Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition of the Input Material
2.2. Experimental Procedure
3. Results
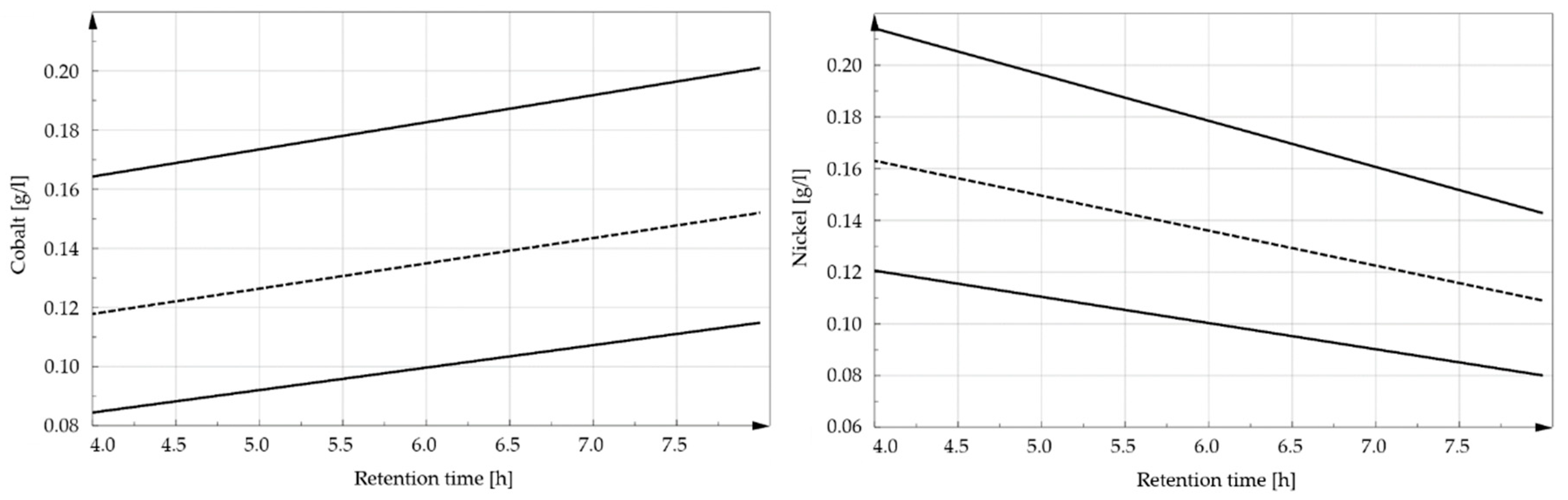
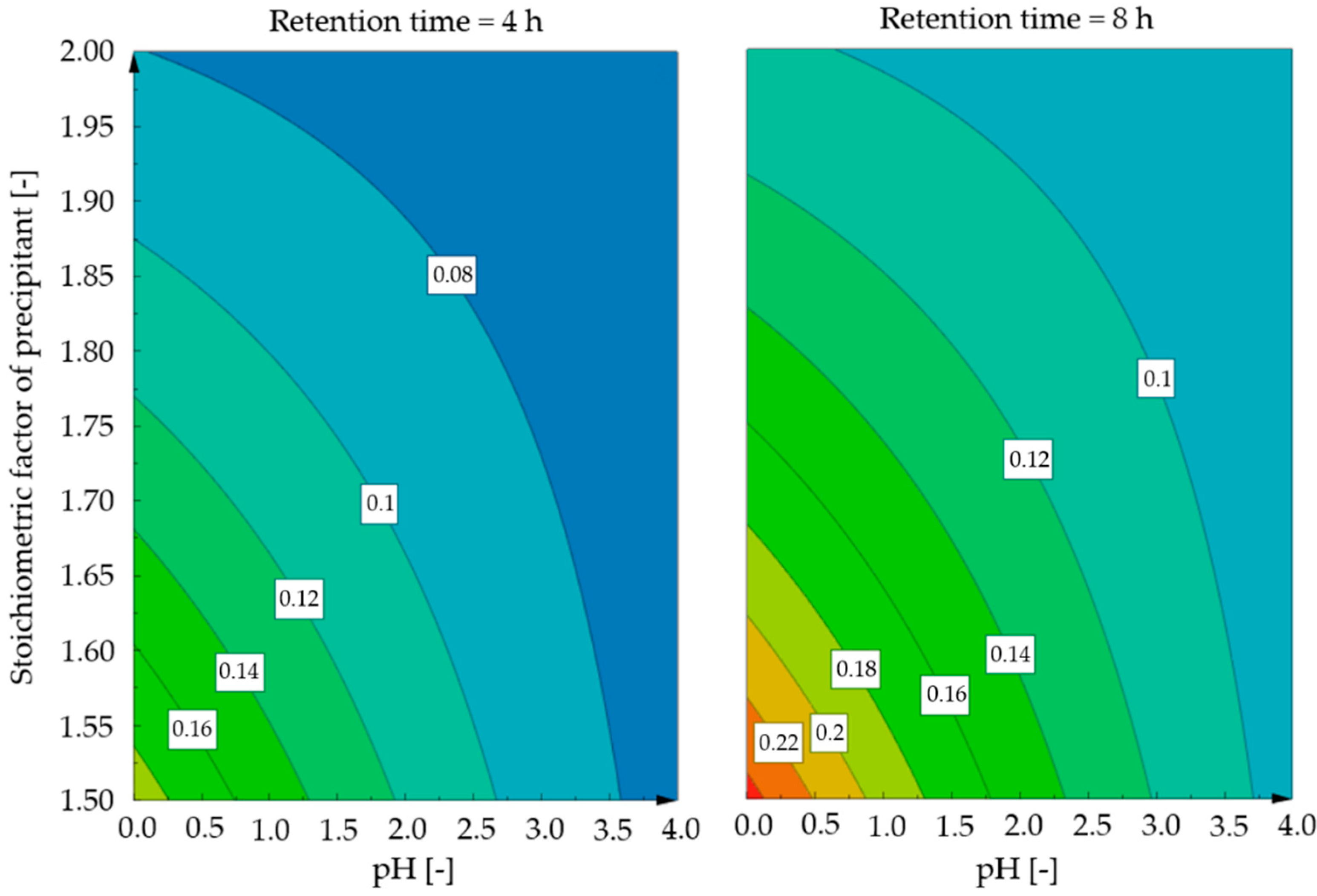
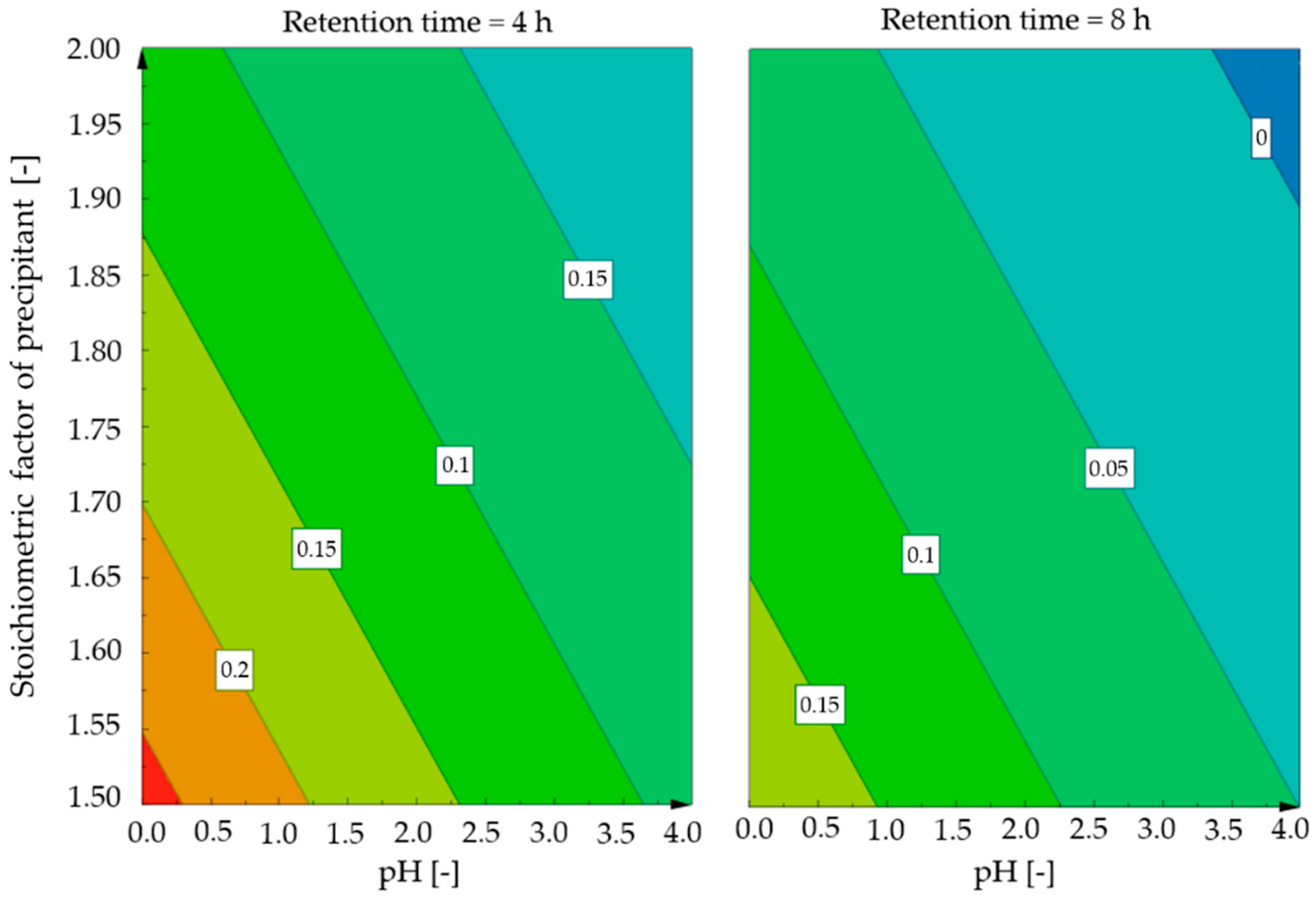
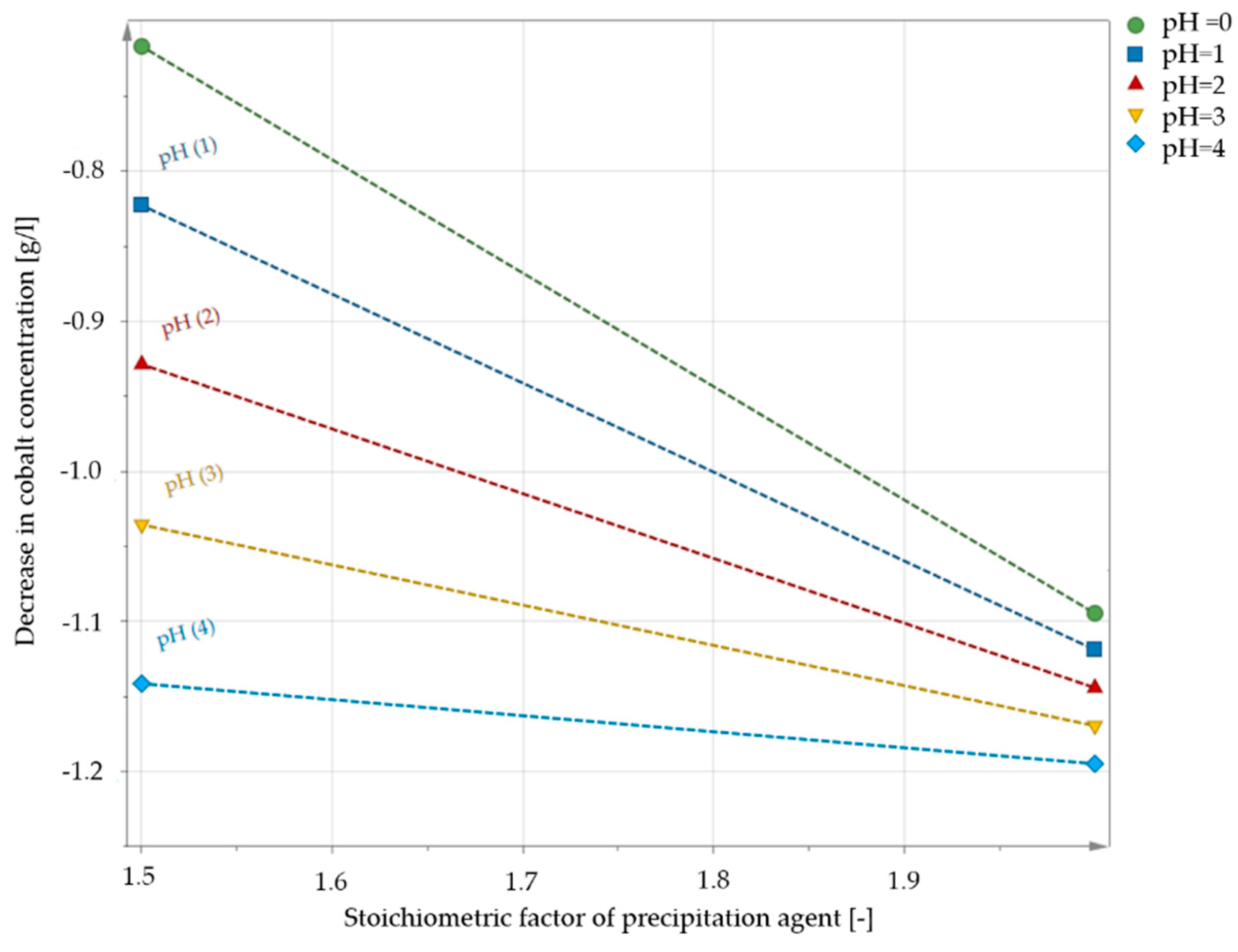
3.1. Influence of the Retention Time
3.2. Influence of the pH Value
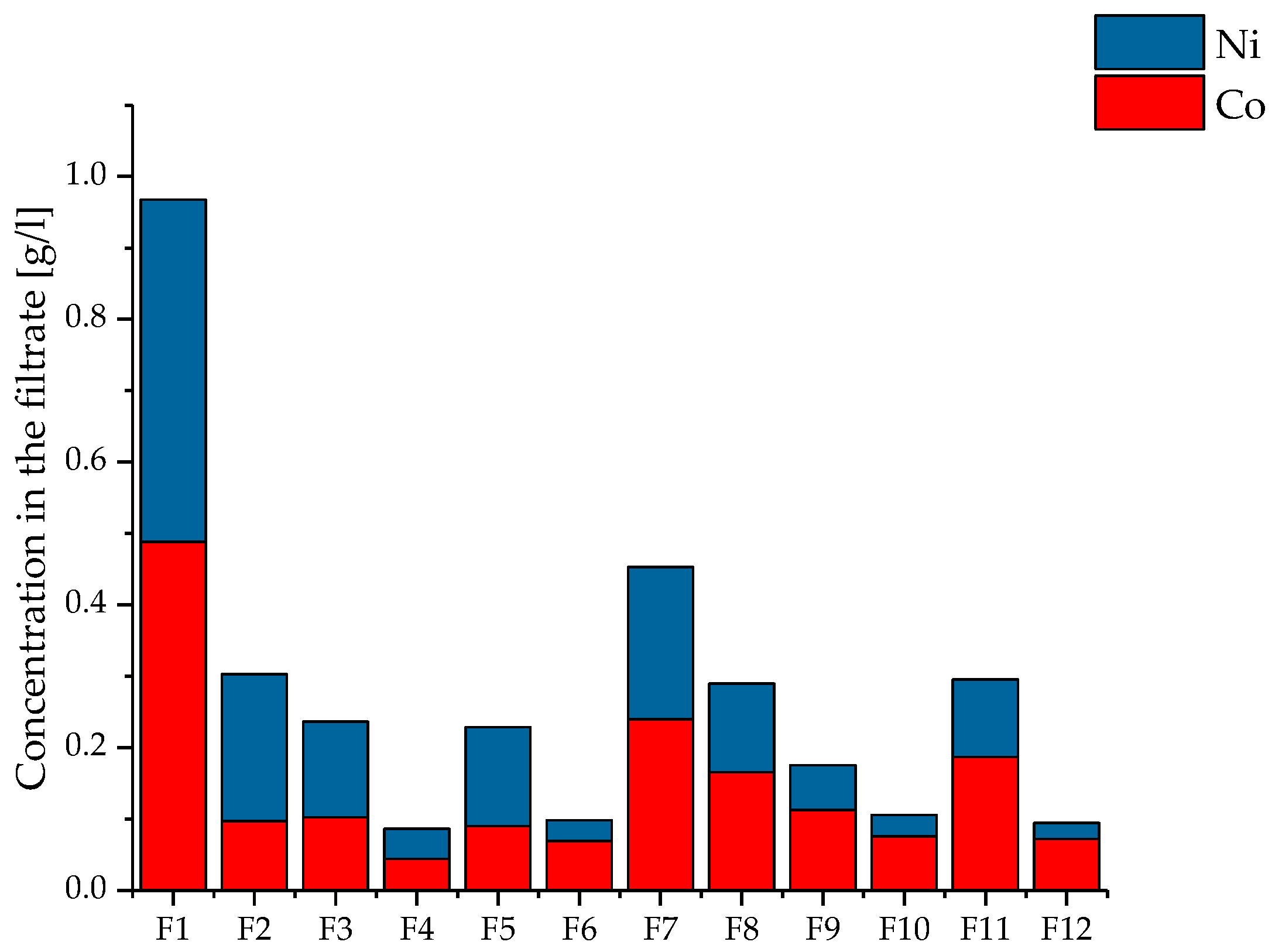
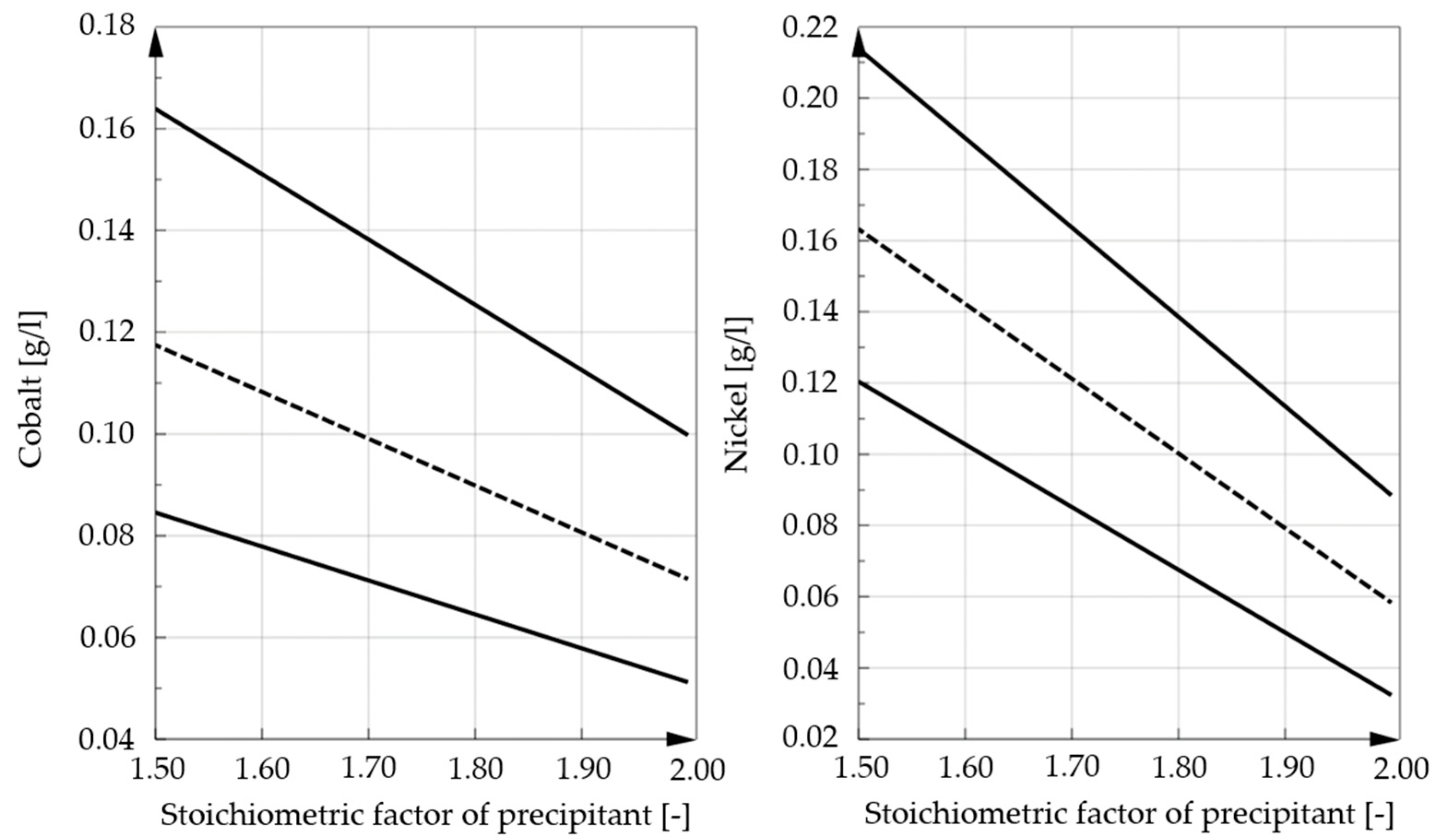
3.3. Influence of the Added Amount of Precipitant
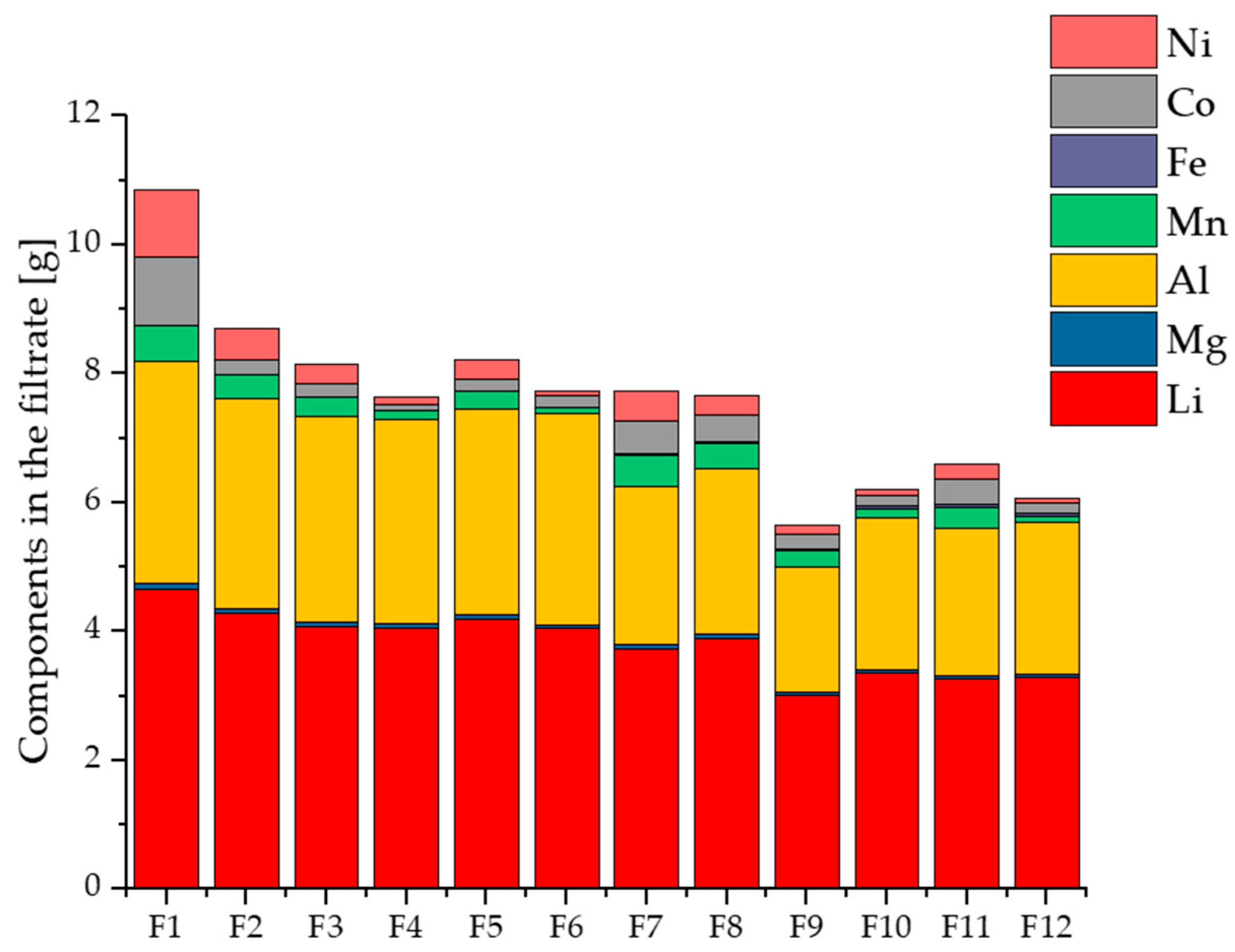
3.4. Composition of the Obtained Product
4. Discussion
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Zhou, T.; Kong, J.; Fang, H.; Chen, Y. Separation and recovery of metal values from leach liquor of waste lithium nickel cobalt manganese oxide based cathodes. Sep. Purif. Technol. 2015, 141, 76–83. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Dutra, A.J.B. Separation of nickel(II), cobalt (II) and lanthanides from spent Ni-MH batteries by hydrochloric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2013, 133, 37–43. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulphuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Sohn, J.; Chang, H.; Senanayake, G.; Shin, S.M. Preparation of cobalt oxide from concentrated material of spent lithium ion batteries by hydrometallurgical method. Adv. Powder Technol. 2010, 21, 175–179. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef]
- European Commission. Study on the Review of the List of Critical Raw Materials: Final Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Community Research and Development Information Service (CORDIS). Economic Importance and Supply Risk of Raw Materials in the European Union. Available online: https://cordis.europa.eu/docs/results/h2020/660/660885_PS/crit-metals.jpg (accessed on 27 October 2020).
- Arpe, H.-J.; Ullmann, F. (Eds.) Part A6-Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Wiley-VCH Verlag GmbH &. Co. KGaA: Weinheim, Germany, 1991; pp. 293–300. [Google Scholar]
- Arpe, H.-J.; Ullmann, F. (Eds.) Part A18-Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Wiley-VCH Verlag GmbH &. Co. KGaA: Weinheim, Germany, 1986; pp. 213–215. [Google Scholar]
- Rahimzei, E.; Sann, K.; Vogel, M.; VDE Verband der Elektrotechnik. Kompendium: Li-Ionen-Batterien. 2015. Available online: https://www.dke.de/resource/blob/933404/3d80f2d93602ef58c6e28ade9be093cf/kompendium-li-ionen-batterien-data.pdf (accessed on 27 October 2020).
- Takacova, Z.; Dzuro, V.; Havlik, T. Cobalt Precipitation from Leachate Originated from Leaching of Spent Li-ion Batteries Active Mass – Characterization of Inputs, Intermediates and Outputs. World Metall. 2017, 70, 336c343. [Google Scholar]
- Ferreira, D.A.; Prados, L.M.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 238–246. [Google Scholar] [CrossRef]
- Korthauer, R. (Ed.) Handbuch Lithium-Ionen-Batterien; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Rath, M.; Behera, L.P.; Dash, B.; Sheik, A.R.; Sanjay, K. Recovery of dimethylglyoxime (DMG) from Ni-DMG complexes. Hydrometallurgy 2018, 176, 229–234. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Godlewska, L.; Grohol, M. Study on the Review of the List of Critical Raw Materials: Critical Raw Materials Factsheets; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Luidold, S.; Honner, M.; Antrekowitsch, H. Leaching of metal-containing residues from the battery sector; Gerold, E. World Metall. 2019, 5, 267–273. [Google Scholar]
- Gerold, E.; Luidold, S.; Scheiber, S.; Honner, M. Synergy effect at the recycling of metal containing waste from the waste industry. Proc. Eur. Metall. Conf. EMC 2019, 2019, 973–982. [Google Scholar]
- Gerold, E.; Luidold, S.; Bartelme, C.; Antrekowitsch, H. Pyrometallurgische Aufarbeitung von Zwischenprodukten beim Batterierecycling. In Proceedings, Recycling und Sekundärrohstoffe, Berlin (2–3 March 2020); Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2020. [Google Scholar]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]




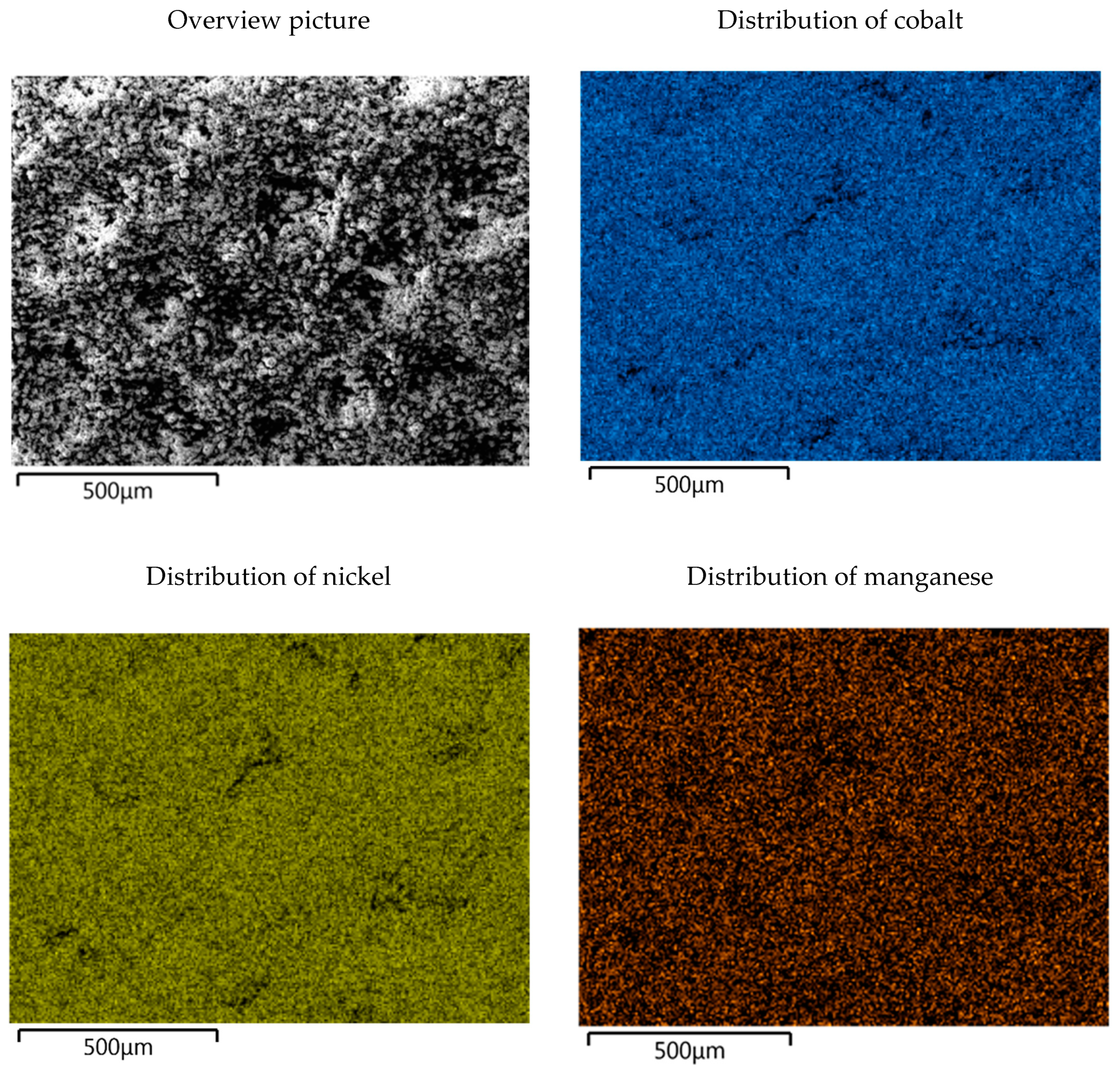

| Element | C | Al | Co | Fe | Li | Mg | Mn | Ni | Si | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| (wt%) | 38.7 | 3.4 | 15.0 | 0.4 | 4.0 | 0.1 | 0.8 | 17.0 | <1.0 | 3.7 |
| Experiment | Stoichiometric Addition of Oxalic Acid | pH | Retention Time (h) |
|---|---|---|---|
| F1 | 1.5× | 0 | 4 |
| F2 | 2× | 0 | 4 |
| F3 | 1.5× | 1 | 4 |
| F4 | 2× | 1 | 4 |
| F5 | 1.5× | 2 | 4 |
| F6 | 2× | 2 | 4 |
| F7 | 1.5× | 0 | 8 |
| F8 | 2× | 0 | 8 |
| F9 | 1.5× | 1 | 8 |
| F10 | 2× | 1 | 8 |
| F11 | 1.5 | 2 | 8 |
| F12 | 2 | 2 | 8 |
| Li (g/L) | Al (g/L) | Mn (g/L) | Fe (g/L) | Mg (g/L) | Co (g/L) | Ni (g/L) |
|---|---|---|---|---|---|---|
| 4.1 | 2.1 | 0.7 | 0.3 | 0.1 | 12.7 | 13.3 |
| No. | Oxalic Acid | pH | Retention Time (h) | Co (wt%) | Ni (wt%) | Cu (wt%) | Al (wt%) | Mn (wt%) | Fe (wt%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.5× | 0 | 4 | 45.7 | 50.5 | 0.0 | 1.3 | 1.0 | 1.5 |
| 2 | 2× | 0 | 4 | 45.2 | 51.2 | 0.0 | 1.1 | 1.5 | 1.1 |
| 3 | 1.5× | 1 | 4 | 46.2 | 50.5 | 0.0 | 1.0 | 1.4 | 0.9 |
| 4 | 2× | 1 | 4 | 46.0 | 50.3 | 0.0 | 0.9 | 1.8 | 1.0 |
| 5 | 1.5× | 2 | 4 | 46.8 | 49.4 | 0.0 | 1.1 | 1.4 | 1.3 |
| 6 | 2× | 2 | 4 | 46.7 | 50.5 | 0.0 | 0.8 | 2.1 | 0.0 |
| 7 | 1.5× | 0 | 8 | 45.1 | 51.7 | 0.0 | 1.2 | 1.2 | 0.8 |
| 8 | 2× | 0 | 8 | 44.8 | 51.4 | 0.0 | 1.2 | 1.6 | 1.0 |
| 9 | 1.5× | 1 | 8 | 46.9 | 49.6 | 0.0 | 1.0 | 1.4 | 1.1 |
| 10 | 2× | 1 | 8 | 46.3 | 50.7 | 0.0 | 1.0 | 2.1 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerold, E.; Luidold, S.; Antrekowitsch, H. Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals 2020, 10, 1435. https://doi.org/10.3390/met10111435
Gerold E, Luidold S, Antrekowitsch H. Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals. 2020; 10(11):1435. https://doi.org/10.3390/met10111435
Chicago/Turabian StyleGerold, Eva, Stefan Luidold, and Helmut Antrekowitsch. 2020. "Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions" Metals 10, no. 11: 1435. https://doi.org/10.3390/met10111435
APA StyleGerold, E., Luidold, S., & Antrekowitsch, H. (2020). Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals, 10(11), 1435. https://doi.org/10.3390/met10111435




