Initial Corrosion Behavior of 12Cr1MoV Steel in Thiosulfate-Containing Sodium Aluminate Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solution
2.2. Corrosion Experiments
2.3. Analysis of Corrosion Products
2.4. Electrochemical Tests
3. Results and Discussion
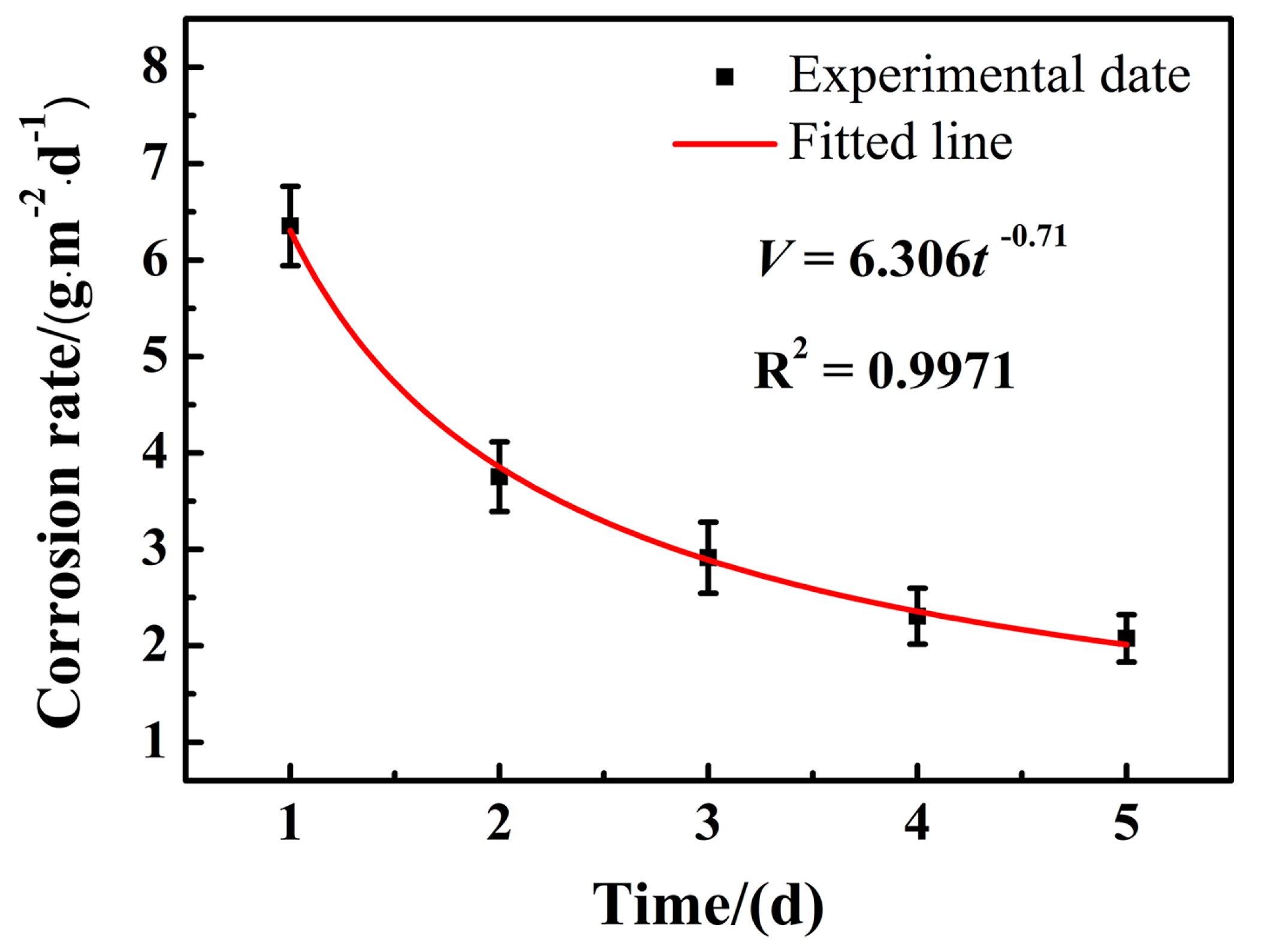
3.1. Corrosion Rate Analysis
3.2. SEM-EDS Analysis
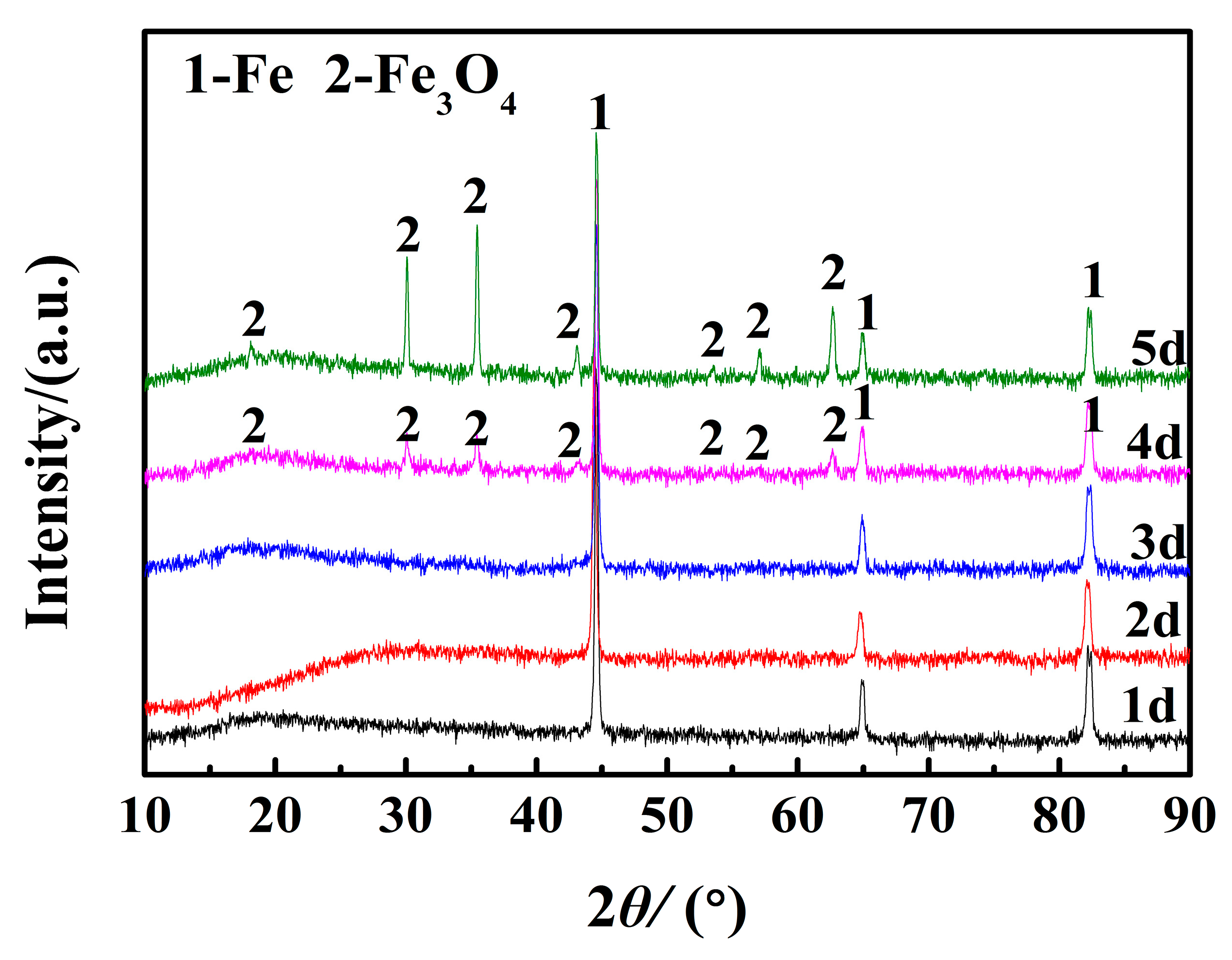
3.3. Analysis of the Phase and Chemical Composition of the Corrosion Products
3.4. Analysis of the Polarization Curves
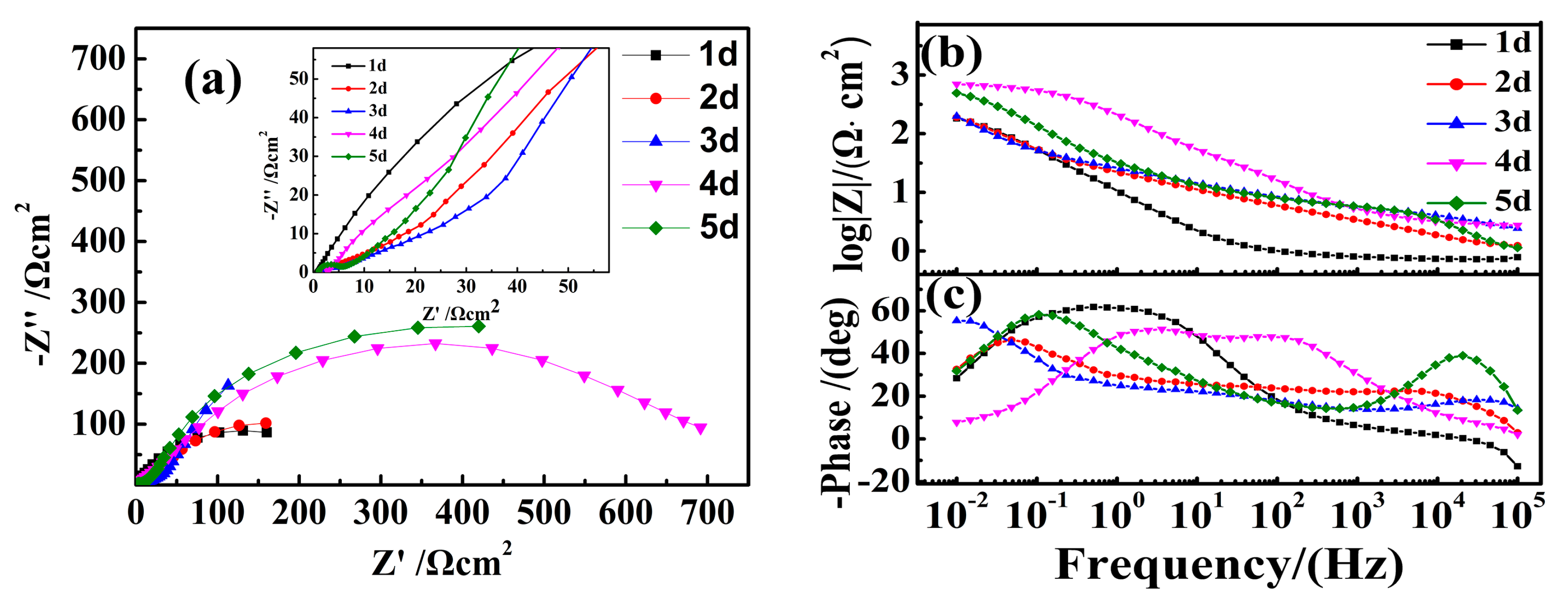
3.5. EIS Analysis
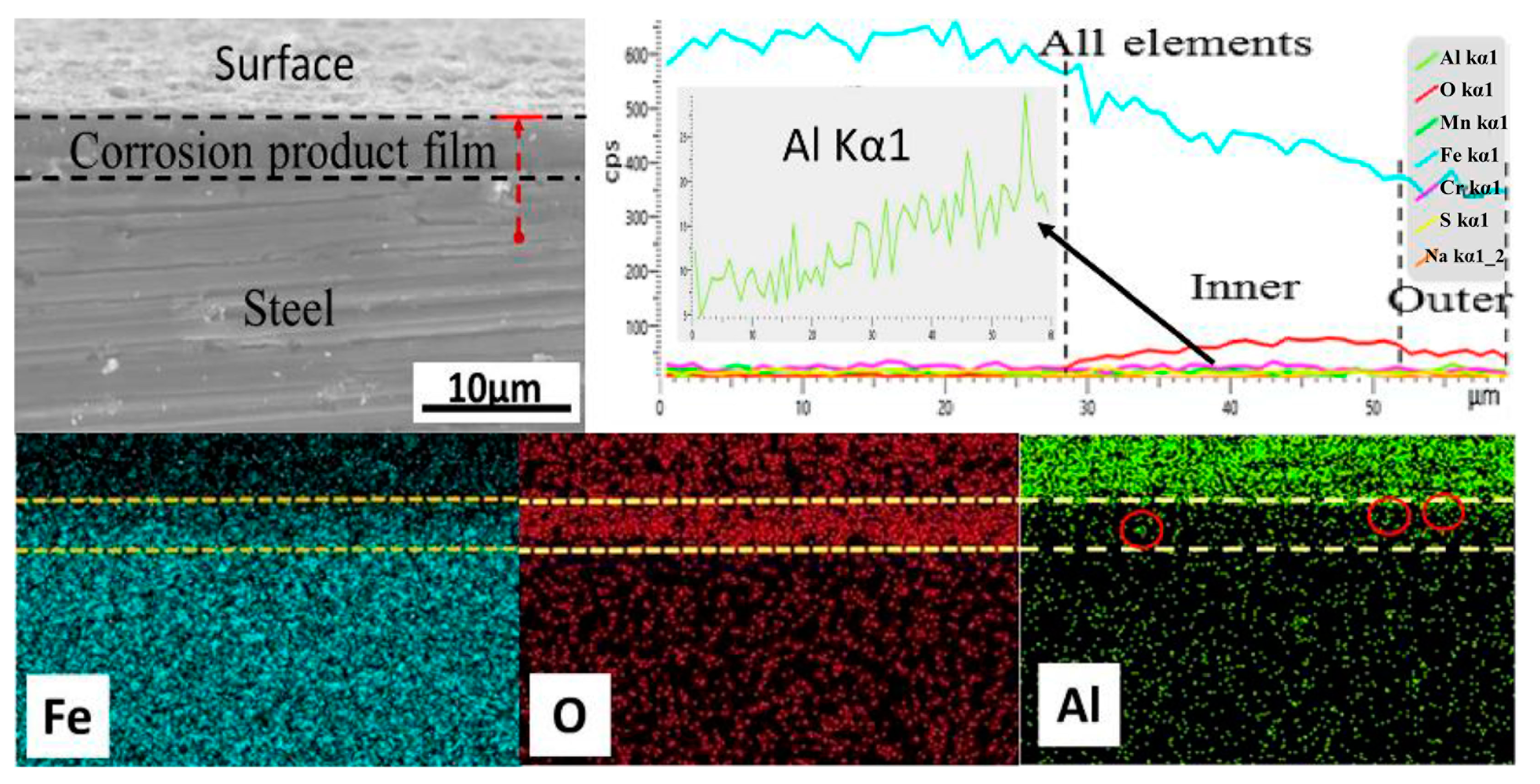
3.6. Analysis of the Cross-Section of the Corrosion Product Film
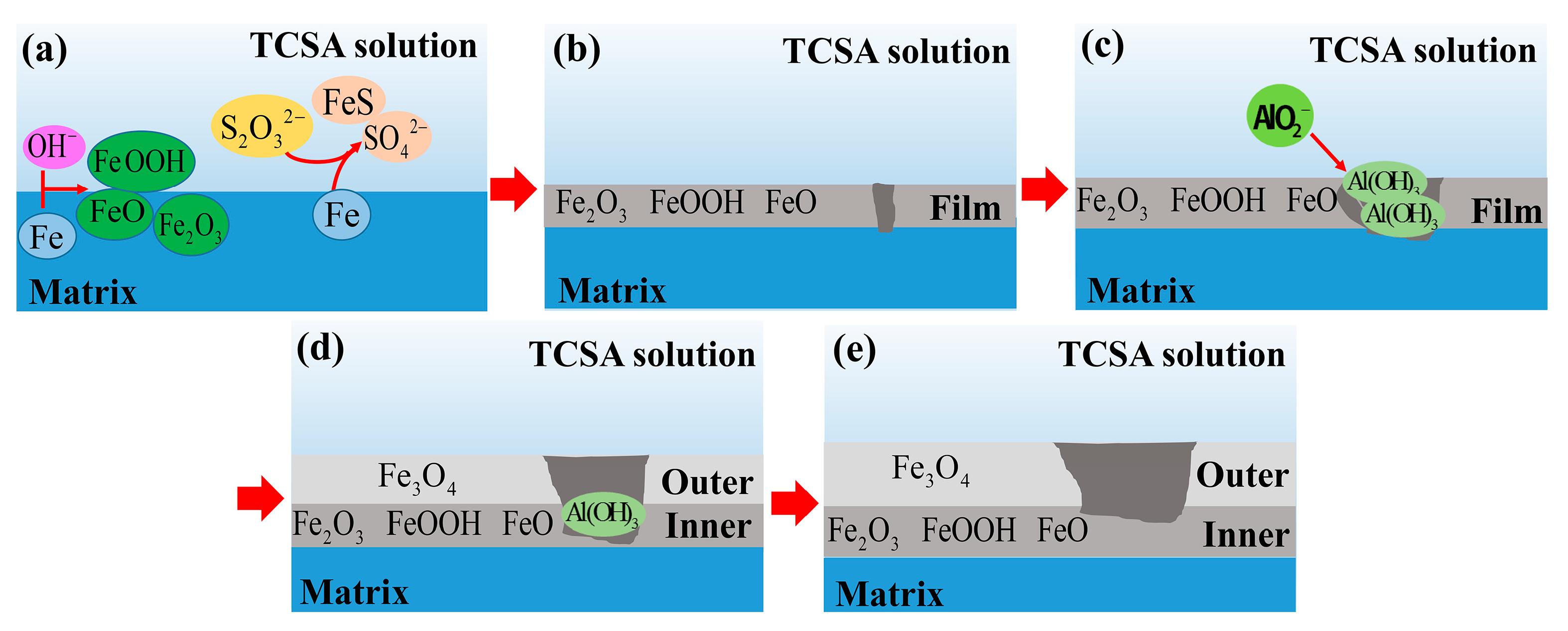
3.7. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.B.; Wang, Y.L.; Zhou, Q.S.; Qi, T.G.; Liu, G.H.; Peng, Z.H.; Wang, H.Y. Reaction Behaviors of Iron and Hematite in Sodium Aluminate Solution at Elevated Temperature. Hydrometallurgy 2018, 175, 257–265. [Google Scholar] [CrossRef]
- Li, X.B.; Li, C.Y.; Peng, Z.H.; Liu, G.H.; Zhou, Q.S.; Qi, T.G. Interaction of Sulfur with Iron Compounds in Sodium Aluminate Solutions. Trans. Nonferr. Metal. Soc. 2015, 25, 608–614. [Google Scholar] [CrossRef]
- Liu, Z.W.; Yan, H.W.; Ma, W.H.; Xie, K.Q.; Xu, B.Q.; Zheng, L.C. Digestion behavior and removal of sulfur in high-sulfur bauxite during bayer process. Miner. Eng. 2020, 149, 106237. [Google Scholar] [CrossRef]
- Li, X.B.; Niu, F.; Tan, J.; Liu, G.H.; Qi, T.G.; Peng, Z.H.; Zhou, Q.S. Removal of S2− Ion From Sodium Aluminate Solutions with Sodium Ferrite. Trans. Nonferr. Metal. Soc. 2016, 26, 1419–1424. [Google Scholar] [CrossRef]
- Yuan, J.J.; Chen, C.; Li, J.; Quan, B.; Wang, L.; Lan, Y.; Du, X.; Fu, H. Growth of Corrosion Product Film on 12Cr1MoV Steel in Sulfur-Containing Sodium Aluminate Solution. Mater. Express 2019, 9, 914–922. [Google Scholar] [CrossRef]
- Lou, Z.N.; Xiong, Y.; Feng, X.D.; Shan, W.J.; Zhai, Y.C. Study on the Roasting and Leaching Behavior of High-Sulfur Bauxite Using Ammonium Bisulfate. Hydrometallurgy 2016, 165, 306–311. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yin, J.G.; Chen, Y.L.; Xia, W.T.; Xiang, X.Y.; Yuan, X.L. Simultaneous Removal of Sulfur and Iron by the Seed Precipitation of Digestion Solution for High-Sulfur Bauxite. Hydrometallurgy 2018, 181, 7–15. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Effects of Thiosulphates and Sulphite Ions on Steel Corrosion. Corros. Sci. 2018, 135, 158–166. [Google Scholar] [CrossRef]
- Gomez-Duran, M.; Macdonald, D.D. Stress Corrosion Cracking of Sensitized Type 304 Stainless Steel in Thiosulfate Solution: I. Fate of the Coupling Current. Corros. Sci. 2003, 45, 1455–1471. [Google Scholar] [CrossRef]
- Wensley, D.A.; Charlton, R.S. Corrosion Studies in Kraft White Liquor Potentiostatic Polarization of Mild Steel in Caustic Solutions Containing Sulfur Species. Corrosion 1980, 36, 385–389. [Google Scholar] [CrossRef]
- Park, J.O.; Verhoff, M.; Alkire, R. Microscopic Behavior of Single Corrosion Pits: The Effect of Thiosulfate on Corrosion of Stainless Steel in NaCl. Electrochim. Acta 1997, 42, 3281–3291. [Google Scholar] [CrossRef]
- Kuo, H.S.; Chang, H.; Tsaia, W.T. The Corrosion Behavior of AISI 209 Stainless Steel in Thiosulfate Ion Containing Saturated Ammonium Chloride Solution. Corros. Sci. 1999, 41, 669–684. [Google Scholar] [CrossRef]
- Laitinen, T. Localized Corrosion of Stainless Steel in Chloride, Sulfate and Thiosulfate Containing Environments. Corros. Sci. 2000, 42, 421–441. [Google Scholar] [CrossRef]
- Xie, Q.L.; Chen, W.M. Corrosion Behavior of 16Mn Low Alloy Steel in Sulfide-Containing Bayer Solutions. Corros. Sci. 2014, 86, 252–260. [Google Scholar] [CrossRef]
- Fu, H.; Chen, C.; Li, J.; Lan, Y.; Wang, L.; Yuan, J. Influence of Na2S On the Corrosion Behavior of Q345 Steel in Sodium Aluminate Solution. Mater. Res. Express 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Quan, B.L.; Li, J.Q.; Chen, C.Y. Synergy Corrosion Effect of Thiosulfate and Sulfide on Q235 Steel in Sodium Aluminate Solution. Mater. Res. Express 2019, 6, 025607. [Google Scholar]
- Zhang, D.Z.; Gao, X.H.; Du, L.X.; Wang, H.X.; Liu, Z.G.; Yang, N.N.; Misra, R.D.K. Corrosion Behavior of High-Strength Steel for Flexible Riser Exposed to CO2-Saturated Saline Solution and CO2-Saturated Vapor Environments. Acta Metall. Sin-Engl. 2019, 32, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.Y.; Wang, L.W.; Ni, H.T.; Hao, W.K.; Man, C.; Chen, S.S.; Wang, X.; Liu, Z.Y.; Li, X.G. Influence of Temperature on the Electrochemical and Passivation Behavior of 2507 Super Duplex Stainless Steel in Simulated Desulfurized Flue Gas Condensates. Corros. Sci. 2017, 118, 31–48. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Brown, J.R.; Bensebaa, F.; Kingston, D. XPS Depth Profiling Study on the Passive Oxide Film of Carbon Steel in Saturated Calcium Hydroxide Solution and the Effect of Chloride on the Film Properties. Appl. Surf. Sci. 2011, 257, 4669–4677. [Google Scholar] [CrossRef]
- Lv, M.Y.; Du, M.; Li, X.; Yue, Y.Y.; Chen, X.C. Mechanism of Microbiologically Influenced Corrosion of X65 Steel in Seawater Containing Sulfate-Reducing Bacteria and Iron-Oxidizing Bacteria. J. Mater. Res. Technol. 2019, 8, 4066–4078. [Google Scholar] [CrossRef]
- Baranwal, P.K.; Rajaraman, P.V. Kinetics of Carbon Steel Dissolution in Ammonium Chloride Solution Containing Sodium Thiosulfate. Int. J. Chem. Kinet. 2019, 51, 497–510. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, H.; Choi, J. Non-Nickel-Based Sealing of Anodic Porous Aluminum Oxide in NaAlO2. Surf. Coat. Tech. 2017, 310, 106–112. [Google Scholar] [CrossRef]
- Krawiec, H.; Stypuła, B.; Stoch, J.; Mikołajczyk, M. Corrosion Behaviour and Structure of the Surface Layer Formed on Austempered Ductile Iron in Concentrated Sulphuric Acid. Corros. Sci. 2006, 48, 595–607. [Google Scholar] [CrossRef]
- Xia, D.H.; Sun, Y.F.; Shen, C.; Chen, X.Y.; Fan, H.Q.; Luo, J.L. A Mechanistic Study on Sulfur-Induced Passivity Degradation on Alloy 800 in Simulated Alkaline Crevice Chemistries at Temperatures Ranging From 21 °C to 300 °C. Corros. Sci. 2015, 100, 504–516. [Google Scholar] [CrossRef]
- Ye, Y.W.; Liu, Z.Y.; Liu, W.; Zhang, D.W.; Zhao, H.C.; Wang, L.P.; Li, X.G. Superhydrophobic Oligoaniline-Containing Electroactive Silica Coating as Pre-Process Coating for Corrosion Protection of Carbon Steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Trdan, U.; Grum, J. Evaluation of Corrosion Resistance of AA6082-T651 Aluminium Alloy After Laser Shock Peening by Means of Cyclic Polarisation and ElS Methods. Corros. Sci. 2012, 59, 324–333. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Zhang, L.; Hu, J.; Zhang, Z.; Lu, M. Effect of pH on the Electrochemical Behaviour and Passive Film Composition of 316L Stainless Steel. Acta Metall. Sin-Engl. 2019, 32, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Liu, Z.Y.; Li, C.T.; Li, X.G. The Mechanism of Stress Corrosion Cracking of Alloy 690TT in a Caustic Solution Containing Lead. Corros. Sci. 2017, 128, 154–163. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.Y.; Lutz, A.; Zhang, F.; Zhao, T.L.; Ma, H.C.; Li, X.G.; Terryn, H. Role of Al2O3 Inclusions on the Localized Corrosion of Q460NH Weathering Steel in Marine Environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Gu, T.Y.; Jia, R.; Unsal, T.; Xu, D.K. Toward a Better Understanding of Microbiologically Influenced Corrosion Caused by Sulfate Reducing Bacteria. J. Mater. Res. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Hwang, E.H.; Park, J.S.; Seong, H.G.; Kim, S.J. Analysis on surface film formed on high-strength carbon steels in acidic phosphate solution and its relationship with localized corrosion in a 3.5% NaCl solution. J. Mater. Res. Technol. 2019, 8, 1419–1426. [Google Scholar] [CrossRef]
- Duret-Thual, C.; Costa, D.; Yang, W.P.; Marcus, P. The role of thiosulfates in the pitting corrosion of Fe-17Cr alloys in neutral chloride solution: Electrochemical and XPS study. Corros. Sci. 1997, 39, 913–933. [Google Scholar] [CrossRef]
- Sipos, P. The structure of Al(III) in strongly alkaline aluminate solutions—A review. J. Mol. Liq. 2009, 146, 1–14. [Google Scholar] [CrossRef]
- Skoufadis, C.; Panias, D.; Paspaliaris, I. Kinetics of boehmite precipitation from supersaturated sodium aluminate solutions. Hydrometallurgy 2003, 68, 57–68. [Google Scholar] [CrossRef]




| C | Cr | Mo | V | Si | Mn | Fe |
|---|---|---|---|---|---|---|
| 0.14 | 0.98 | 0.22 | 0.18 | 0.23 | 0.61 | Balance |
| Time/d | O | S | Cr | Mn | Al | Na | Fe |
|---|---|---|---|---|---|---|---|
| 1 | 6.59 | - | 0.62 | 0.28 | 0.15 | 0.32 | Balance |
| 2 | 4.26 | - | 0.75 | 0.46 | 0.35 | 0.26 | Balance |
| 3 | 6.37 | - | 0.74 | 0.42 | 1.15 | 0.01 | Balance |
| 4 | 27.22 | 0.15 | 1.06 | 0.77 | 1.06 | 0.43 | Balance |
| 5 | 26.80 | 0.18 | 0.83 | 0.62 | 0.37 | 0.25 | Balance |
| Time (d) | Ecorr (mV) | Jcorr (µA·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) |
|---|---|---|---|---|
| 1 | −1208.46 | 265.6 | 55.8 | 83.8 |
| 2 | −1205.59 | 226.392 | 63.2 | 86.3 |
| 3 | −1222.86 | 132.344 | 77.7 | 77.4 |
| 4 | −1267.89 | 49.328 | 252.7 | 19.4 |
| 5 | −1271.53 | 41.192 | 308.8 | 141.8 |
| Time (d) | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (S cm−2 sn) | ndl | Rf (Ω·cm2) | Qf (S cm−2 sn) | nf | X2 (10−4) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.8 | 154 | 0.001 | 0.74 | 122 | 0.024 | 0.74 | 7.54 |
| 2 | 1.0 | 179 | 0.031 | 0.86 | 229 | 0.048 | 0.84 | 6.81 |
| 3 | 1.2 | 491 | 0.026 | 0.74 | 550 | 0.082 | 0.93 | 7.96 |
| 4 | 0.7 | 593 | 0.003 | 0.97 | 690 | 0.002 | 0.59 | 6.35 |
| 5 | 0.8 | 643 | 0.0001 | 0.69 | 1281 | 0.010 | 0.66 | 5.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Chen, C.; Li, J.; Quan, B.; Lan, Y.; Wang, L.; Fu, H.; Gai, J. Initial Corrosion Behavior of 12Cr1MoV Steel in Thiosulfate-Containing Sodium Aluminate Solution. Metals 2020, 10, 1283. https://doi.org/10.3390/met10101283
Yuan J, Chen C, Li J, Quan B, Lan Y, Wang L, Fu H, Gai J. Initial Corrosion Behavior of 12Cr1MoV Steel in Thiosulfate-Containing Sodium Aluminate Solution. Metals. 2020; 10(10):1283. https://doi.org/10.3390/met10101283
Chicago/Turabian StyleYuan, Jingjiu, Chaoyi Chen, Junqi Li, Bianli Quan, Yuanpei Lan, Linzhu Wang, Hui Fu, and Jiaxuan Gai. 2020. "Initial Corrosion Behavior of 12Cr1MoV Steel in Thiosulfate-Containing Sodium Aluminate Solution" Metals 10, no. 10: 1283. https://doi.org/10.3390/met10101283




