svoboda, J. Practical aspects of magnetic methods for materials treatment. In Magnetic Techniques for the Treatment of Materials; Springer: Dordrecht, The Netherlands, 2006; pp. 319–467. [Google Scholar]
- Li, G.; Shi, T.; Rao, M.; Jiang, T.; Zhang, Y. Beneficiation of nickeliferous laterite by reduction roasting in the presence of sodium sulfate. Min. Eng. 2012, 32, 19–26. [Google Scholar] [CrossRef]
- Li, F.; Franzen, H.F. Phase transitions in near stoichiometric iron sulfide. J. Alloys Compd. 1996, 238, 73–80. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.; Cui, Q. Can sodium sulfate be used as an additive for the reduction roasting of high-phosphorus oolitic hematite ore? Int. J. Min. Process. 2014, 133, 119–122. [Google Scholar] [CrossRef]
- Onghena, B.; Binnemans, K. Recovery of Scandium(III) from aqueous solutions by solvent extraction with the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Ind. Eng. Chem. Res. 2015, 54, 1887–1898. [Google Scholar] [CrossRef]
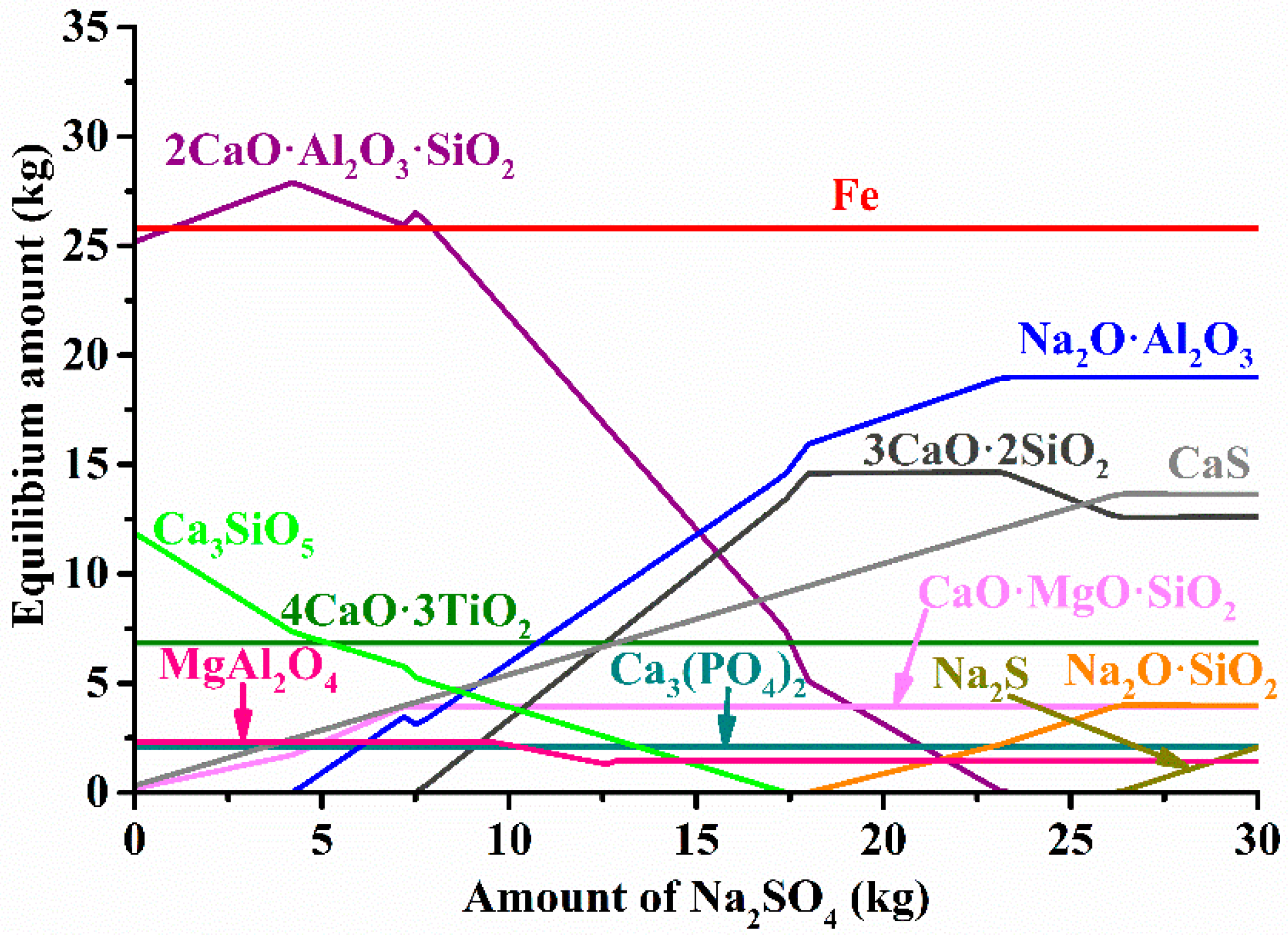
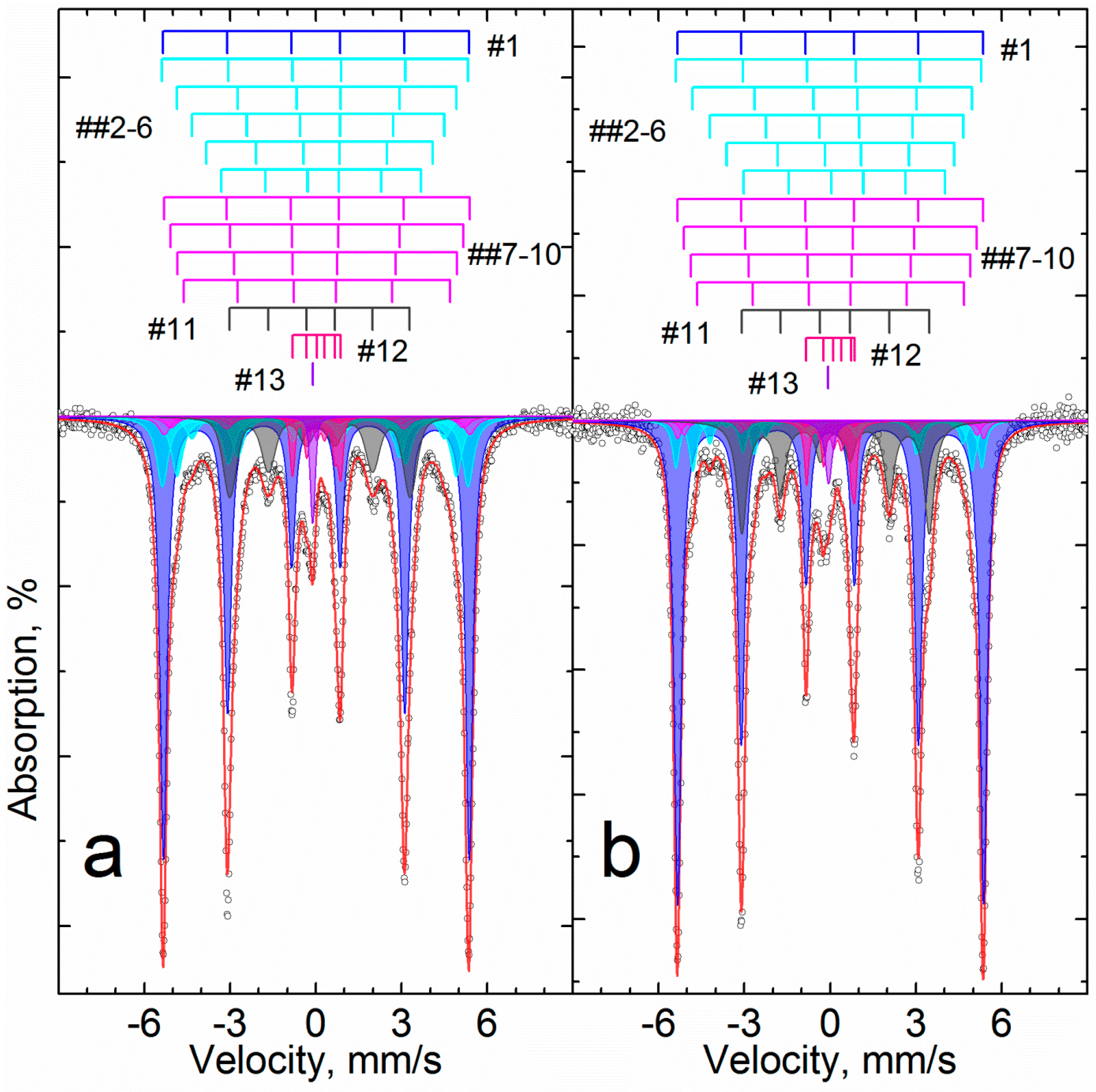
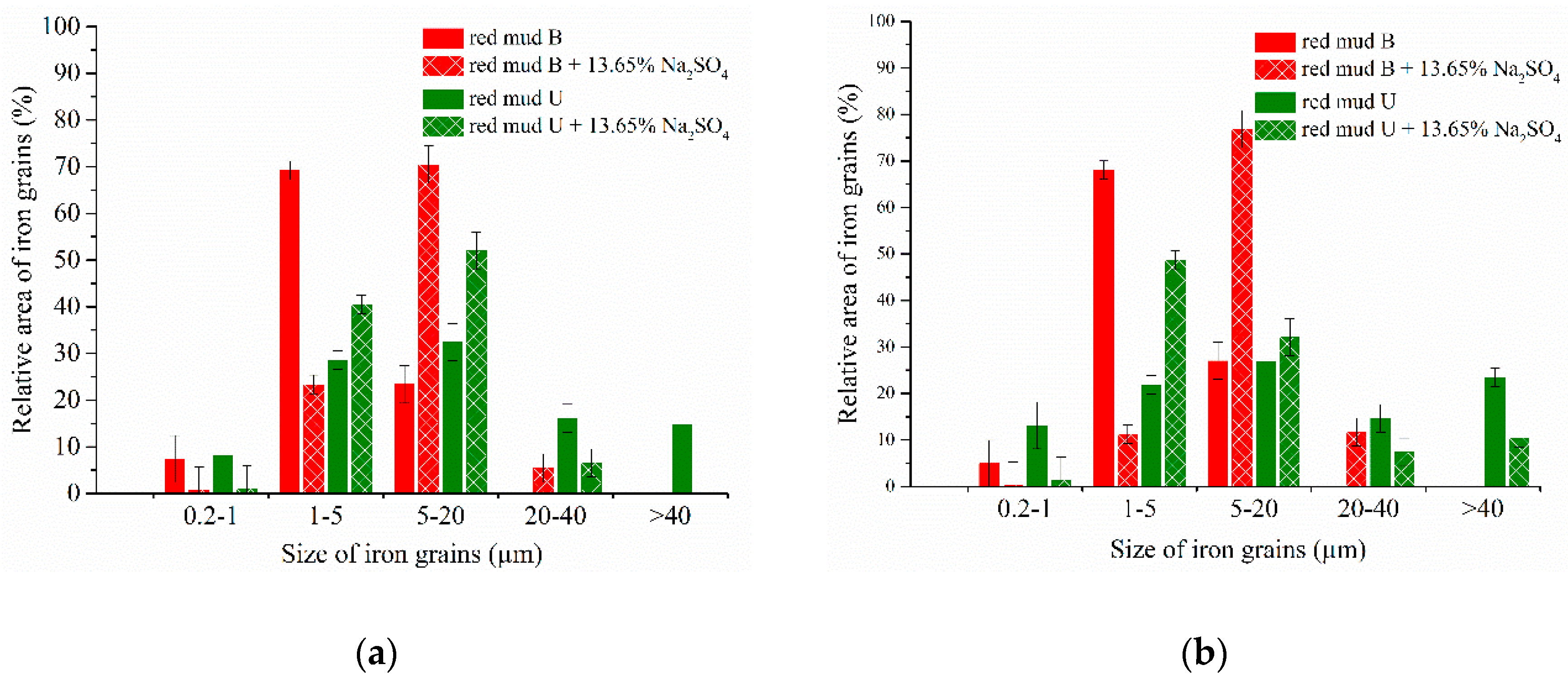
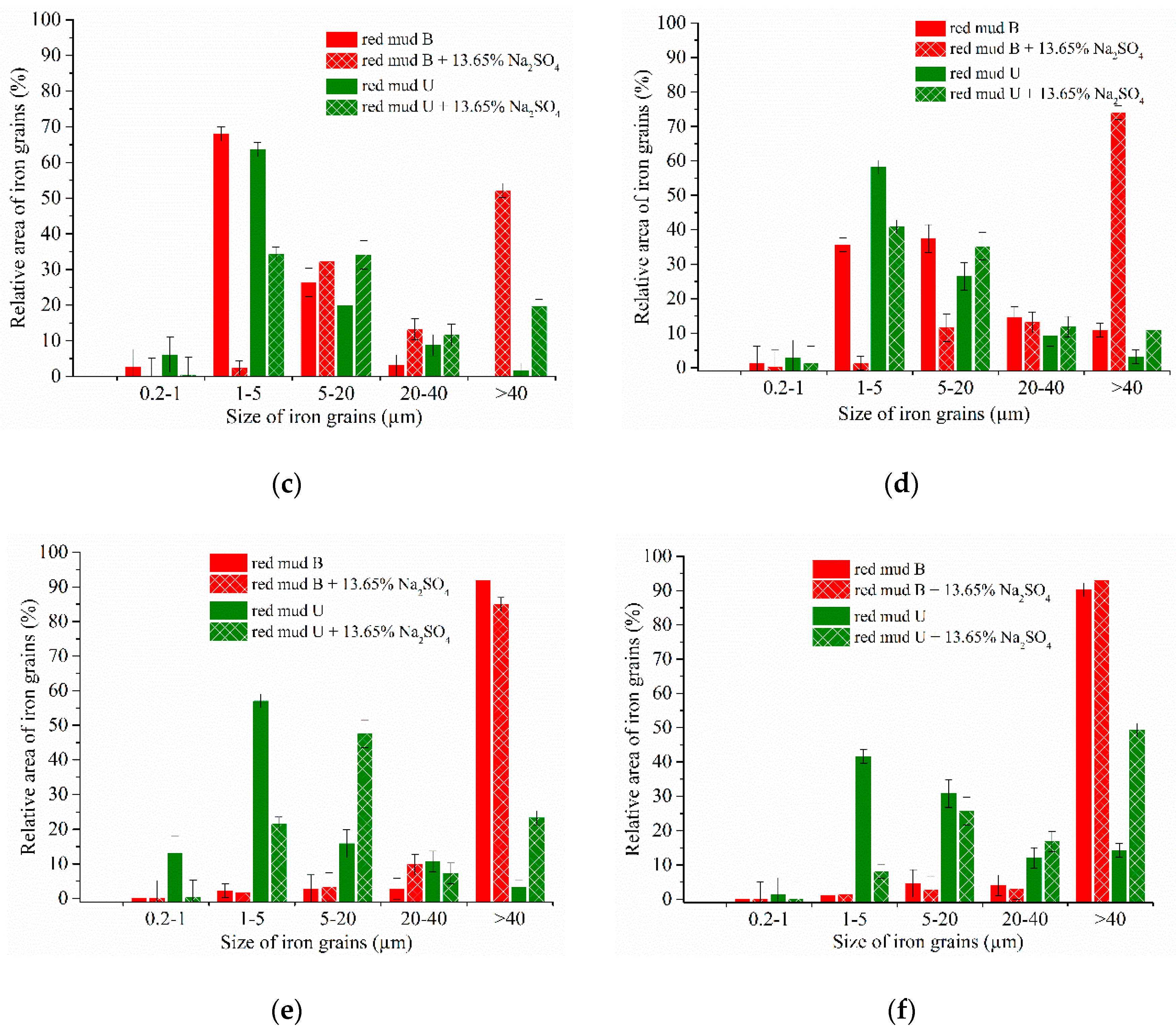
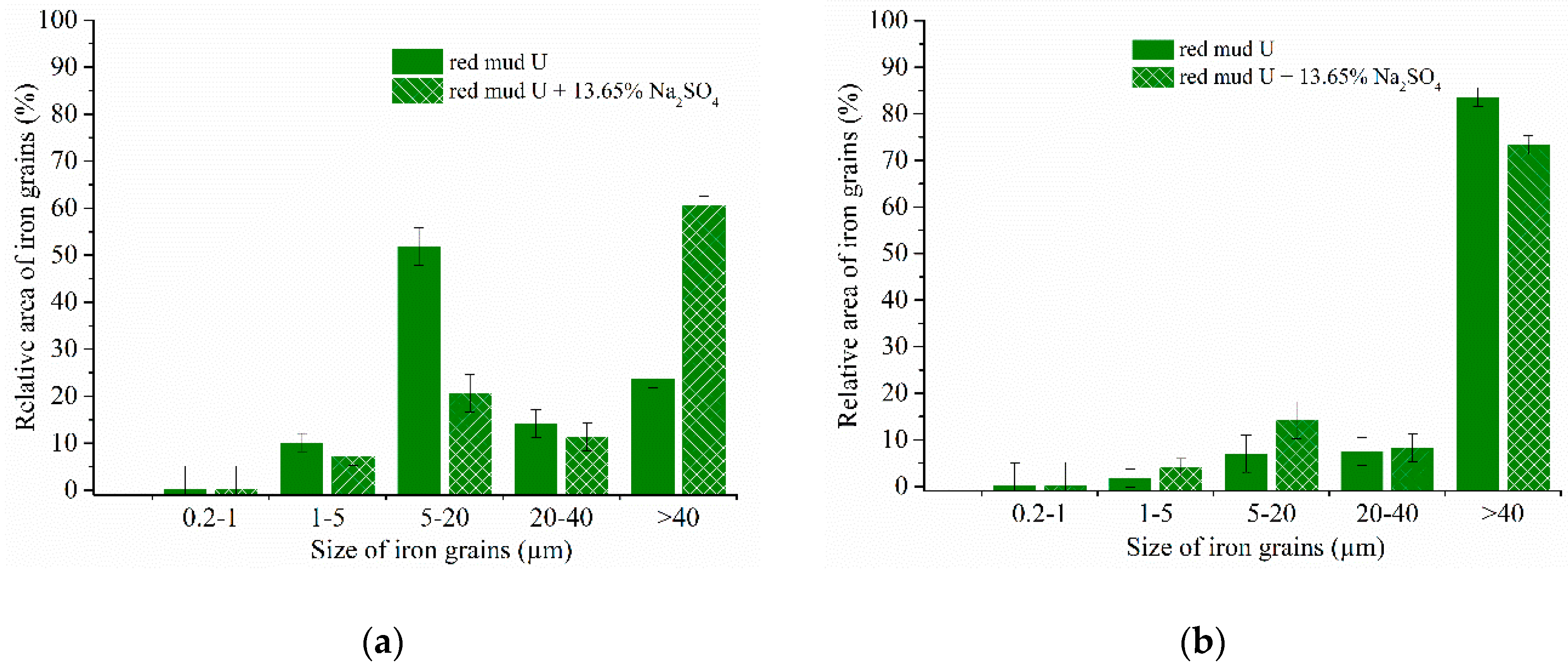
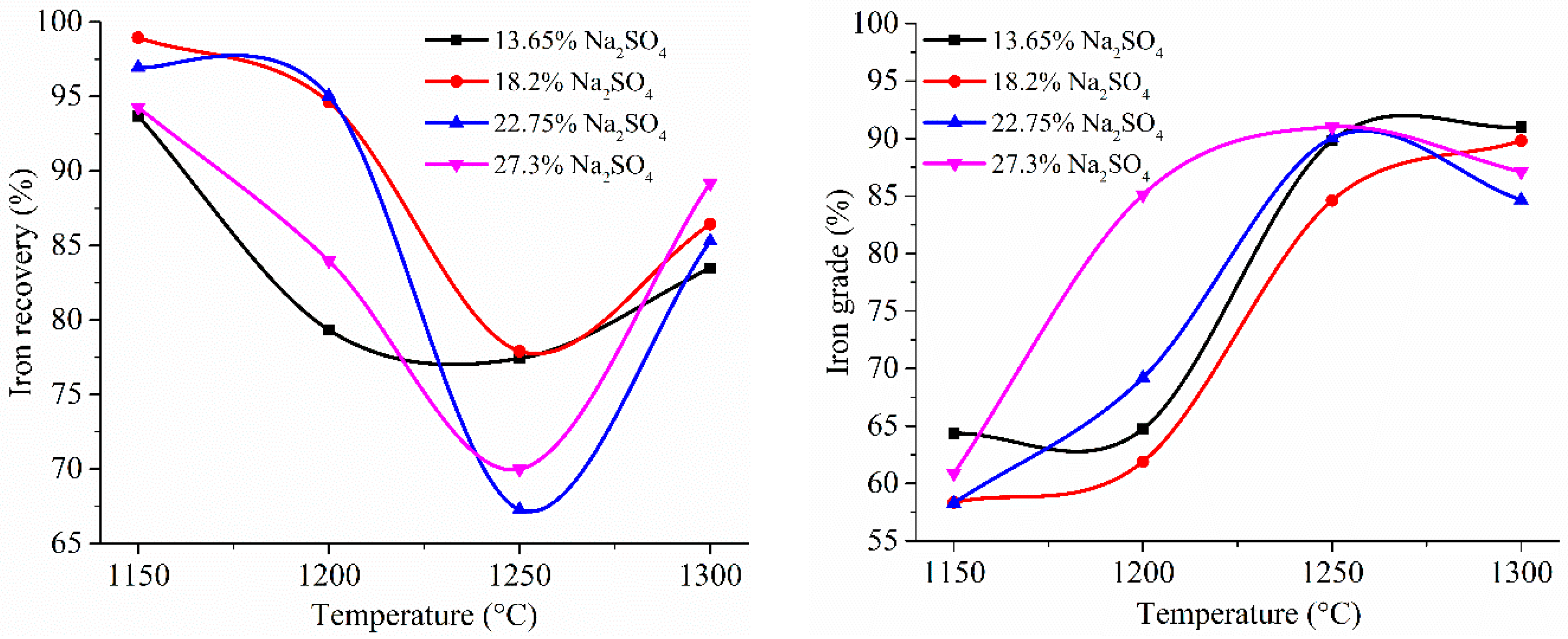
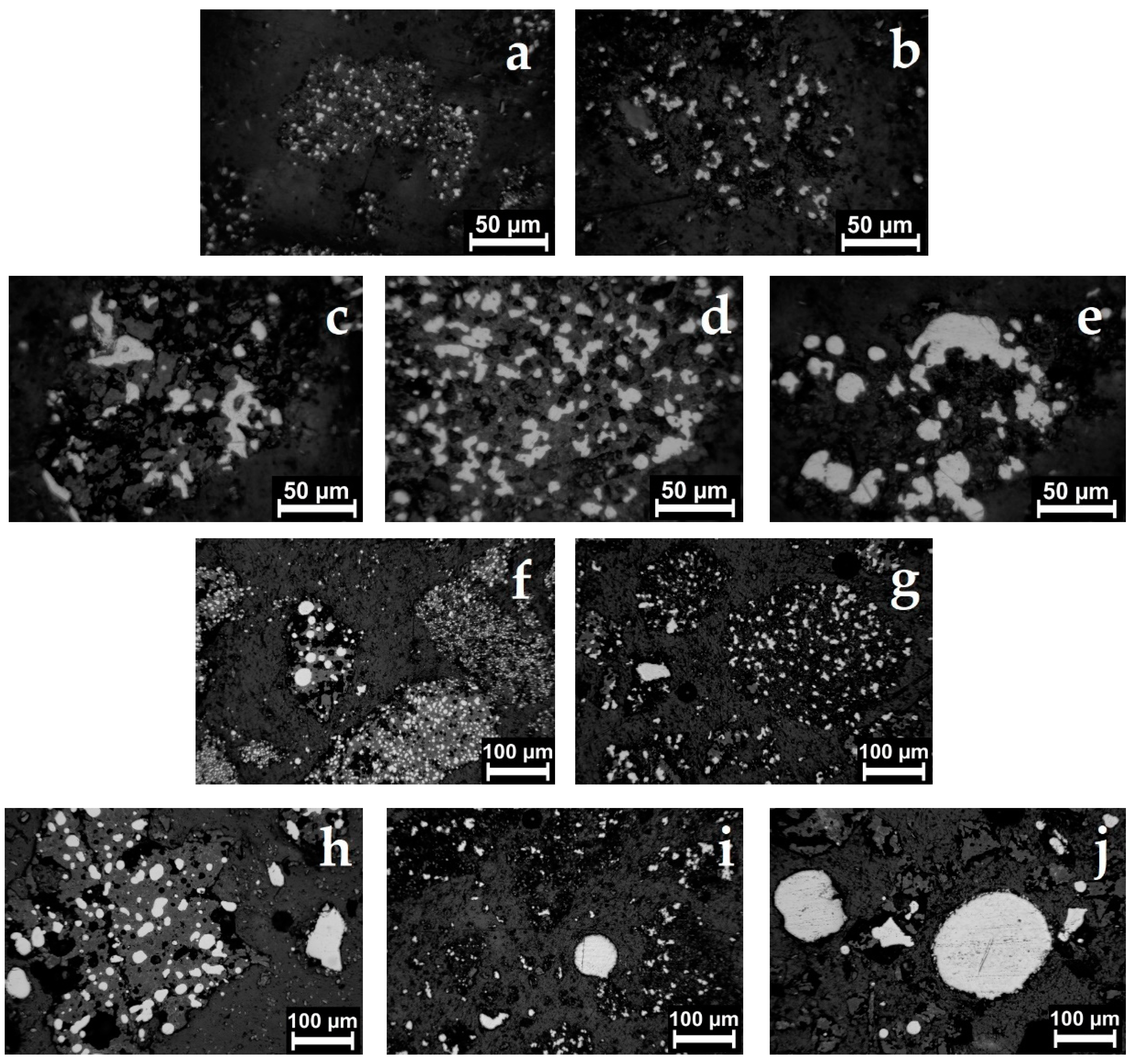
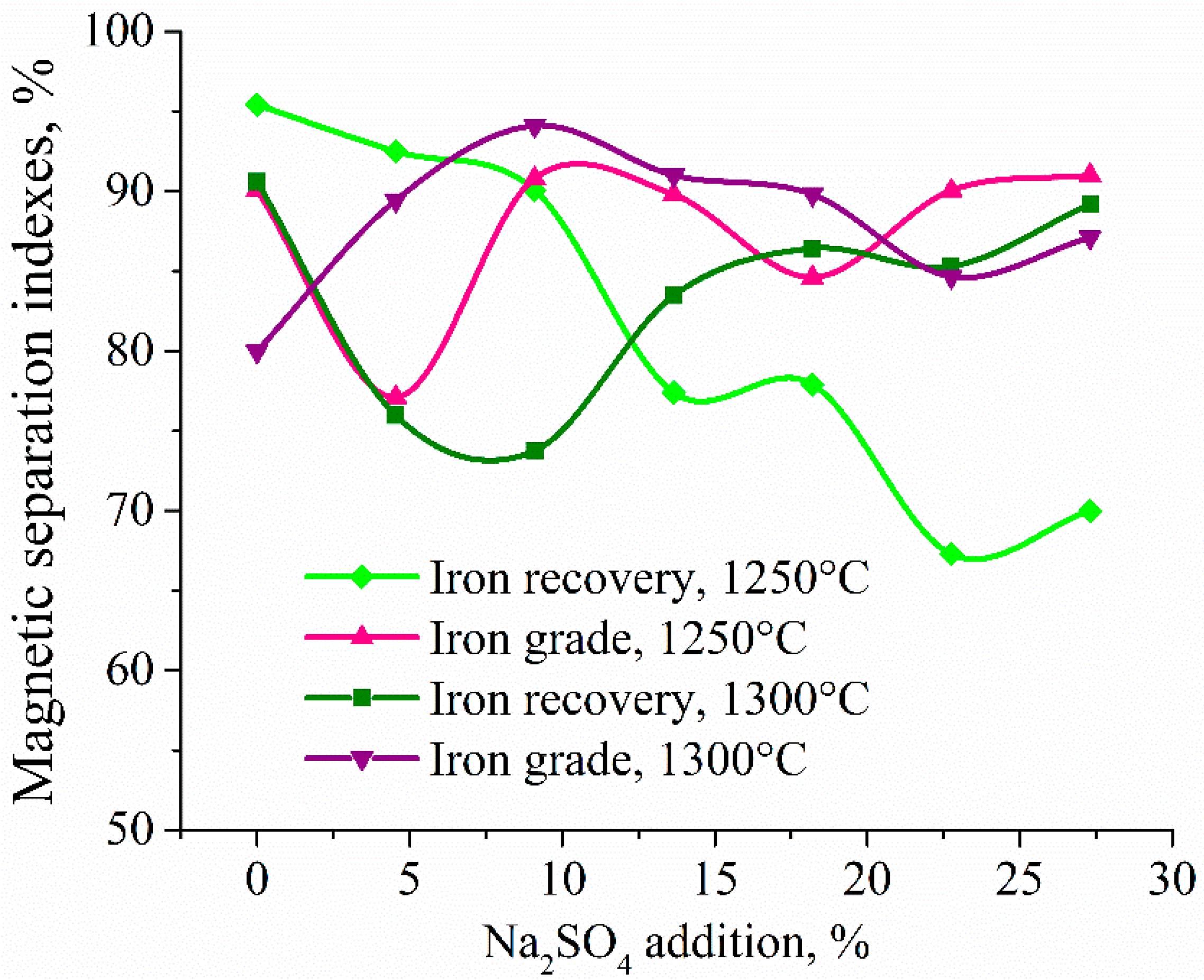
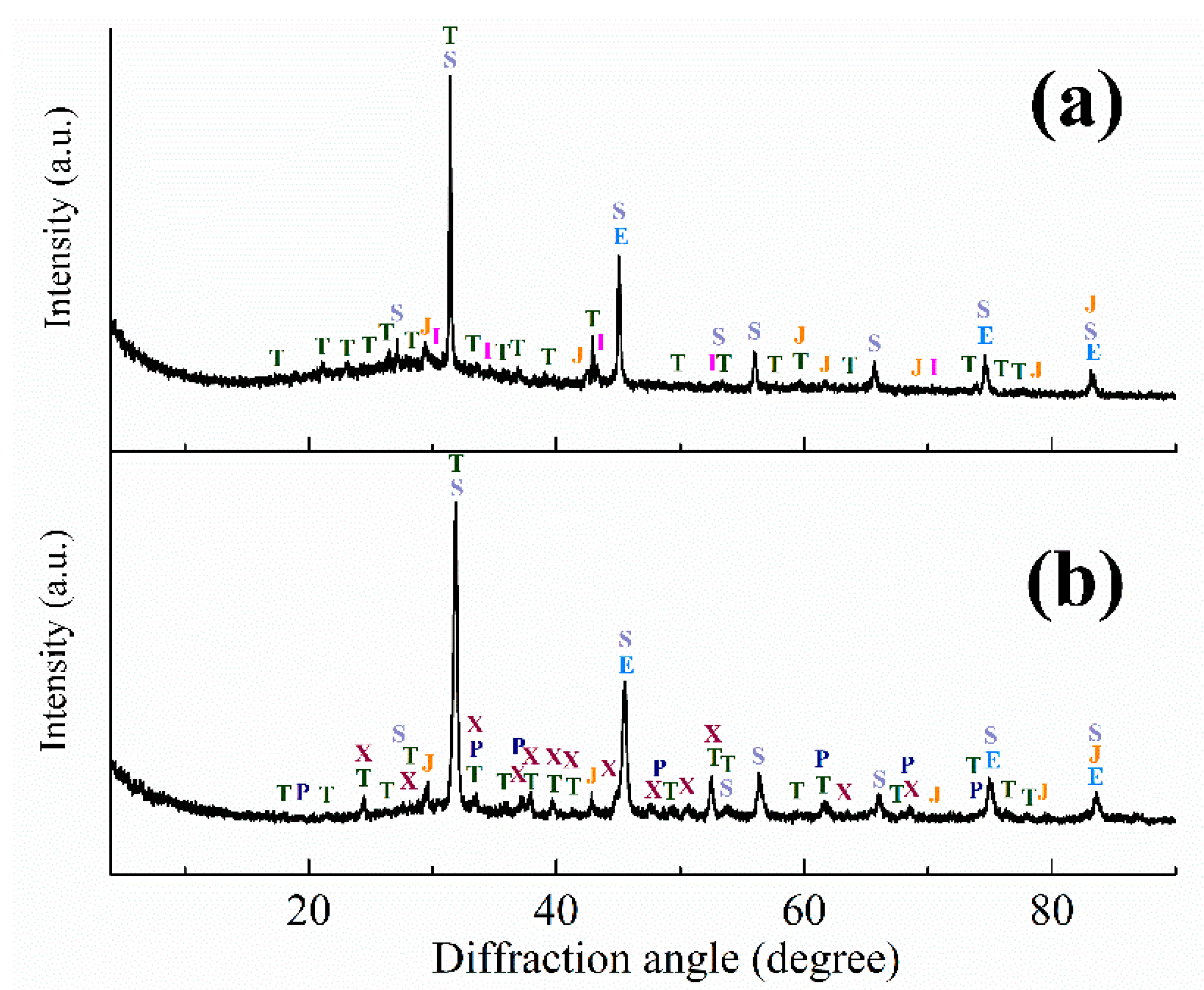
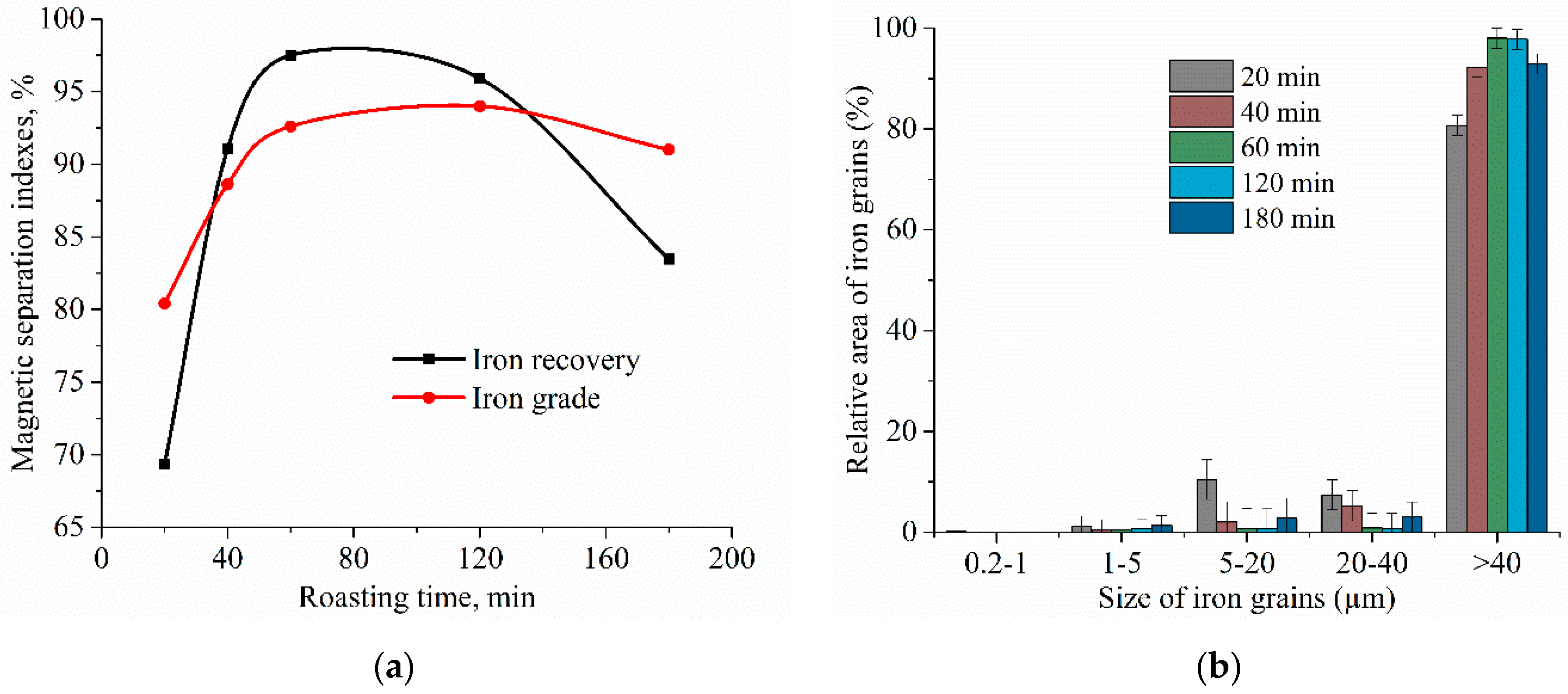
| Aluminum Plant | Codename | Fe2O3 | SiO2 | Al2O3 | TiO2 | CaO | MgO | MnO | Na2O | P2O5 | S | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bogoslovsky | B | 49.81 | 8.71 | 12.77 | 4.67 | 9.26 | 0.65 | 0.26 | 3.30 | 0.85 | 0.48 | 0.014 |
| Ural | U | 36.9 | 8.71 | 11.80 | 3.54 | 23.8 | 1.01 | 0.95 | 0.27 | 0.42 | 0.14 | 0.008 |
| Sample | B | U | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature K | # | Phase | δ | Δ = 2ε | Γexp | Heff | S# | δ | Δ = 2ε | Γexp | Heff | S# |
| mm/s | kOe | % | mm/s | kOe | % | |||||||
| 296 | 1 | α-Fe2O3 | 0.38 | −0.22 | 0.30 | 510.9 | 36 | 0.37 | −0.21 | 0.26 | 513.2 | 25 |
| 2 | α-(Fe1−xAlx)2O3 | 0.38 | −0.22 | 0.32 | 499.7 | 19 | 0.37 | −0.22 | 0.27 | 505.4 | 16 | |
| 3 | 0.38 | −0.20 | 0.48 | 483.1 | 16 | 0.37 | −0.20 | 0.39 | 491.3 | 11 | ||
| 4 | 0.43 | −0.15 | 1.06 | 449 | 10 | 0.37 | −0.18 | 0.72 | 467 | 10 | ||
| 5 | α-Fe1−xAlxOOH | - | - | - | - | - | 0.35 | −0.20 | 1.55 | 362 | 14 | |
| 6 | - | - | - | - | - | 0.35 | −0.20 | 1.55 | 183 | 4 | ||
| 7 | Fe2+Oh | 1.12 | 2.67 | 0.34 | - | 6 | 1.13 | 2.64 | 0.32 | - | 10 | |
| 8 | Fe3+Oh | 0.34 | 0.66 | 0.52 | - | 12 | 0.35 | 0.68 | 0.67 | - | 10 | |
| 77.5 | 9 | α-Fe2O3 | 0.45 | −0.21 | 0.29 | 527.9 | 41 | 0.48 | −0.21 | 0.26 | 530.8 | 32 |
| 10 | α-(Fe1−xAlx)2O3 | 0.46 | −0.21 | 0.36 | 518.2 | 24 | 0.48 | −0.21 | 0.37 | 524.5 | 30 | |
| 11 | 0.45 | −0.18 | 0.76 | 502 | 17 | - | - | - | - | - | ||
| 12 | α-Fe1−xAlxOOH | - | - | - | - | - | 0.47 | −0.23 | 0.67 | 496.7 | 20 | |
| 13 | Fe2+Oh | 1.21 | 2.82 | 0.31 | - | 6 | 1.25 | 2.83 | 0.30 | - | 11 | |
| 14 | Fe3+Oh | 0.41 | 0.70 | 0.53 | - | 12 | 0.46 | 0.69 | 0.58 | - | 8 | |
| Sample | Roasted B | Roasted U | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Phase | δ | dδ | Δ = 2ε | Γexp | Heff | dH | S# | Σ S# | δ | dδ | Δ = 2ε | Γexp | Heff | dH | S# | Σ S# |
| mm/s | kOe | % | mm/s | kOe | % | ||||||||||||
| 1 | α-Fe | 0.00 | - | 0.01 | 0.30 | 331.6 | - | 56 | 56 | 0.01 | - | 0.00 | 0.31 | 331.6 | - | 48 | 48 |
| 2 | α-Fe1−xAx-first coordination sphere | 0.00 | 0.14 | −0.10 | 0.31 | 331.6 | 28.4 | 6 | 19.8 | 0.01 | 0.05 | −0.04 | 0.50 | 331.6 | 28.7 | 12 | 33 |
| 3 | 0.14 | 303.2 | 6.0 | 0.06 | 302.9 | 10.4 | |||||||||||
| 4 | 0.28 | 274.9 | 2.8 | 0.12 | 274.2 | 3.8 | |||||||||||
| 5 | 0.41 | 247 | 0.7 | 0.17 | 245.5 | 0.8 | |||||||||||
| 6 | 0.55 | 218 | 0.1 | 0.22 | 216.8 | 0.1 | |||||||||||
| 7 | α-Fe1−xAx-second coordination sphere | 0.00 | 0.00 | 0.02 | 331.6 | 14.2 | 2.1 | 0.01 | 0.00 | 0.03 | 331.6 | 14.3 | 3 | ||||
| 8 | 0.00 | 303.2 | 1.7 | 0.01 | 317.3 | 2.0 | |||||||||||
| 9 | 0.00 | 289 | 0.6 | 0.01 | 303.0 | 0.5 | |||||||||||
| 10 | 0.00 | 275 | 0.1 | 0.01 | 288.7 | 0.1 | |||||||||||
| 11 | θ-Fe3C | 0.18 | - | 0.02 | 0.42 | 203.2 | - | 18.8 | 18.8 | 0.15 | - | −0.02 | 0.57 | 195.4 | - | 13.5 | 13.5 |
| 12 | Laves phase | 0.13 | - | −0.24 | 0.20 | 52.4 | - | 4.7 | 4.7 | 0.10 | - | −0.07 | 0.21 | 52.4 | - | 3.7 | 3.7 |
| 13 | γ-Fe | −0.06 | - | - | 0.30 | - | - | 1.8 | 1.8 | −0.11 | - | - | 0.21 | - | - | 1.7 | 1.7 |
| Na2SO4 Addition, % | Roasting Temperature (°C) and Codename of a Sample | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1100 | 1150 | 1200 | 1250 | 1300 | 1350 | 1400 | 1450 | |||||||||
| B | U | B | U | B | U | B | U | B | U | B | U | B | U | B | U | |
| 0 | n/s | n/s | n/s | n/s | n/s | n/s | +,m | n/s | +,m | n/s | fm | n/s | fm | + | fm | fm |
| 4.55 | n/s | n/s | n/s | n/s | n/s | n/s | +,m | n/s | +,m | n/s | fm | - | fm | - | fm | fm |
| 9.1 | n/s | n/s | n/s | n/s | n/s | n/s | +,m | n/s | +,m | n/s | fm | - | fm | - | fm | fm |
| 13.65 | n/s | n/s | + | n/s | + | n/s | +,m | n/s | +,m | n/s | fm | + | fm | + | fm | fm |
| 18.2 | n/s | n/s | + | n/s | + | n/s | +,m | n/s | +,m | n/s | fm | - | fm | - | fm | fm |
| 22.75 | n/s | n/s | + | n/s | + | n/s | +,m | n/s | +,m | n/s | fm | - | fm | - | fm | fm |
| 27.3 | n/s | n/s | + | n/s | + | n/s | +,m | n/s | +,m | n/s | fm | + | fm | + | fm | fm |
| Temperature, °C | Addition | Iron Recovery (ε), % | Iron Grade (%Fec), % |
|---|---|---|---|
| 1350 °C | 13.65% Na2SO4 | 83.91 | 83.7 |
| 27.3% Na2SO4 | 87.21 | 51.6 | |
| 1400 °C | Without addition | 82.83 | 83.6 |
| 13.65% Na2SO4 | 86.09 | 78.3 | |
| 27.3% Na2SO4 | 89.08 | 82.7 |
| Addition | Fe | C | S | P |
|---|---|---|---|---|
| – | 90.06 | 1.27 | 0.23 | 0.80 |
| 9.1% Na2SO4 | 90.80 | 1.60 | 1.16 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudinsky, P.; Zinoveev, D.; Pankratov, D.; Semenov, A.; Panova, M.; Kondratiev, A.; Zakunov, A.; Dyubanov, V.; Petelin, A. Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity. Metals 2020, 10, 1571. https://doi.org/10.3390/met10121571
Grudinsky P, Zinoveev D, Pankratov D, Semenov A, Panova M, Kondratiev A, Zakunov A, Dyubanov V, Petelin A. Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity. Metals. 2020; 10(12):1571. https://doi.org/10.3390/met10121571
Chicago/Turabian StyleGrudinsky, Pavel, Dmitry Zinoveev, Denis Pankratov, Artem Semenov, Maria Panova, Alex Kondratiev, Andrey Zakunov, Valery Dyubanov, and Alexander Petelin. 2020. "Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity" Metals 10, no. 12: 1571. https://doi.org/10.3390/met10121571
APA StyleGrudinsky, P., Zinoveev, D., Pankratov, D., Semenov, A., Panova, M., Kondratiev, A., Zakunov, A., Dyubanov, V., & Petelin, A. (2020). Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity. Metals, 10(12), 1571. https://doi.org/10.3390/met10121571







