Electrochemical Corrosion of SAC Alloys: A Review
Abstract
:1. Introduction
2. Techniques to Study Electrochemical Corrosion (ECC)
2.1. Linear Sweep Voltammetry (LSV)
2.2. Electrochemical Impedance Spectroscopy (EIS)
3. Main Corrosion Mechanism of SAC Alloys in Different Environments
3.1. Corrosion Studies of Pure SAC Alloys
3.2. Corrosion Studies Related to the Alloying Elements on SAC Alloys
3.3. Corrosion Studies of SAC Alloys Compared to Other Alloys
3.4. Corrosion Studies of Micro-Alloyed SAC Alloys
3.5. Corrosion Studies of Composite SAC Solder Alloys
3.6. Recently Developed Permanent Magnet Stirring
3.7. Temperature and Cooling Rate Effect on the Corrosion SAC Alloys
4. Effect of Fluxes
5. Conclusions
- LSV is the most frequently applied technique to determine the corrosion parameters. Simple evaluation is possible; for example, the more noble values of Ecorr can indicate a better corrosion behaviour and icorr is a reliable parameter to evaluate the corrosion rate. Although this technique is widely used, it is not recommended for evaluating long-term corrosion performance.
- EIS is the most reliable technique; it evaluates in more detail the resistance of the passive film and the charge transfer resistance at the passive film/metal interface, and it also provides information about the electrolyte layer formation as well.
- Both techniques are also useful for pitting corrosion detection, which is one of the typical types of the solder alloy corrosion processes.
- Within the group of SAC alloys, it is important to study the variation of Ag and Cu content, as these elements are showing potential corrosion accelerating effects.
- Composite solders have great potential to enhance the corrosion performance of SAC alloys. However, this field is not deeply addressed in the literature.
- Slow cooling rates show a significant effect on the grain size, which is directly affecting the corrosion resistance of SAC alloys. The corrosion resistance of solders is expected to decrease when operating under high temperatures and high humidity environments.
- The structure-based corrosion resistance of SAC alloys can be further enhanced by technological improvements, such as the application of PMS during the solidification process.
- Flux residues may impose a high risk of the corrosion even in the presence of no-clean flux, and the risk becomes very high in case of hand soldering applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotadia, H.R.; Howes, P.D.; Mannan, S. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Pecht, M.; Shibutani, T.; Wu, L. A reliability assessment guide for the transition planning to lead-free electronics for companies whose products are RoHS exempted or excluded. Microelectron. Reliab. 2016, 62, 113–123. [Google Scholar] [CrossRef]
- Tao, Q.; Benabou, L.; Le, V.; Hwang, H.; Luu, D. Viscoplastic characterization and post-rupture microanalysis of a novel lead-free solder with small additions of Bi, Sb and Ni. J. Alloys Compd. 2017, 694, 892–904. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Lin, K. Electrochemical corrosion behaviour of lead-free Sn-8.5Zn-XAg-0.1Al-0.5Ga solder in 3.5% NaCl solution. Mater. Sci. Eng. A 2005, 406, 34–42. [Google Scholar] [CrossRef]
- Xiong, M.; Zhang, L. Interface reaction and intermetallic compound growth behavior of Sn-Ag-Cu lead-free solder joints on different substrates in electronic packaging. J. Mater. Sci. 2018, 54, 1741–1768. [Google Scholar] [CrossRef]
- Chen, B.; Li, G. An investigation of effects of Sb on the intermetallic formation in Sn-3.5Ag-0.7Cu solder joints. IEEE Trans. Compon. Packag. Technol. 2005, 28, 534–541. [Google Scholar] [CrossRef]
- Bastecki, B.C. A Benchmark Process for the Lead-Free Assembly of Mixed Technology PCB’s. 1999. Available online: http//www.alphametals.com/products/lead_free/PDF/leadfree.pdf (accessed on 25 August 2020).
- Bílek, J.; Atkinson, J.K.; Wakeham, W.A. Thermal conductivity of molten lead-free solders. Int. J. Thermophys. 2006, 27, 92–102. [Google Scholar] [CrossRef]
- Yamamoto, V.T.; Tsubone, V.K. Assembly technology using lead-free solder. Fujitsu Sci. Tech. J. 2007, 43, 50–58. [Google Scholar]
- Chuang, T.H.; Wu, M.W.; Chang, S.Y.; Ping, S.F.; Tsao, L.C. Strengthening mechanism of nano-Al2O3 particles reinforced Sn3.5Ag0.5Cu lead-free solder. J. Mater. Sci. Mater. Electron. 2010, 22, 1021–1027. [Google Scholar] [CrossRef]
- Lee, N.-C. Future lead-free solder alloys and fluxes—Meeting challenges of miniaturization. In Proceedings of the International Microsystems, Packaging, Assembly and Circuits Technology (IMPACT) Conference, Taipei, Taiwan, 1–3 October 2007. [Google Scholar]
- Muzyczko, T.M. A Review of electrochemical corrosion fundamentals. Ind. Eng. Chem. Prod. Res. Dev. 1978, 17, 169–176. [Google Scholar] [CrossRef]
- Cedex, P. Mechanisms of uniform corrosion under corrosion deposits. J. Mater. Sci. 1993, 28, 2589–2606. [Google Scholar]
- Pryo, M.J.; Keir, D.S. Galvanic corrosion. J. Electrochem. Soc. 1958, 105, 629. [Google Scholar] [CrossRef]
- Akpanyung, K.; Loto, R. Pitting corrosion evaluation: A review. J. Phys. Conf. Ser. 2019, 1378, 022088. [Google Scholar] [CrossRef]
- Abdulsalam, M.I. Behaviour of crevice corrosion in iron. Corros. Sci. 2005, 47, 1336–1351. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, X.; Fan, Y. Study on electrochemical corrosion mechanism of steel foot of insulators for HVDC lines study on electrochemical corrosion mechanism of steel foot of insulators for HVDC lines. IOP Conf. Ser. Mater. Sci. Eng. 2017, 242, 012074. [Google Scholar] [CrossRef]
- Leping, Z.; Shanshan, H.; Baoshuai, W.; Neng, M.; Ruoqian, L.; Xia, X. Electrochemical corrosion in electric energy meters. In Proceedings of the 2019 14th IEEE International Conference on Electronic Measurement & Instruments (ICEMI), Changsha, China, 1–3 November 2019. [Google Scholar]
- Patterson, S.; Daffner, R.H.; Gallo, R.A. Electrochemical corrosion of metal implants. Am. J. Roentgenol. 2005, 184, 1219–1222. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, A.V.R.; Singh, N.; Adya, N.; Saluja, B. Electrochemical corrosion behavior of dental/implant alloys in artificial saliva. J. Mater. Eng. Perform. 2008, 17, 695–701. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, S.; Zeng, G.; Li, L.; Ye, H. Interface reaction between SnAgCu / SnAgCuCe solders and Cu substrate subjected to thermal cycling and isothermal aging. J. Alloys Compd. 2012, 510, 38–45. [Google Scholar] [CrossRef]
- Anderson, I.E.; Foley, J.; Cook, B.A.; Harringa, J.; Terpstra, R.L.; Unal, O. Alloying effects in near-eutectic Sn-Ag-Cu solder alloys for improved microstructural stability. J. Electron. Mater. 2001, 30, 1050–1059. [Google Scholar] [CrossRef]
- Duan, L.; Yu, D.; Han, S.; Ma, H.; Wang, L. Microstructural evolution of Sn–9Zn–3Bi solder/Cu joint during long-term aging at 170°C. J. Alloys Compd. 2004, 381, 202–207. [Google Scholar] [CrossRef]
- Law, C.M.T.; Wu, C.-M.L.; Yu, D.Q.; Wang, L.; Lai, J.K.L. Microstructure, solderability, and growth of intermetallic compounds of Sn-Ag-Cu-RE lead-free solder alloys. J. Electron. Mater. 2006, 35, 89–93. [Google Scholar] [CrossRef]
- Chuang, C.; Tsao, L.C.; Lin, H.; Feng, L. Effects of small amount of active Ti element additions on microstructure and property of Sn3.5Ag0.5Cu solder. Mater. Sci. Eng. A 2012, 558, 478–484. [Google Scholar] [CrossRef]
- Kotadia, H.; Mokhtari, O.; Clode, M.; Green, M.; Mannan, S. Intermetallic compound growth suppression at high temperature in SAC solders with Zn addition on Cu and Ni–P substrates. J. Alloys Compd. 2012, 511, 176–188. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Mongkolwongrojn, M.; Ariga, T. Influence of indium addition on characteristics of Sn–0.3Ag–0.7Cu solder alloy. J. Alloys Compd. 2009, 485, 225–230. [Google Scholar] [CrossRef]
- Lin, L.-W.; Song, J.-M.; Lai, Y.-S.; Chiu, Y.-T.; Lee, N.-C.; Uan, J.-Y. Alloying modification of Sn–Ag–Cu solders by manganese and titanium. Microelectron. Reliab. 2009, 49, 235–241. [Google Scholar] [CrossRef]
- Tsao, L.; Chang, S.; Lee, C.; Sun, W.; Huang, C. Effects of nano-Al2O3 additions on microstructure development and hardness of Sn3.5Ag0.5Cu solder. Mater. Des. 2010, 31, 4831–4835. [Google Scholar] [CrossRef]
- Haseeb, A.; Arafat, M.M.; Johan, M. Stability of molybdenum nanoparticles in Sn–3.8Ag–0.7Cu solder during multiple reflow and their influence on interfacial intermetallic compounds. Mater. Charact. 2012, 64, 27–35. [Google Scholar] [CrossRef]
- Fouzder, T.; Shafiq, I.; Chan, Y.; Sharif, A.; Yung, W.K.C. Influence of SrTiO3 nano-particles on the microstructure and shear strength of Sn–Ag–Cu solder on Au/Ni metallized Cu pads. J. Alloys Compd. 2011, 509, 1885–1892. [Google Scholar] [CrossRef]
- Chang, S.; Jain, C.; Chuang, T.; Feng, L.; Tsao, L. Effect of addition of TiO2 nanoparticles on the microstructure, microhardness and interfacial reactions of Sn3.5AgXCu solder. Mater. Des. 2011, 32, 4720–4727. [Google Scholar] [CrossRef]
- Gain, A.; Chan, Y.; Yung, W.K.C. Effect of additions of ZrO2 nano-particles on the microstructure and shear strength of Sn–Ag–Cu solder on Au/Ni metallized Cu pads. Microelectron. Reliab. 2011, 51, 2306–2313. [Google Scholar] [CrossRef]
- Medgyes, B.; Illés, B.; Harsanyi, G. Electrochemical migration of micro-alloyed low Ag solders in NaCl solution. Period. Polytech. Electr. Eng. 2013, 57, 49. [Google Scholar] [CrossRef]
- Medgyes, B.; Tamasi, P.; Hajdú, F.; Murányi, R.; Lakatos-Varsányi, M.; Gal, L.; Harsanyi, G. Corrosion investigations on lead-free micro-alloyed solder alloys used in electronics. In Proceedings of the 2015 38th International Spring Seminar on Electronics Technology (ISSE), Eger, Hungary, 6–10 May 2015. [Google Scholar]
- Fukuda, Y.; Ganesan, S. Lead-free alloys: Overview. Lead-Free Electron. 2006, 81–100. [Google Scholar] [CrossRef]
- Nazeri, M.F.M.; Yahaya, M.Z.; Gursel, A.; Ani, F.C.; Masri, M.N.; Mohamad, A.A. Corrosion characterization of Sn-Zn solder: A review. Solder. Surf. Mt. Technol. 2019, 31, 52–67. [Google Scholar] [CrossRef]
- Fazal, M.A.; Liyana, N.; Rubaie, S.; Ahmed, A. A critical review on performance, microstructure and corrosion resistance of Pb-free solders. Measurement 2019, 134, 897–907. [Google Scholar] [CrossRef]
- Liu, S.; Xue, S. bai Reliability study of lead-free solders under specific conditions. J. Mater. Sci. Mater. Electron. 2015, 26, 9424–9442. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Liu, Z.; Jiu, Y.; Zhang, S.; Geng, J.; Chen, X.; Wu, S.; He, P.; Long, W. Corrosion behavior of Sn-based lead-free solder alloys: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 9076–9090. [Google Scholar] [CrossRef]
- Li, D.; Conway, P.P.; Liu, C. Corrosion characterization of tin–lead and lead free solders in 3.5 wt.% NaCl solution. Corros. Sci. 2008, 50, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Miernik, A.W.-; Guspiel, J.; Zabdyr, L. Corrosion behavior of lead-free SAC-type solder alloys in liquid media. Arch. Civ. Mech. Eng. 2015, 15, 206–213. [Google Scholar] [CrossRef]
- Rosalbino, F.; Zanicchi, G.; Carlinid, R.; Angelini, E.; Marazza, R. Electrochemical corrosion behaviour of Sn-Ag-Cu (SAC) eutectic alloy in a chloride containing environment. Mater. Corros. 2011, 63, 492–496. [Google Scholar] [CrossRef]
- Zaini, N.B.M.; Nazeri, M.F.B.M. Potentiodynamic polarization effect on phase and microstructure of SAC305 solder in hydrochloric acid solution. In Proceedings of the AIP Conference Proceedings, Penang, Malaysia, 28 May 2016. [Google Scholar]
- Jumali, N.; Mohamad, A.A.; Nazeri, M.F.M. Corrosion properties of Sn-9Zn solder in acidic solution. Mater. Sci. Forum 2017, 888, 365–372. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Huang, H. Effects of Sulfur on the properties of Sn-9Zn as lead-free solder. J. Electron. Mater. 2016, 46, 1067–1071. [Google Scholar] [CrossRef]
- Ahmido, A.; Hajjaji, S.; Ouaki, B.E.; Sebbahi, S. Corrosion behavior of Sn-9Zn-xBi lead-free solder alloys in NaCl 3% solution. Mater. Sci. 2015, 13, 69–76. [Google Scholar]
- Huang, C.; Rao, L.; Ling, H.; Hu, A.; Li, M. Research on the corrosion resistance of SAC305 solder added with Ag3Sn and Cu3Sn nanoparticles. In Proceedings of the 18th International Conference on Electronic Packaging Technology (ICEPT), Harbin, China, 16–19 August 2019. [Google Scholar]
- Wang, M.; Wang, J.; Feng, H.; Ke, W. Effects of microstructure and temperature on corrosion behavior of Sn–3.0Ag–0.5Cu lead-free solder. J. Mater. Sci. Mater. Electron. 2011, 23, 148–155. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Lin, K.-L. Corrosion behavior of Pb-Free Sn-1Ag-0.5Cu-XNi solder alloys in 3.5% NaCl solution. J. Electron. Mater. 2013, 42, 628–638. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Lin, K.-L. Electrochemical corrosion study of Sn–XAg–0.5Cu alloys in 3.5% NaCl solution. J. Mater. Res. 2007, 22, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Chan, Y.; Alam, M.; Jillek, W. Electrochemical corrosion study of Pb-free solders. J. Mater. Res. 2006, 21, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Fayeka, M.; Haseeb, A.S.M.A.; Fazal, M.A. Electrochemical corrosion behaviour of Pb-free SAC 105 and SAC 305 solder alloys: A comparative study. Sains Malays. 2017, 46, 295–302. [Google Scholar] [CrossRef]
- Fayeka, M.; Fazal, M.A.; Haseeb, A.S.M.A. Effect of aluminum addition on the electrochemical corrosion behavior of Sn–3Ag–0.5Cu solder alloy in 3.5 wt% NaCl solution. J. Mater. Sci. Mater. Electron. 2016, 27, 12193–12200. [Google Scholar] [CrossRef]
- Liyana, N.K.; Fazal, M.A.; Haseeb, A.S.M.A.; Rubaie, S. Effect of Zn incorporation on the electrochemical corrosion properties of SAC105 solder alloys. J. Mater. Sci. Mater. Electron. 2019, 30, 7415–7422. [Google Scholar] [CrossRef]
- Han, Y.D.; Chen, L.; Jing, H.Y.; Nai, S.; Wei, J.; Xu, L. Effect of Ni-coated carbon nanotubes on the corrosion behavior of Sn-Ag-Cu solder. J. Electron. Mater. 2013, 42, 3559–3566. [Google Scholar] [CrossRef]
- Nordin, N.I.M.; Ramli, R.; Weide-Zaage, K.; Sabri, M.F.M.; Ibrahim, N.N.S.; Mainal, A.; Datta, R.S.; Said, S.M.; Mamat, A. Impact of aluminium addition on the corrosion behaviour of Sn–1.0Ag–0.5Cu lead-free solder. RSC Adv. 2015, 5, 99058–99064. [Google Scholar] [CrossRef]
- Othman, N.K.; Omar, F.R.; Ani, F.C. Electrochemical migration and corrosion behaviours of SAC305 reinforced by NiO, Fe2O3, TiO2 nanoparticles in NaCl solution. IOP Conf. Series Mater. Sci. Eng. 2019, 701, 012044. [Google Scholar] [CrossRef]
- Subri, N.W.B.; Sarraf, M.; Nasiri-Tabrizi, B.; Ali, B.; Sabri, M.F.M.; Basirun, W.J.; Sukiman, N.L. Corrosion insight of iron and bismuth added Sn–1Ag–0.5Cu lead-free solder alloy. Corros. Eng. Sci. Technol. 2019, 55, 35–47. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; Zanicchi, G.; Carlinid, R.; Marazza, R. Electrochemical corrosion study of Sn–3Ag–3Cu solder alloy in NaCl solution. Electrochim. Acta 2009, 54, 7231–7235. [Google Scholar] [CrossRef]
- Hua, L.; Hou, H. Electrochemical corrosion and electrochemical migration of 64Sn-35Bi-1Ag solder doping with xGe on printed circuit boards. Microelectron. Reliab. 2017, 75, 27–36. [Google Scholar] [CrossRef]
- Bui, Q.V.; Nam, N.D.; Noh, B.-I.; Kar, A.; Kim, J.-G.; Jung, S.-B. Effect of Ag addition on the corrosion properties of Sn-based solder alloys. Mater. Corros. 2009, 61, 30–33. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.-P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Cruz, R.; Nishikata, A.; Tsuru, T. AC impedance monitoring of pitting corrosion of stainless steel under a wet-dry cyclic condition in chloride-containing environment. Corros. Sci. 1996, 38, 1397–1406. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Zohdy, K.M.; Ragab, M. Corrosion and electrochemical behavior of Sn-2Ag-0.5Cu lead-free solders solidified with magnet stirring. J. Mater. Eng. Perform. 2019, 28, 4680–4692. [Google Scholar] [CrossRef]
- Jie, W.; Xue, S.; Jingwen, W.; Jianxin, W.; Deng, Y. Enhancement on the high-temperature joint reliability and corrosion resistance of Sn–0.3Ag–0.7Cu low-Ag solder contributed by Al2O3 Nanoparticles (0.12 wt%). J. Mater. Sci. Mater. Electron. 2018, 29, 19663–19677. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Fekry, A.; Ghoneim, A. Corrosion characterization of new tin–silver binary alloys in nitric acid solutions. Corros. Sci. 2008, 50, 1618–1626. [Google Scholar] [CrossRef]
- Liu, J.-C.; Park, S.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.-S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl− anions in pitting corrosion of Sn–Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Farina, S.; Morando, C. Comparative corrosion behaviour of different Sn-based solder alloys. J. Mater. Sci. Mater. Electron. 2014, 26, 464–471. [Google Scholar] [CrossRef]
- Liao, B.; Cen, H.; Chen, Z.; Guo, X. Corrosion behavior of Sn-3.0Ag-0.5Cu alloy under chlorine-containing thin electrolyte layers. Corros. Sci. 2018, 143, 347–361. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 73th ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Špoták, M.; Drienovsky, M.; Rízeková Trnková, L.; Palcut, M. Corrosion of candidate lead-free solder alloys in saline solution. In Proceedings of the METAL 2015—24th International Conference on Metallurgy and Materials, Brno, Czech Republic, 3–5 June 2015. [Google Scholar]
- Liew, M.C.; Ahmad, I.; Lee, L.M.; Nazeri, M.F.M.; Haliman, H.; Mohamad, A.A. Corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder in potassium hydroxide electrolyte. Met. Mater. Trans. A 2012, 43, 3742–3747. [Google Scholar] [CrossRef]
- Song, F.; Lee, S.W.R. Corrosion of Sn-Ag-Cu Lead-free solders and the corresponding effects on board level solder joint reliability. In Proceedings of the 56th Electronic Components and Technology Conference 2006, San Diego, CA, USA, 30 May–2 June 2006. [Google Scholar]
- Harsanyi, G. Irregular effect of chloride impurities on migration failure reliability: Contradictions or understandable? Microelectron. Reliab. 1999, 39, 1407–1411. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; Zanicchi, G.; Marazza, R. Corrosion behaviour assessment of lead-free Sn–Ag–M (M=In, Bi, Cu) solder alloys. Mater. Chem. Phys. 2008, 109, 386–391. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Ke, W. Corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder joints. Microelectron. Reliab. 2017, 73, 69–75. [Google Scholar] [CrossRef]
- Kaushik, R.K.; Batra, U.; Sharma, J.; Batraa, U. Aging induced structural and electrochemical corrosion behaviour of Sn-1.0Ag-0.5Cu and Sn-3.8Ag-0.7Cu solder alloys. J. Alloys Compd. 2018, 745, 446–454. [Google Scholar] [CrossRef]
- Nurwahida, M.; Mukridz, M.; Ahmad, A.; Muhammad, F. Corrosion properties of SAC305 solder in different solution of HCl and NaCl corrosion properties of SAC305 solder in different solution of HCl and NaCl. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012004. [Google Scholar] [CrossRef]
- Hua, L.; Yang, G.K.; Zhang, H.Q. Effects of Ge doping on electrochemical migration, corrosion behavior and oxidation characteristics of lead-free Sn-3.0Ag-0.5Cu solder for electronic packaging. Adv. Mater. Res. 2010, 146, 953–961. [Google Scholar] [CrossRef]
- Hua, L.; Hou, H.N.; Zhang, H.; Wu, T.; Deng, Y.H. Effects of Zn, Ge doping on electrochemical migration, oxidation characteristics and corrosion behavior of lead-free Sn-3.0Ag-0.5Cu solder for electronic packaging. In Proceedings of the 2010 11th International Conference on Electronic Packaging Technology & High Density Packaging, Xi’an, China, 16–19 August 2010. [Google Scholar]
- Nordin, N.I.M.; Said, S.; Ramli, R.; Sabri, M.F.M.; Sharif, N.; Arifin, N.; Ibrahim, N. Microstructure of Sn–1Ag–0.5Cu solder alloy bearing Fe under salt spray test. Microelectron. Reliab. 2014, 54, 2044–2047. [Google Scholar] [CrossRef]
- Hua, L.; Yang, C. Corrosion behavior, whisker growth, and electrochemical migration of Sn–3.0Ag–0.5Cu solder doping with In and Zn in NaCl solution. Microelectron. Reliab. 2011, 51, 2274–2283. [Google Scholar] [CrossRef]
- Montesperelli, G.; Rapone, M.; Nanni, F.; Travaglia, P.; Riani, P.; Marazza, R.; Gusmano, G. Electrochemical and mechanical behaviour of Sn-2.5Ag-0.5Cu and Sn-48Bi-2Zn solders. Mater. Corros. 2008, 59, 662–669. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Lin, K.-L. Electrochemical corrosion behaviour of Pb-free Sn–8.5Zn–0.05Al–XGa and Sn–3Ag–0.5Cu alloys in chloride containing aqueous solution. Corros. Sci. 2008, 50, 2437–2443. [Google Scholar] [CrossRef]
- Tamási, P.; Kósa, G.; Szabó, B.; Berényi, R.; Medgyes, B. Effect of bismuth and silver on the corrosion behavior of lead-free solders in 3.5 wt% NaCl solution. Period. Polytech. Electr. Eng. Comput. Sci. 2016, 60, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Medgyes, B.; Tamasi, P.; Kosa, G.; Rigler, D.; Gal, L. Morphological and composition study on lead-free micro-alloyed solders after corrosion test. In Proceedings of the 39th International Spring Seminar on Electronics Technology (ISSE), Pilsen, Czech Republic, 18–22 May 2016. [Google Scholar]
- Medgyes, B.; Kósa, G.; Tamási, P.; Szabo, B.; Illés, B.; Lakatos-Varsanyi, M.; Rigler, D.; Gál, L.; Ruszinkó, M.; Harsanyi, G. Corrosion investigations on lead-free solder alloys in MgCl2 and NaCl solutions. In Proceedings of the 2017 IEEE 23rd International Symposium for Design and Technology in Electronic Packaging (SIITME), Constanta, Romania, 26–29 October 2017. [Google Scholar]
- Hu, J.; Luo, T.; Hu, A.; Li, M.; Mao, D. Electrochemical corrosion behaviors of Sn-9Zn-3Bi-xCr solder in 3.5% NaCl solution. J. Electron. Mater. 2011, 40, 1556–1562. [Google Scholar] [CrossRef]
- Tang, S.Y.; Jing, H.Y.; Xu, L.Y.; Han, Y.D.; Lu, G.Q. Effect of Ni-coated carbon nanotubes on the corrosion behavior of Sn-Ag-Cu solders. Adv. Mater. Res. 2011, 418, 1171–1174. [Google Scholar] [CrossRef]
- Han, Y.D.; Nai, S.; Jing, H.Y.; Xu, L.Y.; Tan, C.M.; Wei, J. Development of a Sn–Ag–Cu solder reinforced with Ni-coated carbon nanotubes. J. Mater. Sci. Mater. Electron. 2010, 22, 315–322. [Google Scholar] [CrossRef]
- Xu, L.Y.; Zhang, Z.K.; Jing, H.Y.; Wei, J.; Han, Y. Effect of graphene nanosheets on the corrosion behavior of Sn–Ag–Cu solders. J. Mater. Sci. Mater. Electron. 2015, 26, 5625–5634. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.; Yang, Y.; McLean, A. A review of permanent magnet stirring during metal solidification. Met. Mater. Trans. A 2017, 48, 3083–3100. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Conway, P.P.; Silberschmidt, V.V. Formation of Ag3Sn plates in SnAgCu solder bumps. Mater. Sci. Eng. A 2010, 527, 2588–2591. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, J.; Feng, H.; Ke, W. Effect of Ag3Sn intermetallic compounds on corrosion of Sn-3.0Ag-0.5Cu solder under high-temperature and high-humidity condition. Corros. Sci. 2012, 63, 20–28. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Ke, W. Effect of microstructure and Ag3Sn intermetallic compounds on corrosion behavior of Sn–3.0Ag–0.5Cu lead-free solder. J. Mater. Sci. Mater. Electron. 2014, 25, 5269–5276. [Google Scholar] [CrossRef]
- Zaini, N.M.; Mohtar, M.M.; Mohamad, A.A.; Nazeri, M.F.M. Microstructure and phase analyses on the corrosion of SAC305 solder in NaCl solution. Solid State Phenom. 2018, 273, 91–94. [Google Scholar] [CrossRef]
- Conseil, H.; Verdingovas, V.; Jellesen, M.S.; Ambat, R.; Conseil-Gudla, H. Decomposition of no-clean solder flux systems and their effects on the corrosion reliability of electronics. J. Mater. Sci. Mater. Electron. 2015, 27, 23–32. [Google Scholar] [CrossRef]
- Isaacs, P.; Munson, T. What makes no-clean flux residue benign? In Proceedings of the 2016 Pan Pacific Microelectronics Symposium (Pan Pacific), Big Island, HI, USA, 25–28 January 2016. [Google Scholar]
- Jellesen, M.S.; Minzari, D.; Rathinavelu, U.; Møller, P.; Ambat, R. Corrosion failure due to flux residues in an electronic add-on device. Eng. Fail. Anal. 2010, 17, 1263–1272. [Google Scholar] [CrossRef]
- Verdingovas, V.; Jellesen, M.S.; Ambat, R. Relative effect of solder flux chemistry on the humidity related failures in electronics. Solder. Surf. Mt. Technol. 2015, 27, 146–156. [Google Scholar] [CrossRef]
- Rendl, K.; Wirth, V.; Steiner, F. Impact of no-clean fluxes cleaning on PCB ionic contamination. In Proceedings of the 2015 38th International Spring Seminar on Electronics Technology (ISSE), Eger, Hungary, 6–10 May 2015. [Google Scholar]
- He, X.; Zhou, L.; Shen, J. A study for a typical leakage failure of PCBA with no-cleaning process. In Proceedings of the 2016 17th International Conference on Electronic Packaging Technology (ICEPT), Wuhan, China, 16–19 August 2016. [Google Scholar]
- Hansen, K.S.; Jellesen, M.S.; Moller, P.; Westermann, P.J.S.; Ambat, R. Effect of solder flux residues on corrosion of electronics. In Proceedings of the 2009 Annual Reliability and Maintainability Symposium, Fort Worth, TX, USA, 26–29 January 2009. [Google Scholar]
- Hunt, C.; Zou, L. The impact of temperature and humidity conditions on surface insulation resistance values for various fluxes. Solder. Surf. Mt. Technol. 1999, 11, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Verdingovas, V.; Jellesen, M.S.; Ambat, R. Solder flux residues and humidity-related failures in electronics: Relative effects of weak organic acids used in No-clean flux systems. J. Electron. Mater. 2015, 44, 1116–1127. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, L.; Medgyes, B.; Zhang, Z.; Gao, S.; Jakab, L. Electrochemical migration of Sn and Sn solder alloys: A review. RSC Adv. 2017, 7, 28186–28206. [Google Scholar] [CrossRef] [Green Version]
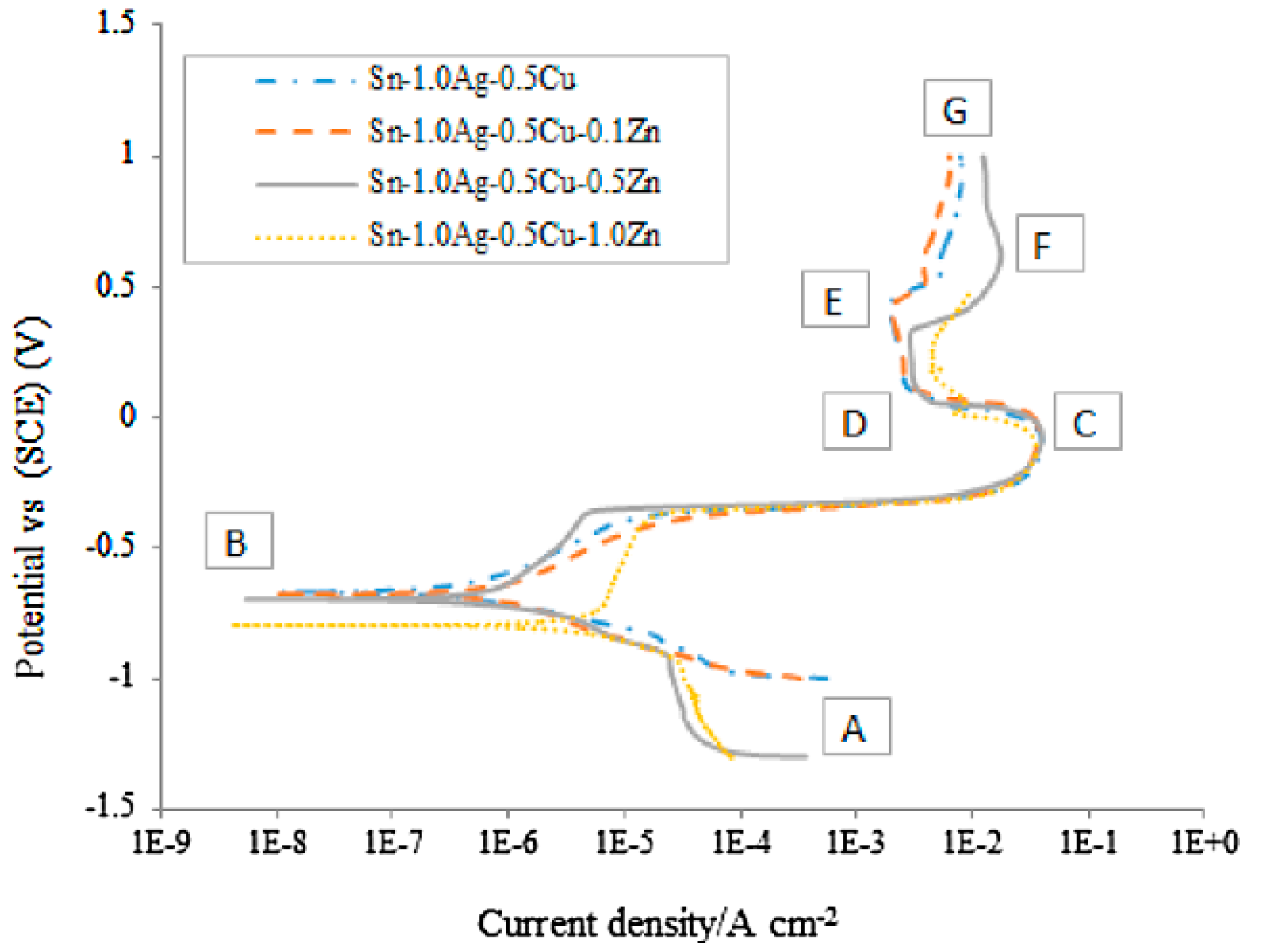
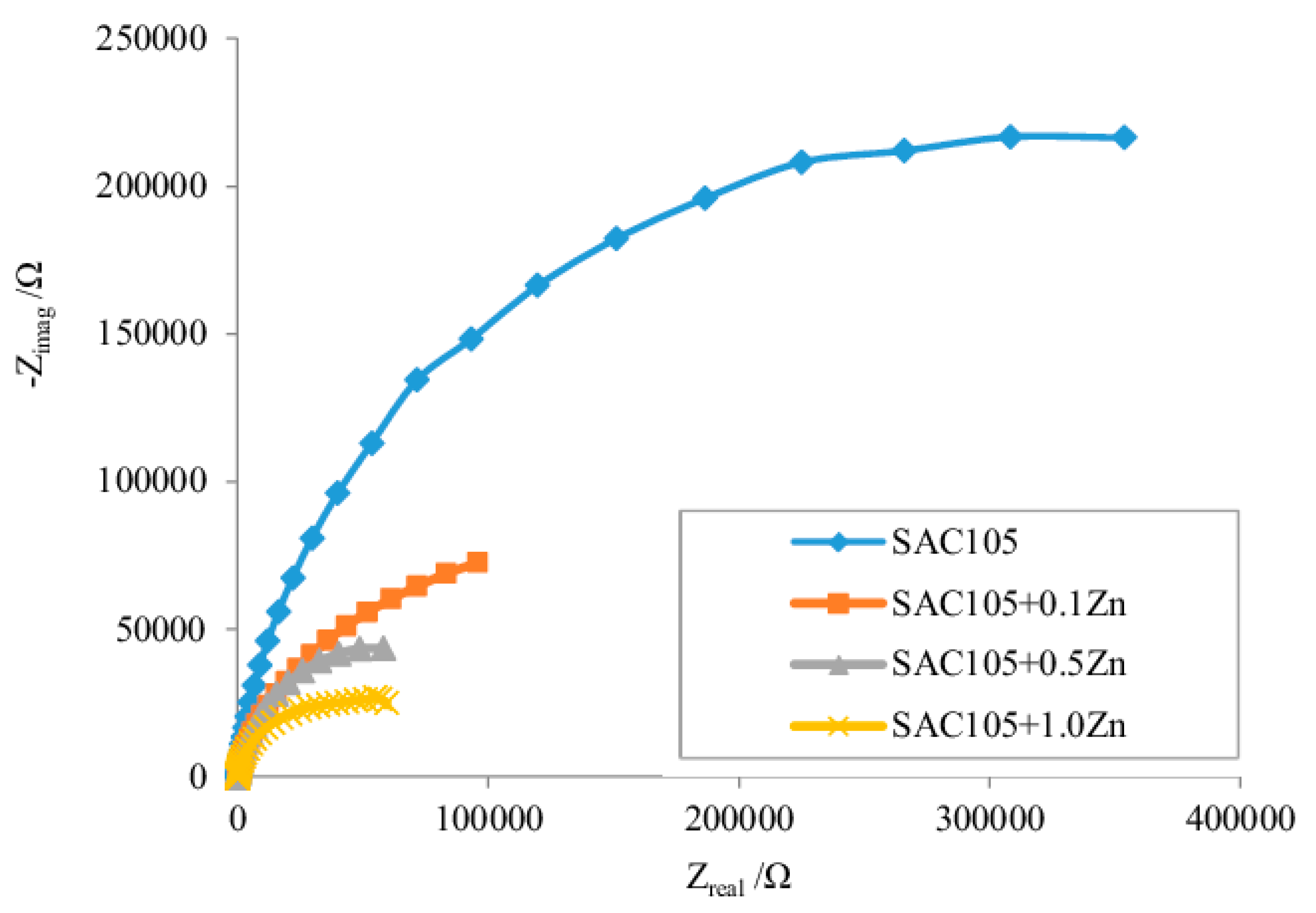
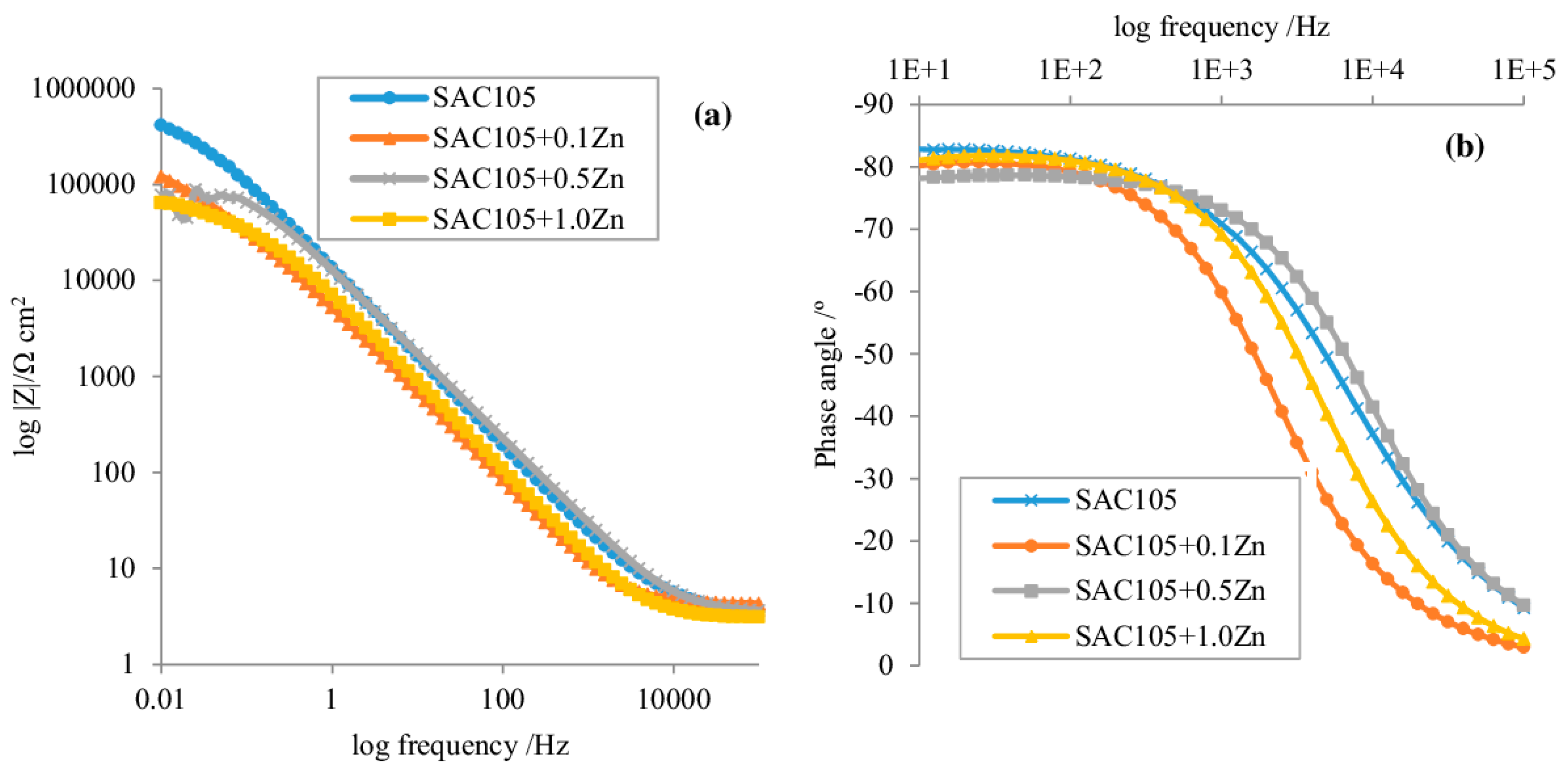

| Element | Half-Reaction | Standard Electrode Potential (V) |
|---|---|---|
| Tin | Sn2+ + 2e− = Sn | −0.1375 |
| Silver | Ag+ + e− = Ag | +0.7996 |
| Copper | Cu+ + e− = Cu | +0.5210 |
| - | Cu2+ + 2e− = Cu | +0.3419 |
| Lead | Pb2+ + 2e− = Pb | −0.1262 |
| Type of SAC | Ecorr (mV) | icorr (mA/cm2) | Reference |
|---|---|---|---|
| SAC305 | −415 (SCE) | 7.175 × 10−5 | [81] |
| SAC305-0.2Ge | −499 (SCE) | 2.213 × 10−4 | [81] |
| SAC305-0.5Ge | −593 (SCE) | 2.842 × 10−4 | [81] |
| SAC305-1Ge | −661 (SCE) | 1.181 × 10−3 | [81] |
| SAC305-2Ge | −598 (SCE) | 3.089 × 10−4 | [81] |
| SAC305-5Ge | −572 (SCE) | 3.859 × 10−4 | [81] |
| SAC305-8Ge | −545 (SCE) | 2.539 × 10−4 | [81] |
| SAC305 | −524 (KCl-Ag/AgCl) | 0.154 | [54] |
| SAC305-0.1Al | −649 (KCl-Ag/AgCl) | 0.54 × 10−3 | [54] |
| SAC305-0.5Al | −719 (KCl-Ag/AgCl) | 1.07 × 10−3 | [54] |
| SAC305 | −524 (Ag/AgCl saturated KCl) | 27.8 × 10−3 | [53] |
| SAC105 | −510 (Ag/AgCl saturated KCl) | 318 × 10−3 | [53] |
| SAC305 | −415 (SCE) | 7.175 × 10−5 | [83] |
| SAC0307 | −840 (saturated Ag/AgCl) | 0.83 × 10−3 | [72] |
| Sn-3.5Ag-0.7Cu | −837 (saturated Ag/AgCl) | 1.03 × 10−3 | [72] |
| Commerical SAC305 | −877 (SCE) | 9.4 × 10−3 | [49] |
| Air-cooled SAC305 | −867 (SCE) | 9.6 × 10−3 | [49] |
| Furnace-cooled SAC305 | −885 (SCE) | 9.5 × 10−3 | [49] |
| SAC305 | −605 (SCE) | 5.37 × 10−4 | [52] |
| Sn-3.5Ag-0.5Cu-9In | −578 (SCE) | 7.413 × 10−3 | [52] |
| SAC105 | −668 (SCE) | 0.451 × 10−3 | [55] |
| SAC105-0.1Zn | −676 (SCE) | 0.705 × 10−3 | [55] |
| SAC105-0.5Zn | −696 (SCE) | 0.891 × 10−3 | [55] |
| SAC105-1Zn | −795 (SCE) | 9.720 × 10−3 | [55] |
| SAC305 | −1052 (SCE) | 2.19 × 10−3 | [79] |
| SAC305 | −1078 (SCE) | 5.2 × 10−3 | [97] |
| SAC305 | −410 (SCE) | 1.469 × 10−2 | [48] |
| SAC105 | −810 (Ag/AgCl) | 4.6 | [51] |
| SAC205 | −814 (Ag/AgCl) | 6.06 | [51] |
| SAC305 | −804 (Ag/AgCl) | 3.14 | [51] |
| SAC405 | −636 (Ag/AgCl) | 2.25 | [51] |
| Sn-3.8Ag-0.7Cu | −727 (SCE) | 0.089 | [41] |
| SAC205 | −989 (Ag/AgCl) | 114 × 10−3 | [65] |
| SAC205 soldified with PMS | −652 (Ag/AgCl) | 31 × 10−3 | [65] |
| Sn-3.5Ag-0.7Cu | −1220 (SCE) | - | [56] |
| Sn-3.5Ag-0.7Cu/0.01 Ni-CNTS | −820 (SCE) | - | [56] |
| Sn-3.5Ag-0.7Cu/0.03 Ni-CNTS | −780 (SCE) | - | [56] |
| Sn-3.5Ag-0.7Cu/0.07 Ni-CNTS | −480 (SCE) | - | [56] |
| SAC305 | −590 (SCE) | 0.14 × 10−3 | [86] |
| SAC0307-0.05Ni | −569 (SCE) | 0.083 × 10−3 | [86] |
| SAC0807-0.1Bi | −597 (SCE) | 0.011 × 10−3 | [86] |
| SAC0807-0.05Ni | −568 (SCE) | 0.024 × 10−3 | [86] |
| SAC0807-3Bi-1.4Sb-0.15Ni | −570 (SCE) | 0.84 × 10−3 | [86] |
| Type of SAC | Type and Concentration of the Solution | Ecorr (mV) | icorr (mA/cm2) | Reference |
|---|---|---|---|---|
| SAC305 | 3% NaCl | −415 (SCE) | 7.175 × 10−5 | [80] |
| SAC105 | 5 wt % NaCl | −741.6 (SCE) | 70.8 × 10−3 | [57] |
| SAC305 | 0.1 M NaCl | −178 | 12.64 × 10−6 | [58] |
| SAC305-0.05Ni | 0.1 M NaCl | −535 | 1.604 × 10−6 | [58] |
| SAC305-0.05Fe2O3 | 0.1 M NaCl | −309 | 1.161 × 10−6 | [58] |
| SAC305-0.05TiO2 | 0.1 M NaCl | −278 | 1.113 × 10−6 | [58] |
| SAC305 | 0.1 M NaCl | −520 (SCE) | - | [60] |
| Sn-3.0Ag-3.0Cu | 0.1 M NaCl | −395 (SCE) | - | [60] |
| Sn-3.1Ag-0.8Cu | 0.1 M NaCl | −670 (SCE) | - | [76] |
| Sn-2.9Ag-6.7Cu | 0.1 M NaCl | −545 (SCE) | - | [76] |
| SAC205 | 3% NaCl | −610 (Ag/AgCl) | - | [84] |
| SAC305 | 6 M KOH | −1108 (Hg/HgO) | 0.1795 | [73] |
| SAC305 | 1 M HCl | −600 (SCE) | 0.01 | [44] |
| SAC305 | 1M HCl | −713.3 (SCE) | 39.83 × 10−3 | [79] |
| SAC105 | 0.1 M NaCl | −599 (SCE) | 0.145 × 10−3 | [59] |
| SAC105-Fe-1Bi | 0.1 M NaCl | −538 (SCE) | 0.128 × 10−3 | [59] |
| SAC105-Fe-2Bi | 0.1 M NaCl | −577 (SCE) | 0.178 × 10−3 | [59] |
| SAC0307 | 0.5 M NaCl | −750 (SCE) | 3.47 × 10−3 | [66] |
| SAC0307-0.12Al2O3 | 0.5 M NaCl | −680 (SCE) | 1.15 × 10−3 | [66] |
| Sn-3.5Ag-0.7Cu | 0.3% Na2SO4 | −685.92 (SCE) | 3.6252 × 10−4 | [90] |
| Sn-3.5Ag-0.7Cu/0.01 Ni-CNTs | 0.3% Na2SO4 | −662.08 (SCE) | 2.3297 × 10−4 | [90] |
| Sn-3.5Ag-0.7Cu/0.03 Ni-CNTs | 0.3% Na2SO4 | −661.59 (SCE) | 12.302 × 10−4 | [90] |
| Sn-3.5Ag-0.7Cu/0.05 Ni-CNTs | 0.3% Na2SO4 | −743.37 (SCE) | 10.462 × 10−4 | [90] |
| SAC105 aged for 4 h at 120 °C | 0.5 M NaCl | −497 (Ag/AgCl 1 M KCl) | 18.45 × 10−3 | [78] |
| SAC105 aged for 72 h at 120 °C | 0.5 M NaCl | −654 (Ag/AgCl 1 M KCl) | 1.99 × 10−3 | [78] |
| SAC387 aged for 4 h at 120 °C | 0.5 M NaCl | −475 (Ag/AgCl 1 M KCl) | 17.48 × 10−3 | [78] |
| SAC387 aged for 72 h at 120 °C | 0.5 M NaCl | −585 (Ag/AgCl 1 M KCl) | 0.37 × 10−3 | [78] |
| Sn-3.8Ag-1.5Cu | 0.1 M NaCl | −390 (SCE) | 7 | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharaibeh, A.; Felhősi, I.; Keresztes, Z.; Harsányi, G.; Illés, B.; Medgyes, B. Electrochemical Corrosion of SAC Alloys: A Review. Metals 2020, 10, 1276. https://doi.org/10.3390/met10101276
Gharaibeh A, Felhősi I, Keresztes Z, Harsányi G, Illés B, Medgyes B. Electrochemical Corrosion of SAC Alloys: A Review. Metals. 2020; 10(10):1276. https://doi.org/10.3390/met10101276
Chicago/Turabian StyleGharaibeh, Ali, Ilona Felhősi, Zsófia Keresztes, Gábor Harsányi, Balázs Illés, and Bálint Medgyes. 2020. "Electrochemical Corrosion of SAC Alloys: A Review" Metals 10, no. 10: 1276. https://doi.org/10.3390/met10101276
APA StyleGharaibeh, A., Felhősi, I., Keresztes, Z., Harsányi, G., Illés, B., & Medgyes, B. (2020). Electrochemical Corrosion of SAC Alloys: A Review. Metals, 10(10), 1276. https://doi.org/10.3390/met10101276






