Removal of Zn(II) in Synthetic Wastewater Using Agricultural Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Site and Physical Treatment of CP, BP, and CC
2.2. Cellulose and Lignin Content in the Three Residues and Analysis IR
2.3. Quantification of Zn(II)
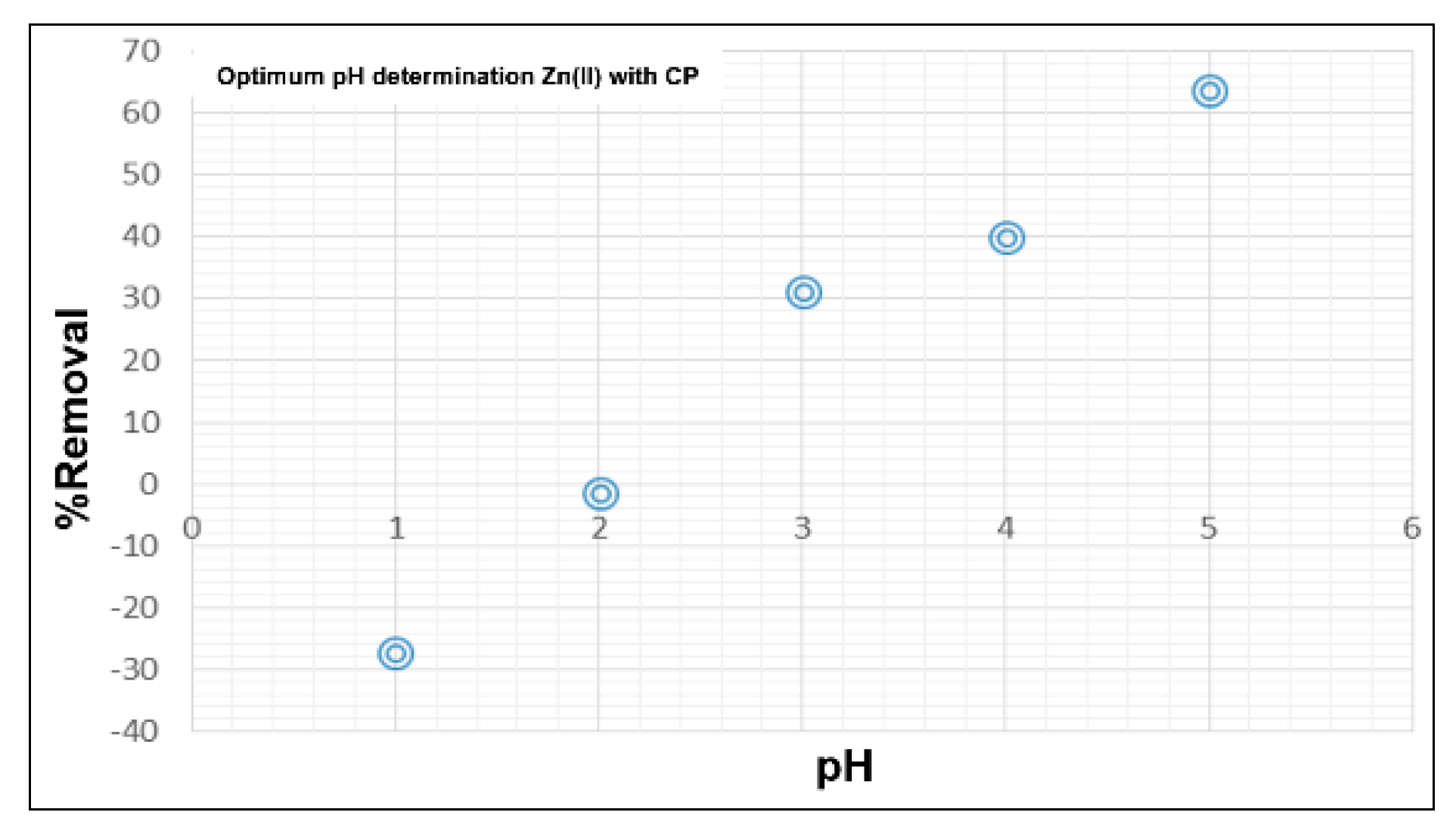
2.4. Determination of the Optimum pH of Adsorption of Zn(II)
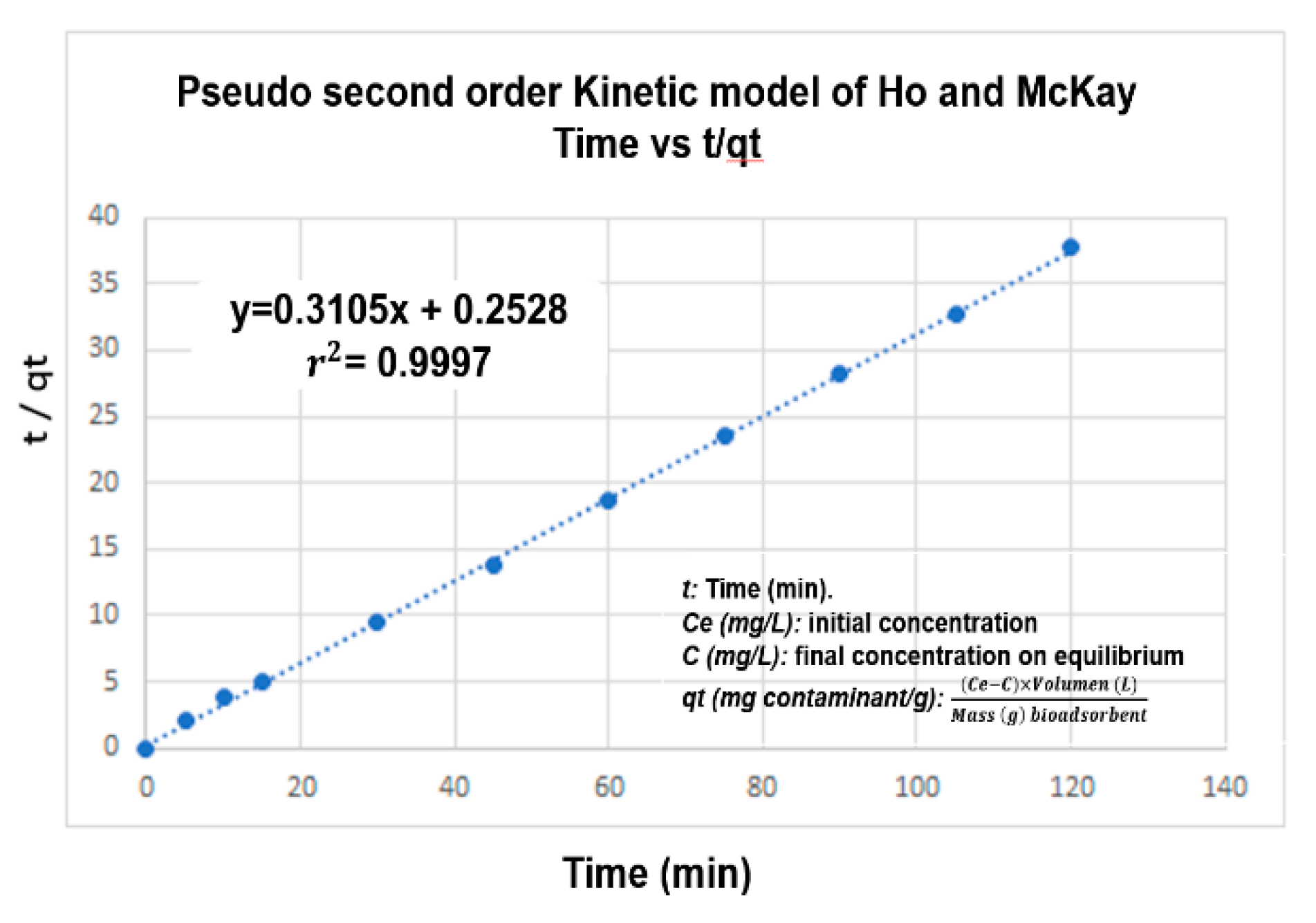
2.5. Determination of the Adsorption Kinetics for Zn(II)
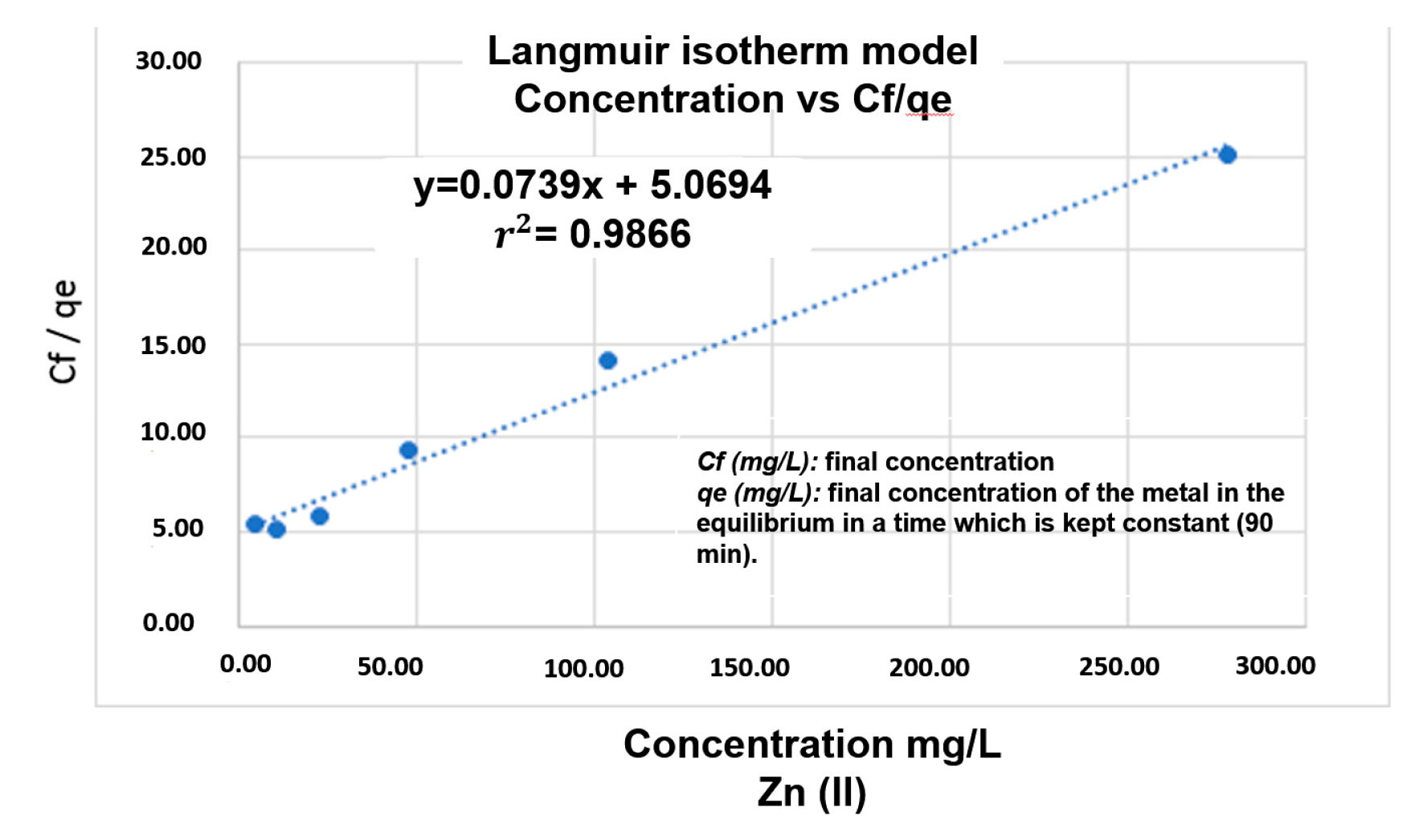
2.6. Determination of the Adsorption Isotherm for Zn(II)
2.7. Determination of the Point of Zero Charge (pHpzc) for the Efficient Biosorbent
3. Results and Discussion
3.1. Lignocelulosic Content for CP, BP, and CC and Elucidation of Organic Functional Groups by Infrared Spectrophotometry (IR)
3.2. Determination of the Most Efficient Biosorbent for the Removal of Zn(II)
3.3. Determination of Optimum pH of Zn(II) with CP
3.4. Kinetics and Adsorption Isotherm
3.5. Determination of Point of Zero Charge (pHpzc) for the CP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Adverse Health Effects of Heavy Metals in Children. 2011. Available online: https://www.who.int/ceh/capacity/heavy_metals.pdf (accessed on 6 May 2020).
- Royal Society of Chemistry. Zinc. 2011. Available online: https://www.rsc.org/periodic-table/element/30/zinc (accessed on 6 May 2020).
- Samanta, D.; Jena, P. Zn in the +III Oxidation State. J. Am. Chem. Soc. 2012, 20, 8400–8403. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.L.; Rodríguez, N. Cáscaras de Frutas Como Bioadsorbentes: Una Alternativa para la Remoción de Metales Pesados en agua Residuales Industriales. En Aproximaciones Teórico-Prácticas al Desarrollo Sostenible; Ediciones: Ciudad de México, Mexico, 2017; pp. 117–138. [Google Scholar]
- Segovia-Sandoval, S.J.; Ocampo-Pérez, R.; Berber-Mendoza, M.S.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Medellin-Castillo, N.A. Alnut shell treated with citric acid and its application as biosorbent in the removal of Zn (II). J. Water Proc. Eng. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Simon-Hettich, B.; Wibbertmann, A.; Wagner, D.; Tomaska, L.; Malcolm. Environmental Health Criteria 221 ZINC. Environmental Health Criteria. 2001, p. 19. Available online: https://www.researchgate.net/publication/235706459_Environmental_Health_Criteria_221_ZINC (accessed on 6 May 2020).
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Biol. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Ambiente y Desarrollo Sostenible (MADS). Resolución 631 de 2015 de Colombia. 2015. Available online: http://www.aguasdebuga.net/intranet/sites/default/files/Resolución0631de2015-Calidadvertimientos.pdf (accessed on 6 May 2020).
- Caviedes, D.I.; Muñoz, R.A.; Perdomo, A.; Rodríguez, D.; Sandoval, I.J. Tratamientos para la Remoción de Metales Pesados Comúnmente Presentes en Aguas Residuales Industriales. Una Revisión. Ing. Reg. 2015, 1, 73–90. [Google Scholar] [CrossRef]
- Nassef, E.; Eltaweel, Y. Removal of Zinc from Aqueous Solution Using Activated Oil Shale. J. Chem. 2019. [Google Scholar] [CrossRef]
- Corredor, Y.; Pérez-Pérez, L.I. Use of Agro-Industrial Waste in Improving the Quality of the Environment. Rev. Fac. Cienc. Básicas 2018, 1, 1–14. [Google Scholar] [CrossRef]
- Gómez Aguilar, D.L.; Rodríguez Miranda, J.P.; Baracaldo Guzmán, D.; Esteban Muñoz, J.A. Using Coffee Pulp as Bioadsorbent for the Removal of Manganese (Mn(II)) from Synthetic Wastewater. Water 2020, 12, 2500. [Google Scholar] [CrossRef]
- United Nations (UN). Goal 3: Ensure a Healthy Life and Promote Well-Being for All People at All Ages. 2017. Available online: https://www.un.org/sustainabledevelopment/es/health/ (accessed on 6 May 2020).
- United Nations (UN). Goal 6: Ensure Water Availability, Sustainable Management and Sanitation for All. 2017. Available online: https://www.un.org/sustainabledevelopment/es/water-and-sanitation/ (accessed on 6 May 2020).
- Escalante, H.; Orduz, J.; Zapata, H.; Cardona, M.; Duarte, M. Atlas del Potencial Energético de la Biomasa Residual en Colombia. 2011; p. 145. Available online: https://bdigital.upme.gov.co/handle/001/1058 (accessed on 6 May 2020).
- Shafiq, M.; Alazaba, A.; Amin, M.T. Removal of Heavy Metals from Wastewater using Date Palm as a Biosorbent: A Comparative Review. Sains Malays. 2018, 47, 35–49. [Google Scholar] [CrossRef]
- Ahmed, H. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar]
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T.V. Removal of hexavalent chromium using coffee husk. International. J. Environ. Pollut. 2010, 43, 106. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2016, 229, 555–565. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Cardona Alzate, C.A. The biorefinery concept for the industrialvalorization of coffee processing by-products. In Handbook of Coffee Processing By-Products; Academic Press: Manizales, Colombia, 2017; pp. 63–92. [Google Scholar]
- Gómez, D. Bioadsorción de Mn (II), Zn (II), Pb (II), Cr (III) y (VI) con Residuos Lignocelulósicos en Aguas Residuales. Una aplicación en Curtiembres. Ph.D. Thesis, Universidad de Manizales, Manizales, Colombia, 2019. [Google Scholar]
- Echeverria, C.; Bazan, G.; Sanchez-Gonzalez, J.; Lescano, L.; Pagador, S.; Linares, G. Pre-treatment by Acidification and Freezing on Corncob Polymers and its Enzymatic Hydrolysis. Asian J. Sci. Res. 2018, 11, 222–231. [Google Scholar] [CrossRef][Green Version]
- ASTM International. ANSI/ASTM D1106-56: Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM International (ASTM): West Conshohocken, PA, USA, 2001. [Google Scholar]
- ASTM International. ANSI/ASTM D1103-60: Method of Test for Alpha-Cellulose in Wood; ASTM International (ASTM): West Conshohocken, PA, USA, 1960. [Google Scholar]
- Yazán, D. Descomposición Microbiológica de Desechos Orgánicos Vegetales Originados en la Universidad Central del Ecuador. Ph.D. Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2013. [Google Scholar]
- Gómez Aguilar, D.L.; Esteban Muñoz, J.A.; Baracaldo Guzmán, D. Tecnologías no convencionales para la remoción de plomo presente en aguas residuales: Una revisión bibliográfica 2010–2019. Tecnura 2020, 64, 97–116. [Google Scholar] [CrossRef]
- Calderón, C. Manual Para la Interpretación de Espectros Infrarrojos; Universidad Nacional de Colombia: Bogotá, Colombia, 1995. [Google Scholar]
- Cooney, D.O. Sólidos Porosos Preparación, Caracterización y Aplicaciones; Moreno, P., Ed.; Ediciones Uniandes: Bogotá, Colombia, 2007. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 1–11. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Guan, S.S.; Chung Hsin, W.; Chao Yin, C.; Shu Shian, G.; Guan, S.S. Adsorption kinetics of lead and zinc ions by coffee residues. Pol. J. Environ. Stud. 2016, 2, 761–767. [Google Scholar] [CrossRef]
- Damal, V.S.; Khanapure, V.U. Removal of Zinc Metal Ions from Electroplating Industrial Waste Water by Using Bio-Sorbent. Int. J. Adv. Eng. Res. Sci. 2017, 7, 113–117. [Google Scholar] [CrossRef]
- Opeolu, B.O.B.; Fatoki, O.S.O. Dynamics of zinc sorption from aqueous matrices using plantain (Musa sp.) peel biomass. Afr. J. Biotechnol. 2012, 68, 13194–13201. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Petrović, J.; Mihajlović, M.; Ćosović, A.; Stankovi, S. Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L.). Ecol. Eng. 2017, 99, 83–90. [Google Scholar] [CrossRef]
- El-Sayed, G.O.; Dessouki, H.; Ibrahiem, S. Removal of Zn (II), Cd (II) and Mn (II) from aqueous solutions by adsorption on maize stalks. Malays. J. Anal. Sci. 2011, 1, 8–21. [Google Scholar]
- Giraldo, L.; Moreno-Piraján, J.C. Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. E J. Chem. 2012, 2, 938–948. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Tapang, K.; Jaroensin, S.; Panphrom, S. Equilibrium and Kinetic Studies of Biosorption of Zn (II) Ions from Wastewater Using Modified Corn Cob. APCBEE Proc. 2012, 3, 60–64. [Google Scholar] [CrossRef]
- Leyva-Ramos, R. Capítulo V Importancia y Aplicaciones de la Adsorción en fase líquida. In Sólidos Porosos; Universidad de los Andes: Bogotá, Colombia, 2007; pp. 55–207. [Google Scholar]
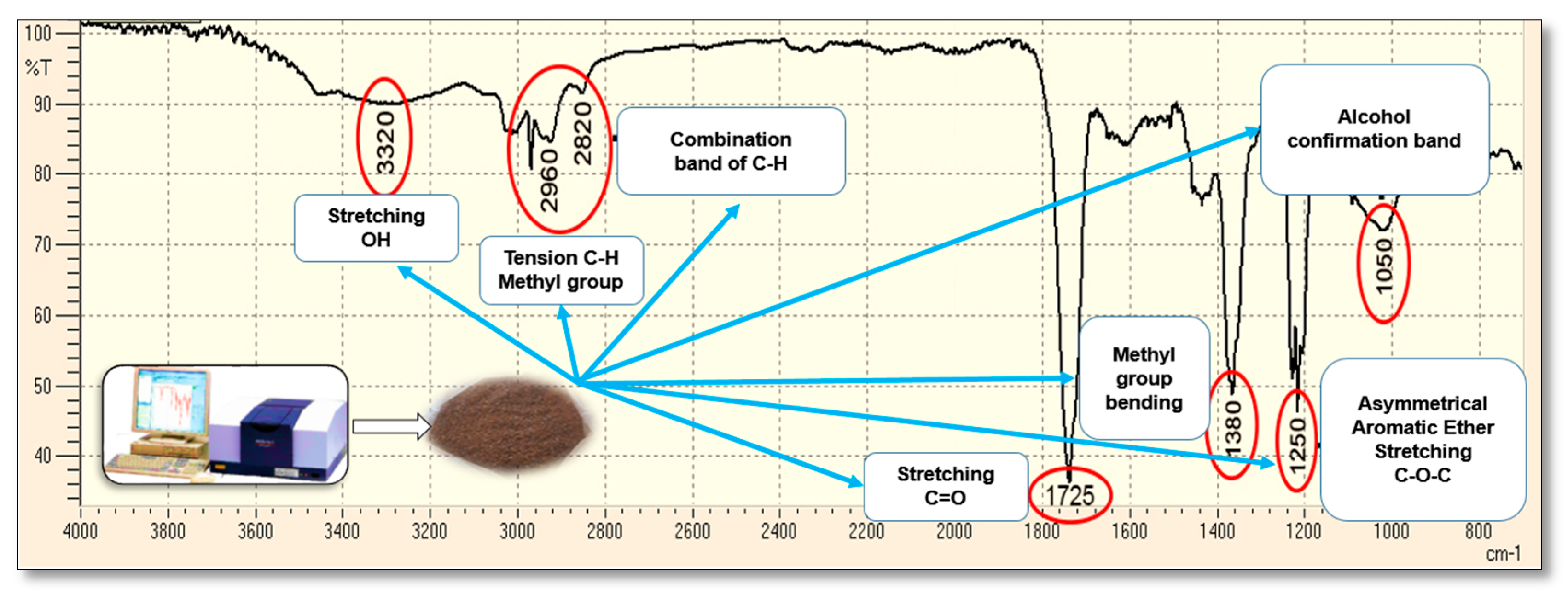
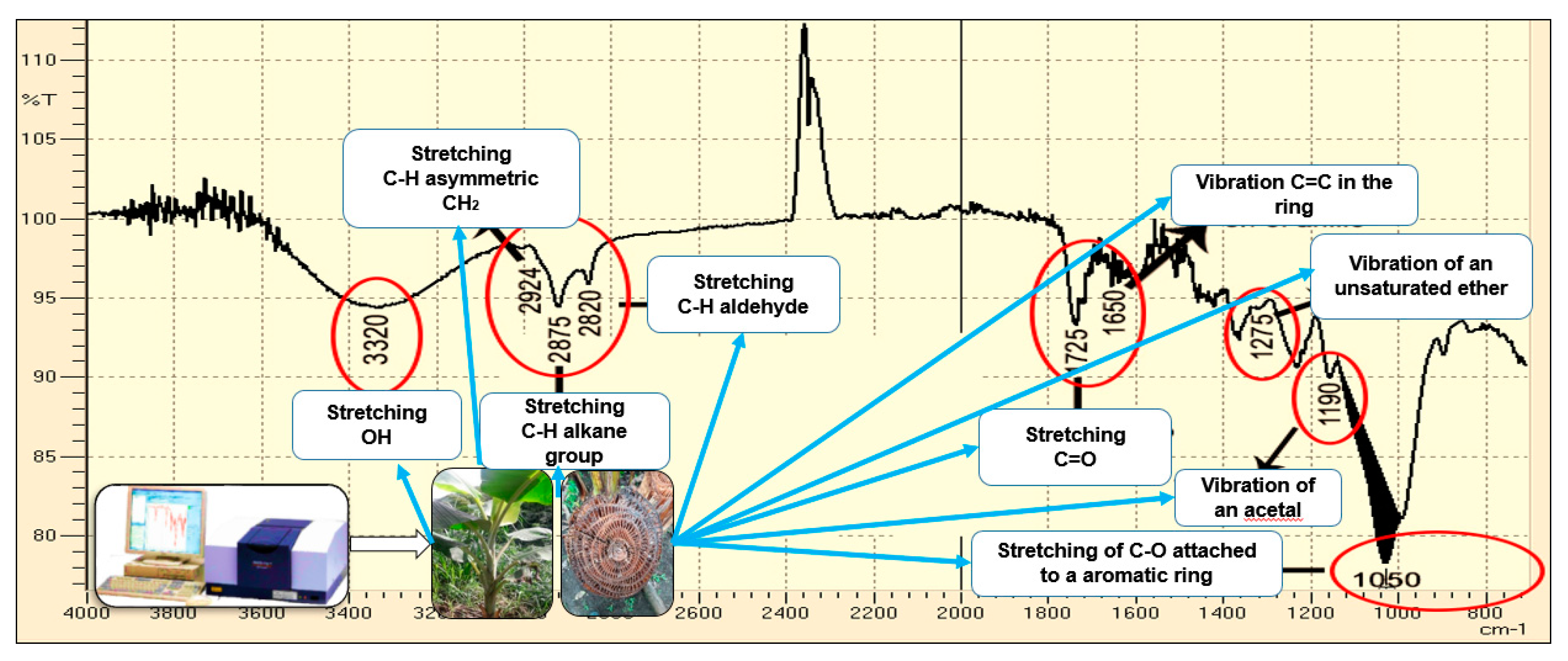
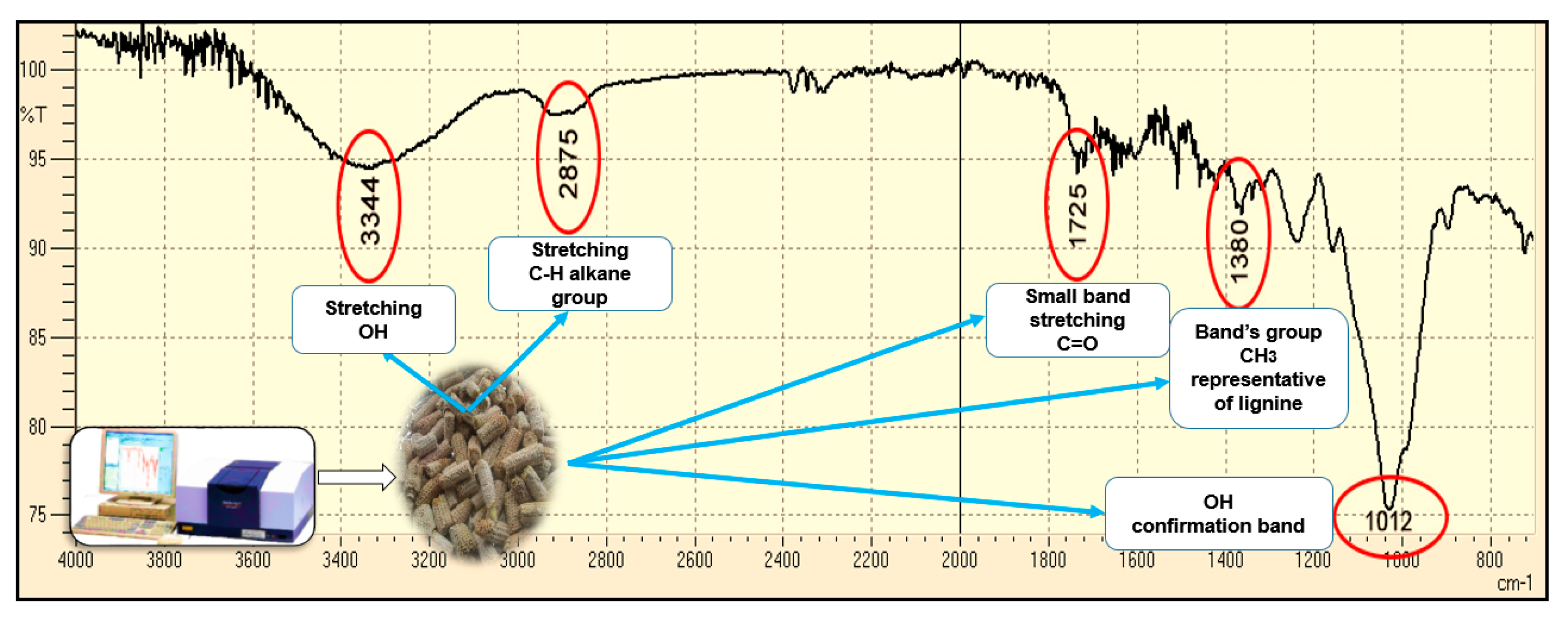

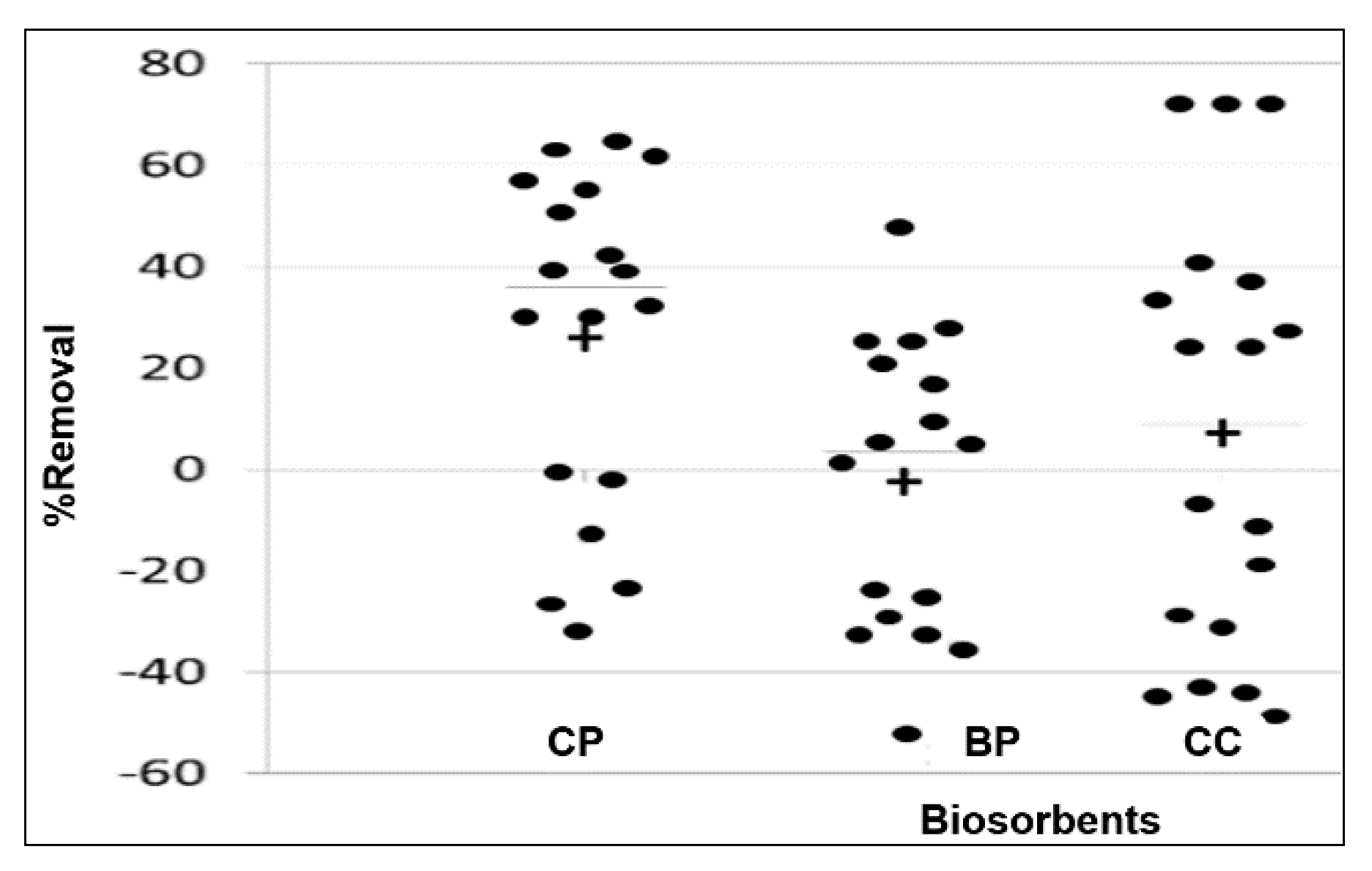

| Agricultural Waste | Parameter | Value of This Study | Comparison Interval | Bibliographic Reference | |
|---|---|---|---|---|---|
| Years | Value | ||||
| CP | % Lignin | 19.25 ± 0.16 | 2017–2020 | 12.20–36.89 | [12,20] |
| % Cellulose | 29.93 ± 0.21 | 16.50–63.00 | |||
| BP | % Lignin | 5.49 ± 0.25 | 2009–2014 | 5.00–37.30 | [21] |
| % Cellulose | 49.24 ± 2.65 | 31.27–65.00 | |||
| CC | % Lignin | 12.23 ± 0.11 | 2008–2018 | 7.39–15.80 | [22] |
| % Cellulose | 47.50 ± 0.60 | 32.30–57.00 | |||
| Order Kinetic | Kinetic Parameters | r2 |
|---|---|---|
| 0 | t vs. C | 0.3035 |
| 1 | t vs. Log C | 0.3767 |
| 2 | t vs. | 0.4489 |
| Pseudo-first order | t vs. Log (qe − qt) | 0.3093 |
| Pseudo-second order | t vs. | 0.9997 |
| Adsorption Isotherms | Langmuir | Freundlich | Henry |
|---|---|---|---|
| r2 | 0.9866 | 0.9516 | 0.8079 |
| Lignocellulosic Material | Characteristics | Adsorption Isotherm | Adsorption Kinetics | Author | |||
|---|---|---|---|---|---|---|---|
| Water Type | pH | Maximum Adsorption Capacity (mg × g−1) | % Efficiency | ||||
| Coffee Pulp | Synthetic | 5.0 | 13.53 | 63.78 | Langmuir | Pseudo second order | Present study |
| Coffee waste | Synthetic | 5.0 | 4.40 | 44.00 | Langmuir | Pseudo second order | [30] |
| Banana peels | Synthetic | 2.0–6.0 | NE | 90.00 | Langmuir | Pseudo second order | [31] |
| Banana waste | Synthetic | 2.0–3.0 | NE | >90.00 | Langmuir | Pseudo second order | [32] |
| Banana pseudo-stem | Synthetic | 6.0 | 0.94 | 97.24 | Langmuir | Pseudo second order | [33] |
| Stigmas/Corn Silk | Synthetic | 5.0 | 13.98 | NE | Langmuir | Pseudo second order | [34] |
| Corncob | Synthetic | 7.0 | 30.30 | 50.00 | Langmuir | Pseudo second order | [35] |
| Lignocellulosic Material | Characteristics | Adsorption Isotherm | Adsorption Kinetics | Author | ||||
|---|---|---|---|---|---|---|---|---|
| Water Type | pH | Maximum Adsorption Capacity (mg × g−1) | % Efficiency | Modification | ||||
| Coffee waste | Synthetic | 5.1 | 19.61 | NE | ZnCl2 and KOH activated with CO2 | Langmuir | Pseudo second order | [36] |
| Corncob | Synthetic | 7.0 | 79.21 | NE | H3PO4 | Langmuir | Pseudo second order | [37] |
| Optimum pH for CP | pHpzc | Chemical Species in Aqueous Solution | Conclusion |
|---|---|---|---|
| 5.0 | 3.95 | Zn2+ [5] | pH > pHpzc The surface of the biosorbent is negatively charged. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Aguilar, D.L.; Rodríguez Miranda, J.P.; Astudillo Miller, M.X.; Maldonado Astudillo, R.I.; Esteban Muñoz, J.A. Removal of Zn(II) in Synthetic Wastewater Using Agricultural Wastes. Metals 2020, 10, 1465. https://doi.org/10.3390/met10111465
Gómez Aguilar DL, Rodríguez Miranda JP, Astudillo Miller MX, Maldonado Astudillo RI, Esteban Muñoz JA. Removal of Zn(II) in Synthetic Wastewater Using Agricultural Wastes. Metals. 2020; 10(11):1465. https://doi.org/10.3390/met10111465
Chicago/Turabian StyleGómez Aguilar, Dora Luz, Juan Pablo Rodríguez Miranda, María Xóchitl Astudillo Miller, Rayma Ireri Maldonado Astudillo, and Javier Andrés Esteban Muñoz. 2020. "Removal of Zn(II) in Synthetic Wastewater Using Agricultural Wastes" Metals 10, no. 11: 1465. https://doi.org/10.3390/met10111465
APA StyleGómez Aguilar, D. L., Rodríguez Miranda, J. P., Astudillo Miller, M. X., Maldonado Astudillo, R. I., & Esteban Muñoz, J. A. (2020). Removal of Zn(II) in Synthetic Wastewater Using Agricultural Wastes. Metals, 10(11), 1465. https://doi.org/10.3390/met10111465






