Joining of Metal to Ceramic Plate Using Super-Spread Wetting
Abstract
1. Introduction
2. Experimental Method
2.1. Metallizing
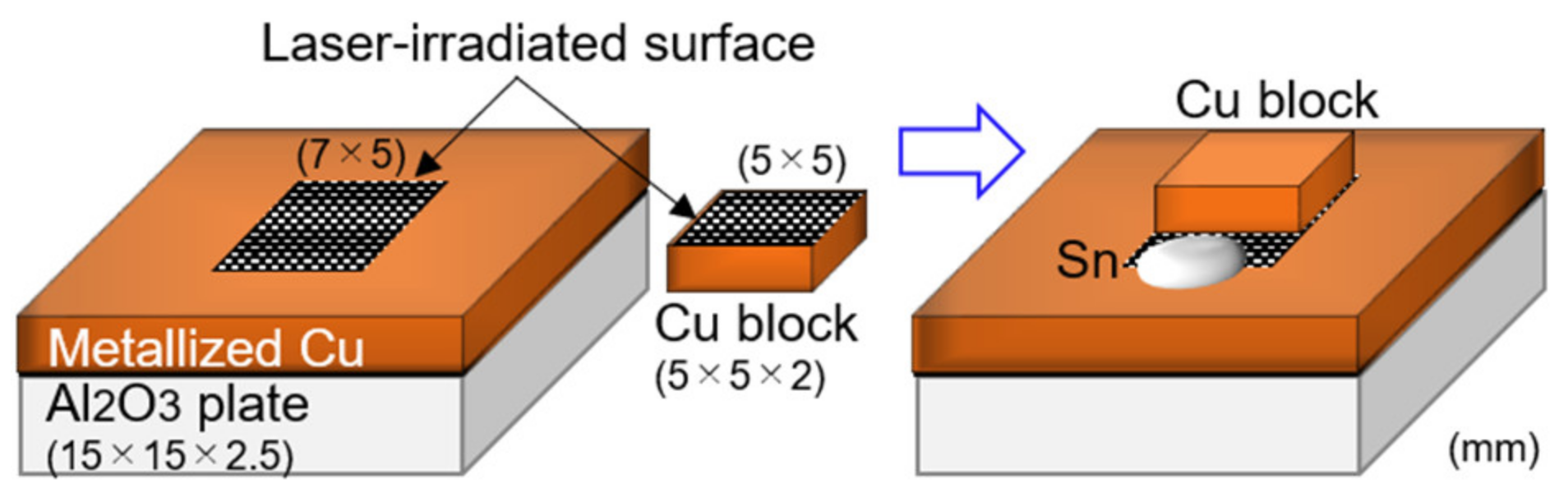
2.2. Joining
3. Results and Discussion
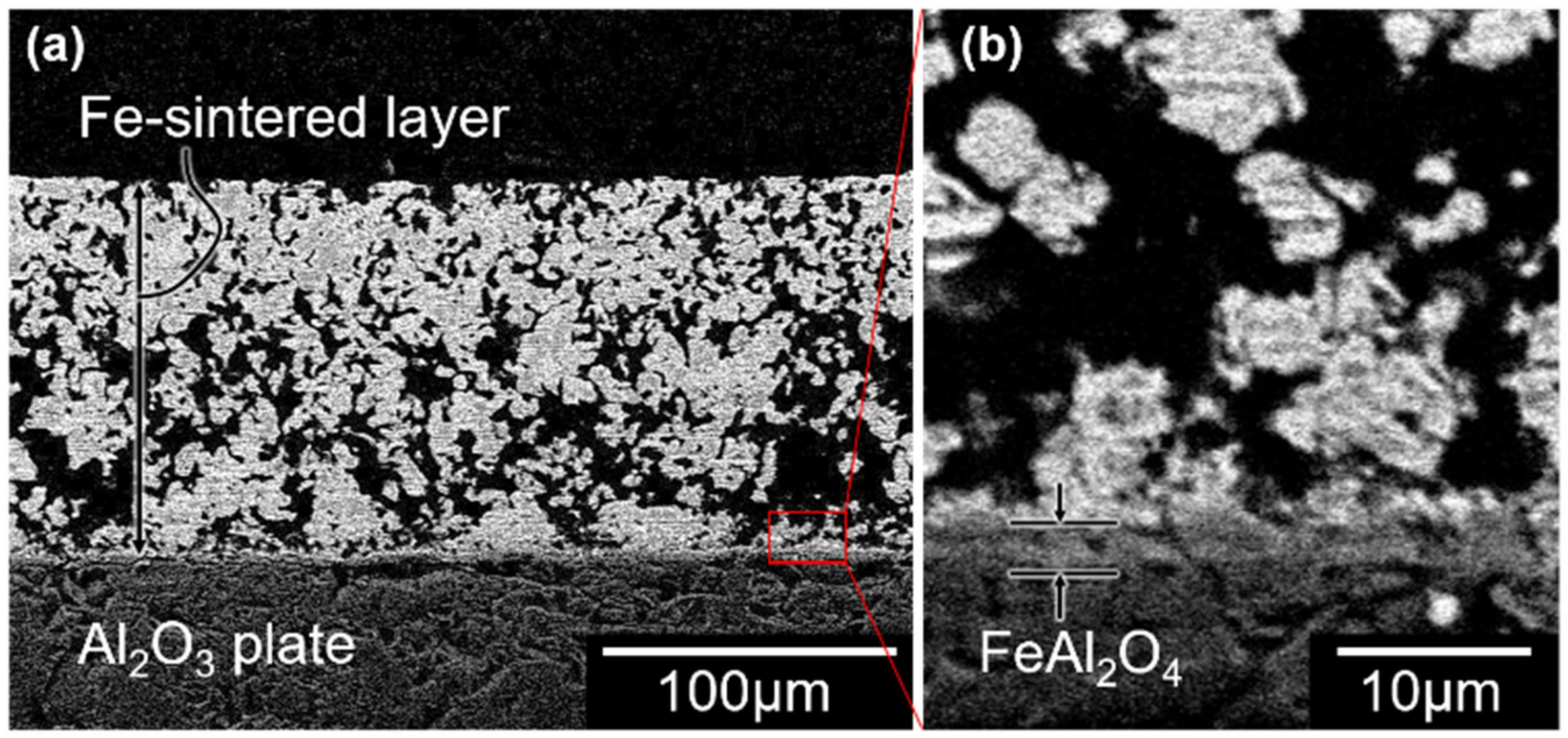
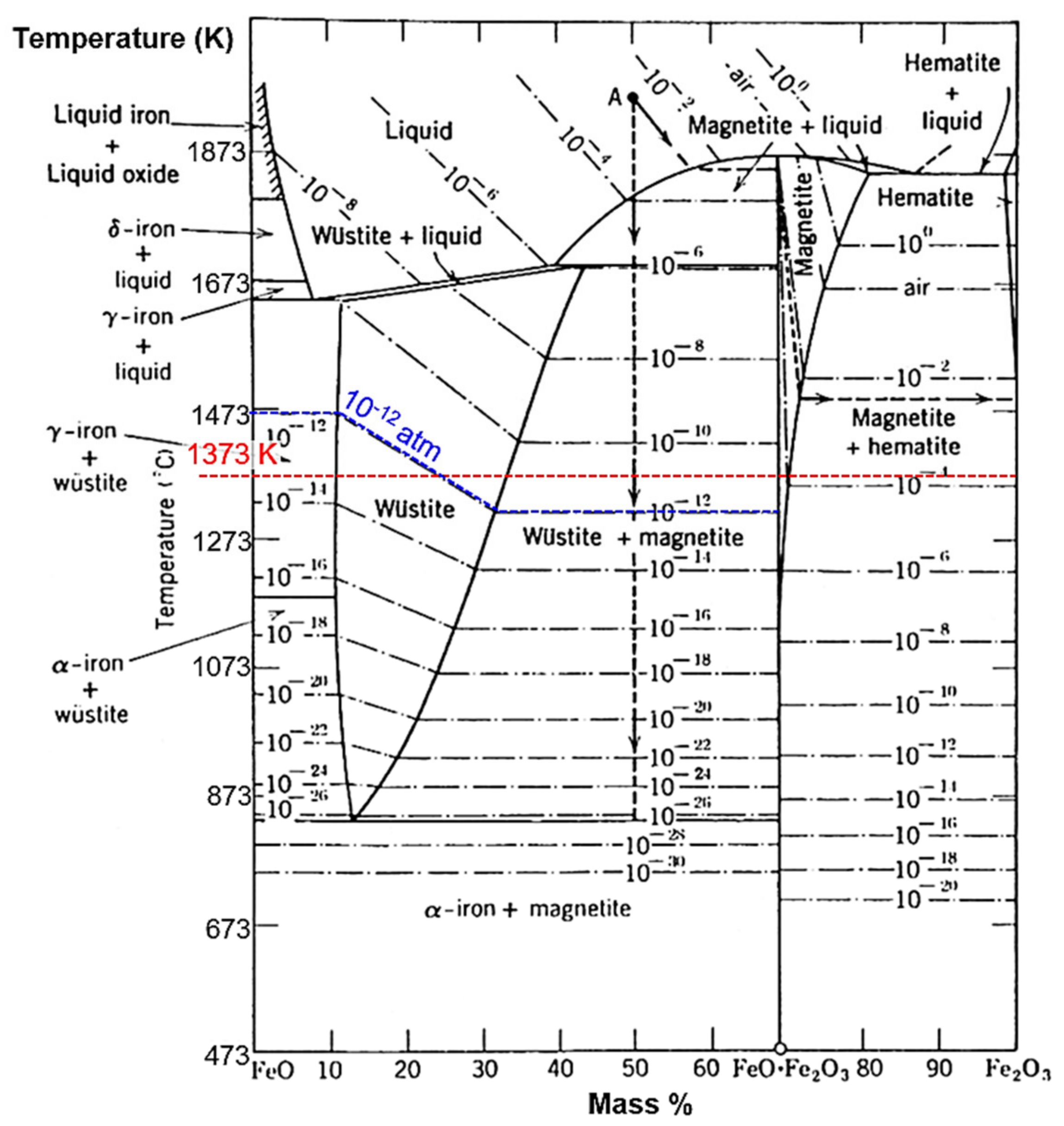
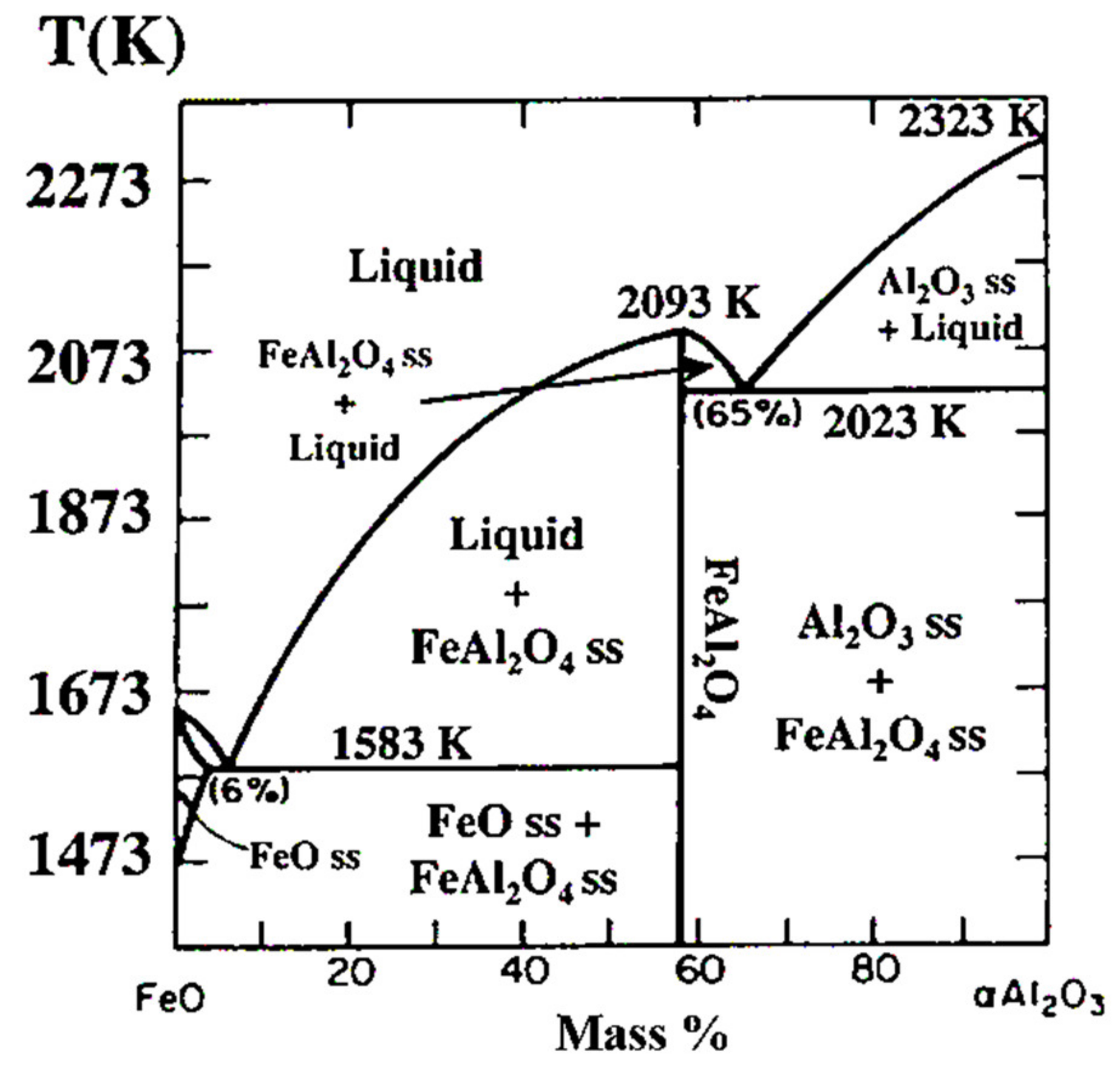
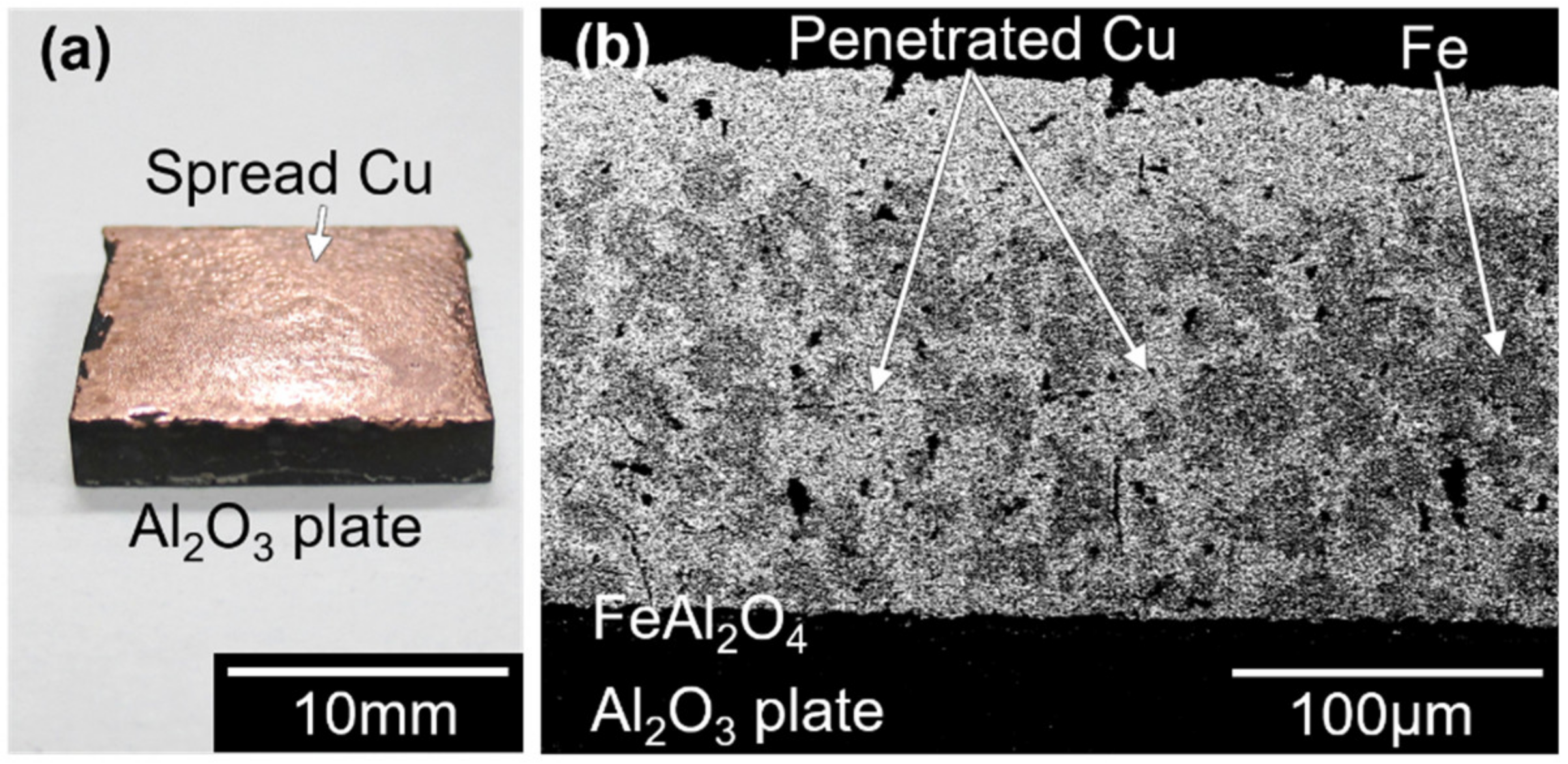
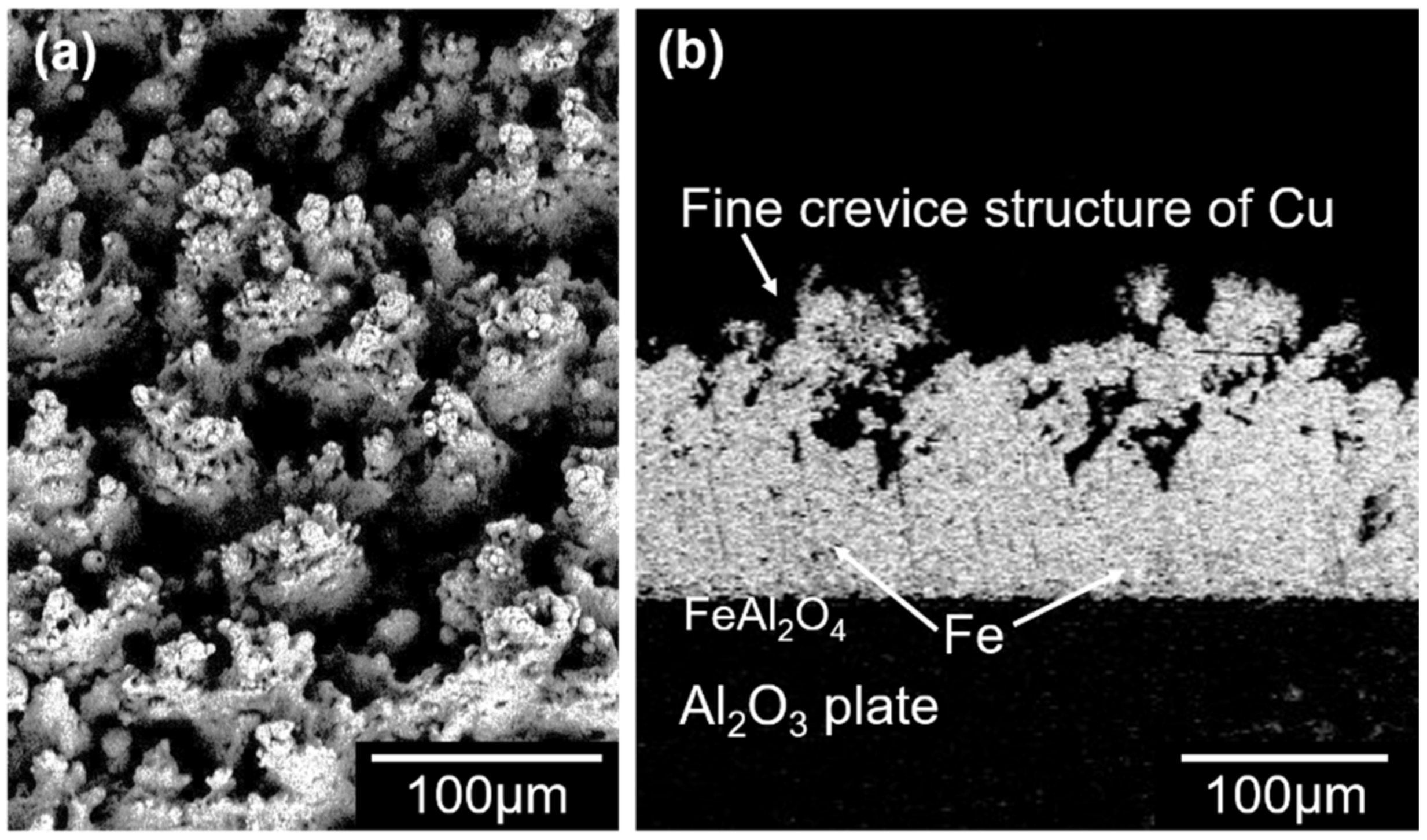
3.1. Copper Metallizing of Al2O3 Plate by Super-Spread Wetting into Powder-Based Metallic Iron Surface Fine Crevice Structure
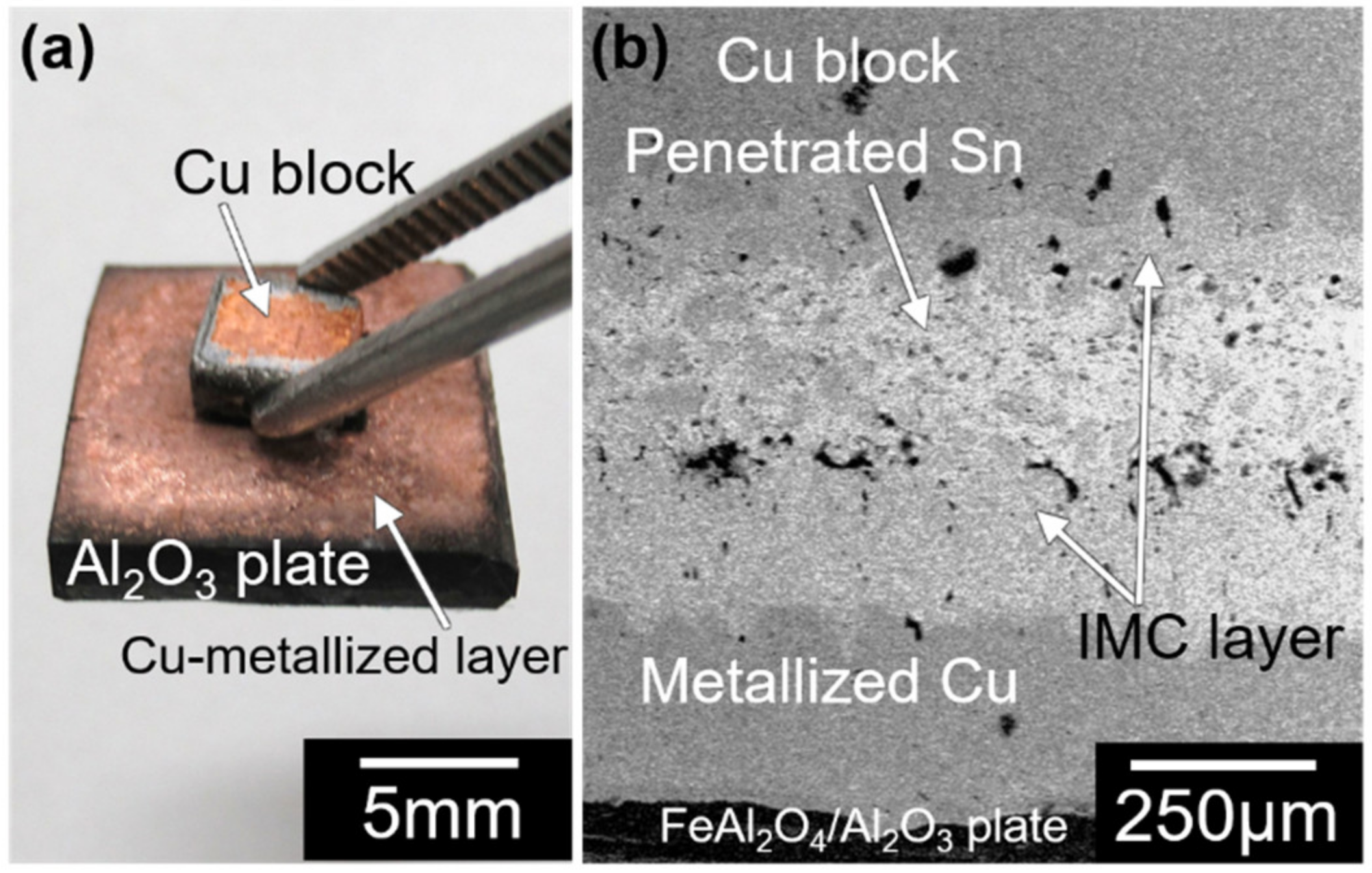
3.2. Joining of Copper Block onto Al2O3 Plate with Surface Fine Crevice Structure Created by Laser Irradiation
4. Conclusions
- (1)
- A powder-based surface fine crevice structure of metallic iron with a high porosity was created by sintering of Fe2O3 powder under reducing conditions. The sintered metallic iron layer bonded well to the surface of an Al2O3 plate due to the FeAl2O4 layer formed at the interface of the sintered metallic iron layer and Al2O3 plate during the heating process of the reduction of Fe2O3 to FeO.
- (2)
- Super-spread wetting of liquid copper occurred on the powder-based surface fine crevice structure of metallic iron, which achieved copper metalizing of the Al2O3 plate surface.
- (3)
- A laser-irradiated surface fine crevice structure was produced on the copper-metalized Al2O3 plate by laser irradiation. Joining of a copper block onto the copper-metalized Al2O3 plate was achieved using super-spread wetting of liquid tin through the structure.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, C.; Huang, C.; Chen, L.; Si, X.; Chen, Z.; Qi, J.; Huang, Y.; Feng, J.; Cao, J. Microstructure and mechanical properties of the SiC/Nb joint brazed using AgCuTi+B4C composite filler metal. Int. J. Refract. Met. Hard Mater. 2019, 85, 105049. [Google Scholar] [CrossRef]
- Way, M.; Willingham, J.; Goodall, R. Brazing filler metals. Int. Mater. Rev. 2020, 65, 257–285. [Google Scholar] [CrossRef]
- Hu, S.P.; Hu, T.Y.; Lei, Y.Z.; Song, X.G.; Liu, D.; Cao, J.; Tang, D.Y. Microstructural evolution and mechanical properties of vacuum brazed Ti2AlNb alloy and Ti60 alloy with Cu75Pt filler metal. Vacuum 2018, 152, 340–346. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, A.; Jung, D.H.; Jung, J.P. Recent Advances in Active Metal Brazing of Ceramics and Process. Met. Mater. Int. 2020, 26, 1087–1098. [Google Scholar] [CrossRef]
- Santuan, Z.; Xiangzhao, Z.; Guiwu, L.; Fabrizio, V.; MariaLuigia, M.; Guanjun, Q.; Alberto, P. Surface Metallization of SiC Ceramic by Mo-Ni-Si Coatings for Improving Its Wettability by Molten Ag. Rare Met. Mater. Eng. 2018, 47, 759–765. [Google Scholar] [CrossRef]
- Shen, Y.-L. Thermal expansion of metal-ceramic composites: A three-dimensional analysis. Mater. Sci. Eng. A 1998, 252, 269–275. [Google Scholar] [CrossRef]
- Kovalev, S.P.; Miranzo, P.; Osendi, M.I. Finite element simulation of thermal residual stresses in joining ceramics with thin metal interlayers. J. Am. Ceram. Soc. 1998, 81, 2342–2348. [Google Scholar] [CrossRef]
- Li, J.Q.; Xiao, P. Joining alumina using an alumina/metal composite. J. Eur. Ceram. Soc. 2002, 22, 1225–1233. [Google Scholar] [CrossRef]
- Asthana, R.; Singh, M. Joining of partially sintered alumina to alumina, titanium, Hastealloy and C-SiC composite using Ag-Cu brazes. J. Eur. Ceram. Soc. 2008, 28, 617–631. [Google Scholar] [CrossRef]
- Shin, J.; Sharma, A.; Jung, D.H.; Jung, J.P. Effect of Sn content on filler and bonding characteristics of active metal brazed Cu/Al2O3 joint. J. Korean Inst. Met. Mater. 2018, 56, 366–374. [Google Scholar] [CrossRef]
- Ghetta, V.; Chatain, D. Morphologies Adopted by Al2O3 Single-Crystal Surfaces in Contact with Cu Droplets. J. Am. Ceram. Soc. 2002, 85, 961–964. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Matsumoto, T.; Nogi, K. Influence of substrate crystallographic orientation on the wettability and adhesion of α-Al2O3 single crystals by liquid Al and Cu. J. Mater. Sci. 2005, 40, 2329–2333. [Google Scholar] [CrossRef]
- Takahira, N.; Tanaka, T.; Hara, S.; Lee, J. Unusual Wetting of Liquid Metals on Iron Substrate with Oxidized Surface in Reduced Atmosphere. Mater. Trans. 2005, 46, 3008–3014. [Google Scholar] [CrossRef]
- Takahira, N.; Yoshikawa, T.; Tanaka, T.; Holappa, L. Wettability of Liquid In and Bi on Flat and Porous Solid Iron Substrate. Mater. Trans. 2007, 48, 2708–2711. [Google Scholar] [CrossRef]
- Takahira, N.; Yoshikawa, T.; Tanaka, T.; Holappa, L. Unusual Wetting of Liquid Bismuth on a Surface-Porous Copper Substrate Fabricated by Oxidation-Reduction Process. Mater. Trans. 2007, 48, 3126–3131. [Google Scholar] [CrossRef]
- Fukuda, A.; Matsukawa, H.; Goto, H.; Suzuki, M.; Nakamoto, M.; Matsumoto, R.; Utsunomiya, H.; Tanaka, T. Metal–Metal Joining by Unusual Wetting on Surface Fine Crevice Structure Formed by Laser Treatment. Mater. Trans. 2015, 56, 1852–1856. [Google Scholar] [CrossRef]
- Nakamoto, M.; Fukuda, A.; Pinkham, J.; Vilakazi, S.; Goto, H.; Matsumoto, R.; Utsunomiya, H.; Tanaka, T. Joining of Copper Plates by Unusual Wetting with Liquid Tin and Tin-Lead Solder on “Surface Fine Crevice Structure”. Mater. Trans. 2016, 57, 973–977. [Google Scholar] [CrossRef]
- Fukuda, A.; Satake, Y.; Goto, H.; Suzuki, M.; Nakamoto, M.; Matsumoto, R.; Utsunomiya, H.; Tanaka, T. Wettability of Liquid Bi, In and Sn on Surface Fine Crevice Sructure of Laser-Irradiated Solid Iron Substrate. J. Smart Process. 2016, 5, 153–158. [Google Scholar] [CrossRef]
- Siboniso, V.; Yeon, J.; Grozescu, C.; Goto, H.; Nakamoto, M.; Matsumoto, R.; Utsunomiya, H.; Tanaka, T. Mechanism of the Unusual Wetting of a Surface Fine Crevice Structure Created by Laser Irradiation. Mater. Trans. 2017, 58, 1227–1230. [Google Scholar] [CrossRef]
- Yeon, J.; Kageyama, T.; Yamada, R.; Ni, P.; Nakamoto, M.; Tanaka, T. Dissimilar Metal Joining of Cu and Fe Using Super-Spread Wetting into Surface Fine Crevice Structures. Mater. Trans. 2020, MT-M2020120. [Google Scholar] [CrossRef]
- Yeon, J.; Ishida, Y.; Nakamoto, M.; Tanaka, T. Joining of Metals by Super-Spread Wetting on Surface Fine Crevice Structure Created by Reduction-Sintering Copper Oxide Powder. Mater. Trans. 2018, 59, 1192–1197. [Google Scholar] [CrossRef]
- Iida, T.; Guthrie, R.I.L. The Thermophysical Properties of Metallic Liquids. Volume 1, Fundamentals, 1st ed.; Oxford University Press: Oxford, UK, 2015; ISBN 9780198729839. [Google Scholar]
- Mei, J.; Halldearn, R.D.; Xiao, P. Mechanisms of the aluminium-iron oxide thermite reaction. Scr. Mater. 1999, 41, 541–548. [Google Scholar] [CrossRef]
- Maitre, A.; Denoirjean, A.; Fauchais, P.; Lefort, P. Plasma-jet coating of preoxidized XC38 steel: Influence of the nature of the oxide layer. Phys. Chem. Chem. Phys. 2002, 4, 3887–3893. [Google Scholar] [CrossRef]
- Fujimura, T.; Tanaka, S.-I. In-situ high temperature X-ray diffraction study of Fe/Al2O3 interface reactions. J. Mater. Sci. 1999, 34, 425–429. [Google Scholar] [CrossRef]
- Lukyanov, A.; Churakova, A.; Filatov, A.; Levin, E.; Valiev, R.; Gunderov, D.; Antipov, E. Microstructure refinement in Cu-Fe alloy using high pressure torsion. IOP Conf. Ser. Mater. Sci. Eng. 2014, 63. [Google Scholar] [CrossRef]
- Fields, R.J.; Low, S.R.; Lucey, G.K. Physical and Mechanical Properties of intermetallic compounds Commonly Found in Solder Joints. In Metal Science of Joining. In Proceedings of the TMS Symposium, Cincinnati, OH, USA, 20–24 October 1991. [Google Scholar]
- Sales, B.C.; Yan, J.; Meier, W.R.; Christianson, A.D.; Okamoto, S.; McGuire, M.A. Electronic, magnetic, and thermodynamic properties of the kagome layer compound FeSn. Phys. Rev. Mater. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Snipes, E.K.; Flowers, G.T.; Bozack, M.J. Influence of Systematic Coefficient of Thermal Expansion (CTE) Variations on Sn Whiskering. In Proceedings of the 2016 IEEE 62nd Holm Conference on Electrical Contacts (Holm), Clearwater Beach, FL, USA, 9–12 October 2016; pp. 205–208. [Google Scholar]
- Yunus, M.; Srihari, K.; Pitarresi, J.M.; Primavera, A. Effect of voids on the reliability of BGA/CSP solder joints. Microelectron. Reliab. 2003, 43, 2077–2086. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeon, J.; Yamamoto, M.; Ni, P.; Nakamoto, M.; Tanaka, T. Joining of Metal to Ceramic Plate Using Super-Spread Wetting. Metals 2020, 10, 1377. https://doi.org/10.3390/met10101377
Yeon J, Yamamoto M, Ni P, Nakamoto M, Tanaka T. Joining of Metal to Ceramic Plate Using Super-Spread Wetting. Metals. 2020; 10(10):1377. https://doi.org/10.3390/met10101377
Chicago/Turabian StyleYeon, Jaebong, Michiru Yamamoto, Peiyuan Ni, Masashi Nakamoto, and Toshihiro Tanaka. 2020. "Joining of Metal to Ceramic Plate Using Super-Spread Wetting" Metals 10, no. 10: 1377. https://doi.org/10.3390/met10101377
APA StyleYeon, J., Yamamoto, M., Ni, P., Nakamoto, M., & Tanaka, T. (2020). Joining of Metal to Ceramic Plate Using Super-Spread Wetting. Metals, 10(10), 1377. https://doi.org/10.3390/met10101377




