The Mini Blast Furnace Process: An Efficient Reactor for Green Pig Iron Production Using Charcoal and Hydrogen-Rich Gas: A Study of Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. The Multiphase and Multicomponent Model Equations Applied to the Charcoal Mini Blast Furnace Reactor
2.2. Numerical Solution of the Conservation Equations
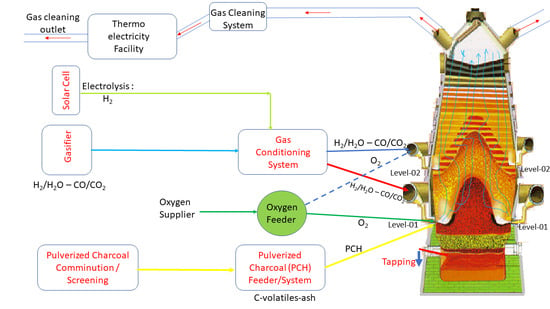
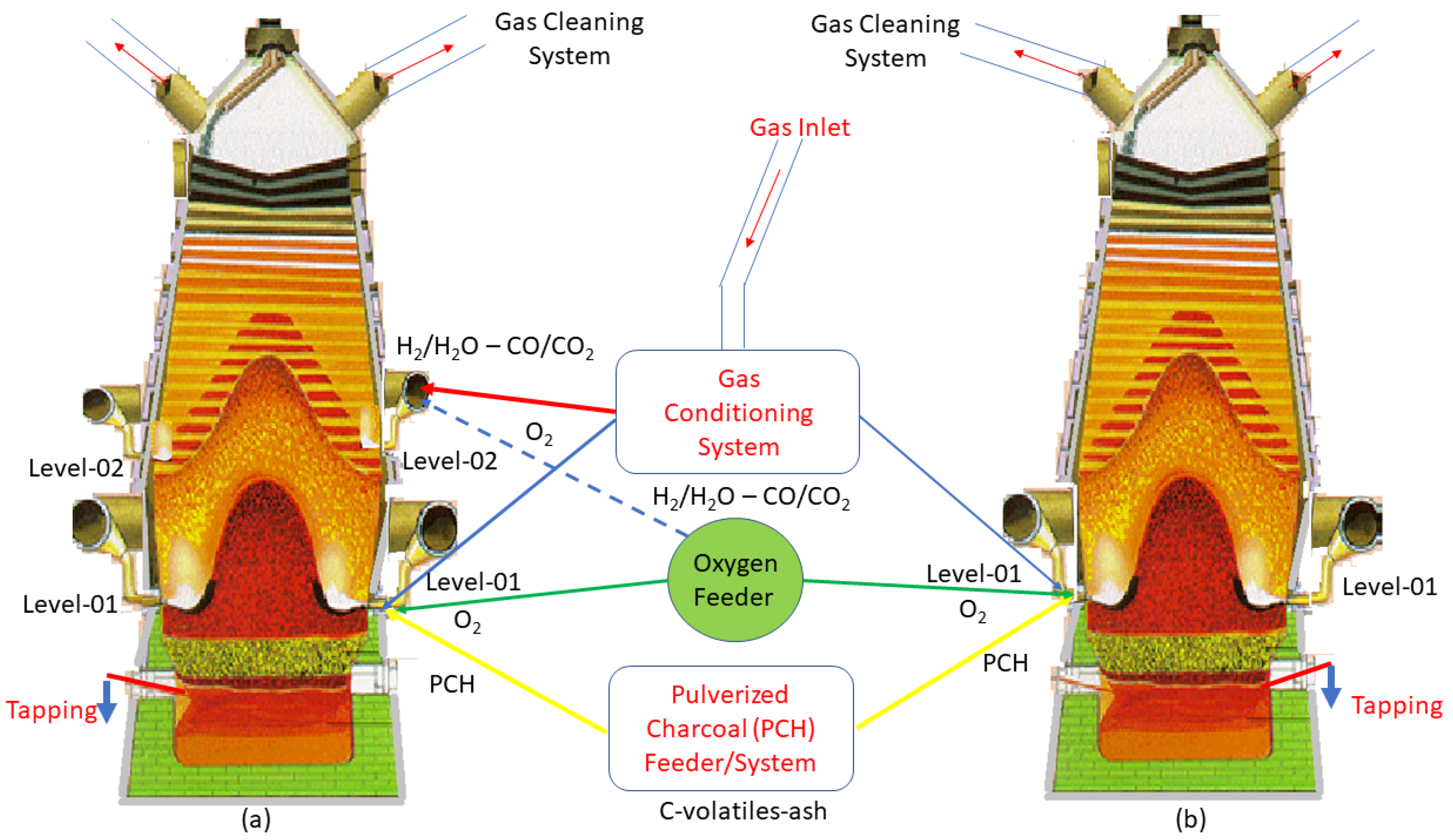
2.3. A New Concept for Hydrogen-Rich Gas Injections in Two Tuyeres Levels of the Charcoal Mini Blast Furnace
3. Results and Discussion
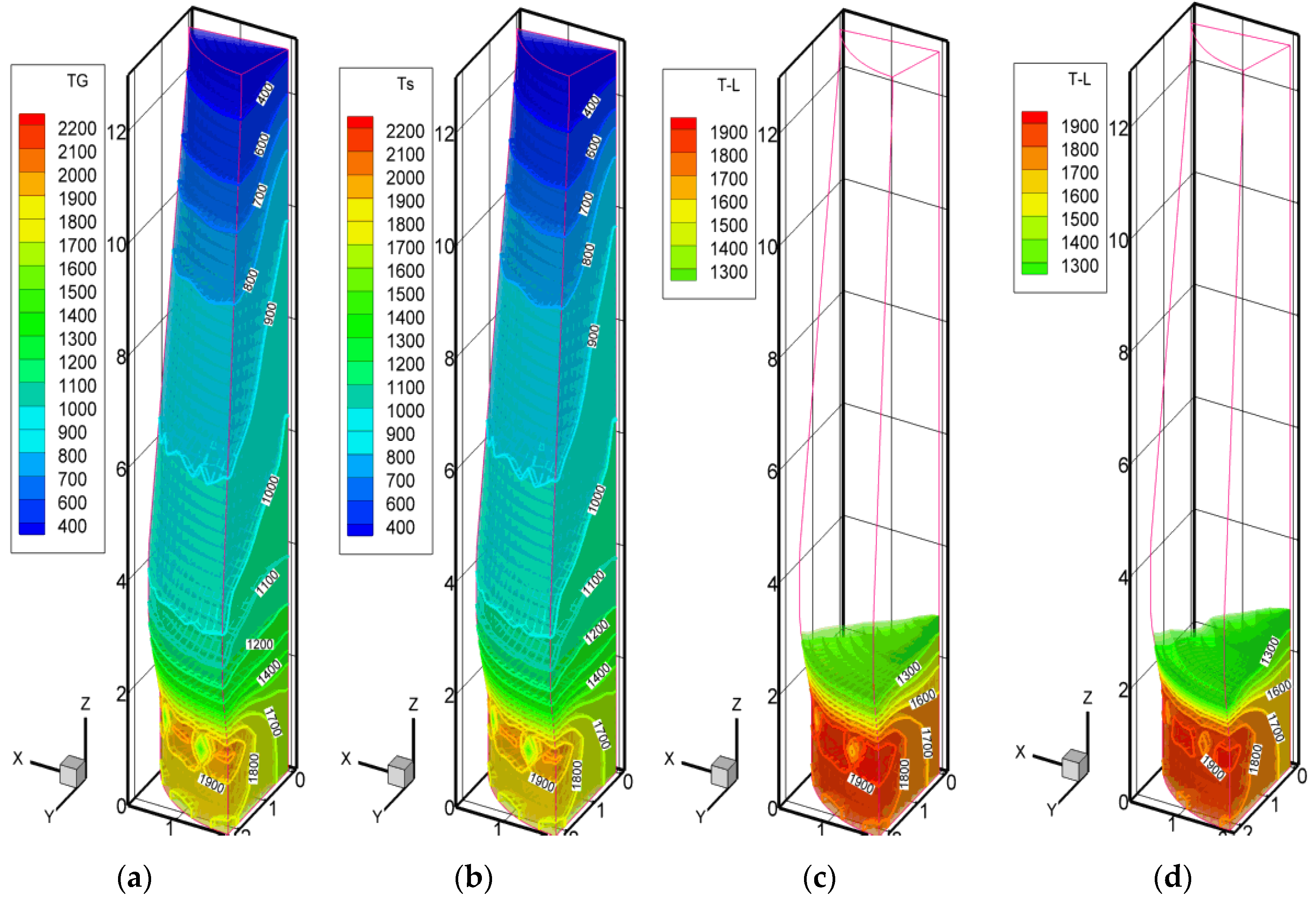
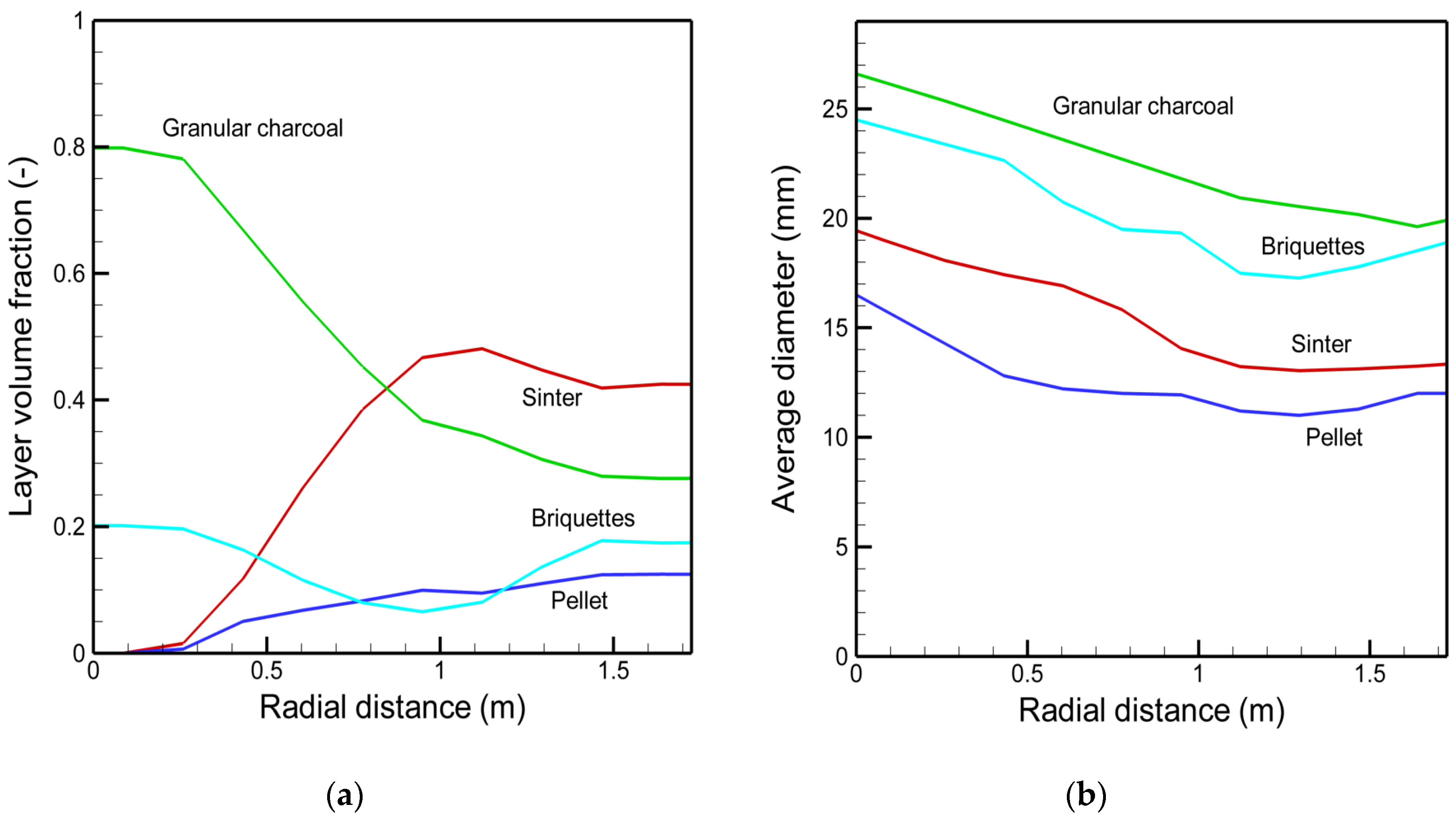
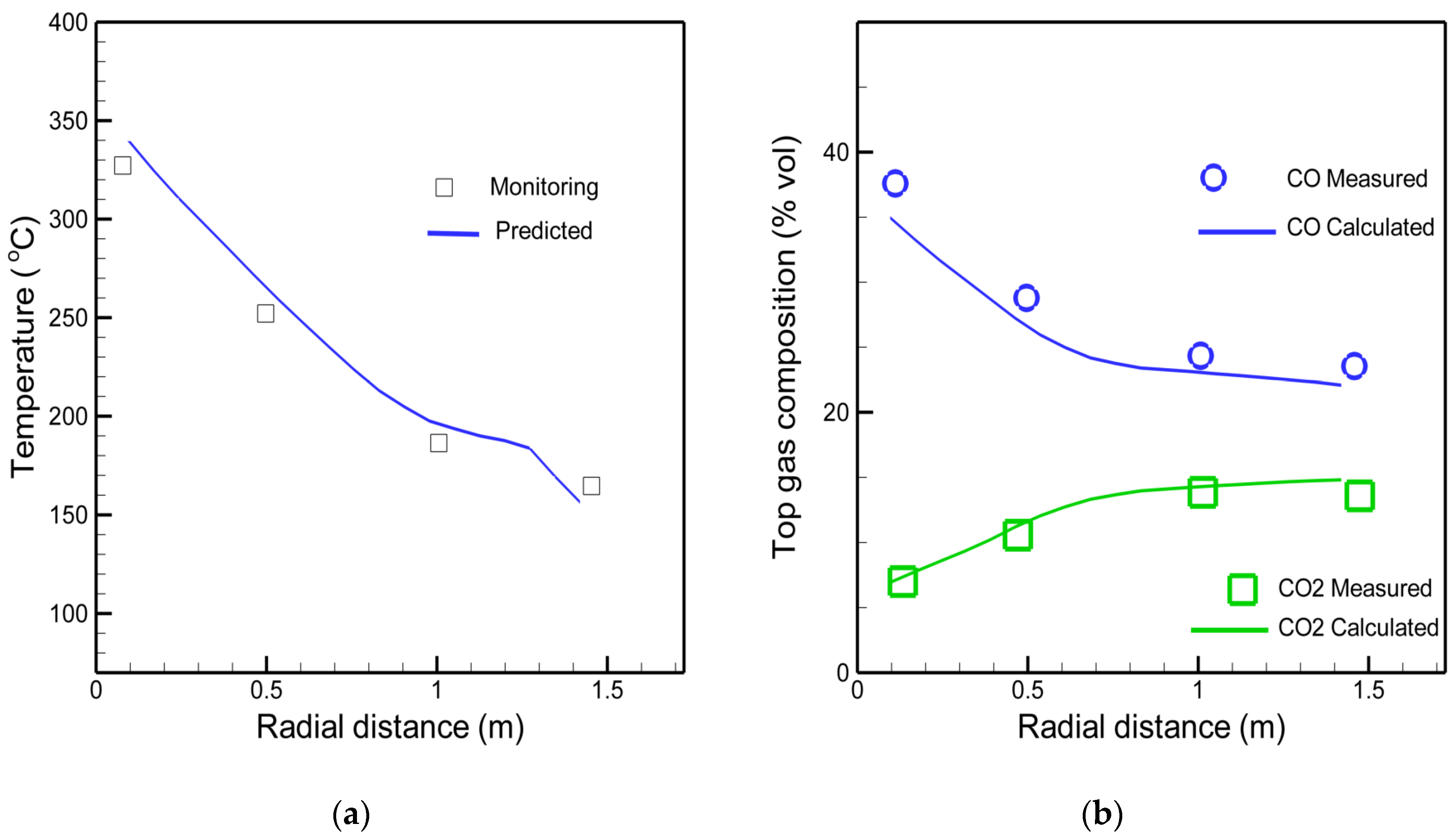
3.1. The Actual Mini Blast Furnace Operation and Model Comparison
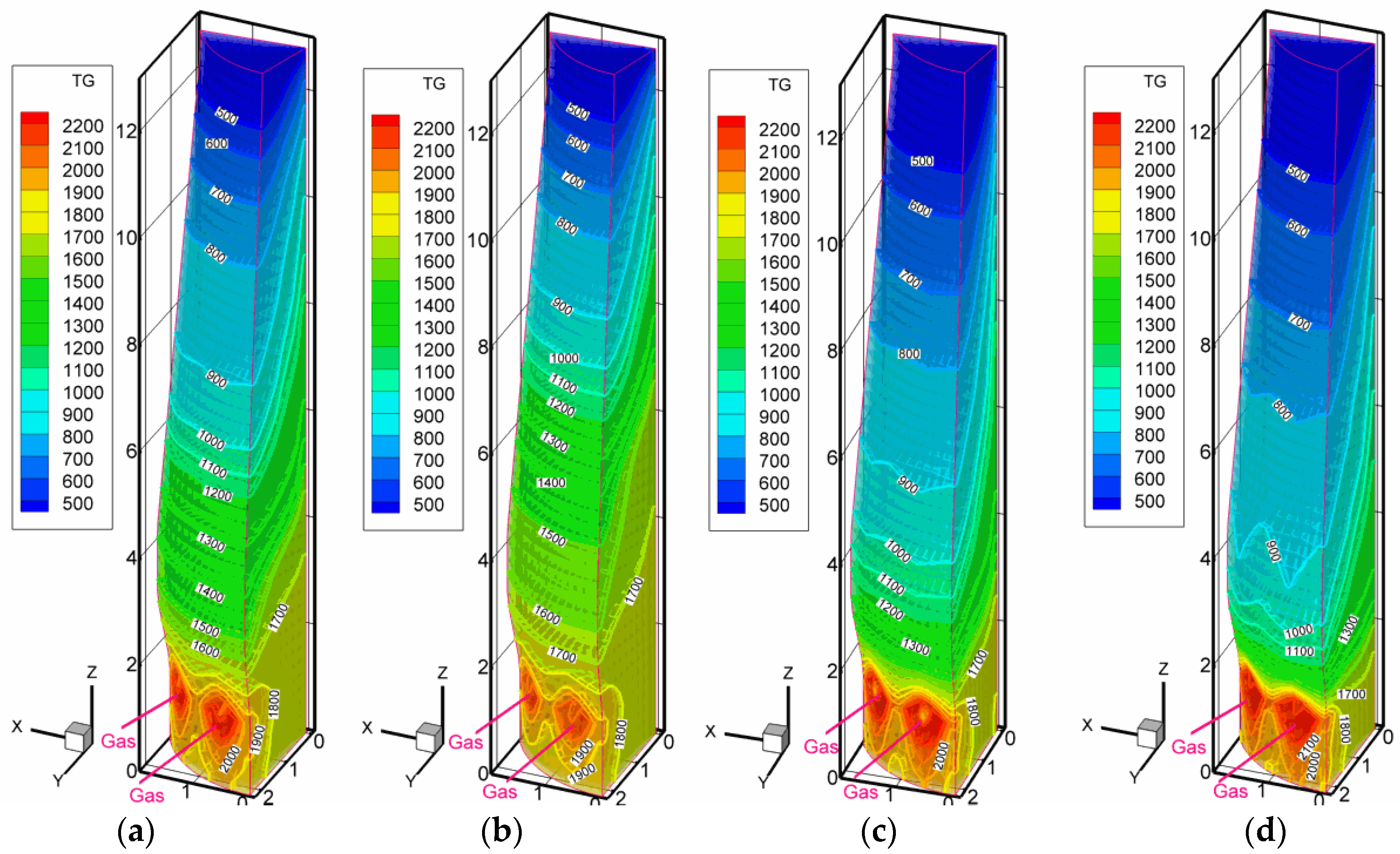
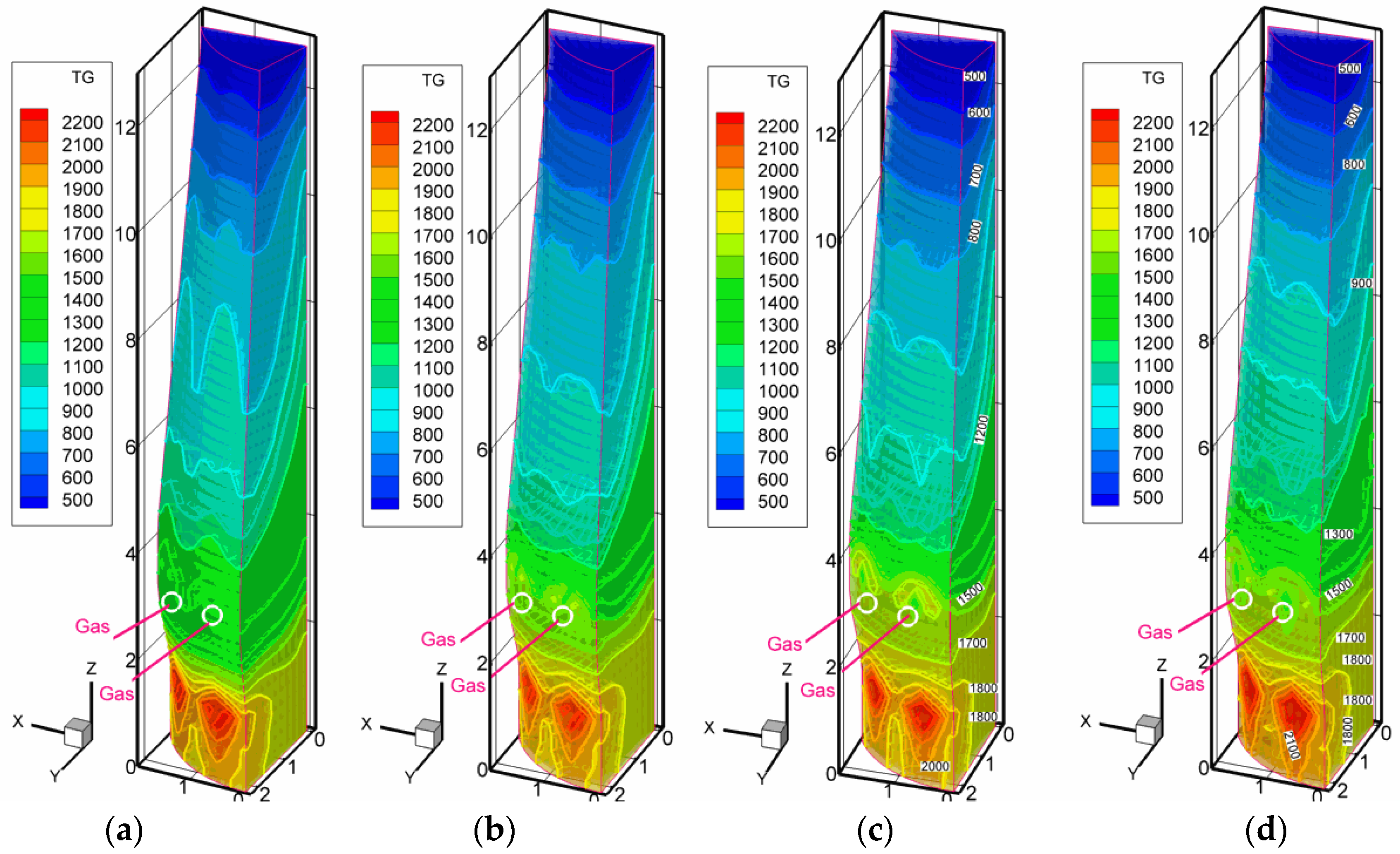
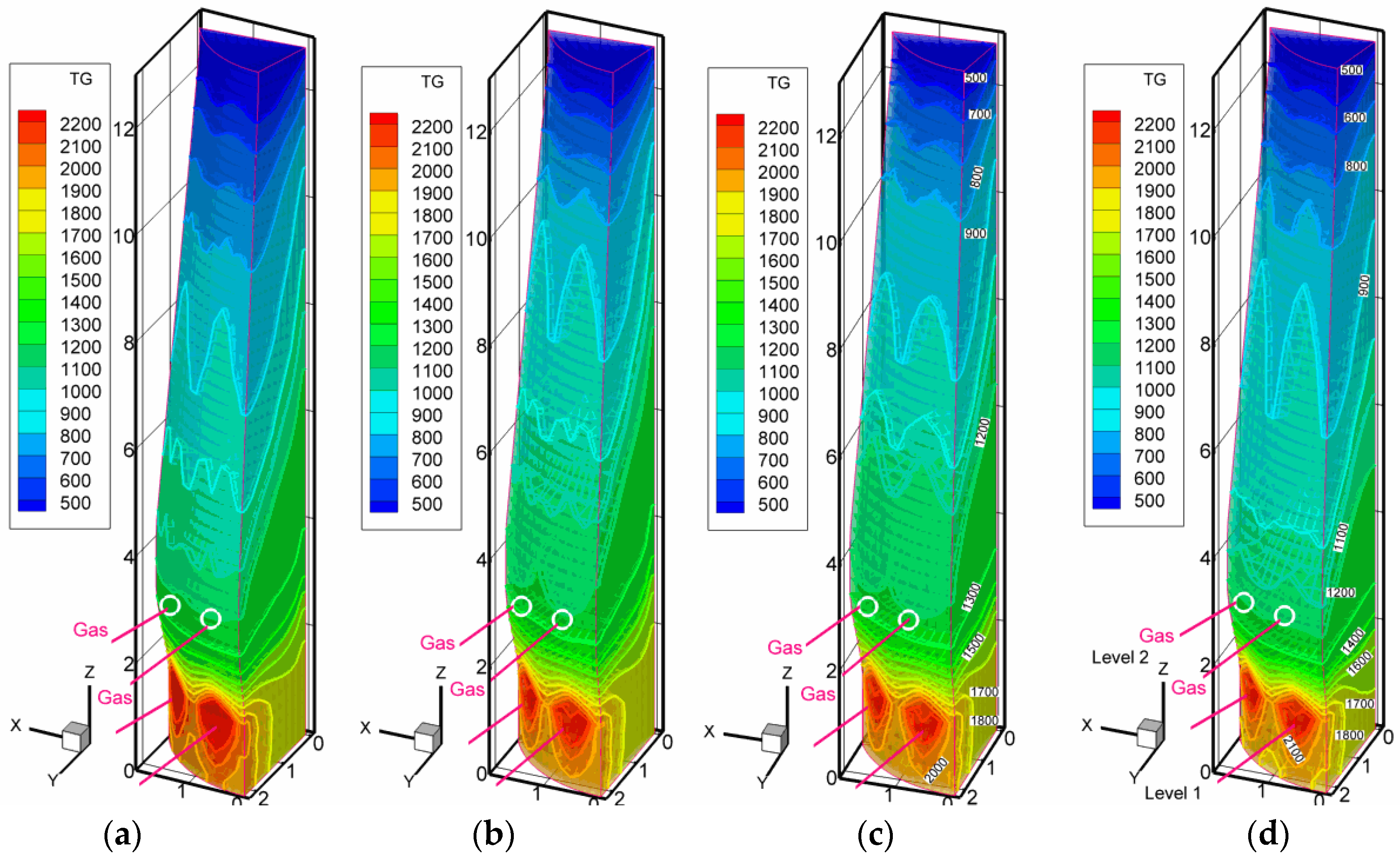
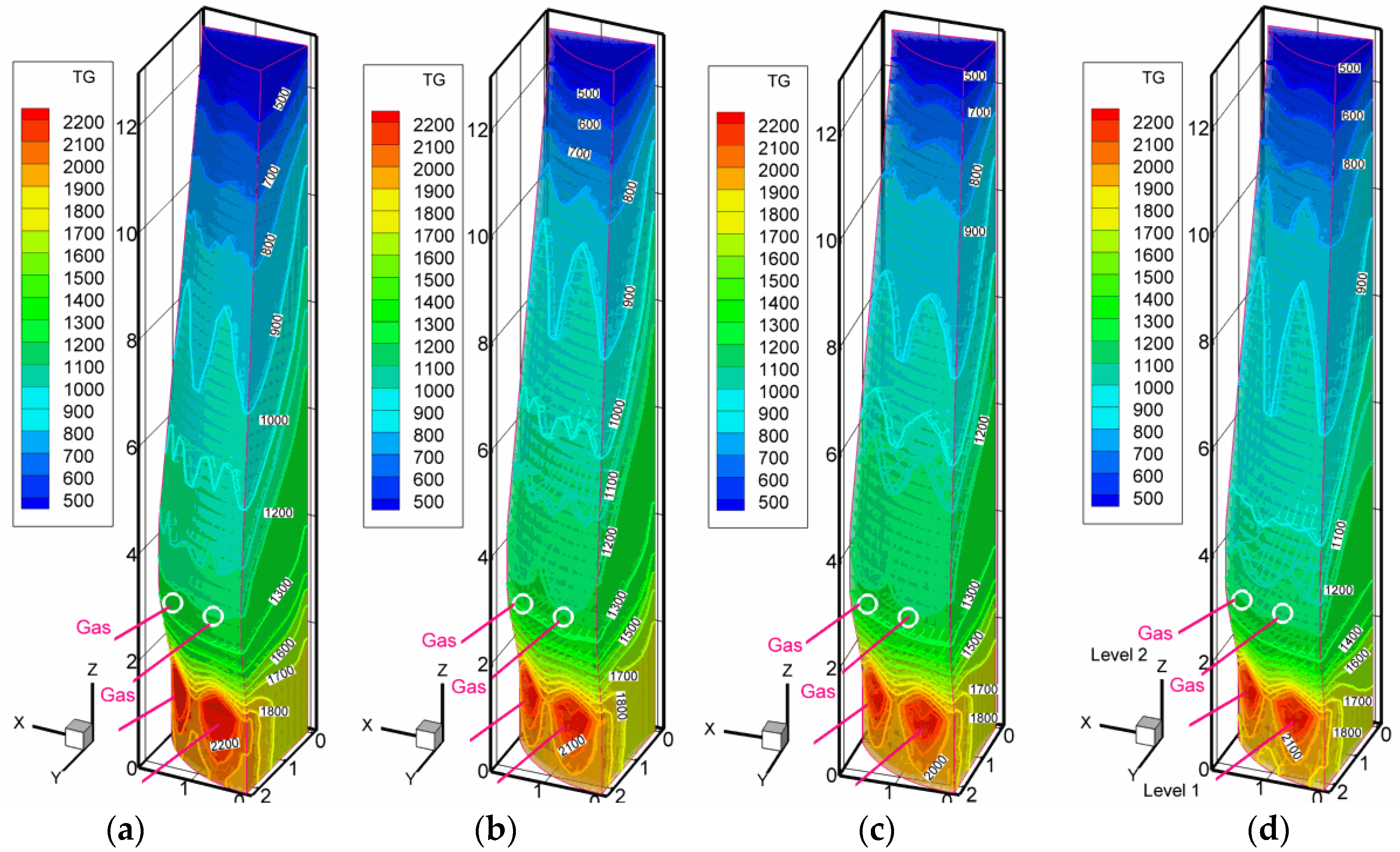
3.2. Scenarios with High Pulverized Charcoal Combined with Hydrogen-Rich Gas Injections in the Raceway and Oxygen Enrichment
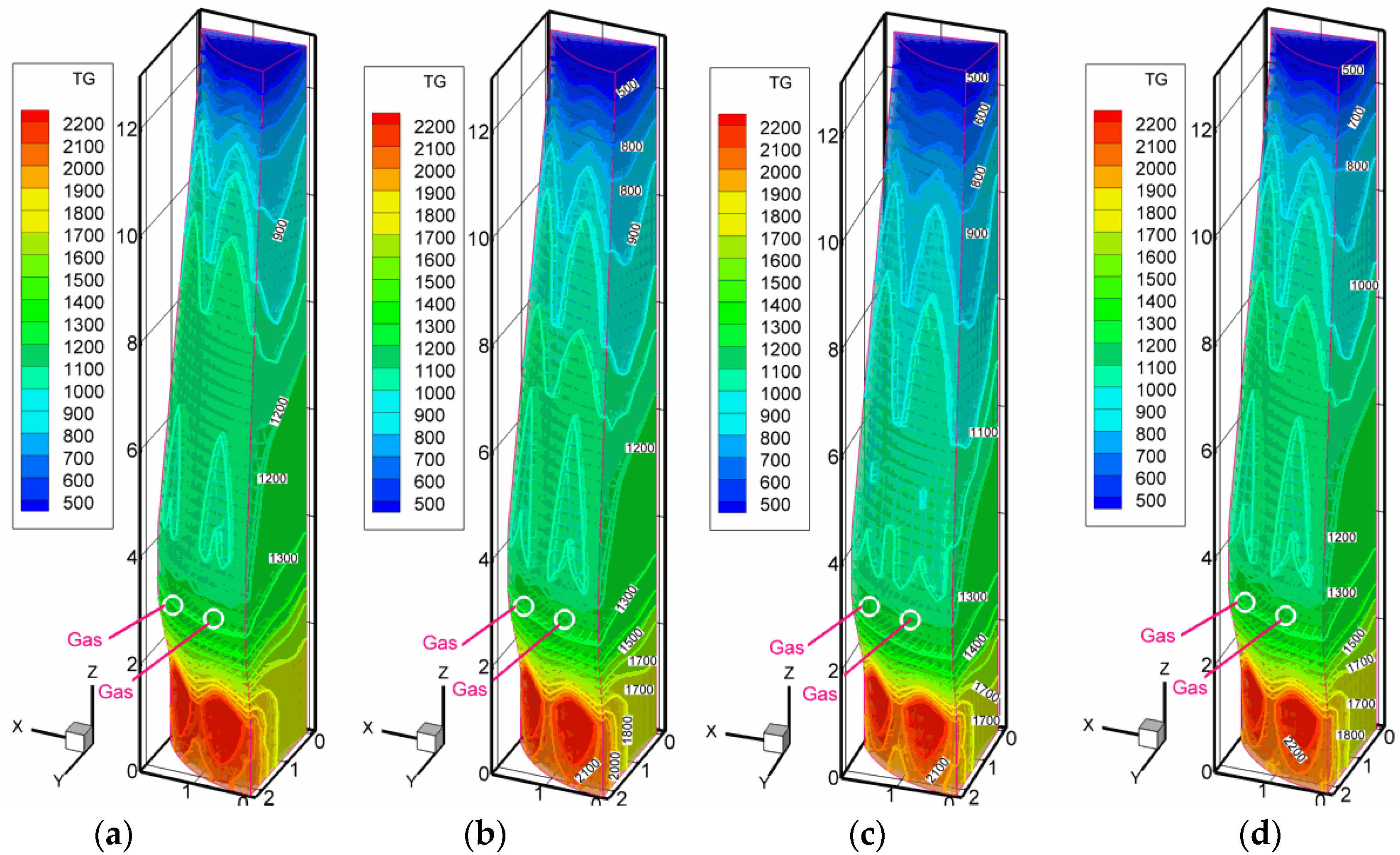
3.3. Scenarios with High Rates of Hydrogen-Rich Gas Injections in the Shaft Zone
3.4. Discussion on the Enhancement of the Reduction Kinetics under the Conditions of the Injection
4. Summary, Outlook, and Future Trends
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, X.; Li, H.; Guo, J.; Gu, B.; Zeng, Y. Energy-saving and emission-reduction technology selection and CO2 emission reduction potential of China’s iron steel industry under energy substitution policy. J. Clean. Prod. 2019, 222, 823–834. [Google Scholar] [CrossRef]
- Rocha, E.P.; Guilherme, V.S.; Castro, J.A.; Sasaki, Y.; Yagi, J. Analysis of synthetic natural gas injection into charcoal blast furnace. J. Mater. Res. Technol. 2013, 2, 255–262. [Google Scholar] [CrossRef][Green Version]
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How it Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Castro, J.A.; Medeiros, G.A.; Oliveira, E.M.; Silva, L.M.; Nogami, H. A Comprehensive Modeling as a Tool for Developing New Mini Blast Furnace Technologies Based on Biomass and Hydrogen Operation. J. Sustain. Metall. 2020, 6, 281–293. [Google Scholar] [CrossRef]
- Yilmaz, C.; Jens Wendelstorf, J.; Turek, T. Modeling and simulation of hydrogen injection into a blast furnace to reduce carbon dioxide emissions. J. Clean. Prod. 2017, 154, 488–501. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Abdul Quader, M.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Castro, J.A. A Multi-Dimensional Transient Mathematical Model of Blast Furnace Based on Multi-Fluid Model. Ph.D. Thesis, Tohoku University, Sendai, Japan, 15 March 2001. [Google Scholar]
- Omori, Y. The Blast Furnace Phenomena and Modelling, 1st ed.; Elsevier Applied Sciences: London, UK, 1987; pp. 20–480. [Google Scholar]
- Castro, J.A.; Araujo, G.M.; Mota, I.O.; Sasaki, Y.; Yagi, J. Analysis of the combined injection of pulverized coal and charcoal into large blast furnaces. J. Mater. Res. Technol. 2013, 2, 308–314. [Google Scholar] [CrossRef]
- Castro, J.A.; Nogami, H.; Yagi, J. Three-dimensional multiphase mathematical modeling of the blast furnace based on the multifluid model. ISIJ Int. 2002, 42, 44–52. [Google Scholar] [CrossRef]
- Li, H.; Saxén, H.; Liu, W.; Zou, Z.; Shao, L. Model-Based Analysis of Factors Affecting the Burden Layer Structure in the Blast Furnace Shaft. Metals 2019, 9, 1003. [Google Scholar] [CrossRef]
- Castro, J.A.; Silva, A.J.; Sasaki, Y.; Yagi, J. A six-phases 3-D model to study simultaneous injection of high rates of pulverized coal and charcoal into the blast furnace with oxygen enrichment. ISIJ Int. 2011, 51, 748–758. [Google Scholar] [CrossRef]
- Austin, P.R.; Nogami, H.; Yagi, J. A mathematical model for blast furnace reactions analysis based on the four-fluid model. ISIJ Int. 1997, 37, 748–755. [Google Scholar] [CrossRef]
- Castro, J.A.; Nogami, H.; Yagi, J. Numerical investigation of simultaneous injection of pulverized coal and natural gas with oxygen enrichment to the blast furnace. ISIJ Int. 2002, 42, 1203–1211. [Google Scholar] [CrossRef]
- Castro, J.A.; Takano, C.; Yagi, J. A theoretical study using the multiphase numerical simulation technique for effective use of H2 as blast furnaces fuel. J. Mater. Res. Technol. 2017, 6, 258–270. [Google Scholar] [CrossRef]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef]
- Castro, J.A.; Nogami, H.; Yagi, J. Numerical analysis of multiple injections of pulverized coal, pre-reduced iron ore and flux with oxygen enrichment to the blast furnace. ISIJ Int. 2001, 41, 18–24. [Google Scholar] [CrossRef]
- Ueda, S.; Natsui, S.; Nogami, H.; Yagi, J.; Ariyama, T. Recent progress and future perspective on mathematical modeling of blast furnace. ISIJ Int. 2010, 50, 914–923. [Google Scholar] [CrossRef]
- Ariyama, T.; Natsui, S.; Kon, T.; Ueda, S.; Kikuchi, S.; Nogami, H. Recent Progress on Advanced Blast Furnace Mathematical Models Based on Discrete Method. ISIJ Int. 2014, 54, 1457–1471. [Google Scholar] [CrossRef]
- Castro, J.A.; Silva, A.J.; Nogami, H.; Yagi, J. Simulação computacional da injeção de carvão pulverizado nas ventaneiras de mini altos-fornos. TMMM Tecnol. Metal. Mater. Min. 2004, 1, 59–62. [Google Scholar] [CrossRef]
- Li, M.; Wei, H.; Ge, Y.; Xiao, G.; Yu, Y. A Mathematical Model Combined with Radar Data for Bell-Less Charging of a Blast Furnace. Processes 2020, 8, 239. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Okamoto, Y.; Kaise, T.; Sawa, Y.; Kano, M. Practical Operation Guidance on Thermal Control of Blast Furnace. ISIJ Int. 2019, 59, 1573–1581. [Google Scholar] [CrossRef]
- Castro, J.A.; Nogami, H.; Yagi, J. Transient mathematical model of blast furnace based on multi-fluid concept, with application to high PCI operation. ISIJ Int. 2001, 40, 637–646. [Google Scholar] [CrossRef]
- Kou, M.; Zhou, H.; Hong, Z.; Shun Yao, S.; Wu, S.; Xu, H.; Xu, J. Numerical Analysis of Effects of Different Blast Parameters on the Gas and Burden Distribution Characteristics Inside Blast Furnace. ISIJ Int. 2020, 60, 905–914. [Google Scholar] [CrossRef]
- Castro, J.A.; Silva, L.M.; Medeiros, G.A.; Oliveira, E.M.; Nogami, H. Analysis of a compact iron ore sintering process based on agglomerated biochar and gaseous fuels using a 3D multiphase multicomponent mathematical model. J. Mater. Res. Technol. 2020, 9, 6001–6013. [Google Scholar] [CrossRef]
- Da Rocha, E.P.; de Castro, J.A.; Silva, L.; de Souza Caldas, R. Computational Analysis of The Performance of Shaft Furnaces with Partial Replacement of The Burden with Self-Reducing Pellets Containing Biomass. Mater. Res. 2019, 22, e20190533. [Google Scholar] [CrossRef]
- Castro, J.A.; Rocha, E.P.; Oliveira, E.M.; Campos, M.F.; Francisco, A.S. Mathematical modeling of the shaft furnace process for producing DRI based on the multiphase theory. REM Int. Eng. J. 2018, 71, 81–87. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Fu, G.; Li, W.; Zhu, M. Numerical Analysis of the Effect of Water Gas Shift Reaction on Flash Reduction Behavior of Hematite with Syngas. ISIJ Int. 2019, 59, 2193–2204. [Google Scholar] [CrossRef]
- Matos, U.F.; Castro, J.A. Modeling of self-reducing agglomerates charging in the mini blast furnace with top gas recycling. REM Int. Eng. J. 2012, 65, 65–71. [Google Scholar]
- Yagi, J. Mathematical modeling of the flow of four fluids in a packed bed. ISIJ Int. 1993, 33, 619–639. [Google Scholar] [CrossRef]
- Zare Ghadi, A.; Valipour, M.S.; Vahedi, S.M.; Sohn, H.S. A Review on the Modeling of Gaseous Reduction of Iron Oxide Pellets. Steel Res. Int. 2020, 91, 1900270. [Google Scholar] [CrossRef]
- Bonalde, A.; Henriquez, A.; Manrique, M. Kinetic analysis of the iron oxide reduction using hydrogencarbon monoxide mixtures as reducing agent. ISIJ Int. 2005, 45, 155–1260. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D. Perry’s Chemical Engineers Handbook, 6th ed.; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Ergun, S. Fluid Flow through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from drops. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- El-Tawil, A.A.; Ahmed, H.M.; Ökvist, L.S.; Björkman, B. Self-Reduction Behavior of Bio-Coal Containing Iron Ore Composites. Metals 2020, 10, 133. [Google Scholar] [CrossRef]
- Lemos, L.R.; Rocha, S.H.S.F.; Castro, L.F.A.; Assunção, G.B.M.; Silva, G.L.R. Mechanical strength of briquettes for use in blast furnaces. REM Int. Eng. J. 2019, 72, 63–69. [Google Scholar] [CrossRef][Green Version]
- Paknahad, P.; Askari, M.; Shahahmadi, S.A. Cold-Briquetted Iron and Carbon (CBIC): Investigation of the Influence of Environmental Condition on Its Chemical and Physical Properties. J. Sustain. Metall. 2019, 5, 497–509. [Google Scholar] [CrossRef]
- Castro, J.A.; Baltazar, A.W.S.; Silva, A.J.; DAbreu, J.C.; Yagi, J. Evaluation of the performance of the blast furnace operating with self-reducing pellet using the computational simulation. TMMM Tecnol. Metal. Mater. Min. 2005, 2, 45–50. [Google Scholar]
- Tang, H.; Sun, Y.; Rong, T. Experimental and Numerical Investigation of Reaction Behavior of Carbon Composite Briquette in Blast Furnace. Metals 2020, 10, 49. [Google Scholar] [CrossRef]
- Chu, M.; Nogami, H.; Yagi, J. Numerical Analysis on Blast Furnace Performance under Operation with Top Gas Recycling and Carbon Composite Agglomerates Charging. ISIJ Int. 2004, 44, 2159–2167. [Google Scholar] [CrossRef]
- Mousa, E.; Lundgren, M.; Ökvist, L.S.; From, L.E.; Robles, A.; Hällsten, S.; Sundelin, B.; Friberg, H.; El-Tawil, A. Reduced Carbon Consumption and CO2 Emission at the Blast Furnace by Use of Briquettes Containing Torrefied Sawdust. J. Sustain. Metall. 2019, 5, 391–401. [Google Scholar] [CrossRef]
- Tang, H.; Rong, T.; Fan, K. Numerical investigation of applying high-carbon metallic briquette in blast furnace ironmaking. ISIJ Int. 2019, 59, 810–819. [Google Scholar] [CrossRef]
- Natsui, S.; Kikuchi, T.; Suzuki, R.O. Numerical analysis of carbon monoxide–hydrogen gas reduction of iron ore in a packed bed by an Euler–Lagrange approach. Metall. Mater. Trans. B 2014, 45, 2395–2413. [Google Scholar] [CrossRef]
- Patankar, S.V.; Spalding, D.B. A calculation procedure for heat, mass and momentum transfer in three-dimensional parabolic flows. Int. J. Heat Mass Transf. 1972, 15, 1787–1806. [Google Scholar] [CrossRef]
- Karki, K.C.; Patankar, S.V. Calculation procedure for viscous incompressible flows in complex geometries. Numer. Heat Transf. B 1988, 14, 295–307. [Google Scholar] [CrossRef]
- Melaen, M.C. Calculation of fluid flows with staggered and nonstaggered curvilinear non-orthogonal grids-the theory. Numer. Heat Transf. B 1992, 21, 1–19. [Google Scholar] [CrossRef]
- Castro, J.A.; Dabreu, J.C.; Silva, A.J. Two-phase model and computational simulation of self-reduction in shaft furnaces. TMMM Tecnol. Metal. Mater. Min. 2006, 2, 45–50. [Google Scholar]
- Noldin, J.H., Jr.; Cox, I.J.; DAbreu, J.C. The Tecnored ironmaking process part 1-competitiveness and pilot development work. Ironmak. Steelmak. 2008, 37, 334–340. [Google Scholar]
- Santos, D.M.; Mourão, M.B.; Takano, C. Reaction rate and product morphology in carbon composite iron ore pellets with and without portland cement. Ironmak. Steelmak. 2010, 35, 245–250. [Google Scholar] [CrossRef]
- Dabreu, J.C.; Kohler, H.M.; Noldin, J.H., Jr. Mathematical model for descending self-reducing agglomerates in lumpy zone of Tecnored furnace. Ironmak. Steelmak. 2013, 40, 484–490. [Google Scholar] [CrossRef]
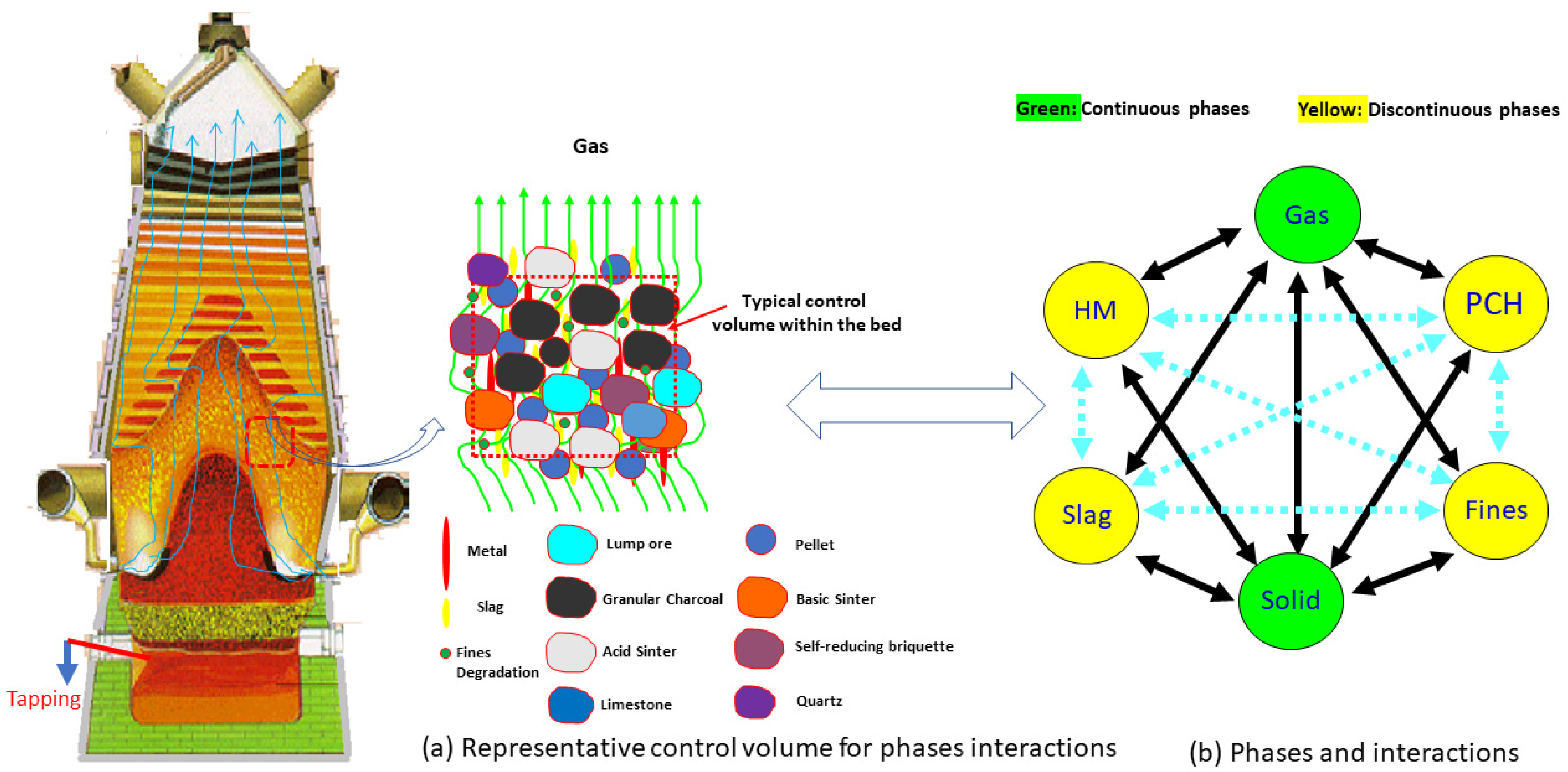
| Phases | Chemical Species |
|---|---|
| Gas | N2, O2, CO, CO2, H2, H2O, NOx, SOx, H2S, SiO, fuel and reducing gas (H2, CO, CO2, H2O, CH4, C2H6, C3H8, C4H10, CnHm) |
| Granular charcoal: C, volatile, H2O, Ash (SiO2, CaO, Al2O3, MgO, MnO, P2O5, S) | |
| Sinter: Fe2O3, Fe3O4, FeO, Fe, H2O, SiO2, CaO, Al2O3, MgO, MnO, P2O5, FeS | |
| Solid | Pellet: Fe2O3, Fe3O4, FeO, Fe, H2O, SiO2, CaO, Al2O3, MgO, MnO, P2O5, FeS |
| Lump ore: Fe2O3, Fe3O4, FeO, Fe, H2O, SiO2, CaO, Al2O3, MgO, MnO, P2O5, FeS | |
| Briquette: C, volatile, Fe2O3, Fe3O4, FeO, Fe, H2O, SiO2, CaO, Al2O3, MgO, MnO, P2O5, FeS | |
| Quartz: SiO2, H2O, gangue (Al2O3, MgO, MnO, CaO, P2O5, Fe2O3, Fe3O4, FeO) | |
| Limestone: CaCO3, MgCO3, H2O, gangue (SiO2, Al2O3, MgO, MnO, CaO, P2O5, Fe2O3, Fe3O4, FeO) | |
| PCH 1 | C, volatile, H2O, ash (SiO2, CaO, Al2O3, MgO, MnO, P2O5, S) |
| Fines 2 | C, volatile, H2O, Fe2O3, Fe3O4, FeO, Fe, SiO2, CaO, Al2O3, MgO, MnO, P2O5, FeS |
| Hot metal | Fe, C, Si, Mn, P, S, Ti. TiC |
| Slag | SiO2, CaO, Al2O3, MgO, MnO, TiO2, MnS, P2O5 |
| Reactions | Reactions |
|---|---|
| Combustion and Gasification | |
| i = granular charcoal, pulverized charcoal, briquettes, biomasses, and fines | |
| Gas-Solid Gaseous Reduction | |
| i = lump ore, sinter, pellets, briquette, and fines | |
| Melting/Slagging | |
| i = lump ore, sinter, pellets, briquette, granular charcoal, pulverized charcoal, biomasses, and fines | |
| Hot Metal Incorporation Elements | |
| i = granular charcoal, pulverized charcoal, briquettes, biomasses, and fines | |
| Liquid-Solid Direct Reduction | |
| i = Granular charcoal, briquette, pulverized charcoal, and fines | |
| Gas (%vol) | CO | CO2 | H2 | H2O | CH4 | CmHn | N2 | SO2 |
|---|---|---|---|---|---|---|---|---|
| Shaft | 16.1 | 3.9 | 76.4 | 0.1 | 1.2 | 0.4 | 0.8 | 0.1 |
| Tuyere | 22.0 | 3.2 | 51 | 0.5 | 14.8 | 0.9 | 7.6 | 0.0 |
| Species | Granular Charcoal | Pulverized Charcoal (PCH) | Sinter | Pellets | Briquette | Lump Ore |
|---|---|---|---|---|---|---|
| C (fix) | 75.5 | 73.4 | 0.0 | 0.0 | 27.1 | 0.0 |
| Volatiles | 22.4 | 25.3 | 0.0 | 0.0 | 1.2 | 0.0 |
| H2O | 1.5 | 0.3 | 1.3 | 0.4 | 0.2 | 2.5 |
| Fe2O3 | 0.0 | 0.0 | 71.3 | 87.3 | 36.5 | 92.6 |
| Fe3O4 | 0.0 | 0.0 | 15.2 | 2.0 | 21.5 | 0.0 |
| Fe0.95O | 0.0 | 0.0 | 0.2 | 1.1 | 0.0 | 0.0 |
| FeS | 0.3 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 |
| S2 | 0.05 | 0.02 | 0.0 | 0.0 | 0.0 | 0.0 |
| P2O5 | 0.05 | 0.08 | 0.01 | 0.06 | 0.04 | 0.05 |
| CaO | 0.1 | 0.2 | 4.1 | 1.1 | 2.7 | 0.0 |
| SiO2 | 0.6 | 0.4 | 5.6 | 3.7 | 4.3 | 3.1 |
| MnO | 0.01 | 0.01 | 0.11 | 0.12 | 0.10 | 0.05 |
| MgO | 0.01 | 0.02 | 0.89 | 0.04 | 0.03 | 0.01 |
| TiO2 | 0.00 | 0.00 | 0.06 | 0.02 | 0.02 | 0.01 |
| Operational Parameters | Measured | Calculated | Error (%) 1 |
|---|---|---|---|
| Productivity (tHM/day/m3) | 2.80 | 2.82 | 0.71 |
| Granular charcoal (kg/tHM) | 398.5 | 395.4 | 0.77 |
| Blast rate (Nm3/tHM) | 1042 | 1037 | 0.48 |
| O2 rate (Nm3/tHM) | 334.5 | 331.4 | 0.93 |
| O2 enrichment (%) | 9.4 | 9.5 | 1.06 |
| Outlet gas (Nm3/tHM) | 1548 | 1531 | 1.09 |
| CO (kg/tHM) | 632.1 | 653.7 | 2.18 |
| CO2 (kg/tHM) | 722.3 | 701.9 | 3.59 |
| H2 (kg/tHM) | 1.8 | 1.9 | 5.55 |
| H2O (kg/tHM) | 64.8 | 62.7 | 3.32 |
| Temperature (°C) | 202.5 | 200.4 | 1.04 |
| Slag rate (kg/tHM) | 132.0 | 135.2 | 2.42 |
| Slag basicity (B2 = CaO/SiO2) | 1.05 | 1.10 | 4.76 |
| Silicon pig iron (%) | 1.73 | 1.65 | 4.62 |
| Carbon intensity (kg C/tHM) | 499.5 | 495.8 | 0.74 |
| Scenarios Analyzed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 |
| Productivity (tHM/day/m3) | 2.8 | 2.9 | 3.3 | 3.6 | 3.0 | 3.2 | 3.7 | 3.2 | 2.9 | 3.1 |
| Granular charcoal (kg/tHM) | 395.4 | 400.6 | 392.1 | 401.7 | 283.1 | 283.3 | 273.1 | 283.2 | 314.3 | 278.1 |
| Blast (Nm3/tHM) | 1037 | 1018 | 903 | 832 | 825 | 822 | 792 | 822 | 633 | 536.3 |
| Outlet gas (Nm3/tHM) | 1663 | 1679 | 1676 | 1593 | 2135 | 2066 | 1950 | 2086 | 1692 | 1750 |
| Outlet gas temperature (°C) | 200 | 205 | 167 | 152 | 391 | 368 | 339 | 371 | 291 | 312 |
| CO (kg/tHM) | 654 | 717 | 1031 | 926 | 888 | 855 | 791 | 866 | 779 | 788 |
| CO2 (kg/tHM) | 702 | 655 | 512 | 379 | 330 | 356 | 389 | 349 | 481 | 406 |
| H2 (kg/tHM) | 1.9 | 2.4 | 4.4 | 3.8 | 35.6 | 32.7 | 29.3 | 33.7 | 17.2 | 25.3 |
| H2O (kg/tHM) | 62.7 | 63.1 | 86.5 | 60.6 | 244.1 | 226.1 | 207.5 | 230.8 | 164.4 | 215.2 |
| Slag (kg/tHM) | 135 | 118 | 108 | 143 | 137 | 132 | 135 | 133 | 124 | 126 |
| Basicity (-) | 1.15 | 1.18 | 1.10 | 1.06 | 1.21 | 1.17 | 1.19 | 1.20 | 1.35 | 1.26 |
| Carbon intensity (kg C/tHM) | 472 | 486 | 582 | 500 | 471 | 464 | 446 | 466 | 465 | 448 |
| Parameters | Case 11 | Case 12 | Case 13 | Case 14 | Case 15 | Case 16 | Case 17 | Case 18 | Case 19 | Case 20 |
| Productivity (tHM/day/m3) | 4.6 | 4.9 | 2.8 | 2.8 | 3.4 | 3.6 | 3.8 | 4.4 | 6.1 | 6.5 |
| Granular charcoal (kg/tHM) | 299 | 278 | 315 | 300 | 299 | 279 | 278 | 273 | 263 | 263 |
| Blast (Nm3/tHM) | 329 | 313 | 845 | 858 | 901 | 814 | 753 | 679 | 550 | 515 |
| Outlet gas (Nm3/tHM) | 1442 | 1391 | 1827 | 2050 | 2218 | 2080 | 1928 | 1793 | 1840 | 1930 |
| Outlet gas temperature (°C) | 233 | 217 | 299 | 345 | 374 | 337 | 329 | 315 | 327 | 346 |
| CO (kg/tHM) | 717 | 664 | 791 | 843 | 836 | 793 | 771 | 725 | 793 | 850 |
| CO2 (kg/tHM) | 501 | 529 | 469 | 403 | 415 | 425 | 425 | 453 | 380 | 321 |
| H2 (kg/tHM) | 17.8 | 17.2 | 18.4 | 31.6 | 38.9 | 35.8 | 32.7 | 26.6 | 33.9 | 41.8 |
| H2O (kg/tHM) | 167.9 | 162.1 | 150.7 | 228.6 | 299.7 | 265.0 | 255.5 | 251.3 | 277.6 | 315.8 |
| Slag (kg/tHM) | 143 | 134 | 130 | 131 | 130 | 132 | 135 | 124 | 129 | 121 |
| Basicity (-) | 1.12 | 1.05 | 1.31 | 1.25 | 1.23 | 1.08 | 1.14 | 1.26 | 1.18 | 1.32 |
| Carbon intensity 1 (IC= kg C/tHM) | 444 | 429 | 467 | 449 | 471 | 456 | 446 | 434 | 443 | 452 |
| Reactions 1 | |||||||||||
| (R1C) | (R1H) | ||||||||||
| (R2C) | (R2H) | ||||||||||
| (R3C) | (R3H) | ||||||||||
| Reactions | Reference | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 |
| (R1C) 1 | 61.5 | 62.3 | 57.2 | 60.7 | 71.1 | 63.2 | 63.2 | 63.1 | 62.7 | 63.2 | 62.9 |
| (R1H) 2 | 0.017 | 0.019 | 0.050 | 0.046 | 0.046 | 0.027 | 0.032 | 0.036 | 0.028 | 0.029 | 0.039 |
| (R2C) 1 | 124.9 | 125.6 | 125.8 | 162.5 | 173.1 | 51.6 | 57.2 | 63.8 | 236.24 | 57.3 | 43.1 |
| (R2H) 2 | 0.015 | 0.017 | 0.049 | 0.013 | 0.060 | 4.953 | 5.130 | 4.659 | 5.207 | 6.14 | 6.15 |
| (R3C) 1 | 393.6 | 388.2 | 391.7 | 401.2 | 428.8 | 272.9 | 91.3 | 302.1 | 85.6 | 372.9 | 247.2 |
| (R3H) 2 | 0.000 | 0.001 | 0.002 | 0.000 | 0.000 | 14.050 | 12.458 | 10.816 | 12.859 | 7.645 | 16.540 |
| Reactions | Reference | Case 11 | Case 12 | Case 13 | Case 14 | Case 15 | Case 16 | Case 17 | Case 18 | Case 19 | Case 20 |
| (R1C) 1 | 61.5 | 62.9 | 62.8 | 63.2 | 63.3 | 63.2 | 63.2 | 63.0 | 63.1 | 62.9 | 64.9 |
| (R1H) 2 | 0.017 | 0.042 | 0.045 | 0.036 | 0.038 | 0.038 | 0.044 | 0.049 | 0.048 | 0.046 | 0.044 |
| (R2C) 1 | 124.9 | 57.9 | 59.4 | 73.6 | 54.5 | 52.2 | 58.8 | 59.2 | 58.6 | 43.6 | 40.3 |
| (R2H) 2 | 0.015 | 6.06 | 4.96 | 3.97 | 5.35 | 5.53 | 5.05 | 4.97 | 5.03 | 6.13 | 7.93 |
| (R3C ) 1 | 393.6 | 359.9 | 363.0 | 346.5 | 301.7 | 315.1 | 301.1 | 305.6 | 312.6 | 260.8 | 227.8 |
| (R3H) 2 | 0.000 | 7.15 | 5.69 | 7.70 | 11.53 | 10.07 | 10.58 | 10.21 | 9.63 | 13.65 | 15.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adilson de Castro, J.; Medeiros, G.A.d.; Oliveira, E.M.d.; de Campos, M.F.; Nogami, H. The Mini Blast Furnace Process: An Efficient Reactor for Green Pig Iron Production Using Charcoal and Hydrogen-Rich Gas: A Study of Cases. Metals 2020, 10, 1501. https://doi.org/10.3390/met10111501
Adilson de Castro J, Medeiros GAd, Oliveira EMd, de Campos MF, Nogami H. The Mini Blast Furnace Process: An Efficient Reactor for Green Pig Iron Production Using Charcoal and Hydrogen-Rich Gas: A Study of Cases. Metals. 2020; 10(11):1501. https://doi.org/10.3390/met10111501
Chicago/Turabian StyleAdilson de Castro, Jose, Giulio Antunes de Medeiros, Elizabeth Mendes de Oliveira, Marcos Flavio de Campos, and Hiroshi Nogami. 2020. "The Mini Blast Furnace Process: An Efficient Reactor for Green Pig Iron Production Using Charcoal and Hydrogen-Rich Gas: A Study of Cases" Metals 10, no. 11: 1501. https://doi.org/10.3390/met10111501
APA StyleAdilson de Castro, J., Medeiros, G. A. d., Oliveira, E. M. d., de Campos, M. F., & Nogami, H. (2020). The Mini Blast Furnace Process: An Efficient Reactor for Green Pig Iron Production Using Charcoal and Hydrogen-Rich Gas: A Study of Cases. Metals, 10(11), 1501. https://doi.org/10.3390/met10111501